Investigation of SARS-CoV-2 Infection among Companion Animals in Households with Confirmed Human COVID-19 Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Sample Collection
2.2. RT-PCR, Serology, and Genomic Sequencing
2.3. Data Analysis
3. Results
3.1. Descriptive Results
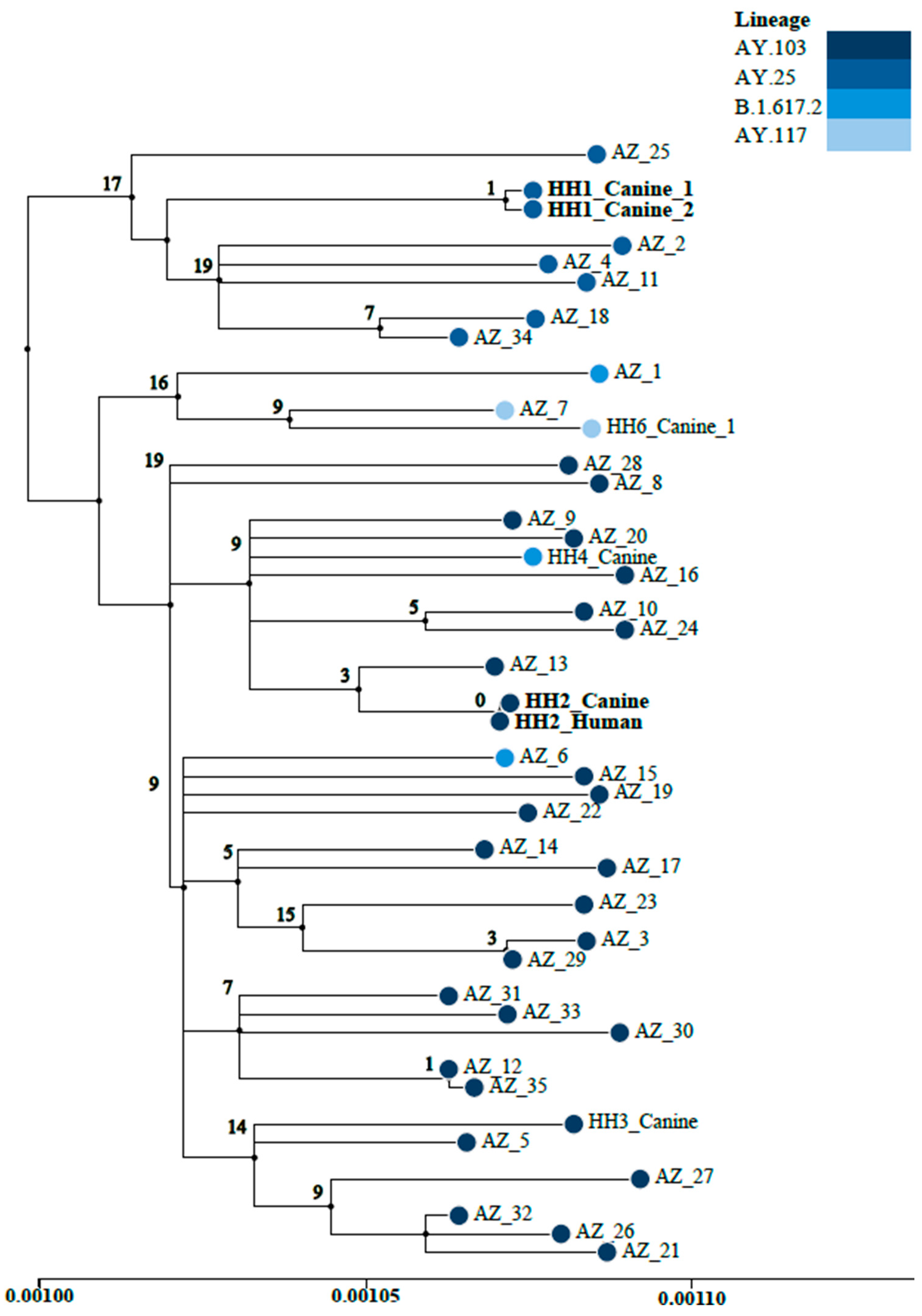
3.2. Whole-Genome Sequencing
3.3. Risk Factor Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimers
References
- Sit, T.H.; Brackman, C.J.; Ming Ip, S.M.; Tam, K.W.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Jairack, W.; Chamsai, E.; Udom, K.; Charoenkul, K.; Chaiyawong, S.; Techakriengkrai, N.; Tangwangvivat, R.; Suwannakarn, K.; Amonsin, A. SARS-CoV-2 delta variant infection in domestic dogs and cats, Thailand. Sci. Rep. 2022, 12, 8403. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.S.; Peloquin, S.; Proia, K.; Hart, E.; Lee, J.; Vyhnal, K.K.; Sasaki, E.; Balamayooran, G.; Asin, J.; Southard, T.; et al. Investigation of SARS-CoV-2 infection and associated lesions in exotic and companion animals. Vet. Path. 2022, 59, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Smith, D.; Ghai, R.R.; Wallace, R.M.; Torchetti, M.K.; Loiacono, C.; Murrell, L.S.; Carpenter, A.; Moroff, S.; Rooney, J.A.; et al. First reported cases of SARS-CoV-2 infection in companion animals—New York, March-April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.I.; Elia, G.; Grassi, A.; Giordano, A.; Desario, C.; Medardo, M.; Smith, S.L.; Anderson, E.R.; Prince, T.; Patterson, G.T.; et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Commun. 2020, 11, 6231. [Google Scholar] [CrossRef] [PubMed]
- Oude Munnink, B.B.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2021, 371, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Pickering, B.; Lung, O.; Maguire, F.; Kruczkiewicz, P.; Kotwa, J.D.; Buchanan, T.; Gagnier, M.; Guthrie, J.L.; Jardine, C.M.; Marchand-Austin, A.; et al. Highly divergent white-tailed deer SARS-CoV-2 with potential deer-to-human transmission. Nat. Microbiol. 2022, 7, 2011–2024. [Google Scholar] [CrossRef] [PubMed]
- Sila, T.; Sunghan, J.; Laochareonsuk, W.; Surasombatpattana, S.; Kongkamol, C.; Ingviya, T.; Siripaitoon, P.; Siripaitoon, P.; Kanchanasuwan, S.; Hortiwakul, T.; et al. Suspected Cat-to-Human Transmission of SARS-CoV-2, Thailand, July–September 2021. Emerg. Infect. Dis. 2022, 28, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, A.A.; Richardson, K.L.; Ghai, R.R.; Pope, B.; Yeadon, J.; Culp, B.; Behravesh, C.B.; Liu, L.; Brown, J.A.; Boyer, L.V. Probable Transmission of SARS-CoV-2 from African Lion to Zoo Employees, Indiana, USA, 2021. Emerg. Infect. Dis. 2023, 29, 1102–1108. [Google Scholar] [CrossRef]
- Goraichuk, I.V.; Arefiev, V.; Stegniy, B.T.; Gerilovych, A.P. Zoonotic and Reverse Zoonotic Transmissibility of SARS-CoV-2. Virus Res. 2021, 302, 198473. [Google Scholar] [CrossRef]
- Teixeira, A.I.P.; de Brito, R.N.; Gontijo, C.C.; Romero, G.A.S.; Ramalho, W.M.; Haddad, R.; Noronha, E.F.; de Araújo, W.N. The role of pets in SARS-CoV-2 transmission: An exploratory analysis. Infection 2022, 51, 455–458. [Google Scholar] [CrossRef]
- Agüero, B.; Berrios, F.; Pardo-Roa, C.; Ariyama, N.; Bennett, B.; Medina, R.A.; Neira, V. First detection of Omicron variant BA.4.1 lineage in dogs, Chile. Vet. Q. 2024, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goryoka, G.W.; Cossaboom, C.M.; Gharpure, R.; Dawson, P.; Tansey, C.; Rossow, J.; Mrotz, V.; Rooney, J.; Torchetti, M.; Loiacono, C.M.; et al. One Health Investigation of SARS-CoV-2 Infection and Seropositivity among Pets in Households with Confirmed Human COVID-19 Cases-Utah and Wisconsin, 2020. Viruses 2021, 13, 1813. [Google Scholar] [CrossRef]
- Chan, T.; Klaus, J.; Meli, M.L.; Hofmann-Lehmann, R. SARS-CoV-2 infections in cats, dogs, and other animal species: Findings on infection and data from Switzerland. Schweiz Arch. Tierheilkd 2021, 163, 821–835. [Google Scholar] [CrossRef]
- Michelitsch, A.; Allendorf, V.; Conraths, F.J.; Gethmann, J.; Schulz, J.; Wernike, K.; Denzin, N. SARS-CoV-2 Infection and Clinical Signs in Cats and Dogs from Confirmed Positive Households in Germany. Viruses 2023, 15, 837. [Google Scholar] [CrossRef]
- Giraldo-Ramirez, S.; Rendon-Marin, S.; Jaimes, J.A.; Martinez-Gutierrez, M.; Ruiz-Saenz, J. SARS-CoV-2 clinical outcome in domestic and wild cats: A systematic review. Animals 2021, 11, 2056. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.; Ghai, R.R.; Gary, J.; Ritter, J.M.; Carvallo, F.R.; Diel, D.G.; Martins, M.; Murphy, J.; Schroeder, B.; Brightbill, K.; et al. Determining the role of natural SARS-CoV-2 infection in the death of domestic pets: 10 cases (2020–2021). J. Am. Vet. Med. Assoc. 2021, 259, 1032–1039. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Santana, D.S.; Lima, M.B.M.; Silva, C.S.; de Oliveira, L.G.; Monteiro, E.O.L.; Dias, R.d.S.; Pereira, B.d.K.B.; Nery, P.A.d.S.; Ferreira, M.A.S.; et al. Assessment of the Risk Impact of SARS-CoV-2 Infection Prevalence between Cats and Dogs in America and Europe: A Systematic Review and Meta-Analysis. Pathogens 2024, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.-J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microb. Inf. 2020, 9, 2394–2403. [Google Scholar] [CrossRef]
- Yaglom, H.Y.; Hecht, G.; Goedderz, A.; Jasso-Selles, D.; Ely, J.L.; Ruberto, I.; Bowers, J.R.; Engelthaler, D.M.; Venkat, H. Genomic investigation of a household SARS-CoV-2 disease cluster in Arizona involving a cat, dog, and pet owner. One Health 2021, 13, 100333. [Google Scholar] [CrossRef]
- Ladner, J.T.; Larsen, B.B.; Bowers, J.R.; Hepp, C.M.; Bolyen, E.; Folkerts, M.; Sheridan, K.; Pfeiffer, A.; Yaglom, H.; Yaglom, D.; et al. An Early Pandemic Analysis of SARS-CoV-2 Population Structure and Dynamics in Arizona. mBio 2020, 11, e02107-20. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, M.L.; Lemmer, D.; Pfeiffer, A.; Vazquez, D.; French, C.; Jones, A.; Nguyen, M.; Larsen, B.; Porter, W.T.; Sheridan, K.; et al. Sequencing the pandemic: Rapid and high-throughput processing and analysis of COVID-19 clinical samples for 21st century public health. F1000Research 2021, 10, 48. [Google Scholar] [CrossRef]
- O’Toole, Á.; Scher, E.; Underwood, A.; Jackson, B.; Hill, V.; McCrone, J.T.; Colquhoun, R.; Ruis, C.; Abu-Dahab, K.; Taylor, B.; et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus. Evol. 2021, 7, veab064. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Boyles, A.; Shankar, A.; Kim, J.; Knyazev, S.; Cintron, R.; Switzer, W.M. MicrobeTrace: Retooling molecular epidemiology for rapid public health response. PLoS Comput. Biol. 2021, 17, e1009300. [Google Scholar] [CrossRef] [PubMed]
- Hamer, S.A.; Pauvolid-Corrêa, A.; Zecca, I.B.; Davila, E.; Auckland, L.D.; Roundy, C.M.; Tang, W.; Torchetti, M.K.; Killian, M.L.; Jenkins-Moore, M.; et al. SARS-CoV-2 Infections and Viral Isolations among Serially Tested Cats and Dogs in Households with Infected Owners in Texas, USA. Viruses 2021, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Pourbagher-Shahri, A.M.; Mohammadi, G.; Ghazavi, H.; Forouzanfar, F. Susceptibility of domestic and companion animals to SARS-CoV-2: A comprehensive review. Trop. Anim. Health Prod. 2023, 55, 60. [Google Scholar] [CrossRef]
- Schimmoller, B.J.; Trovao, N.S.; Isbell, M.; Goel, C.; Heck, B.F.; Archer, T.C.; Cardinal, K.D.; Naik, N.B.; Dutta, S.; Daniel, A.R.; et al. COVID-19 Exposure Assessment Tool (CEAT): Exposure quantification based on ventilation, infection prevalence, group characteristics, and behavior. Sci. Adv. 2022, 8, eabq0593. [Google Scholar] [CrossRef]
- Allen, J.G.; Ibrahim, A.M. Indoor Air Changes and Potential Implications for SARS-CoV-2 Transmission. JAMA 2021, 325, 2112–2113. [Google Scholar] [CrossRef]
- Kannekens-Jager, M.M.; de Rooij, M.M.T.; de Groot, Y.; Biesbroeck, E.; de Jong, M.K.; Pijnacker, T.; Schuurman, N.; Broekhuizen-Stins, M.J.; Zhao, S.; Duim, B.; et al. SARS-CoV-2 infection in dogs and cats is associated with contact to COVID-19-positive household members. Transbound. Emerg. Dis. 2022, 69, 4034–4040. [Google Scholar] [CrossRef]
- Bienzle, D.; Rousseau, J.; Marom, D.; MacNicol, J.; Jacobson, L.; Sparling, S.; Prystajecky, N.; Fraser, E.; Weese, J.S. Risk Factors for SARS-CoV-2 Infection and Illness in Cats and Dogs. Emerg. Infect. Dis. 2022, 28, 1154–1162. [Google Scholar] [CrossRef]
- Calvet, G.A.; Pereira, S.A.; Ogrzewalska, M.; Pauvolid-Correa, A.; Resende, P.C.; de Souza Tassinari, W.; de Pina Costa, A.; Keidel, L.O.; da Rocha, A.S.B.; da Silva, M.F.B.; et al. Investigation of SARS-COV-2 Infection in Dogs and Cats of Humans Diagnosed with Covid-19 in Rio De Janeiro, Brazil. PLoS ONE 2021, 16, e0250853. [Google Scholar] [CrossRef]
- Liew, A.Y.; Carpenter, A.; Moore, T.A.; Wallace, R.M.; Hamer, S.A.; Hamer, G.L.; Fischer, R.S.B.; Zecca, I.B.; Davila, E.; Auckland, L.D.; et al. Clinical and epidemiologic features of SARS-CoV-2 in dogs and cats compiled through national surveillance in the United States. J. Am. Vet. Med. Assoc. 2023, 261, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Alberto-Orlando, S.; Calderon, J.L.; Leon-Sosa, A.; Patiño, L.; Zambrano-Alvarado, M.N.; Pasquel-Villa, L.D.; Rugel-Gonzalez, D.O.; Flores, D.; Mera, M.D.; Valencia, P.; et al. SARS-CoV-2 transmission from infected owner to household dogs and cats is associated with food sharing. Int. J. Infect. Dis. 2022, 122, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, E.C.; Reid, T.J. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound. Emerg. Dis. 2021, 68, 1850–1867. [Google Scholar] [CrossRef] [PubMed]
- Ferasin, L.; Fritz, M.; Ferasin, H.; Bequart, P.; Corbet, S.; Gouilh, M.A.; Legros, V.; Leroy, E.M. Infection with SARS-CoV-2 variant B.1.1.7 detected in a group of dogs and cats with suspected myocarditis. Vet. Rec. 2021, 189, 1–9. [Google Scholar] [CrossRef]
- De Morais, H.A.; Dos Santos, A.P.; Do Nascimento, N.C.; Kmetiuk, L.B.; Barbosa, D.S.; Brandão, P.E.; Guimaraes, A.M.S.; Pettan-Brewer, C.; Barbosa, D.S.; Brandão, P.E.; et al. Natural Infection by SARS-CoV-2 in Companion Animals: A Review of Case Reports and Current Evidence of Their Role in the Epidemiology of COVID-19. Front. Vet. Sci. 2020, 7, 591216. [Google Scholar] [CrossRef] [PubMed]
- Jarrah, S.A.; Kmetiuk, L.B.; Valleriani, F.; Bonfini, B.; Lorusso, A.; Vasinioti, V.; Decaro, N.; dos Santos, M.T.; Spohr, K.A.H.; Pratelli, A.; et al. SARS-CoV-2 antibodies in dogs and cats in a highly infected area of Brazil during the pandemic. Front. Vet. Sci. 2023, 10, 1111728. [Google Scholar] [CrossRef]
- Barroso, R.; Vieira-Pires, A.; Antunes, A.; Fidalgo-Carvalho, I. Susceptibility of Pets to SARS-CoV-2 Infection: Lessons from a Seroepidemiologic Survey of Cats and Dogs in Portugal. Microorganisms 2022, 10, 345. [Google Scholar] [CrossRef]
- Decaro, N.; Grassi, A.; Lorusso, E.; Patterson, E.I.; Lorusso, A.; Desario, C.; Anderson, E.R.; Vasinioti, V.; Wastika, C.E.; Hughes, G.L.; et al. Long-term persistence of neutralizing SARS-CoV-2 antibodies in pets. Transbound. Emerg. Dis. 2021, 69, 3073–3076. [Google Scholar] [CrossRef]
- Kang, S.W.; Park, H.; Yeun Kim, J.; Bae, J.Y.; Park, M.S.; Kim, S.H. Comparison of culture-competent virus shedding duration of SARS-CoV-2 Omicron variant in regard to vaccination status: A prospective cohort study. Vaccine 2023, 41, 2769–2772. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.Y.; Park, H.; Park, S.; Lim, J.S.; Lim, S.Y.; Lim, J.S.; Lim, S.Y.; Bae, S.; Lim, Y.-J.; et al. Transmission and Infectious SARS-CoV-2 Shedding Kinetics in Vaccinated and Unvaccinated Individuals. JAMA Netw. Open 2022, 5, e2213606. [Google Scholar] [CrossRef]
| Characteristic | Number of Pets (%) |
|---|---|
| Species | |
| Dog | 71 (64.5%) |
| Cat | 39 (35.5%) |
| Breed/Breed Group | |
| Dog (n = 71) | |
| Terrier | 14 (19.7%) |
| Herding | 11 (15.5%) |
| Toy | 11 (15.5%) |
| Sporting | 8 (11.3%) |
| Mixed breed | 8 (11.3%) |
| Working | 7 (9.9%) |
| Hound | 5 (7.0%) |
| Non-sporting | 4 (5.6%) |
| Foundation stock service | 3 (4.2%) |
| Cat (n = 39) | |
| Domestic Shorthair | 28 (71.8%) |
| Domestic Longhair | 5 (12.8%) |
| Siamese | 3 (7.7%) |
| American Curl | 1 (2.6%) |
| Devon Rex | 1 (2.6%) |
| Maine Coon | 1 (2.6%) |
| Age | 3 months–15 years (mean 5.8 years) |
| Range | |
| <1 year (pediatric) | 15 (13.6%) |
| 1–8 years (adult) | 63 (57.3%) |
| >8 years (senior) | 32 (29.1%) |
| Sex | |
| Male | 60 (54.5%) |
| Female | 50 (45.5%) |
| Spay/Neuter Status | |
| Spayed/neutered | 94 (85.5%) |
| Not spayed/neutered | 16 (14.5%) |
| SARS-CoV-2 laboratory testing | |
| Negative | 70 (63.6%) |
| Positive | 40 (36.4%) |
| PCR+ only | 9 |
| Seropositive only | 21 |
| PCR+ and seropositive | 10 |
| Health Status | |
| Asymptomatic | 77 (70%) |
| Recent clinical signs | 17 (15.5%) |
| Current clinical signs | 16 (14.5%) |
| Clinical signs 1 (n = 33) | |
| Sneezing | 26 (78.8%) |
| Lethargy | 15 (45.5%) |
| Ocular discharge | 14 (42.4%) |
| Nasal discharge | 13 (39.4%) |
| Coughing | 13 (39.4%) |
| Difficulty breathing | 8 (24.2%) |
| Anorexia/inappetence | 6 (18.2%) |
| Fever | 4 (12.1%) |
| Vomiting | 2 (6.1%) |
| Diarrhea | 2 (6.1%) |
| Time (in days) between case onset and pet sample collection | 4–114 (mean 31, median 17) |
| Variable | Number of Positive Pets/ Total (%) | Relative Risk (95% Confidence Interval); p-Value (Fisher Exact) |
|---|---|---|
| Cat | 13/39 (33.3%) | RR 0.88 (0.51–1.50) |
| Dog | 27/71 (38.0%) | |
| Pediatric/old (<1 or >8 yrs.) | 14/48 (29.2%) | RR 1.22 (0.92–1.61) |
| Adult (1–8 years old) | 26/62 (41.9%) | |
| Male | 17/50 (34.0%) | RR 0.89 (0.54–1.47) |
| Female | 23/60 (38.3%) | |
| Spayed/neutered | 31/94 (33.0%) | RR 0.58 (0.35–0.99); p = 0.09 |
| Not spayed/neutered | 9/16 (56.3%) | |
| Pet immunocompromised | 14/25 (56.0%) | RR 1.83 (1.14–2.94); p = 0.03 * |
| Pet not immunocompromised | 26/85 (30.6%) | |
| Pet ever symptomatic | 13/33 (39.4%) | RR 1.12 (0.67–1.89) |
| Pet asymptomatic | 27/77 (35.1%) | |
| More than one individual in household tested positive | 32/61 (52.5%) | RR 3.21 (1.63–6.33); p = 0.0001 * |
| Only one individual in household tested positive | 8/49 (16.3%) | |
| Multiple pets residing in the household | 34/95 (35.8%) | RR 0.89 (0.46–1.76) |
| Single pet household | 6/15 (40.0%) | |
| Household residence type: | RR 0.97 (0.38–2.45) | |
| Private home (free-standing)/townhouse | 37/102 (36.3%) | |
| Apartment/condo | 3/8 (37.5%) | |
| Household risk (vaccination status): | RR 0.77 (0.47–1.27) | |
| Low/Medium | 20/62 (32.3%) | |
| High | 20/48 (41.7%) | |
| PCR samples: | RR 3.54 (1.36–9.22); p = 0.01 * | |
| ≤14 days between case onset and pet sample collection | 8/26 (30.8%) | |
| >14 days between case onset and pet sample collection | 6/69 (8.7%) | |
| Virus neutralization (serology) samples: | RR 3.30 (0.86–12.64) | |
| ≤30 days between case onset and pet sample collection | 19/46 (41.3%) | |
| >30 days between case onset and pet sample collection | 2/16 (14.3%) | |
| Pet contact w/symptomatic case: | RR 1.78 (0.98–3.26) | |
| Slept in same bed | 30/69 (43.5%) | |
| Did not sleep in same bed | 10/41 (24.4%) | |
| Licking face and/or hands | 17/49 (34.7%) | RR 0.92 (0.56–1.52) |
| Did not lick face and/or hands | 23/61 (37.7%) | |
| Sharing food and/or utensils | 0/5 | RR 0.37 (0.06–2.33) |
| Not sharing food and/or utensils | 40/105 (38.1%) | |
| Providing medical care to pet | 4/7 (57.1%) | RR 1.63 (0.82–3.27) |
| Not providing medical care to pet | 36/103 (35.0%) | |
| Taking walks (dogs only) | 13/33 (39.4%) | RR 1.07 (0.59–1.94) |
| Not taking walks (dogs only) | 14/38 (36.8%) | |
| Lying on couch together/pet sits on lap | 27/84 (32.1%) | RR 0.64 (0.39–1.05) |
| No lying on couch together/pet does not sit on lap | 13/26 (50.0%) | |
| Cleaning pet waste | 11/26 (42.3%) | RR 1.22 (0.72–2.10) |
| No cleaning pet waste | 29/84 (34.5%) | |
| Petting/cuddling | 35/100 (35.0%) | RR 0.70 (0.36–1.37) |
| No petting/cuddling | 5/10 (50.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkat, H.; Yaglom, H.D.; Hecht, G.; Goedderz, A.; Ely, J.L.; Sprenkle, M.; Martins, T.; Jasso-Selles, D.; Lemmer, D.; Gesimondo, J.; et al. Investigation of SARS-CoV-2 Infection among Companion Animals in Households with Confirmed Human COVID-19 Cases. Pathogens 2024, 13, 466. https://doi.org/10.3390/pathogens13060466
Venkat H, Yaglom HD, Hecht G, Goedderz A, Ely JL, Sprenkle M, Martins T, Jasso-Selles D, Lemmer D, Gesimondo J, et al. Investigation of SARS-CoV-2 Infection among Companion Animals in Households with Confirmed Human COVID-19 Cases. Pathogens. 2024; 13(6):466. https://doi.org/10.3390/pathogens13060466
Chicago/Turabian StyleVenkat, Heather, Hayley D. Yaglom, Gavriella Hecht, Andrew Goedderz, Jennifer L. Ely, Michael Sprenkle, Taylor Martins, Daniel Jasso-Selles, Darrin Lemmer, Jordan Gesimondo, and et al. 2024. "Investigation of SARS-CoV-2 Infection among Companion Animals in Households with Confirmed Human COVID-19 Cases" Pathogens 13, no. 6: 466. https://doi.org/10.3390/pathogens13060466





