Abstract
The eradication of the hepatitis C virus (HCV) has revolutionized the hepatology paradigm, halting the progression of advanced liver disease in patients with chronic infection and reducing the risk of hepatocarcinoma. In addition, treatment with direct-acting antivirals can reverse the lipid and carbohydrate abnormalities described in HCV patients. Although HCV eradication may reduce the overall risk of vascular events, it is uncertain whether altered lipid profiles increase the risk of cerebrovascular disease in certain patients. We have conducted a review on HCV and lipid and carbohydrate metabolism, as well as new scientific advances, following the advent of direct-acting antivirals.
1. Introduction
The hepatitis C virus (HCV) is recognized as a significant human pathogen that initially causes acute hepatitis. However, it has the potential to evolve into chronic hepatitis, leading to severe liver complications such as cirrhosis and hepatocellular carcinoma, posing a substantial global public health challenge. As a single-stranded RNA virus belonging to the Flaviviridae family, HCV’s mechanism of infection and replication is complex. It involves evading the host’s immune response, contributing to its chronicity in infected individuals. The virus’s genetic diversity, marked by multiple genotypes and subtypes, complicates vaccine development and treatment strategies. The progression from acute to chronic HCV infection stresses the importance of early detection and effective antiviral therapies to prevent long-term liver damage and reduce the risk of liver cancer. Despite advances in treatment, HCV remains a leading cause of liver transplantation worldwide, highlighting the need for continued research and public health efforts to combat this virus [1].
HCV transmission occurs primarily through blood-to-blood contact. In healthcare environments, reusing or inadequately sterilizing medical equipment, notably syringes and needles, presents a significant risk. Additionally, the transfusion of blood and blood products that have not undergone thorough screening processes can serve as a conduit for HCV transmission. Another prevalent route is through the sharing of injection equipment among individuals using injectable drugs [2].
HCV is classified into seven genotypes, with multiple subtypes, which are unevenly distributed geographically and differ in response to treatment [3].
2. Epidemiology of HCV Infection and Clinical Course
The prevalence of HCV infection has been declining since the second half of the 20th century [4]. This is due to improved hygienic and dietary conditions in developing countries and active surveillance in high-incidence countries. Together, these strategies have played a pivotal role in reducing the global burden of HCV, showcasing the importance of comprehensive public health initiatives in combating infectious diseases.
However, accurate estimates of global HCV prevalence are difficult to establish due to underdiagnosis, underreporting, and a lack of routine surveillance in most countries [5]. The estimated global prevalence of HCV viremia in early 2020 was 0.7 percent, reflecting 56.8 million people with chronic HCV infection. These data reflect a decrease in prevalence compared to 2015 when there were 63.6 million chronic HCV infections, representing 0.9 percent of the global population.
In Europe, the main incidence areas are the Eastern Mediterranean countries (62.5 per 100,000), where it is associated with healthcare, and the Eastern European region (61.8 per 100,000), where it is associated with injectable drug use [6].
Hepatitis C often progresses stealthily, mirroring other liver diseases with an initial asymptomatic phase in most cases. Over time, it may lead to cirrhosis, presenting complications such as ascites, variceal bleeding, and hepatic encephalopathy. A notable distinction of hepatitis C from other liver diseases is its propensity to cause extrahepatic manifestations, including joint pain (arthralgias), cryoglobulinemia, and various metabolic changes. This broad spectrum of potential effects highlights the complexity of hepatitis C, affecting not just liver function but also other bodily systems and requiring comprehensive management strategies.
3. Biology of Hepatitis C Virus and Its Association with Lipoproteins
Characteristics of the Hepatitis C Virus
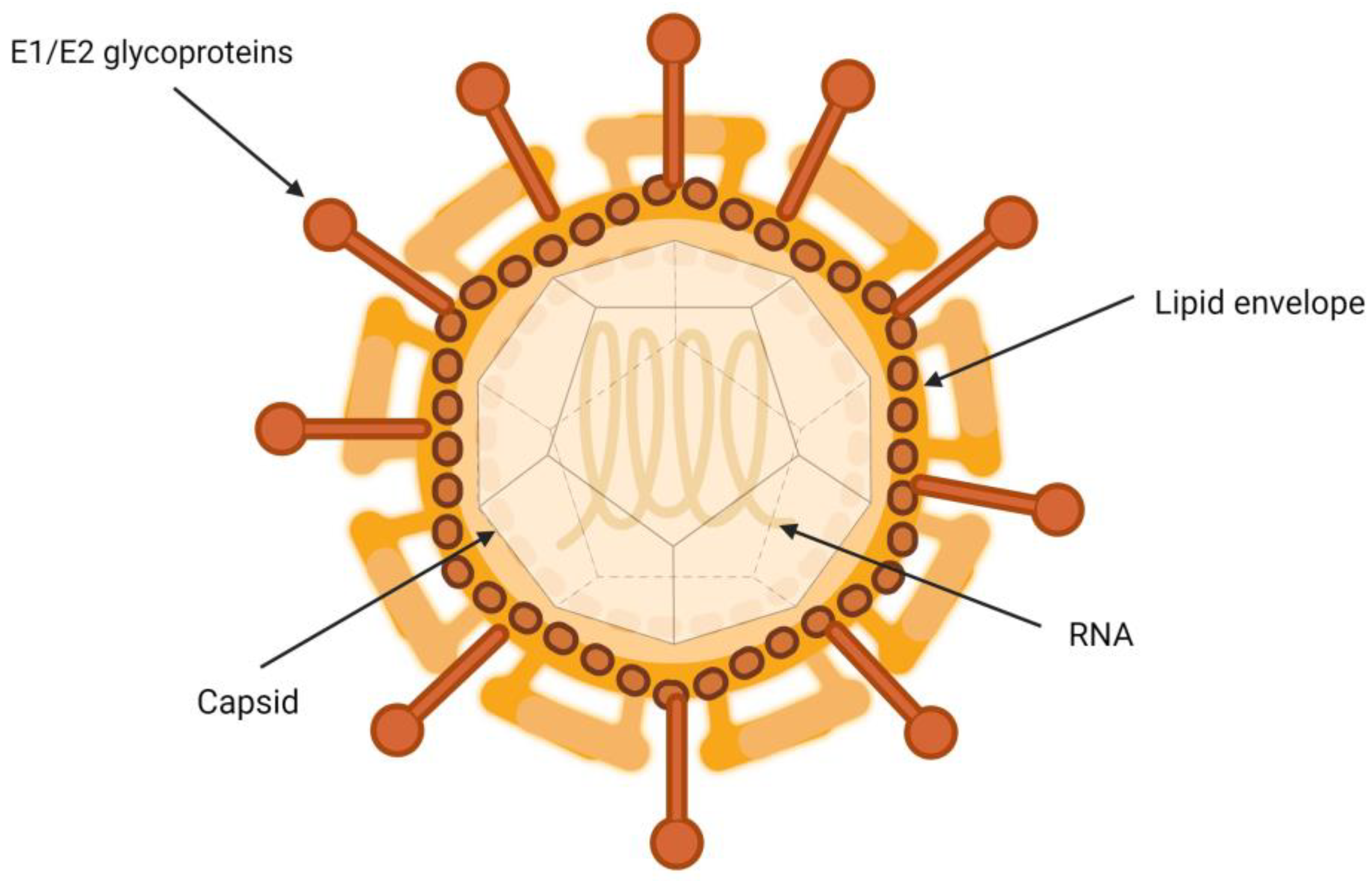
HCV is a particle between 50 and 80 nm in diameter containing a single-stranded RNA genome, nucleus, E1 and E2 glycoproteins, and type I transmembrane proteins, which form covalent bonds with infected hepatocytes [7]. They are closely associated with lipoproteins, which gives them a very low density [8]. The interactions governing the relationship between HCV virions and the different lipoproteins involved remain to be fully characterized [9].
It has been suggested that HCV virion is a hybrid, consisting of a viral part merged with a lipoprotein capsule (Figure 1) [10]. Another hypothesis is that the relationship occurs through the interaction of apolipoproteins and lipid molecules that are part of the HCV envelope [11]. In both cases, the interaction with host lipoproteins could contribute to protecting and concealing the virion particles, covering their surface. This glycoprotein coat is essential in the process of inclusion of the viral particle into the target cells. It plays a crucial role in the binding and fusion process between the viral envelope and the endosomal membrane of the host cells [12].
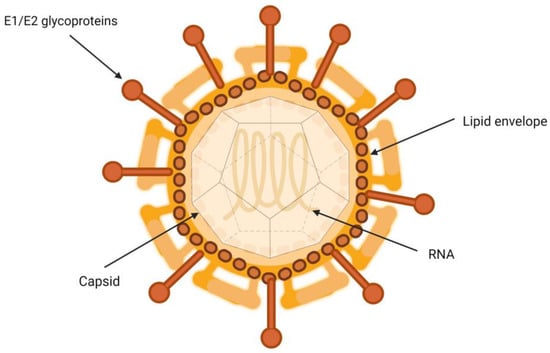
Figure 1.
Structure of hepatitis C virion.
In viral replication, HCV relies on the host cellular mechanism, which is associated with endoplasmic reticulum-derived membranes and various proteins [13]. HCV induces a massive reorganization of intracellular membranes, creating a membranous network [14].
Several electron microscopic studies have shown that the predominant structure is a double membrane vesicle consisting of proteins and cholesterol, as well as deposits of triglycerides (TGs) and cholesterol esters [15,16,17]. HCV alters the expression of genes involved in lipid metabolism, resulting in the accumulation of intracellular lipids [18].
4. Lipoproteins
Lipoproteins serve as vehicles for lipid transport, consisting of a nonpolar core filled with triglycerides (TGs) and esterified cholesterol, encased in a polar outer layer composed of apoproteins, phospholipids, and free cholesterol. This diverse group includes chylomicrons, very low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), low-density lipoproteins (LDLs), and high-density lipoproteins (HDLs). The diversity among these lipoproteins lies in their free cholesterol and TG content and their unique compositions of apolipoproteins, reflecting their varied roles in lipid transport and metabolism within the body [19]. This variation emphasizes the complexity of lipid dynamics and their critical functions in maintaining cellular and systemic health.
Lipoprotein metabolism encompasses both exogenous and endogenous pathways. The exogenous pathway involves the absorption of dietary lipids through intestinal enterocytes, which are packaged into chylomicrons and enter the lymphatic system before reaching the bloodstream. On the other hand, the endogenous pathway occurs primarily in the liver (hepatocytes), where lipoproteins such as VLDL are synthesized and released into the circulation. These pathways are crucial for distributing lipids across different tissues for energy use, storage, or membrane synthesis.
4.1. Exogenous Pathway of Lipoprotein Metabolism
The lipids we obtain from the diet are mainly TGs. Once in the intestine, they bind to apoprotein B-48 in enterocytes, forming chylomicrons. These are secreted into the lymphatic vessels, reaching the general circulation via the thoracic duct. Chylomicrons become mature once they receive APOCII and APOE from HDL particles. There is also an exchange of TG with LDL particles located in the vascular endothelium, becoming remnant chylomicrons, taken up by hepatocytes through an interaction with APOE [20].
4.2. Endogenous Pathway of Lipoprotein Metabolism
The liver is the main organ involved in the endogenous lipoprotein metabolism pathway. Hepatocytes secrete VLDL, the formation of which is initiated in the sarcoplasmic reticulum by the incorporation of TG into APOB100 particles through the action of microsomal TG transfer protein. Cholesterol esters and APOE are incorporated into this particle. This is followed by the exocytosis of VLDL lipoproteins into the bloodstream, acquiring more APOE and APOC from the HDL particles [21]. Mature VLDLs are catabolized by the APOCII-activated enzyme lipoprotein lipase and renamed remnant VLDL or IDL. They are incorporated back into the liver through the interaction of APOE [22]. Alternatively, they are again hydrolyzed by hepatic lipase, whereby IDLs are transformed into LDLs, depleted of TGs and high in cholesterol. These particles transport cholesterol to peripheral tissues or the liver via APOB100 interactions with LDL receptors [23].
On the other hand, APOAI is the primary apolipoprotein of HDL particles [24]. It is again synthesized in the liver and intestine and is involved in forming these molecules through the esterification of cholesterol and phospholipids. During this process, the HDL molecules progressively lose part of their cholesterol load until they return to the hepatocyte or enterocyte, where they replenish their cholesterol stores [25].
5. Lipoprotein Profile Assessment
Dyslipidemia is a quantitative or qualitative alteration in circulating lipoproteins in plasma, notably an increase in the concentration of low-density lipoprotein cholesterol (LDL-cholesterol) [26]. However, episodes of atherothrombotic pathology are still observed in patients with normal or low cholesterol levels and without other known cardiovascular risk factors [27]. This suggests that there are other lipid alterations, beyond LDL cholesterol levels, that also increase cardiovascular risk [28]. The atherogenic potential of lipoproteins should be hence defined not just by their quantity but by their characteristics, including their number, size, and composition. Therefore, analyzing these aspects of lipoproteins provides a more comprehensive assessment of a patient’s lipid profile, offering insights beyond traditional cholesterol measurements [29,30].
As previously explained, lipoprotein particles differ from each other in terms of their free cholesterol and TG content. The relationship between density and size is inverse, with the smallest particles having the highest density [31].
These differences in the composition of the same class of particles influence the atherosclerotic process. Healthy vascular endothelium can be freely traversed by particles with diameters of less than 70 nm. These particles, especially smaller LDL particles, can be retained and are the origin of the atherogenic process [32].
It has also been observed that HDL particles can undergo modifications that change their structure and composition, thereby altering their function. For example, in diseases such as type II diabetes (T2DM), chronic kidney disease, sarcoidosis, and inflammatory processes, HDL particles lose their protective function and acquire an atherogenic effect [33].
The size of LDL lipoproteins is variable and depends on their core’s lipid content, which determines the particles’ density. This variability, which can be influenced by various alterations in lipoprotein metabolism, can lead to a discrepancy between the serum LDL cholesterol concentration and the number of circulating LDL particles [34]. Thus, many LDL particles may be associated with a normal LDL cholesterol concentration. This situation is known as c-LDL/p-LDL mismatch.
In these cases, different studies have found that particle number measurement is a better indicator than LDL concentration for assessing cardiovascular risk [35]. The prognostic ability of LDL particle number has been evaluated in different studies. For example, in the Framingham cohort, it was shown that an LDL particle concentration below the 25th percentile was a more reliable predictor of cardiovascular risk than an equivalent serum LDL cholesterol concentration [36]. Studies have even shown that treatment based on LDL particle number targets improves clinical outcomes over that based on LDL cholesterol concentration [37,38].
6. Alterations in Lipid Metabolism Associated with HCV Infection
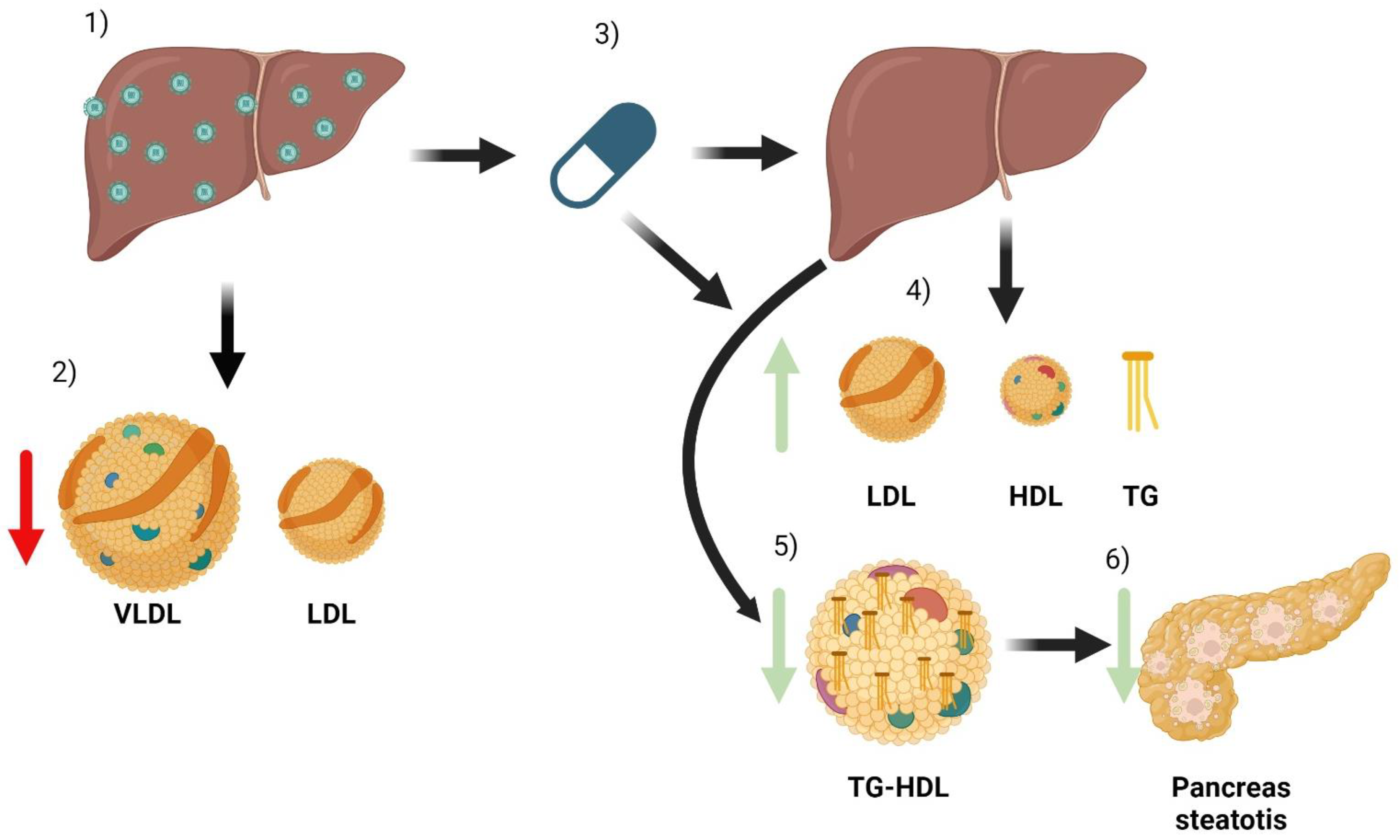
The most important complications associated with chronic HCV infection are liver cirrhosis and liver cancer. However, there are many extrahepatic manifestations that cause high morbidity and mortality [39]. Most are immunological or lymphoproliferative in origin, but alterations in the lipid profile have also been identified, leading to metabolic and cardiovascular complications [40]. The lipid profile’s modifications related to HCV infection and treatment are shown in Figure 2.
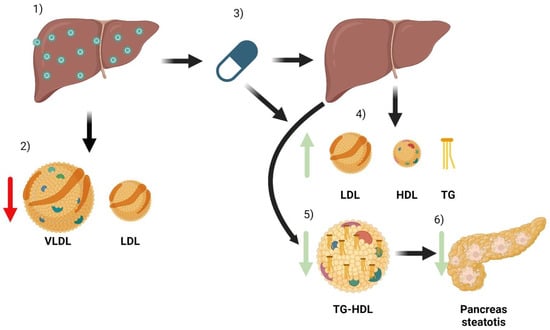
Figure 2.
Summary of the main effects of HCV and its treatment on a patient’s lipid metabolism. When a patient has HCV infection, the main organ affected is the liver (1). Infection causes some changes in lipid metabolism, especially a decrease in the number of VLDL and LDL particles (2). After treatment with DAA (3), the infection is cured. Due to the combination of liver healing and the direct effect of antivirals, there are changes in lipoparticles. There is an increase in serum LDL, HDL, and triglyceride particles (4). In addition, improved liver function reduces the triglyceride content of HDL particles (5). HDL particles can now better mobilize lipids from tissues, which can reduce pancreatic steatosis and thus improve insulin resistance (6).
It is very striking that some studies have even been able to link the development of hepatocarcinoma with an alteration in oncogenesis. Moreover, this phenomenon is much more marked in HCV-infected patients than in HBV-infected patients. Some of the mediators involved could be AKT2, SREBP1c, and PPARγ. Also, some regulatory enzymes such as ACC and FAS may be involved [41].
Chronic HCV infection results in low levels of VLDL and LDL. Despite this apparently beneficial change, these patients have an increased development of atherosclerosis, leading to an increased cardiovascular risk [42]. This occurs independently of other risk factors, such as the development of T2DM or the presence of hepatic steatosis. Interestingly, HCV eradication leads to an increase in serum cholesterol and LDL levels, creating a combination of circumstances that may exacerbate the risk of atherosclerosis injury [43].
Another finding observed in patients with chronic HCV infection is the existence of abnormal lipoproteins, including VLDL particles enriched with TG, which increase atherogenic risk. These particles disappear after successful HCV treatment and cure.
However, it appears that the extent of this interaction is related to certain host polymorphisms and hepatitis C virus genotypes. Both factors are highly variable [44,45]. Evidence highlights that genotype 3 of the hepatitis C virus, accounting for 20–30% of infections, is particularly associated with the development of hepatic steatosis, exhibiting a more pronounced degree of steatosis in patients, even those without obesity, compared to other genotypes. This association extends to a direct correlation between viral load and steatosis severity, exclusively in genotype 3, a phenomenon not observed in other genotypes. Moreover, genotype 3 is linked to several adverse disease progression outcomes, such as increased treatment resistance and a higher risk of developing HCC [46].
The underlying mechanisms, though not fully understood, suggest that genotype 3 impacts key metabolic pathways involving microsomal triglyceride transfer protein (MTTP), sterol regulatory element-binding protein 1c (SREBP-1c), and peroxisome proliferator-activated receptor alpha (PPAR-α) [47]. This insight emphasizes the need for a genotype-specific approach in managing HCV infections, considering the unique challenges posed by genotype 3.
6.1. Diabetes Mellitus and Insulin Resistance
The development of T2DM is one of the most common HCV-related complications [48]. This relationship stems from a complex interplay between insulin resistance, hepatic steatosis, and inflammatory processes [49]. HCV-core transcription leads to an increased expression of TNF-alpha and thus to the induction of insulin resistance. This explains why the prevalence of T2DM is higher in patients with HCV liver disease compared to other etiologies of liver disease [50].
The development of T2DM can occur at any stage of liver disease, even with low degrees of fibrosis [51]. However, it is more prevalent in patients with advanced fibrosis or even liver cirrhosis [52]. As previously described, a genotype-dependent factor must be considered. Patients with genotype 3 have a higher risk of developing insulin resistance and diabetes. On the other hand, patients with genotype 1 would be more likely to improve their carbohydrate metabolism after a viral cure compared to genotypes 2 and 3 [53].
The development of T2DM correlates directly with the severity of liver fibrosis. Although it can occur in patients with mild fibrosis, the highest incidence is observed in those with liver cirrhosis. In addition, patients with HCV-associated T2DM have an increased risk of developing HCC. Regarding the relationship between T2DM and HCV treatment, early interferon treatment showed a worse response in patients with T2DM and HCV [54]. A decrease in the risk of de novo T2DM has been observed in several studies with the newer treatments, direct-acting analogues (DAAs) [55,56].
DAAs prevent the future onset of T2DM and improve glucose metabolism in patients who achieve sustained viral response (SVR). During follow-up, a decrease in glycated hemoglobin and an improvement in insulin resistance-related parameters have been observed. However, their long-term duration after achieving SVR needs to be better established [57].
6.2. Cardiovascular Diseases
HCV infection confers increased cardiovascular morbidity and mortality [58]. Early studies showed a relationship between HCV seropositivity and reduced carotid artery intima/media ratio. Subsequently, HCV was also found to cause an increased expression of pro-atherogenic cytokines [59,60].
Cardiovascular involvement appears to predominate in HCV patients compared to patients with other similar conditions, such as hepatitis B virus (HBV) [61]. This indicates that the cardiovascular risk is not solely due to liver damage but is an inherent effect of HCV itself.
In studies with large cohorts of patients with very long follow-up periods, it became evident that those patients who received antiviral treatment and achieved HCV eradication had lower mortality rates than those patients who did not receive treatment, not only due to hepatic but also extrahepatic causes, especially cardiovascular. Other studies showed improved myocardial perfusion in those patients who had SVR [62].
Another aspect to consider in the relationship between cardiovascular disease and hepatitis C is the interaction between their respective treatments. Antihypertensive drugs and statins are among the most frequently used simultaneously in patients receiving direct-acting antivirals. About 10% of patients took a statin before starting antiviral treatment [63]. Therefore, it is particularly important to consider interactions between these drugs [64]. Not all statins interact in the same way with all antivirals, although the most common complication is the development of myopathies and the need to lower the dose. Specific combinations, such as glecaprevir/pibrentasvir with atorvastatin, lovastatin, or simvastatin, as well as ledipasvir/sofosbuvir with rosuvastatin are formally contraindicated [65].
Author Contributions
All authors have participated in the literature review and analysis of the results. A.P.-O., D.C.-D. and V.B.-M. drafted the initial manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
D.C.-D. is partially funded by a Rio Hortega Grant from Instituto de Salud Carlos III [grant number CM21/00067].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Simmonds, P. The origin of hepatitis C virus. Curr. Top. Microbiol. Immunol. 2013, 369, 1–15. [Google Scholar]
- Suryaprasad, A.G.; White, J.Z.; Xu, F.; Eichler, B.A.; Hamilton, J.; Patel, A.; Bel-Hamdounia, S.; R-Church, D.; Barton, K.; Fisher, C.; et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin. Infect. Dis. 2014, 59, 1411–1419. [Google Scholar] [CrossRef]
- Zein, N.N. Clinical significance of hepatitis C virus genotypes. Clin. Microbiol. Rev. 2000, 13, 223–235. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. The lancet. Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Frieden, T.R.; Harold Jaffe, D.W.; Rasmussen, S.A.; Leahy, M.A.; Martinroe, J.C.; Spriggs, S.R.; Doan, Q.M.; King Terraye MStarr, P.H.; Roper, W.L.; Hill, C.; et al. Sexually Transmitted Diseases Treatment Guidelines. Morb. Mortal Wkly Rep. 2015, 64, 1–140. [Google Scholar]
- Catanese, M.T.; Uryu, K.; Kopp, M.; Edwards, T.J.; Andrus, L.; Rice, W.J.; Silvestry, M.; Kuhn, R.J.; Rice, C.M. Ultrastructural analysis of hepatitis C virus particles. Proc. Natl. Acad. Sci. USA 2013, 110, 9505–9510. [Google Scholar] [CrossRef]
- Vieyres, G.; Dubuisson, J.; Pietschmann, T. Incorporation of Hepatitis C Virus E1 and E2 Glycoproteins: The Keystones on a Peculiar Virion. Viruses 2014, 6, 1149–1187. [Google Scholar] [CrossRef]
- André, P.; Komurian-Pradel, F.; Deforges, S.; Perret, M.; Berland, J.L.; Sodoyer, M.; Pol, S.; Chébrot, C.; Paranhos-Baccalà, G.; Lotteau, V. Characterization of Low- and Very-Low-Density Hepatitis C Virus RNA-Containing Particles. J. Virol. 2002, 76, 6919–6928. [Google Scholar] [CrossRef] [PubMed]
- Bartenschlager, R.; Penin, F.; Lohmann, V.; André, P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011, 19, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D. Virion assembly and release. Curr. Top. Microbiol. Immunol. 2013, 369, 199–218. [Google Scholar] [CrossRef]
- Pileri, P.; Uematsu, Y.; Campagnoli, S.; Galli, G.; Falugi, F.; Petracca, R.; Weiner, A.J.; Houghton, M.; Rosa, D.; Grandi, G.; et al. Binding of hepatitis C virus to CD81. Science 1998, 282, 938–941. [Google Scholar] [CrossRef]
- Lohmann, V. Hepatitis C virus RNA replication. Curr. Top. Microbiol. Immunol. 2013, 369, 167–198. [Google Scholar] [CrossRef]
- Ferraris, P.; Blanchard, E.; Roingeard, P. Ultrastructural and biochemical analyses of hepatitis C virus-associated host cell membranes. J. Gen. Virol. 2010, 91, 2230–2237. [Google Scholar] [CrossRef]
- Paul, D.; Hoppe, S.; Saher, G.; Krijnse-Locker, J.; Bartenschlager, R. Morphological and Biochemical Characterization of the Membranous Hepatitis C Virus Replication Compartment. 2013. Available online: http://jvi.asm.org/ (accessed on 10 January 2024).
- Diamond, D.L.; Syder, A.J.; Jacobs, J.M.; Sorensen, C.M.; Walters, K.A.; Proll, S.C.; McDermott, J.E.; Gritsenko, M.A.; Zhang, Q.; Zhao, R.; et al. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010, 6, e1000719. [Google Scholar] [CrossRef]
- Targett-Adams, P.; Boulant, S.; McLauchlan, J. Visualization of Double-Stranded RNA in Cells Supporting Hepatitis C Virus RNA Replication. J. Virol. 2008, 82, 2182–2195. [Google Scholar] [CrossRef]
- Miyanari, Y.; Atsuzawa, K.; Usuda, N.; Watashi, K.; Hishiki, T.; Zayas, M.; Bartenschlager, R.; Wakita, T.; Hijikata, M.; Shimotohno, K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007, 9, 1089–1097. [Google Scholar] [CrossRef]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef]
- O’Connell, B.J.; Denis, M.; Genest, J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation 2004, 110, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Da Silva Sanchez, A.J.; Lokugamage, M.P.; Loughrey, D.; Echeverri, E.S.; Cristian, A.; Hatit, M.Z.C.; Santangelo, P.J.; Zhao, K.; Dahlman, J.E. The Extent to Which Lipid Nanoparticles Require Apolipoprotein E and Low-Density Lipoprotein Receptor for Delivery Changes with Ionizable Lipid Structure. Nano Lett. 2022, 22, 10025–10033. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux. J. Clin. Med. 2023, 12, 4399. [Google Scholar] [CrossRef]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific Binding of ApoA-I, Enhanced Cholesterol Efflux, and Altered Plasma Membrane Morphology in Cells Expressing ABC1*. 2000. Available online: http://www.jbc.org (accessed on 11 January 2024).
- Giammanco, A.; Cefalù, A.B.; Noto, D.; Averna, M.R. The pathophysiology of intestinal lipoprotein production. Front. Physiol. 2015, 6, 61. [Google Scholar] [CrossRef]
- Ferrari, R.; Aguiar, C.; Alegria, E.; Bonadonna, R.C.; Cosentino, F.; Elisaf, M.; Farnier, M.; Ferrières, J.; Filardi, P.P.; Hancu, N.; et al. Current practice in identifying and treating cardiovascular risk, with a focus on residual risk associated with atherogenic dyslipidaemia. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2016, 18 (Suppl. SC), C2–C12. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Fazio, S.; Hussain, M.M.; Kontush, A.; Krauss, R.M.; Otvos, J.D.; Remaley, A.T.; Schaefer, E.J. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011, 57, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.C.; Davignon, J.; Hermans, M.P.; Al-Rubeaan, K.; Amarenco, P.; Assmann, G.; Barter, P.; Betteridge, J.; Bruckert, E.; Cuevas, A.; et al. Residual macrovascular risk in 2013: What have we learned? Cardiovasc. Diabetol. 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.C.; Sacks, F.M.; Hermans, M.P.; Assmann, G.; Brown, W.V.; Ceska, R.; Chapman, M.J.; Dodson, P.M.; Fioretto, P.; Ginsberg, H.N.; et al. The Residual Risk Reduction Initiative: A call to action to reduce residual vascular risk in dyslipidaemic patient. Diabetes Vasc. Dis. Res. 2008, 5, 319–335. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Underberg, J.A. Systematic review: Evaluating the effect of lipid-lowering therapy on lipoprotein and lipid values. Cardiovasc. Drugs Ther. 2013, 27, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterolrich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr Opin Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Pintó, X.; Masana, L.; Civeira, F.; Real, J.; Ibarretxe, D.; Candas, B.; Puzo, J.; Díaz, J.; Amigó, N.; Esteban, M.; et al. Documento de consenso de un grupo de expertos de la Sociedad Española de Arteriosclerosis (SEA) sobre el uso clínico de la resonancia magnética nuclear en el estudio del metabolismo lipoproteico (Liposcale). Clínica Investig. Arterioscler. 2020, 32, 219–229. [Google Scholar] [CrossRef]
- Otvos, J.D.; Mora, S.; Shalaurova, I.; Greenland, P.; MacKey, R.H.; Goff, D.C. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J. Clin. Lipidol. 2011, 5, 105–113. [Google Scholar] [CrossRef]
- Mora, S.; Caulfield, M.P.; Wohlgemuth, J.; Chen, Z.; Superko, H.R.; Rowland, C.M.; Glynn, R.J.; Ridker, P.M.; Krauss, R.M. Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circulation 2015, 132, 2220–2229. [Google Scholar] [CrossRef]
- Bermudez-Lopez, M.; Perpiñan, H.; Amigo, N.; Castro, E.; Alonso, N.; Mauricio, D.; Fernandez, E.; Valdivieso, J.M. Advanced Lipoprotein Parameters Could Better Explain Atheromatosis in Non-Diabetic Chronic Kidney Disease Patients. Available online: https://academic.oup.com/ckj/article/14/12/2591/6316255 (accessed on 10 January 2023).
- Tehrani, D.M.; Zhao, Y.; Blaha, M.J.; Mora, S.; Mackey, R.H.; Michos, E.D.; Budoff, M.J.; Cromwell, W.; Otvos, J.D.; Rosenblit, P.D.; et al. Discordance of Low-Density Lipoprotein and High-Density Lipoprotein Cholesterol Particle Versus Cholesterol Concentration for the Prediction of Cardiovascular Disease in Patients with Metabolic Syndrome and Diabetes Mellitus (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2016, 117, 1921–1927. [Google Scholar] [CrossRef]
- Cantey, E.P.; Wilkins, J.T. Discordance between lipoprotein particle number and cholesterol content: An update. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 130–136. [Google Scholar] [CrossRef]
- Gumber, S.C.; Chopra, S. Hepatitis C: A multifaceted disease-Review of extrahepatic manifestations. Ann. Intern. Med. 1995, 123, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Zignego, A.L.; Giannini, C.; Monti, M.; Gragnani, L. Hepatitis C virus lymphotropism: Lessons from a decade of studies. Dig. Liver Dis. 2007, 39 (Suppl. S1), S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Pan, Q.; Wang, G.; Cheng, D.; Yang, J.; Chen, H.; Xu, G. De novo lipogenesis is elicited dramatically in human hepatocellular carcinoma especially in hepatitis C virus-induced hepatocellular carcinoma. MedComm 2020, 1, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kang, R.; Zhao, Z. Is Hepatitis C Associated with Atherosclerotic Burden? A Systematic Review and Meta-Analysis. Available online: www.plosone.org (accessed on 29 December 2023).
- Bassendine, M.F.; Nielsen, S.U.; Bridge, S.H.; Felmlee, D.J.; Sheridan, D.A.; Packard, C.J.; Neely, R.D. Hepatitis C virus and atherosclerosis: A legacy after virologic cure? Clin. Res. Hepatol. Gastroenterol. 2017, 41, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Miyaaki, H.; Miuma, S.; Motoyoshi, Y.; Narita, S.; Toda, S.; Takahashi, Y.; Honda, T.; Yajima, H.; Uehara, R.; et al. Carotid Intima-media Thickness and Small Dense Low-density Lipoprotein Cholesterol Increase after One Year of Treatment with Direct-acting Antivirals in Patients with Hepatitis C Virus Infection. Intern Med. 2019, 58, 1209–1215. [Google Scholar] [CrossRef]
- Drazilova, S.; Gazda, J.; Janicko, M.; Jarcuska, P. Chronic Hepatitis C Association with Diabetes Mellitus and Cardiovascular Risk in the Era of DAA Therapy. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6150861. [Google Scholar] [CrossRef]
- Huang, J.F.; Huang, C.F.; Yeh, M.L.; Dai, C.Y.; Yu, M.L.; Chuang, W.L. Updates in the management and treatment of HCV genotype 3, what are the remaining challenges? Expert Rev. Anti-Infect. Ther. 2018, 16, 907–912. [Google Scholar] [CrossRef]
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs 2017, 77, 131–144. [Google Scholar] [CrossRef]
- Serfaty, L.; Capeau, J. Hepatitis C, insulin resistance and diabetes: Clinical and pathogenic data. Liver Int. 2009, 29 (Suppl. S2), 13–25. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.Y.; Yeh, M.L.; Huang, C.F.; Hou, C.H.; Hsieh, M.Y.; Huang, J.F.; Lin, I.L.; Lin, Z.Y.; Chen, S.C.; Wang, L.Y.; et al. Chronic hepatitis C infection is associated with insulin resistance and lipid profiles. J. Gastroenterol. Hepatol. 2015, 30, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.C.; Cacoub, P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: A contemporary review. World J. Gastroenterol. 2017, 23, 1697. [Google Scholar] [CrossRef] [PubMed]
- Drazilova, S.; Janicko, M.; Skladany, L.; Kristian, P.; Oltman, M.; Szantova, M.; Krkoska, D.; Mazuchova, E.; Piesecka, L.; Vahalova, V.; et al. Glucose Metabolism Changes in Patients with Chronic Hepatitis C Treated with Direct Acting Antivirals. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6095097. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Fujie, H.; Miyoshi, H.; Tsutsumi, T.; Tsukamoto, K.; Kimura, S.; Moriya, K.; Koike, K. Hepatitis C Virus Infection and Diabetes: Direct Involvement of the Virus in the Development of Insulin Resistance. Gastroenterology 2004, 126, 840–848. [Google Scholar] [CrossRef]
- Thompson, A.J.; Patel, K.; Chuang, W.L.; Lawitz, E.J.; Rodriguez-Torres, M.; Rustgi, V.K.; Flisiak, R.; Pianko, S.; Diago, M.; Arora, S.; et al. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut 2012, 61, 128. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Del Mar Viloria, M.; Andrade, R.J.; Salmerón, J.; Diago, M.; Fernández-Rodríguez, C.M.; Corpas, R.; Cruz, M.; Grande, L.; Vázquez, L.; et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005, 128, 636–641. [Google Scholar] [CrossRef]
- Li, J.; Gordon, S.C.; Rupp, L.B.; Zhang, T.; Trudeau, S.; Holmberg, S.D.; Moorman, A.C.; Spradling, P.R.; Teshale, E.H.; Boscarino, J.A.; et al. Sustained virological response does not improve long-term glycaemic control in patients with type 2 diabetes and chronic hepatitis C. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 1027–1032. [Google Scholar] [CrossRef]
- Nevola, R.; Rinaldi, L.; Zeni, L.; Sasso, F.C.; Pafundi, P.C.; Guerrera, B.; Marrone, A.; Giordano, M.; Adinolfi, L.E. Metabolic and renal changes in patients with chronic hepatitis C infection after hepatitis C virus clearance by direct-acting antivirals. JGH Open Open Access J. Gastroenterol. Hepatol. 2020, 4, 713–721. [Google Scholar] [CrossRef]
- Arase, Y.; Suzuki, F.; Suzuki, Y.; Akuta, N.; Kobayashi, M.; Kawamura, Y.; Yatsuji, H.; Sezaki, H.; Hosaka, T.; Hirakawa, M.; et al. Sustained Virological Response Reduces Incidence of Onset of Type 2 Diabetes in Chronic Hepatitis C. 2008. Available online: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.22703 (accessed on 20 January 2024).
- Voulgaris, T.; Sevastianos, V.A. Atherosclerosis as Extrahepatic Manifestation of Chronic Infection with Hepatitis C Virus. Hepat. Res. Treat. 2016, 2016, 7629318. [Google Scholar] [CrossRef] [PubMed]
- Boddi, M.; Abbate, R.; Chellini, B.; Giusti, B.; Solazzo, V.; Soft, F.; Pratesi, G.; Pratesi, C.; Gensini, G.; Zignego, A.L. HCV infection facilitates asymptomatic carotid atherosclerosis: Preliminary report of HCV RNA localization in human carotid plaques. Dig. Liver Dis. 2007, 39 (Suppl. S1), S55–S60. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Zampino, R.; Restivo, L.; Lonardo, A.; Guerrera, B.; Marrone, A.; Nascimbeni, F.; Florio, A.; Loria, P. Chronic hepatitis C virus infection and atherosclerosis: Clinical impact and mechanisms. World J. Gastroenterol. WJG 2014, 20, 3410. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.C.; Chen, T.H.; Wu, M.; Cheng, C.W.; Chen, S.W.; Chang, C.W.; Chen, C.C.; Chang, S.H.; Hung, K.C.; Chern, M.S.; et al. Comparison of cardiovascular outcomes and all-cause mortality in patients with chronic hepatitis B and C: A 13-year nationwide population-based study in Asia. Atherosclerosis 2018, 269, 178–184. [Google Scholar] [CrossRef]
- Maruyama, S.; Koda, M.; Oyake, N.; Sato, H.; Fujii, Y.; Horie, Y.; Murawaki, Y. Myocardial injury in patients with chronic hepatitis C infection. J. Hepatol. 2013, 58, 11–15. [Google Scholar] [CrossRef]
- Rodríguez-Osorio, I.; Cid, P.; Morano, L.; Castro, Á.; Suárez, M.; Delgado, M.; Margusino, L.; Meijide, H.; Pernas, B.; Tabernilla, A.; et al. Real life experience with direct-acting antivirals agents against hepatitis C infection in elderly patients. J. Clin. Virol. 2017, 88, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Ottman, A.A.; Townsend, M.L.; Hashem, M.G.; DiMondi, V.P.; Britt, R.B. Incidence of Drug Interactions Identified by Clinical Pharmacists in Veterans Initiating Treatment for Chronic Hepatitis C Infection. Ann. Pharmacother. 2018, 52, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.H.; Tseng, C.W.; Lee, C.H.; Tseng, K.C. Drug-drug interactions between direct-acting antivirals and statins in the treatment of chronic hepatitis C. Tzu Chi Med. J. 2020, 32, 331–338. [Google Scholar]
- Meissner, E.G.; Lee, Y.J.; Osinusi, A.; Sims, Z.; Qin, J.; Sturdevant, D.; McHutchison, J.; Subramanian, M.; Sampson, M.; Naggie, S.; et al. Effect of Sofosbuvir and Ribavirin Treatment on Peripheral and Hepatic Lipid Metabolism in Chronic HCV, Genotype-1 Infected Patients. Hepatology 2015, 61, 790. [Google Scholar] [CrossRef]
- Inoue, T.; Goto, T.; Iio, E.; Matsunami, K.; Fujiwara, K.; Shinkai, N.; Matsuura, K.; Matsui, T.; Nojiri, S.; Tanaka, Y. Changes in serum lipid profiles caused by three regimens of interferon-free direct-acting antivirals for patients infected with hepatitis C virus. Hepatol. Res. 2018, 48, E203–E212. [Google Scholar] [CrossRef] [PubMed]
- Mauss, S.; Berger, F.; Wehmeyer, M.H.; Ingiliz, P.; Hueppe, D.; Lutz, T.; Simon, K.G.; Schewe, K.; Rockstroh, J.K.; Baumgarten, A.; et al. Effect of Antiviral Therapy for HCV on Lipid Levels. Antivir. Ther. 2017, 22, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.L.; Junga, Z.; Singla, M.B.; Sjogren, M.; Torres, D.; Singal, M.B.; Sjogren, M.; Torres, D. Hepatitis C eradication with Sofosbuvir Leads to Significant Metabolic Changes. Retrosp. Cohort Study 2016, 8, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Cheng, P.N.; Tseng, C.Y.; Tsai, W.J.; Chiu, Y.C.; Young, K.C. Favouring modulation of circulating lipoproteins and lipid loading capacity by direct antiviral agents grazoprevir/elbasvir or ledipasvir/sofosbuvir treatment against chronic HCV infection. Gut 2018, 67, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Girona, J.; Amigó, N.; Ibarretxe, D.; Plana, N.; Rodríguez-Borjabad, C.; Heras, M.; Ferré, R.; Gil, M.; Correig, X.; Masana, L. HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. Available online: www.mdpi.com/journal/ijms (accessed on 25 January 2024).
- Bartolomei, G.; Cevik, R.E.; Marcello, A. Modulation of hepatitis C virus replication by iron and hepcidin in Huh7 hepatocytes. J. Gen. Virol. 2011, 92, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.M.; Bour, J.B.; Galland-Jos, C.; Minello, A.; Verges, B.; Guiguet, M.; Brun, J.M.; Hillon, P. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J. Hepatol. 2001, 35, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.Y.; Chuang, W.L.; Ho, C.K.; Hsieh, M.Y.; Huang, J.F.; Lee, L.P.; Hou, N.J.; Lin, Z.Y.; Chen, S.C.; Hsieh, M.Y.; et al. Associations between hepatitis C viremia and low serum triglyceride and cholesterol levels: A community-based study. J. Hepatol. 2008, 49, 9–16. [Google Scholar] [CrossRef]
- Kim, A.Y. Clinical Infectious Diseases AASLD-IDSA HCV Guidance Panel a. Available online: www.HCVGuidelines.org (accessed on 12 December 2023).
- Gitto, S.; Cicero, A.F.G.; Loggi, E.; Giovannini, M.; Conti, F.; Grandini, E.; Guarneri, V.; Scuteri, A.; Vitale, G.; Cursaro, C.; et al. Worsening of Serum Lipid Profile after Direct Acting Antiviral Treatment. Ann. Hepatol. 2018, 17, 64–75. [Google Scholar] [CrossRef]
- Huang, C.F.; Dai, C.Y.; Yeh, M.L.; Huang, C.I.; Lee, H.C.; Lai, W.T.; Liang, P.C.; Lin, Y.H.; Hsieh, M.Y.; Hou, N.J.; et al. Cure or curd: Modification of lipid profiles and cardio-cerebrovascular events after hepatitis C virus eradication. Kaohsiung J. Med. Sci. 2020, 36, 920–928. [Google Scholar] [CrossRef]
- Casas-Deza, D.; Martínez-Sapiña, A.; Espina, S.; Garcia-Rodriguez, B.; Fernandez-Bonilla, E.M.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Evaluation of Cardiovascular Risk Factors after Hepatitis C Virus Eradication with Direct-Acting Antivirals in a Cohort of Treatment-Naïve Patients without History of Cardiovascular Disease. J. Clin. Med. 2022, 11, 4049. [Google Scholar] [CrossRef]
- Chida, T.; Kawata, K.; Ohta, K.; Matsunaga, E.; Ito, J.; Shimoyama, S.; Yamazaki, S.; Noritake, H.; Suzuki, T.; Suda, T.; et al. Rapid Changes in Serum Lipid Profiles during Combination Therapy with Daclatasvir and Asunaprevir in Patients Infected with Hepatitis C Virus Genotype 1b. Gut Liver 2018, 12, 201–207. [Google Scholar] [CrossRef]
- Endo, D.; Satoh, K.; Shimada, N.; Hokari, A.; Aizawa, Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J. Gastroenterol. 2017, 23, 2355–2364. [Google Scholar] [CrossRef][Green Version]
- Chaudhury, C.S.; Sheehan, J.; Chairez, C.; Akoth, E.; Gross, C.; Silk, R.; Kattakuzhy, S.; Rosenthal, E.; Kottilil, S.; Masur, H.; et al. No Improvement in Hemoglobin A1c Following Hepatitis C Viral Clearance in Patients with and without HIV. J. Infect. Dis. 2017, 217, 47–50. [Google Scholar] [CrossRef]
- Townsend, K.; Meissner, E.G.; Sidharthan, S.; Sampson, M.; Remaley, A.T.; Tang, L.; Kohli, A.; Osinusi, A.; Masur, H.; Kottilil, S. Interferon-Free Treatment of Hepatitis C Virus in HIV/Hepatitis C Virus-Coinfected Subjects Results in Increased Serum Low-Density Lipoprotein Concentration. AIDS Res. Hum. Retroviruses 2016, 32, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Beig, J.; Orr, D.; Harrison, B.; Gane, E. Hepatitis C Virus Eradication with New Interferon-Free Treatment Improves Metabolic Profile in Hepatitis C Virus-Related Liver Transplant Recipients. Liver Transpl. 2018, 24, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.R.; Velosa, J.; Serejo, F. Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication comparison of the new direct-acting antiviral agents with the old regimens. Scand J Gastroenterol. 2018, 53, 857–863. [Google Scholar] [CrossRef] [PubMed]
- El-Sagheer, G.; Soliman, E.; Ahmad, A.; Hamdy, L. Study of changes in lipid profile and insulin resistance in Egyptian patients with chronic hepatitis C genotype 4 in the era of DAAs. Libyan J. Med. 2018, 13, 1435124. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Colominas, E.; Batlle, M.; Monge-Escartin, I.; Duran, X.; Viu, A.; de Antonio-Cusco, M.; Grau, S.; Bessa, X.; Carrión, J.A. Impact of HCV cure with drug-acting antivirals in the use of concomitant medication and lipid profile: Follow-up data 2 years after the sustained virological response. Eur. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Doyle, M.A.; Galanakis, C.; Mulvihill, E.; Crawley, A.; Cooper, C.L. Hepatitis C Direct Acting Antivirals and Ribavirin Modify Lipid but not Glucose Parameters. Cells 2019, 8, 252. [Google Scholar] [CrossRef]
- Ichikawa, T.; Miyaaki, H.; Miuma, S.; Taura, N.; Motoyoshi, Y.; Akahoshi, H.; Nakamura, J.; Takahashi, Y.; Honda, T.; Yajima, H.; et al. Changes in serum LDL, PCSK9 and microRNA-122 in patients with chronic HCV infection receiving Daclatasvir/Asunaprevir. Biomed. Rep. 2019, 10, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Soroida, Y.; Sato, M.; Hikita, H.; Kobayashi, T.; Endo, M.; Sato, M.; Gotoh, H.; Iwai, T.; Tateishi, R.; et al. Eradication of hepatitis C virus is associated with the attenuation of steatosis as evaluated using a controlled attenuation parameter. Sci. Rep. 2018, 8, 7845. [Google Scholar] [CrossRef]
- Cheng, P.N.; Chen, J.Y.; Chiu, Y.C.; Chiu, H.C.; Tsai, L.M. Augmenting central arterial stiffness following eradication of HCV by direct acting antivirals in advanced fibrosis patients. Sci. Rep. 2019, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kalra, B.S.; Srivastava, S.; Chawla, S. Effect of sofosbuvir and daclatasvir on lipid profile, glycemic control and quality of life index in chronic hepatitis C, genotype 3 patients. Indian J. Gastroenterol. 2019, 38, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Maida, M.; Macaluso, F.S.; Barbara, M.; Licata, A.; Craxì, A.; Cammà, C. Hepatitis C Virus Infection Is Associated with Increased Cardiovascular Mortality: A Meta-Analysis of Observational Studies. Gastroenterology 2016, 150, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Casas-Deza, D.; Espina, S.; Martínez-Sapiña, A.; del Moral-Bergos, R.; Garcia-Sobreviela, M.P.; Lopez-Yus, M.; Calmarza, P.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Triglyceride-rich lipoproteins and insulin resistance in patients with chronic hepatitis C receiving direct-acting antivirals. Atherosclerosis 2023, 375, 59–66. [Google Scholar] [CrossRef]
- Hashimoto, S.; Yatsuhashi, H.; Abiru, S.; Yamasaki, K.; Komori, A.; Nagaoka, S.; Saeki, A.; Uchida, S.; Bekki, S.; Kugiyama, Y.; et al. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS ONE 2016, 11, e0163644. [Google Scholar] [CrossRef]
- Corey, K.E.; Kane, E.; Munroe, C.; Barlow, L.L.; Zheng, H.; Chung, R.T. Hepatitis C Virus Infection and Its Clearance Alter Circulating Lipids: Implications for Long-Term Follow-Up. 2009. Available online: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.23219 (accessed on 13 January 2024).
- Butt, A.A.; Yan, P.; Shuaib, A.; Abou-Samra, A.B.; Shaikh, O.S.; Freiberg, M.S. Direct-Acting Antiviral Therapy for HCV Infection Is Associated with a Reduced Risk of Cardiovascular Disease Events. Gastroenterology 2019, 156, 987–996. [Google Scholar] [CrossRef]
- Mahale, P.; Engels, E.A.; Li, R.; Torres, H.A.; Hwang, L.Y.; Brown, E.L.; Kramer, J.R. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut 2018, 67, 553. [Google Scholar] [CrossRef]
- Daskalopoulou, S.S.; Delaney, J.A.C.; Filion, K.B.; Brophy, J.M.; Mayo, N.E.; Suissa, S. Discontinuation of Statin Therapy Following an Acute Myocardial Infarction: A Population-Based Study. Available online: https://academic.oup.com/eurheartj/article/29/17/2083/578019 (accessed on 24 January 2024).
- Petta, S.; Adinolfi, L.E.; Fracanzani, A.L.; Rini, F.; Caldarella, R.; Calvaruso, V.; Cammà, C.; Ciacco, M.; Di-Marco, V.; Grimaudo, S.; et al. Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J. Hepatol. 2018, 69, 18–24. [Google Scholar] [CrossRef]
- A Systematic Review and Meta-Analysis of the Therapeutic Equivalence of Statins. Available online: www.hiv-druginteractions.org (accessed on 25 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).