Investigation of Synergistic Influence of Ultrasound and Co-Doping to Degrade Toluene from Polluted Air in Construction Sites—An Experimental Approach
Abstract
1. Introduction
- The setting up of a continuous air purification reactor suitable for the real conditions of working environments;
- Increasing the contact surface of the pollutant with the photocatalyst and the retention time by creating sloping surfaces;
- Synthesis of a photocatalytic with better properties using an ultrasonic bath technique;
- Achieving a high rate of toluene degradation under continuous conditions;
- Improvement of photocatalyst performance under visible light compared to ultraviolet light.
2. Materials and Methods
2.1. Materials
2.2. Nanophotocatalyst Preparation Procedure
2.3. Nanophotocatalysts Characterization Techniques
2.4. Experimental Setup for Photocatalytic Performance Test
3. Results and Discussions
3.1. Nanophotocatalysts Characterization
3.1.1. XRD Analysis
3.1.2. FESEM Analysis
3.1.3. EDX Analysis
3.1.4. BET–BJH Analysis
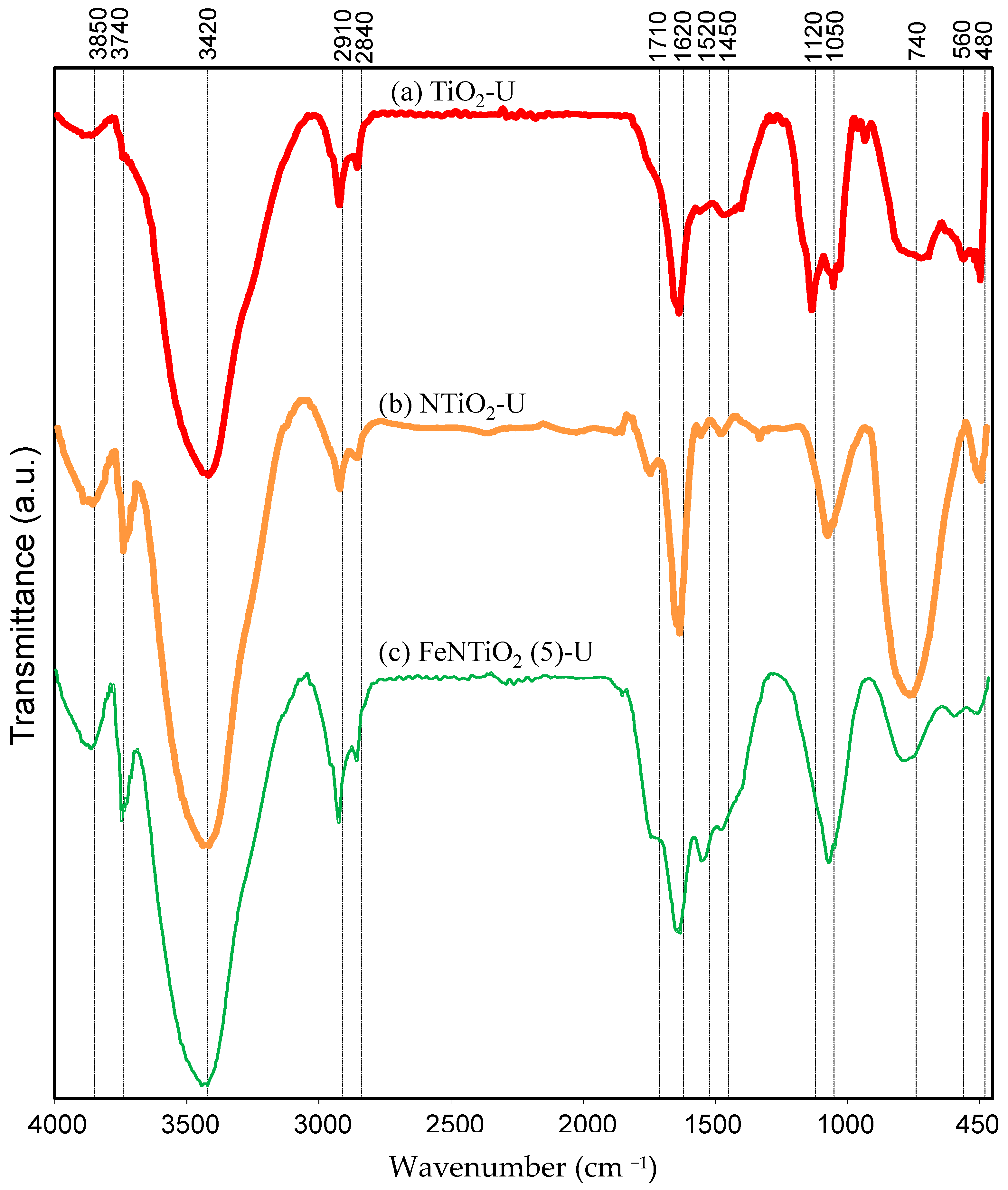
3.1.5. FTIR Analysis
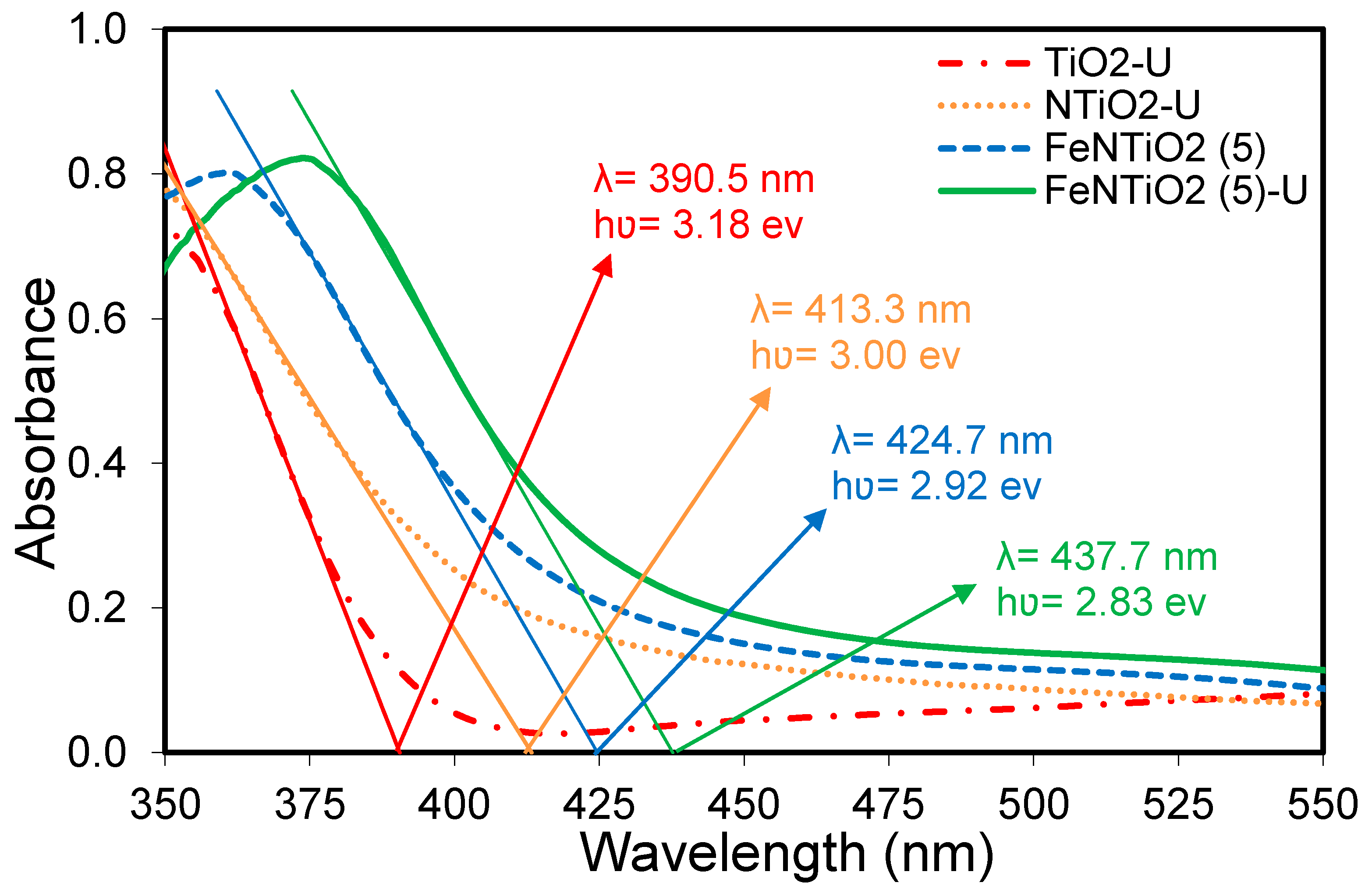
3.1.6. DRS Analysis
3.2. Photocatalytic Degradation of Toluene
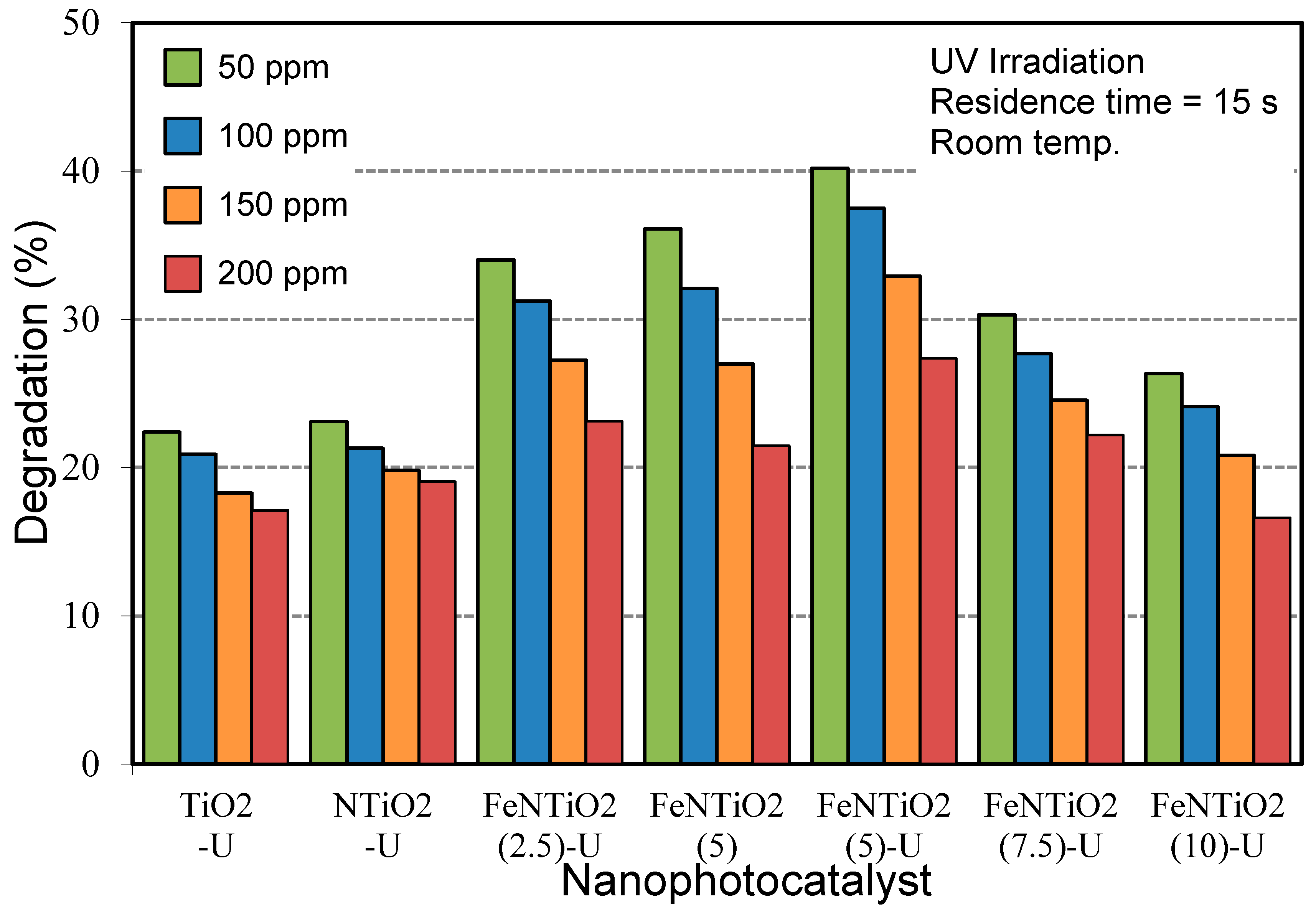
3.2.1. Influence of Doping Composition
3.2.2. Influence of Ultrasound Irradiation
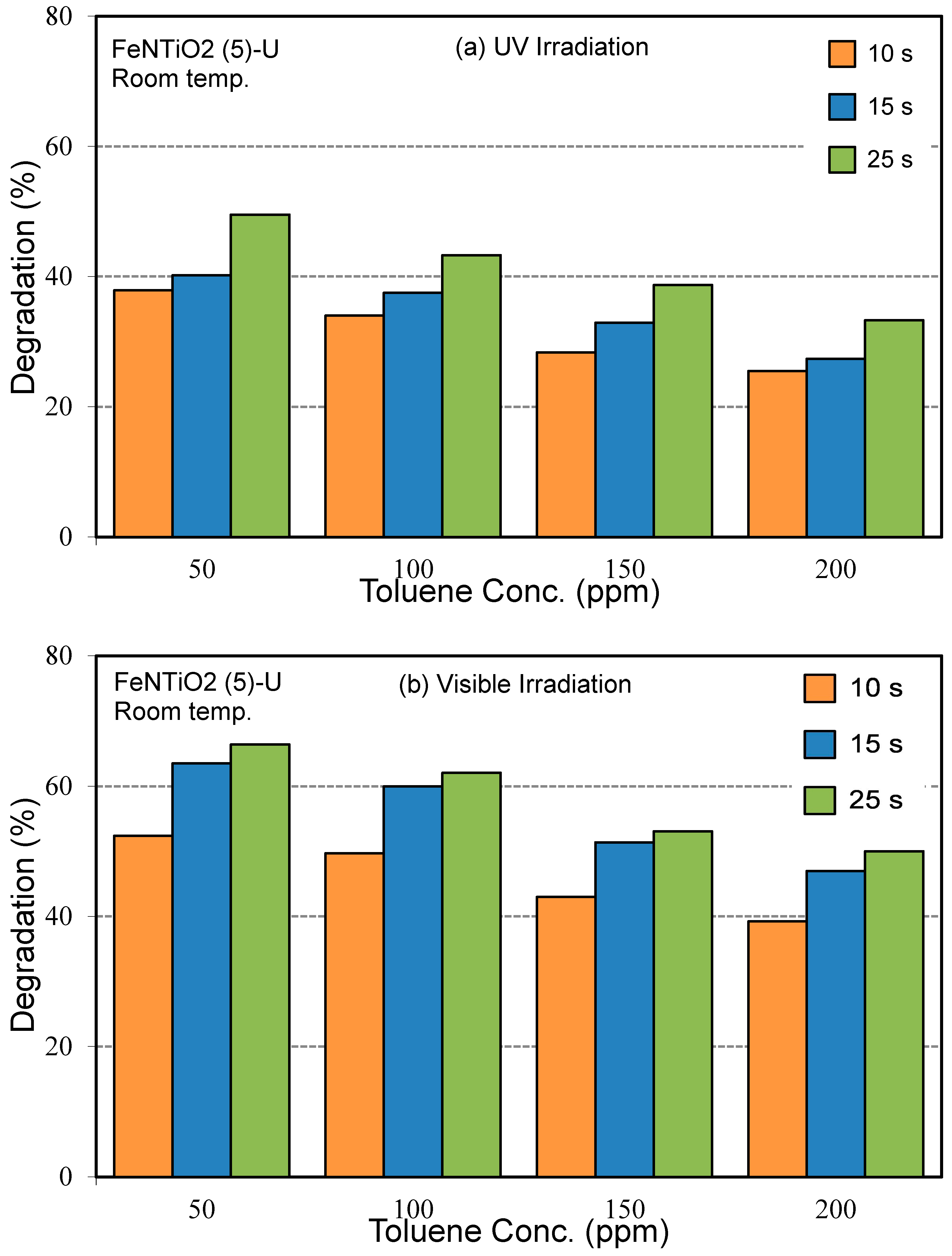
3.2.3. Influence of Light Source
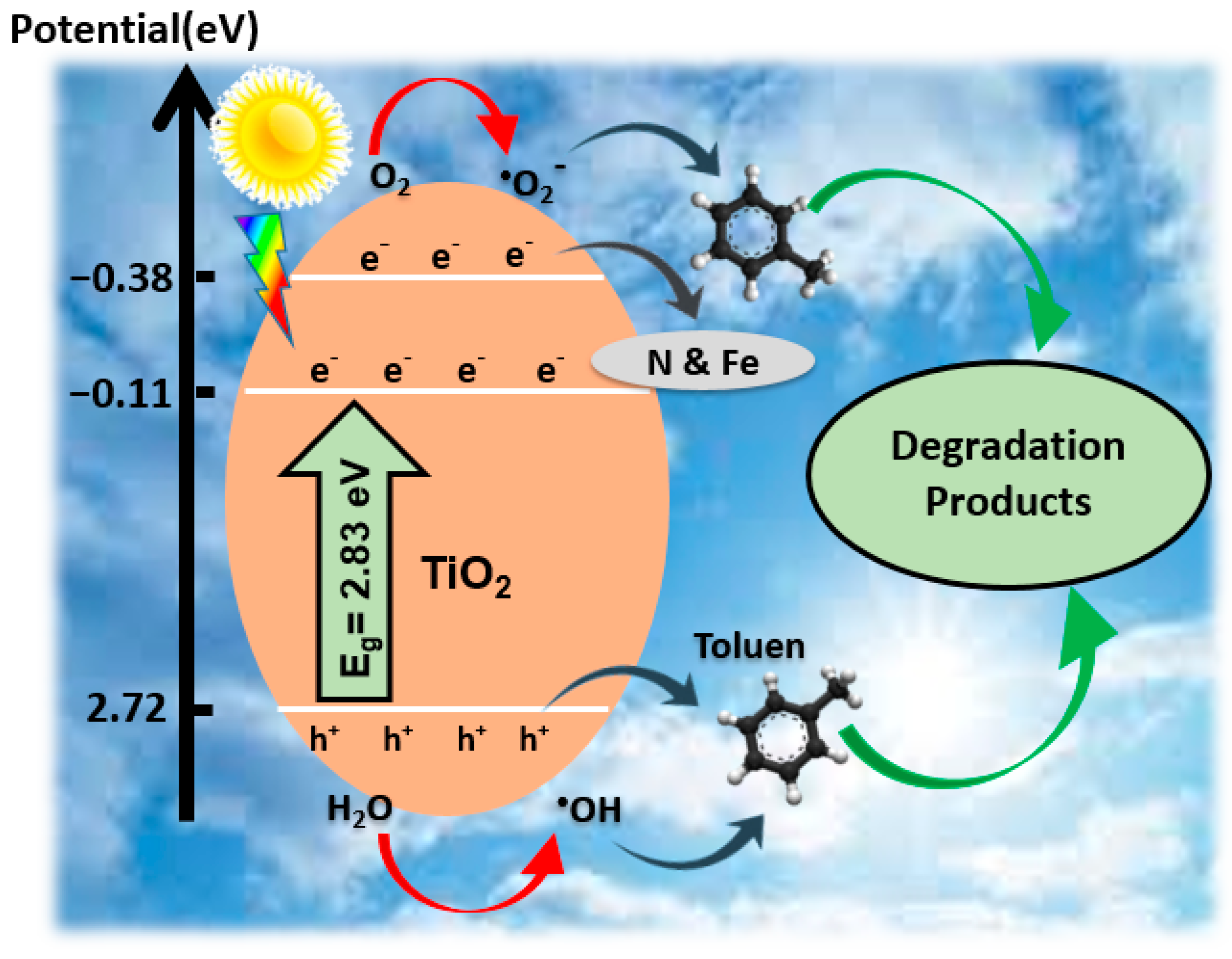
3.3. Reaction Pathway for Toluene Photocatalytic Degradation
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Complete Term |
| PTFE | Poly Tetra Fluoro Ethylene |
| XRD | X-ray Diffraction |
| BET | Brunauer–Emmett–Teller |
| DRS | Diffuse Reflection Spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| EDX | Energy Dispersive X-ray Spectroscopy |
| SEM | Scanning Electron Microscope |
| VOCs | Volatile Organic Compounds |
| IRAC | International Agency of Research and Cancer |
| EPA | Environmental Protection Agency |
| NIOSH | National Institute for Occupational Safety and Health |
| OSHA | Occupational Safety and Health Administration |
| PPE | Personal Protective Equipment |
| UV | Ultraviolet irradiation |
| PCO | Photocatalytic oxidation |
References
- Campos-Candel, A.; Llobat-Estellés, M.; Mauri-Aucejo, A. Comparative evaluation of liquid chromatography versus gas chromatography using a β-cyclodextrin stationary phase for the determination of BTEX in occupational environments. Talanta 2009, 78, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Hinwood, A.L.; Rodriguez, C.; Runnion, T.; Farrar, D.; Murray, F.; Horton, A.; Glass, D.; Sheppeard, V.; Edwards, J.W.; Denison, L.; et al. Risk factors for increased BTEX exposure in four Australian cities. Chemosphere 2007, 66, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Shakerkhatibi, M.; Benis, K.Z.; Asghari-Jafarabadi, M.; Sadeghi-Bazarghani, H.; Allahverdipour, H.; Oskouei, D.S.; Fatehifar, E.; Farajzadeh, M.; Yadeghari, A.; Ansarin, K.; et al. Air pollution-related asthma profiles among children/adolescents: A multi-group latent class analysis. Ecotoxicol. Environ. Saf. 2021, 219, 112344. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.P.; Hill, F.N.; Garst, R.H. Production of Ethylbenzene and Benzene from Toluene. U.S. Patent No. 3,557,235, 19 January 1971. [Google Scholar]
- Zhou, Z.-H.; Deng, Y.; Wu, K.-Y.; Tan, Q.-W.; Zhou, X.-L.; Song, D.-L.; Huang, F.-X.; Zeng, W.-H. Source Profiles of VOCs Associated with Typical Industrial Processes in Chengdu. Huan Jing Ke Xue = Huanjing Kexue 2019, 40, 3949–3961. [Google Scholar]
- Cho, M.-H.; Jung, S.-H.; Kim, J.-S. Pyrolysis of mixed plastic wastes for the recovery of benzene, toluene, and xylene (BTX) aromatics in a fluidized bed and chlorine removal by applying various additives. Energy Fuels 2009, 24, 1389–1395. [Google Scholar] [CrossRef]
- Souza, E.C.; Vessoni-Penna, T.C.; Al Arni, S.; Domínguez, J.M.; Converti, A.; Oliveira, R.P.d.S. Influence of toluene and salinity on biosurfactant production by Bacillus sp.: Scale up from flasks to a bench-scale bioreactor. Braz. J. Chem. Eng. 2017, 34, 395–405. [Google Scholar] [CrossRef]
- Cruz, S.L.; Rivera-García, M.T.; Woodward, J.J. Review of toluene action: Clinical evidence, animal studies and molecular targets. J. Drug Alcohol Res. 2014, 3, 235840. [Google Scholar] [CrossRef]
- Masekameni, M.D.; Moolla, R.; Gulumian, M.; Brouwer, D. Risk Assessment of Benzene, Toluene, Ethyl Benzene, and Xylene Concentrations from the Combustion of Coal in a Controlled Laboratory Environment. Int. J. Environ. Res. Public Health 2019, 16, 95. [Google Scholar] [CrossRef]
- Barros, N.; Carvalho, M.; Silva, C.; Fontes, T.; Prata, J.C.; Sousa, A.; Manso, M.C. Environmental and biological monitoring of benzene, toluene, ethylbenzene and xylene (BTEX) exposure in residents living near gas stations. J. Toxicol. Environ. Health Part A 2019, 82, 550–563. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Tong, D.; Wang, Q.; Wu, M.; Sun, B.; Su, G.; Tan, L. Environmental impact and health risk assessment of volatile organic compound emissions during different seasons in Beijing. J. Environ. Sci. 2019, 93, 1–12. [Google Scholar] [CrossRef]
- Filley, C.M.; Halliday, W.; Kleinschmidt-DeMasters, B. The effects of toluene on the central nervous system. J. Neuropathol. Exp. Neurol. 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.; Takagi, M.; Walterfang, M.; Lubman, D.I. Toluene misuse and long-term harms: A systematic review of the neuropsychological and neuroimaging literature. Neurosci. Biobehav. Rev. 2008, 32, 910–926. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Mitsui, T.; Matsumura, M. Photocatalytic activity of S-doped TiO2 photocatalyst under visible light. Chem. Lett. 2003, 32, 364–365. [Google Scholar] [CrossRef]
- Tang, H.; Berger, H.; Schmid, P.; Lévy, F.; Burri, G. Photoluminescence in TiO2 anatase single crystals. Solid State Commun. 1993, 87, 847–850. [Google Scholar] [CrossRef]
- Ikehata, K.; El-Din, M.G. Aqueous pesticide degradation by ozonation and ozone-based advanced oxidation processes: A review (Part II). Ozone Sci. Eng. 2005, 27, 173–202. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. B Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Neppolian, B.; Anpo, M. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2. Catal. Today 2003, 84, 191–196. [Google Scholar] [CrossRef]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Transition metal ion impregnated mesoporous TiO2 for photocatalytic degradation of organic contaminants in water. Ind. Eng. Chem. Res. 2006, 45, 5231–5238. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C 2009, 114, 783–792. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Yu, J.; Yip, H.; Wong, P.K.; Zhao, J. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 2005, 39, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Yu, J.C.; Lee, S. Low-temperature hydrothermal synthesis of S-doped TiO2 with visible light photocatalytic activity. J. Solid State Chem. 2006, 179, 1171–1176. [Google Scholar] [CrossRef]
- Litter, M.; Navio, J.A. Photocatalytic properties of iron-doped titania semiconductors. J. Photochem. Photobiol. A Chem. 1996, 98, 171–181. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Ranjit, K.; Viswanathan, B. Synthesis, characterization and photocatalytic properties of iron-doped TiO2 catalysts. J. Photochem. Photobiol. A Chem. 1997, 108, 79–84. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Bahnemann, D.W.; Dohrmann, J.K. A novel preparation of iron-doped TiO2 nanoparticles with enhanced photocatalytic activity. Chem. Commun. 2000, 16, 1539–1540. [Google Scholar] [CrossRef]
- Soria, J.; Conesa, J.C.; Augugliaro, V.; Palmisano, L.; Schiavello, M.; Sclafani, A. Dinitrogen photoreduction to ammonia over titanium dioxide powders doped with ferric ions. J. Phys. Chem. 1991, 95, 274–282. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Wang, C.; Li, S.; Lai, Y.; Chen, H.; Lin, C. Ultrasound aided photochemical synthesis of Ag loaded TiO2 nanotube arrays to enhance photocatalytic activity. J. Hazard. Mater. 2009, 171, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, S.; Tanaka, J.; Sato, S.; Ibusuki, T. Photocatalytic oxidation of dibenzothiophenes in acetonitrile using TiO2: Effect of hydrogen peroxide and ultrasound irradiation. J. Photochem. Photobiol. A Chem. 2002, 149, 183–189. [Google Scholar] [CrossRef]
- Su, W.; Chen, J.; Wu, L.; Wang, X.; Wang, X.; Fu, X. Visible light photocatalysis on praseodymium (III)-nitrate-modified TiO2 prepared by an ultrasound method. Appl. Catal. B Environ. 2008, 77, 264–271. [Google Scholar] [CrossRef]
- Wu, Q.; Ouyang, J.; Xie, K.; Sun, L.; Wang, M.; Lin, C. Ultrasound-assisted synthesis and visible-light-driven photocatalytic activity of Fe-incorporated TiO2 nanotube array photocatalysts. J. Hazard. Mater. 2012, 199, 410–417. [Google Scholar] [CrossRef]
- Niksefat, V.; Ghorbani, M. Mechanical and electrochemical properties of ultrasonic-assisted electroless deposition of Ni–B–TiO2 composite coatings. J. Alloys Compd. 2015, 633, 127–136. [Google Scholar] [CrossRef]
- Zhang, S. Synergistic effects of C–Cr codoping in TiO2 and enhanced sonocatalytic activity under ultrasonic irradiation. Ultrason. Sonochem. 2012, 19, 767–771. [Google Scholar] [CrossRef]
- Teh, C.Y.; Wu, T.Y.; Juan, J.C. Facile sonochemical synthesis of N, Cl-codoped TiO2: Synthesis effects, mechanism and photocatalytic performance. Catal. Today 2015, 256, 365–374. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and characterization of nitrogen-doped TiO2 nanophotocatalyst with high visible light activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Anpo, M.; Ichihashi, Y.; Takeuchi, M.; Yamashita, H. Design of unique titanium oxide photocatalysts by an advanced metal ion-implantation method and photocatalytic reactions under visible light irradiation. Res. Chem. Intermed. 1998, 24, 143–149. [Google Scholar] [CrossRef]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, H. Origin of low photocatalytic activity of rutile TiO2. Electron. Mater. Lett. 2009, 5, 73–76. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, S.; Sato, T. The influence of synthesis method on the properties of iron contained N doped TiO2 photocatalysts. Appl. Catal. B Environ. 2011, 103, 462–469. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Influence of synthesis method on leaching of the Cr-TiO2 catalyst for visible light liquid phase photocatalysis and their stability. Appl. Catal. B Environ. 2016, 180, 351–361. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Guo, X.; He, T.; Feng, Y. Preparation of highly visible light active Fe–N co-doped mesoporous TiO2 photocatalyst by fast sol–gel method. J. Nanoparticle Res. 2014, 16, 2246. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Rasheed, A.; Ahmad, M.; Ramzan, M.; Shakoor, A.; Elahi, A.; Abbas, S.M.; Hussain, R.; Niaz, N.A. Co-doping effect of carbon and yttrium on photocatalytic activity of TiO2 nanoparticles for methyl orange degradation. J. Ovonic Res. 2015, 11, 107–112. [Google Scholar]
- Lachom, V.; Poolcharuansin, P.; Laokul, P. Preparation, characterizations and photocatalytic activity of a ZnO/TiO2 nanocomposite. Mater. Res. Express 2017, 4, 035006. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Ziarani, G.M. Ultrasonic-assisted sol–gel synthesis of samarium, cerium co-doped TiO2 nanoparticles with enhanced sonocatalytic efficiency. Ultrason. Sonochem. 2015, 26, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Enhancing the photocatalytic oxidation of dibenzothiophene using visible light responsive Fe and N co-doped TiO2 nanoparticles. Ceram. Int. 2017, 43, 973–981. [Google Scholar] [CrossRef]
- Shirsath, S.; Pinjari, D.; Gogate, P.; Sonawane, S.; Pandit, A. Ultrasound assisted synthesis of doped TiO2 nano-particles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochem. 2013, 20, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Gogate, P.R. Ultrasound assisted synthesis of iron doped TiO2 catalyst. Ultrason. Sonochem. 2018, 40, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, I.; Kumar, P.; Gupta, A.; Sekhar, P.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Fe-doped TiO2 powders for solar light response and photocatalytic applications. Process. Appl. Ceram. 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Zarrabi, M.; Haghighi, M.; Alizadeh, R. Sonoprecipitation dispersion of ZnO nanoparticles over graphene oxide used in photocatalytic degradation of methylene blue in aqueous solution: Influence of irradiation time and power. Ultrason. Sonochem. 2018, 48, 370–382. [Google Scholar] [CrossRef]
- Nawawi, W.; Nawi, M. Electron scavenger of thin layer Carbon coated and Nitrogen doped P25 with enhanced photocatalytic activity under visible light fluorescent lamp. J. Mol. Catal. A Chem. 2013, 374, 39–45. [Google Scholar] [CrossRef]
- Asl, E.A.; Haghighi, M.; Talati, A. Sono-solvothermal fabrication of flowerlike Bi7O9I3-MgAl2O4 pn nano-heterostructure photocatalyst with enhanced solar-light-driven degradation of methylene blue. Sol. Energy 2019, 184, 426–439. [Google Scholar]
- Chen, X.; Kuo, D.-H.; Lu, D. N-doped mesoporous TiO2 nanoparticles synthesized by using biological renewable nanocrystalline cellulose as template for the degradation of pollutants under visible and sun light. Chem. Eng. J. 2016, 295, 192–200. [Google Scholar] [CrossRef]
- He, K.; Zhao, C.; Zhao, G.; Han, G. Effects of pore size on the photocatalytic activity of mesoporous TiO2 prepared by a sol–gel process. J. Sol-Gel Sci. Technol. 2015, 75, 557–563. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.B.; Ogale, S.B. N-doped TiO2 nanoparticle based visible light photocatalyst by modified peroxide sol−gel method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Ropero-Vega, J.L.; Aldana-Pérez, A.; Gómez, R.; Niño-Gómez, M.E. Sulfated titania [TiO2/SO42−]: A very active solid acid catalyst for the esterification of free fatty acids with ethanol. Appl. Catal. A: Gen. 2010, 379, 24–29. [Google Scholar] [CrossRef]
- Shouxin, L.; Xiaoyun, C.; Xi, C. Preparation of N-doped visible-light response nanosize TiO2 photocatalyst using the acid-catalyzed hydrolysis method. Chin. J. Catal. 2006, 27, 697–702. [Google Scholar]
- Cheng, X.; Yu, X.; Xing, Z. Enhanced photoelectric property and visible activity of nitrogen doped TiO2 synthesized from different nitrogen dopants. Appl. Surf. Sci. 2013, 268, 204–208. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Zhang, Y.; Liu, S. An efficient two-step technique for nitrogen-doped titanium dioxide synthesizing: Visible-light-induced photodecomposition of methylene blue. J. Phys. Chem. C 2007, 111, 1010–1014. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Rodríguez-González, V.; Gyawali, G.; Cho, S.-H.; Sekino, T.; Lee, S.-W. Synthesis of solar light responsive Fe, N co-doped TiO2 photocatalyst by sonochemical method. Catal. Today 2013, 212, 75–80. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Synthesis and characterization of N-doped TiO2 nanoparticles and their application in photocatalytic oxidation of dibenzothiophene under visible light. Ceram. Int. 2016, 42, 14834–14842. [Google Scholar] [CrossRef]
- Karvinen, S.; Hirva, P.; Pakkanen, T.A. Ab initio quantum chemical studies of cluster models for doped anatase and rutile TiO2. J. Mol. Struct. Theochem 2003, 626, 271–277. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hu, S.-H.; Fu, Y.-P. Effects of surface hydroxyl group density on the photocatalytic activity of Fe3+-doped TiO2. J. Alloys Compd. 2015, 632, 326–334. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, photocatalytic activity, and mechanism of nano-TiO2 co-doped with nitrogen and iron (III). J. Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Adán, C.; Bahamonde, A.; Oller, I.; Malato, S.; Martínez-Arias, A. Influence of iron leaching and oxidizing agent employed on solar photodegradation of phenol over nanostructured iron-doped titania catalysts. Appl. Catal. B Environ. 2014, 144, 269–276. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Shi, Y.; Liu, Y. Preparation of N, Fe co-doped TiO2 with visible light response. Powder Technol. 2011, 207, 165–169. [Google Scholar] [CrossRef]
- Neppolian, B.; Wang, Q.; Jung, H.; Choi, H. Ultrasonic-assisted sol-gel method of preparation of TiO2 nano-particles: Characterization, properties and 4-chlorophenol removal application. Ultrason. Sonochem. 2008, 15, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.V.; Saini, C.; Ambati, R.; Gogate, P.R. Synthesis of ultrasound assisted nanostuctured photocatalyst (NiO supported over CeO2) and its application for photocatalytic as well as sonocatalytic dye degradation. Catal. Today 2018, 300, 50–57. [Google Scholar] [CrossRef]
- Akly, C.; Chadik, P.A.; Mazyck, D.W. Photocatalysis of gas-phase toluene using silica–titania composites: Performance of a novel catalyst immobilization technique suitable for large-scale applications. Appl. Catal. B Environ. 2010, 99, 329–335. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Beeldens, A.; Van Langenhove, H. Heterogeneous photocatalytic removal of toluene from air on building materials enriched with TiO2. Build. Environ. 2008, 43, 406–414. [Google Scholar] [CrossRef]
- Korologos, C.A.; Philippopoulos, C.J.; Poulopoulos, S.G. The effect of water presence on the photocatalytic oxidation of benzene, toluene, ethylbenzene and m-xylene in the gas-phase. Atmos. Environ. 2011, 45, 7089–7095. [Google Scholar] [CrossRef]
- Dolat, D.; Mozia, S.; Ohtani, B.; Morawski, A.W. Nitrogen, iron-single modified (N-TiO2, Fe-TiO2) and co-modified (Fe, N-TiO2) rutile titanium dioxide as visible-light active photocatalysts. Chem. Eng. J. 2013, 225, 358–364. [Google Scholar] [CrossRef]
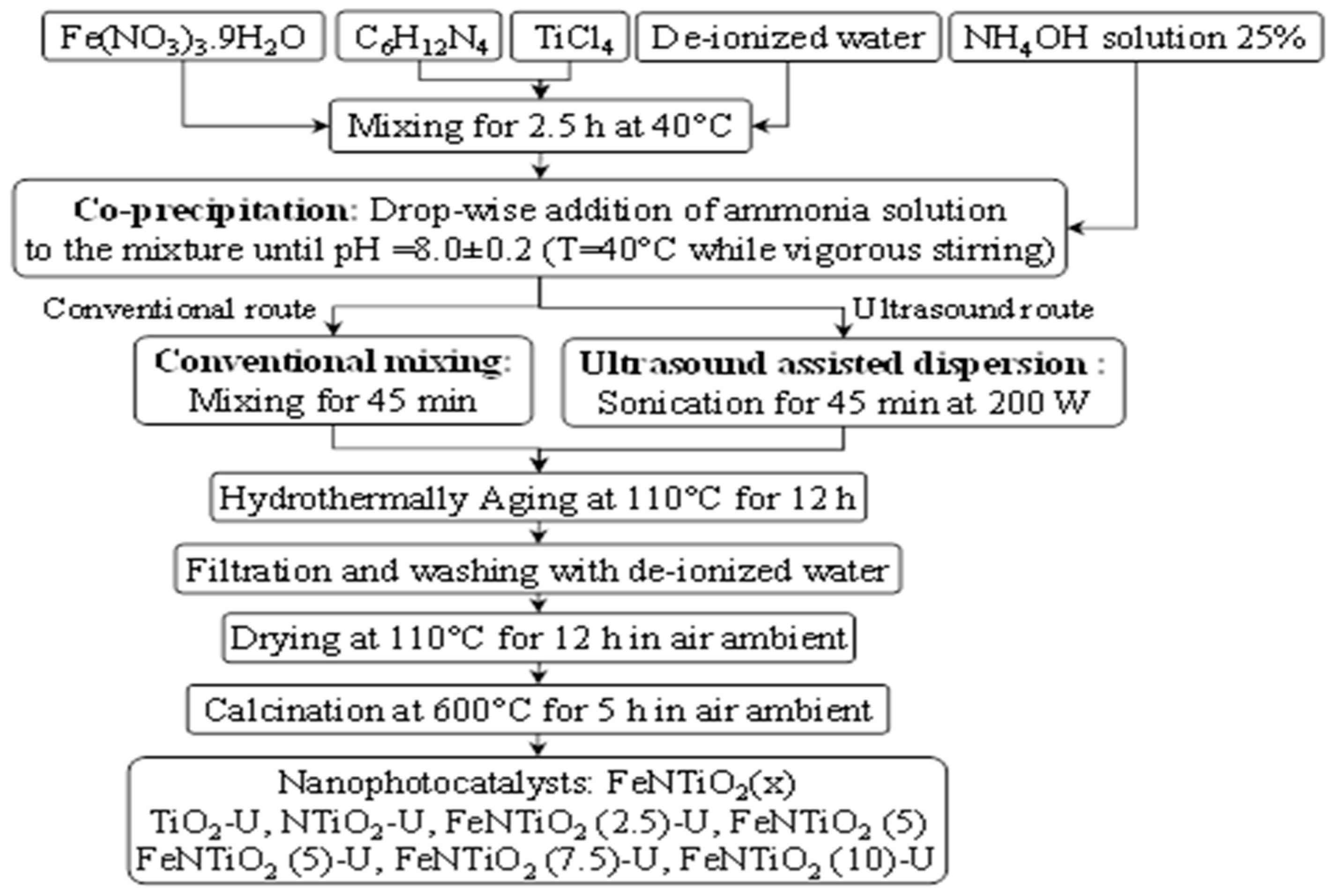
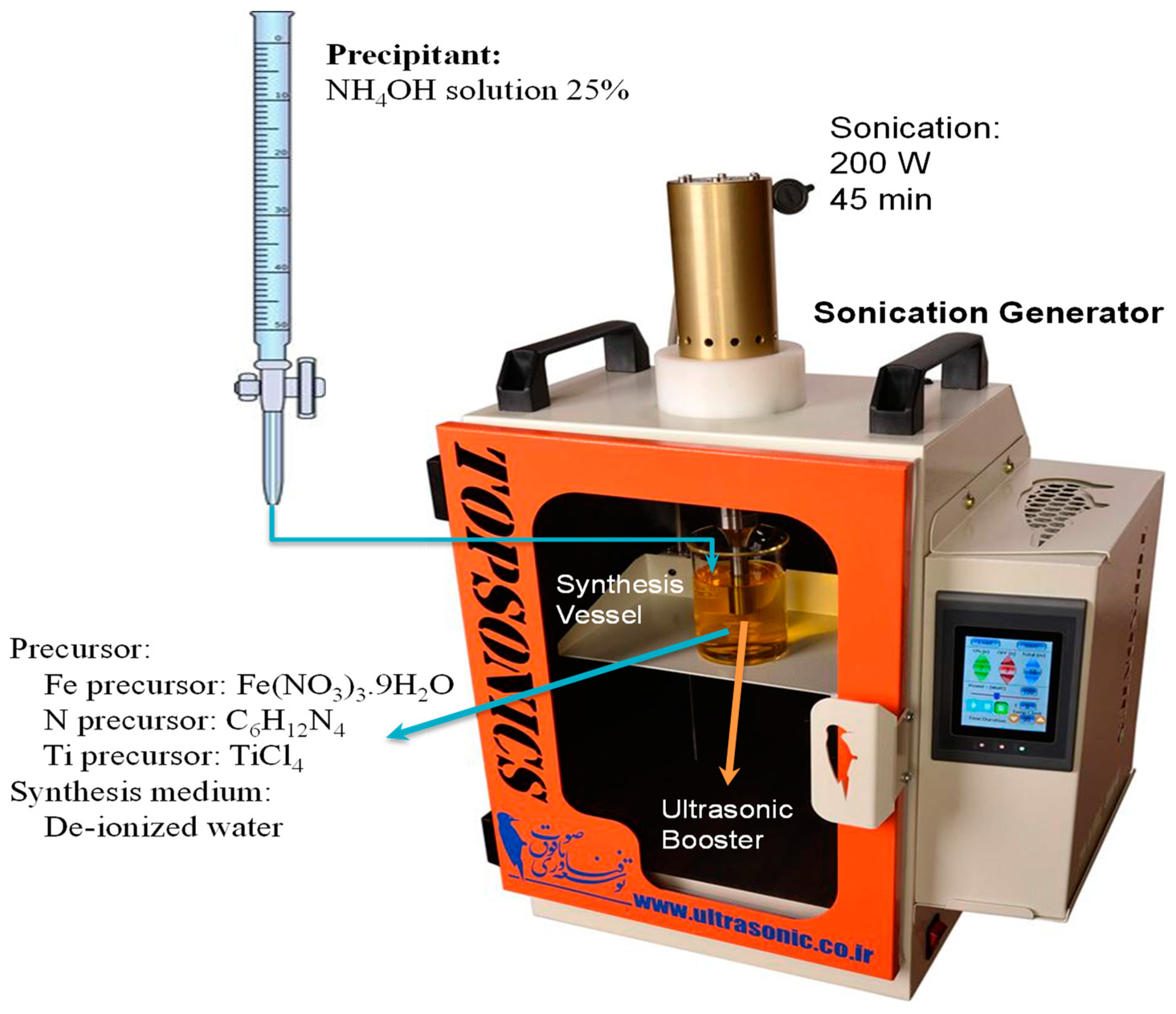
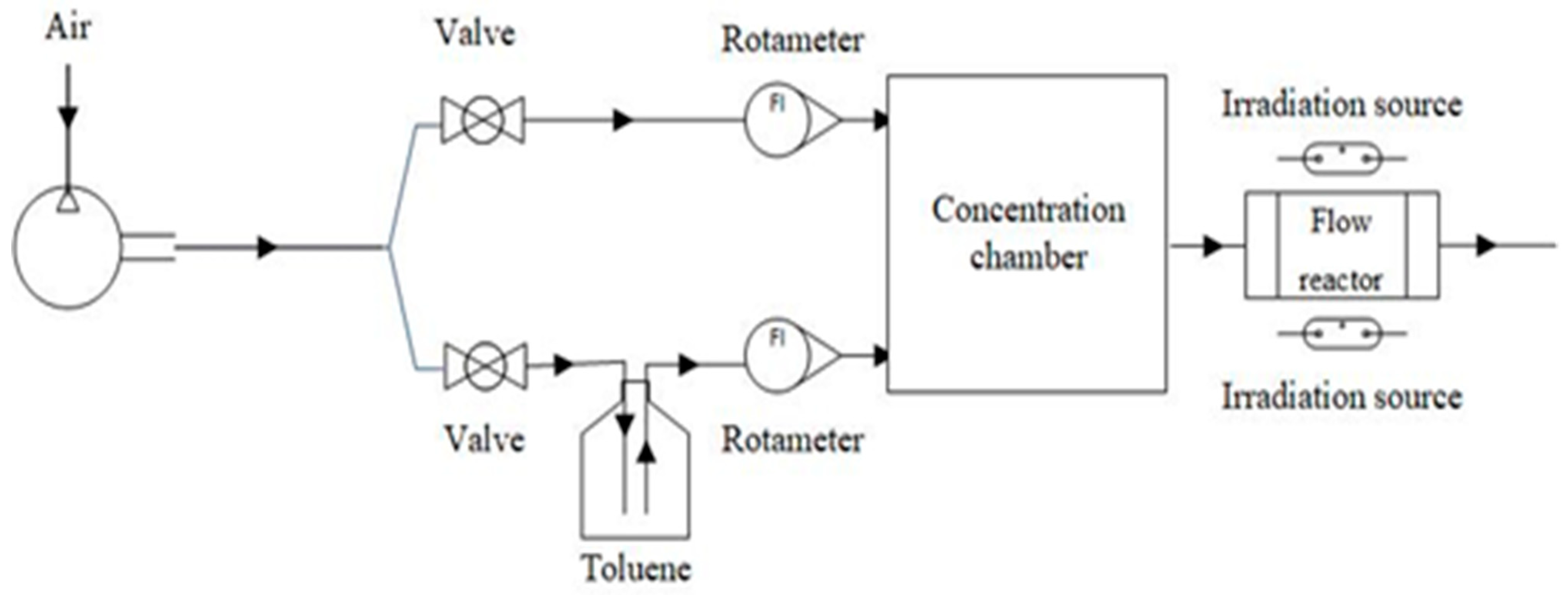
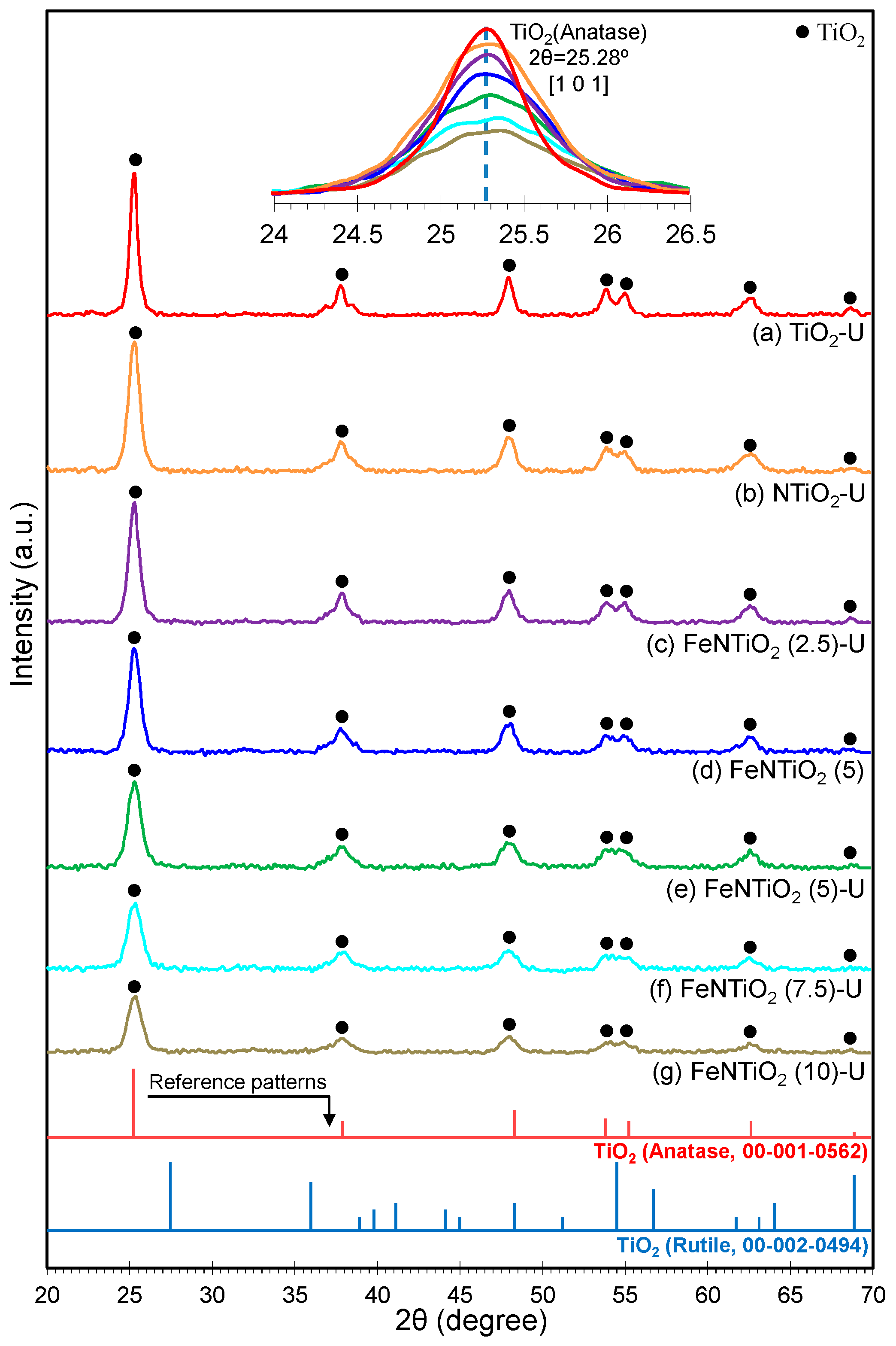
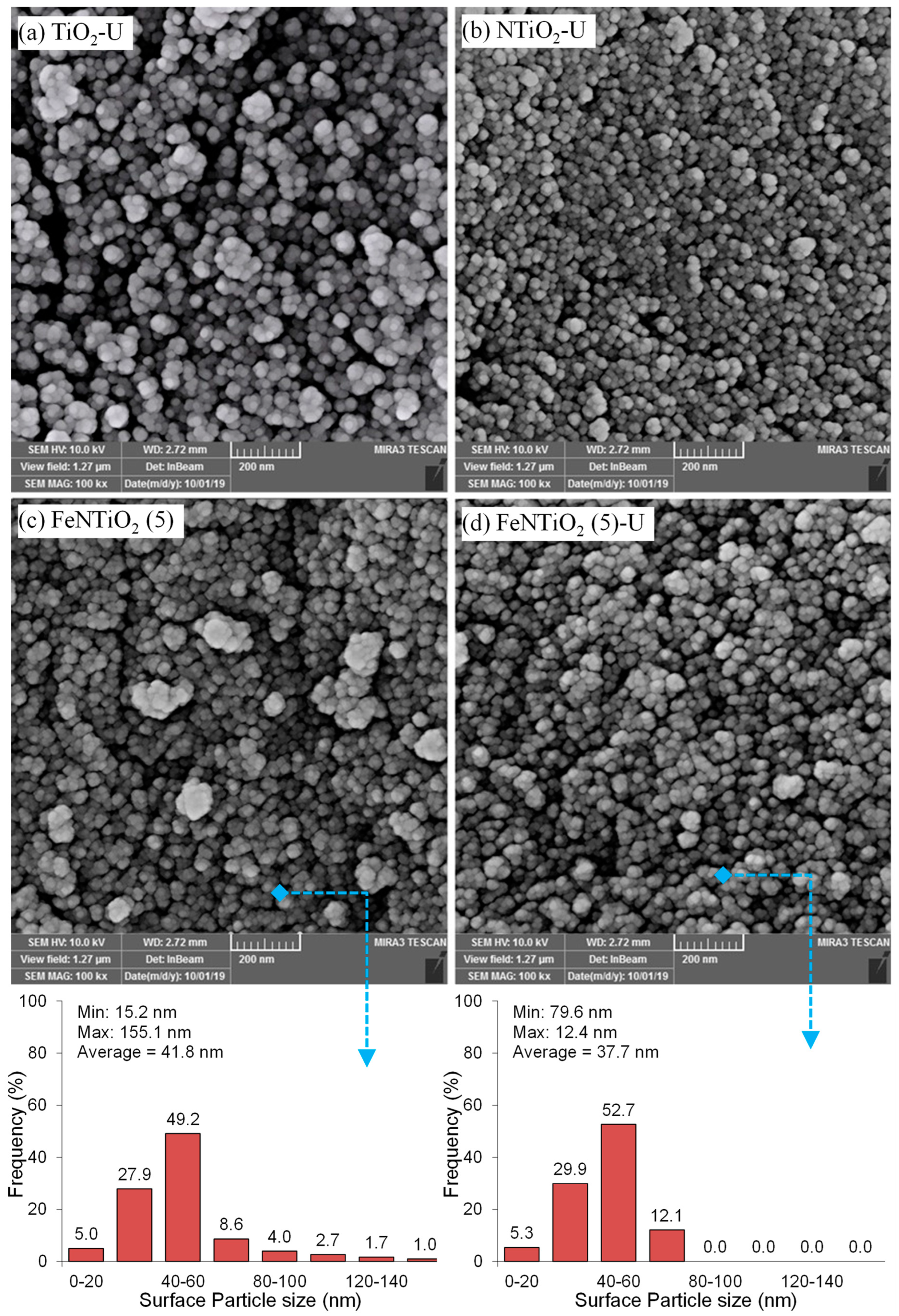

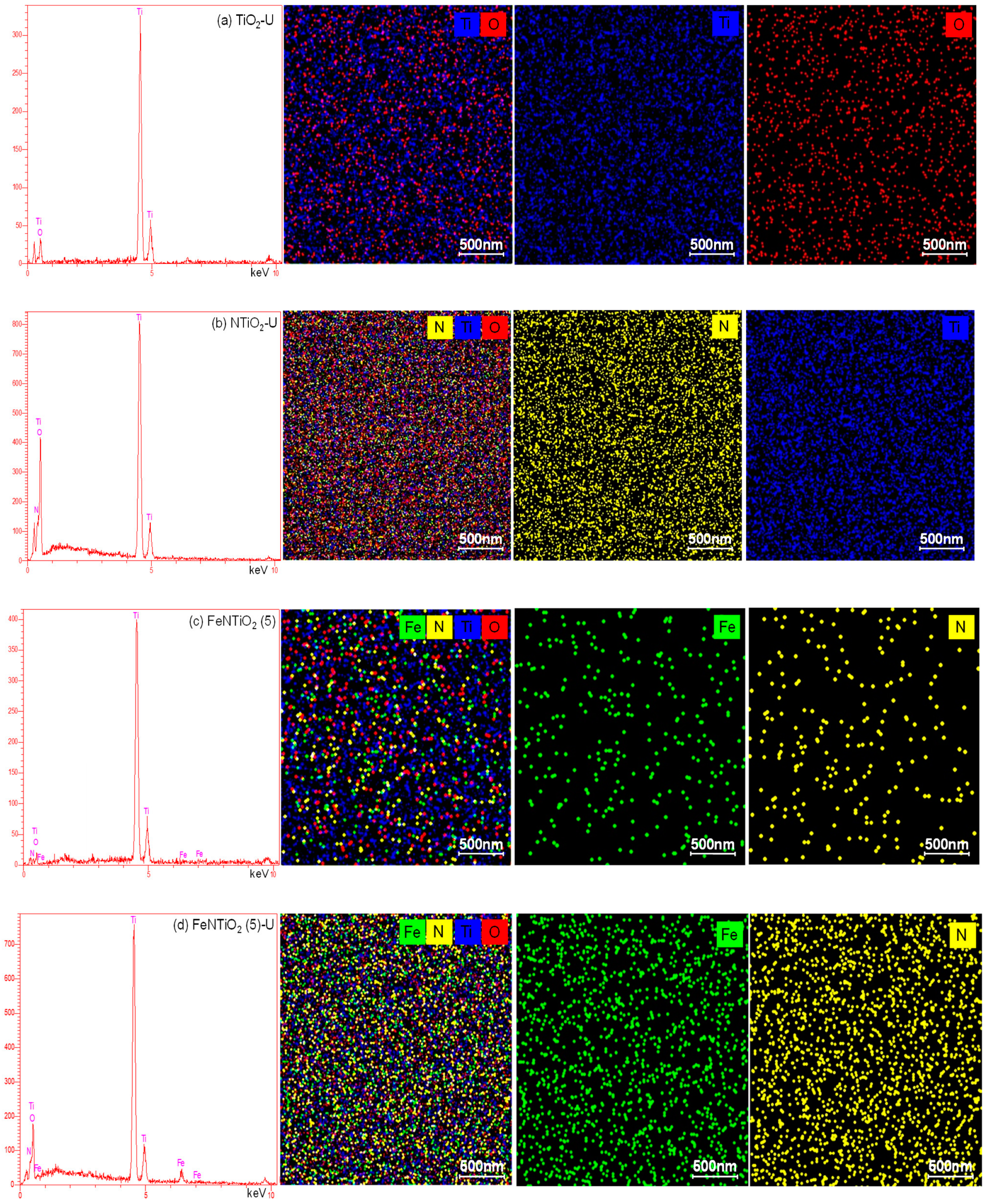
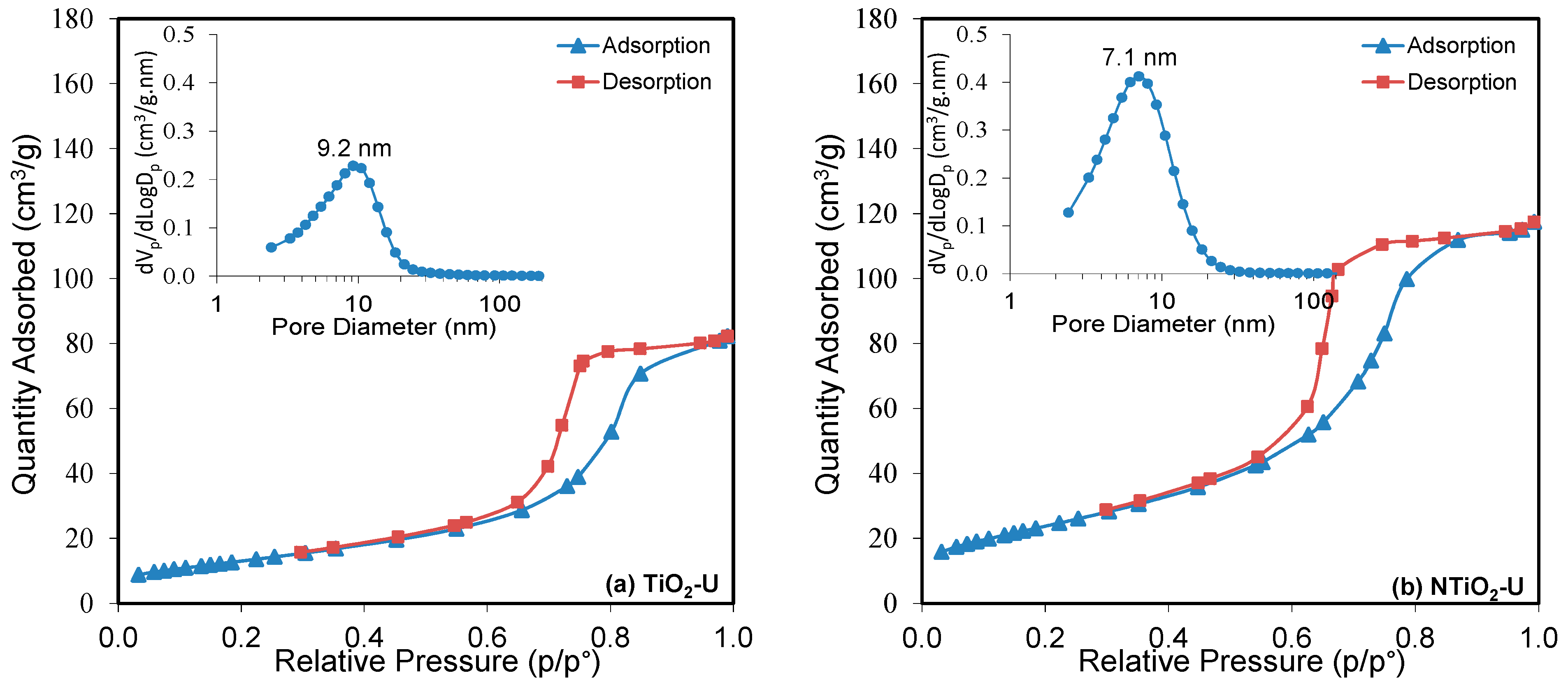
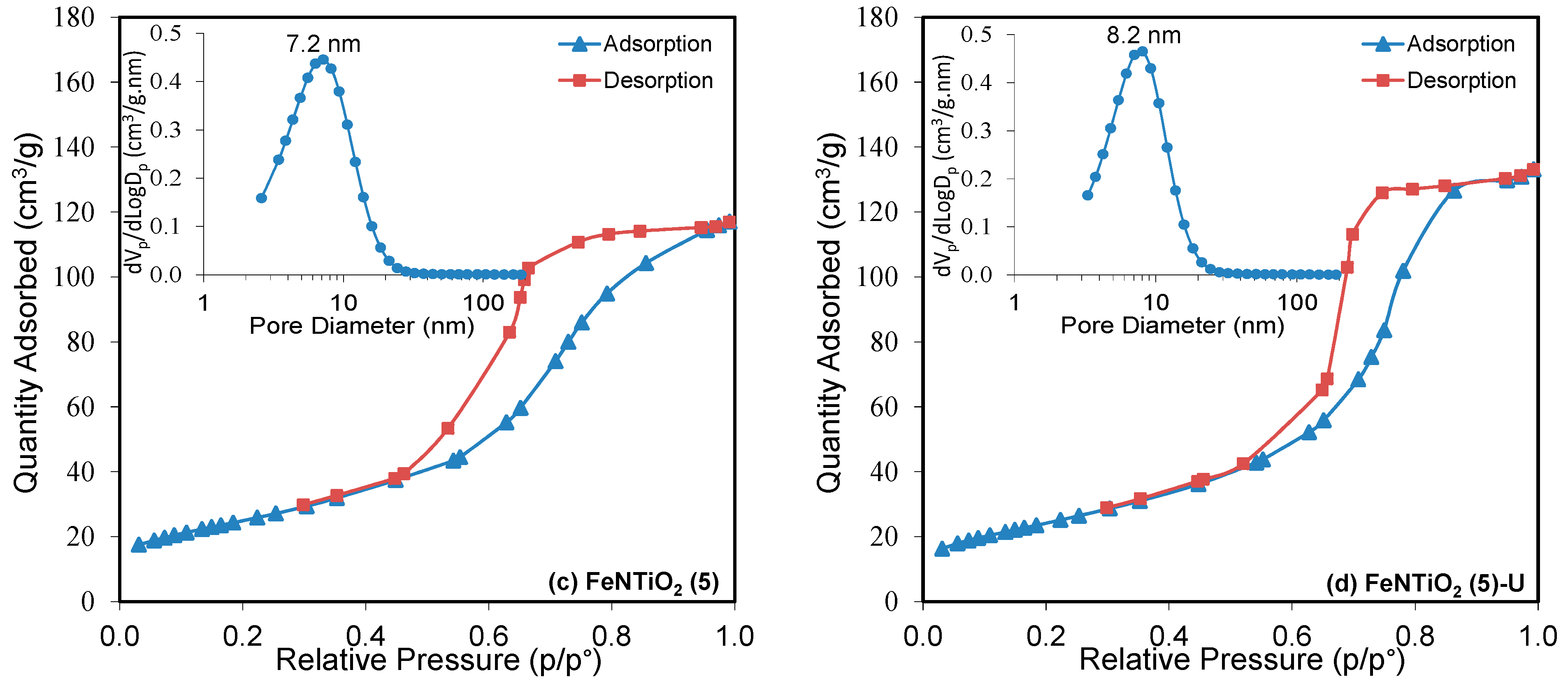

| No | Substance | JCPDS No | Phase | XRD Major Peaks |
|---|---|---|---|---|
| 1 | TiO2 | 00-001-0562 | Anatase | 25.3, 37.9, 48.4, 53.9, 55.3, 62.7, 69.0, 70.2, 75.4, 83.2 |
| Nanophotocatalyst | N (wt. %) | Fe/TiO2 (wt. %) | Ultrasound Irradiation | SBET (m2/g) | VP (cm3/g) | DP (nm) | λ0 (nm) | Band Gap (eV) | Relative Crystallinity | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Power (W) | TiO2 a | ||||||||
| TiO2-U | - | 0 | 45 | 300 | 47.8 | 0.1310 | 10.6 | 390.5 | 3.18 | 100 |
| NTiO2-U | 30 | 0 | 45 | 300 | 88.0 | 0.1880 | 8.2 | 413.3 | 3 | 92.6 |
| FeNTiO2(2.5)-U | 30 | 2.5 | 45 | 300 | - | - | - | - | - | 86.2 |
| FeNTiO2(5) | 30 | 5 | - | - | 89.4 | 0.1940 | 8.1 | 424.7 | 2.92 | 75.5 |
| FeNTiO2(5)-U | 30 | 5 | 45 | 300 | 88.3 | 0.2070 | 9.3 | 437.7 | 2.83 | 61.7 |
| FeNTiO2(7.5)-U | 30 | 7.5 | 45 | 300 | - | - | - | - | - | 48.9 |
| FeNTiO2(10)-U | 30 | 10 | 45 | 300 | - | - | - | - | - | 40.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarzadeh, O.; Rasoulzadeh, Y.; Haghighi, M.; Talati, A.; Golzad, H. Investigation of Synergistic Influence of Ultrasound and Co-Doping to Degrade Toluene from Polluted Air in Construction Sites—An Experimental Approach. Buildings 2024, 14, 2876. https://doi.org/10.3390/buildings14092876
Akbarzadeh O, Rasoulzadeh Y, Haghighi M, Talati A, Golzad H. Investigation of Synergistic Influence of Ultrasound and Co-Doping to Degrade Toluene from Polluted Air in Construction Sites—An Experimental Approach. Buildings. 2024; 14(9):2876. https://doi.org/10.3390/buildings14092876
Chicago/Turabian StyleAkbarzadeh, Omid, Yahya Rasoulzadeh, Mohammad Haghighi, Azadeh Talati, and Hamed Golzad. 2024. "Investigation of Synergistic Influence of Ultrasound and Co-Doping to Degrade Toluene from Polluted Air in Construction Sites—An Experimental Approach" Buildings 14, no. 9: 2876. https://doi.org/10.3390/buildings14092876
APA StyleAkbarzadeh, O., Rasoulzadeh, Y., Haghighi, M., Talati, A., & Golzad, H. (2024). Investigation of Synergistic Influence of Ultrasound and Co-Doping to Degrade Toluene from Polluted Air in Construction Sites—An Experimental Approach. Buildings, 14(9), 2876. https://doi.org/10.3390/buildings14092876







