Oropouche Virus Exposure in Febrile Patients during Chikungunya Virus Introduction in the State of Amapá, Amazon Region, Brazil
Abstract
:1. Introduction
2. Materials and Methods
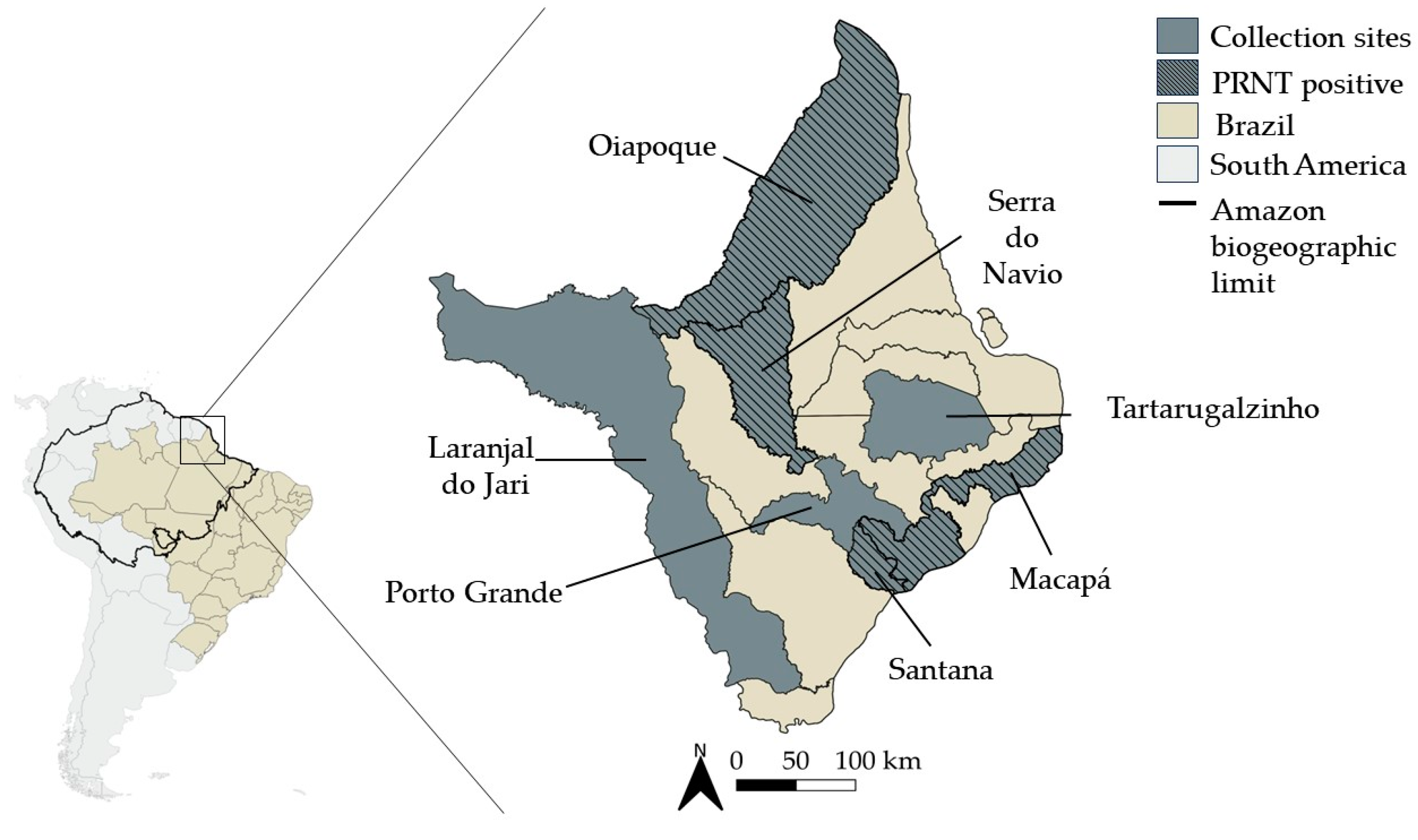
2.1. Study Site and Samples Collection
2.2. Molecular Investigation of CHIKV and OROV
2.3. Serological Investigation of CHIKV
2.4. Neutralizing Antibodies Investigation of OROV
3. Results
Oropouche Surveillance in Febrile Patients during Chikungunya Outbreak
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, N.I.O.; Sacchetto, L.; de Rezende, I.M.; Trindade, G.d.S.; LaBeaud, A.D.; de Thoisy, B.; Drumond, B.P. Recent sylvatic yellow fever virus transmission in Brazil: The news from an old disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Franco, O. História da Febre Amarela No Brasil. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/0110historia_febre.pdf (accessed on 16 December 2023).
- Schatzmayr, H.G.; Nogueira, R.M.R.; Rosa, A.P.A.T. An outbreak of dengue virus at Rio de Janeiro—1986. Mem. Inst. Oswaldo Cruz 1986, 81, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.C.G.; de Filippis, A.M.B.; Lima, M.; Faria, N.; de Bruycker-Nogueira, F.; Santos, J.B.; Heringer, M.; Chouin-Carneiro, T.; Couto-Lima, D.; de Santis Gonçalves, B.; et al. 30 years of dengue fatal cases in Brazil: A laboratorial-based investigation of 1047 cases. BMC Infect. Dis. 2018, 18, 346. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, M.; Fischer, M.; Staples, J.E. Zika Virus Spreads to New Areas—Region of the Americas, May 2015–January 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 55–58. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; Faria, N.R.; Vasconcelos, J.M.d.; Golding, N.; Kraemer, M.U.; de Oliveira, L.F.; Azevedo, R.D.S.D.S.; da Silva, D.E.A.; da Silva, E.V.P.; da Silva, S.P.; et al. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W. The Newala epidemic. III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J. Hyg. 1956, 54, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Brault, A.C.; Tesh, R.B.; Weaver, S.C. Re-emergence of chikungunya and o’nyong-nyong viruses: Evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000, 81, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Gasque, P.; Couderc, T.; Lecuit, M.; Roques, P.; Ng, L.F. Chikungunya virus pathogenesis and immunity. Vector-Borne Zoonotic Dis. 2015, 15, 241–249. [Google Scholar] [CrossRef]
- Amaral, J.K.; Taylor, P.C.; Schoen, R.T. Brazil at the Center of Chikungunya Outbreaks. J. Glob. Infect. Dis. 2023, 15, 131–132. [Google Scholar] [CrossRef]
- Imad, H.A.; Phadungsombat, J.; Nakayama, E.E.; Suzuki, K.; Ibrahim, A.M.; Afaa, A.; Azeema, A.; Nazfa, A.; Yazfa, A.; Ahmed, A.; et al. Clinical Features of Acute Chikungunya Virus Infection in Children and Adults during an Outbreak in the Maldives. Am. J. Trop. Med. Hyg. 2021, 105, 946–954. [Google Scholar] [CrossRef]
- de Souza, T.M.A.; de Lima, R.C.; Solórzano, V.E.F.; Damasco, P.V.; de Souza, L.J.; Sanchez-Arcila, J.C.; Guimarães, G.M.C.; Paiva, I.A.; da Rocha Queiroz Lima, M.; de Bruycker-Nogueira, F.; et al. Was It Chikungunya? Laboratorial and Clinical Investigations of Cases Occurred during a Triple Arboviruses’ Outbreak in Rio de Janeiro, Brazil. Pathogens 2022, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, A.; Dominguez, M.; Helynck, B.; Sissoko, D.; Wichmann, O.; Quenel, P.; Germonneau, P.; Quatresous, I. Atypical Chikungunya virus infections: Clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Réunion. Epidemiol. Infect. 2009, 137, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Deeba, I.M.; Hasan, M.M.; Al Mosabbir, A.; Siam, M.H.B.; Islam, M.S.; Raheem, E.; Hossain, M.S. Manifestations of Atypical Symptoms of Chikungunya during the Dhaka Outbreak (2017) in Bangladesh. Am. J. Trop. Med. Hyg. 2019, 100, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.K.; Alera, M.T.; Lago, C.B.; Tac-An, I.A.; Villa, D.; Fernandez, S.; Thaisomboonsuk, B.; Klungthong, C.; Levy, J.W.; Velasco, J.M.; et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Neglected Trop. Dis. 2015, 9, e0003764. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.V.; Trinta, K.S.; Montalbano, C.A.; Sucupira, M.V.; de Lima, M.M.; Marques, E.; Romanholi, I.H.; Croda, J. Seroprevalence of chikungunya virus in a rural community in Brazil. PLoS Neglected Trop. Dis. 2017, 11, e0005319. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H. Oropouche virus: A new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961, 10, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.D.P.; Bensabath, G.; Causey, O.R.; Shope, R. Epidemia de vírus Oropouche em Belém. Rev. Serviço Espec. Saúde Pública 1962, 2, 15–23. [Google Scholar]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.; Zuchi, N.; Souza, V.C.; Naveca, F.G.; Santos, M.A.; Slhessarenko, R.D. Detection of Oropouche virus segment S in patients and in Culex quinquefasciatus in the state of Mato Grosso, Brazil. Memórias Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef]
- de Souza Luna, L.K.; Rodrigues, A.H.; Santos, R.I.; Sesti-Costa, R.; Criado, M.F.; Martins, R.B.; Silva, M.L.; Delcaro, L.S.; Proença-Modena, J.L.; Figueiredo, L.T.; et al. Oropouche virus is detected in peripheral blood leukocytes from patients. J. Med. Virol. 2017, 89, 1108–1111. [Google Scholar] [CrossRef]
- Durango-Chavez, H.V.; Toro-Huamanchumo, C.J.; Silva-Caso, W.; Martins-Luna, J.; Aguilar-Luis, M.A.; Del Valle-Mendoza, J.; Puyen, Z.M. Oropouche virus infection in patients with acute febrile syndrome: Is a predictive model based solely on signs and symptoms useful? PLoS ONE 2022, 17, e0270294. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Chiaravalloti-Neto, F. Brazil reports an increased incidence of oropouche and mayaro fever in the amazon region. Travel Med. Infect. Dis. 2024, 58, 102692. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO. Atualização Epidemiológica—Oropouche na Região das Américas—6 de Março de 2024. Available online: https://reliefweb.int/report/world/epidemiological-alert-oropouche-region-americas-2-february-2024?gad_source=1&gclid=EAIaIQobChMI0560lOC-hgMVERZ7Bx2lPAItEAAYASAAEgJA4vD_BwE (accessed on 9 March 2024).
- Filho, A.C.; Souza, O.B.d. Atlas de Pressões e Ameaças às Terra Indígenas na Amazônia Brasileira. Available online: https://acervo.socioambiental.org/sites/default/files/publications/I4L00018.pdf (accessed on 10 March 2024).
- IBGE. Cidades e Estados—Amapá. Available online: https://www.ibge.gov.br/cidades-e-estados/ap.html (accessed on 17 February 2024).
- Costa, J.S.S.; Borges, W.L. Caracterização dos municípios do Amapá, amazônia oriental brasileira, quanto às diferenças nos níveis de desenvolvimento. Rev. Bras. Gestão Desenvolv. Reg. 2022, 18, 361–374. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya Virus in US Travelers Returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Nascimento, V.A.D.; Souza, V.C.; Nunes, B.T.D.; Rodrigues, D.S.G.; Vasconcelos, P. Multiplexed reverse transcription real-time polymerase chain reaction for simultaneous detection of Mayaro, Oropouche, and Oropouche-like viruses. Memórias Inst. Oswaldo Cruz 2017, 112, 510–513. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Malhas Territoriais. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais.html (accessed on 26 February 2024).
- RAISG. Cartographic Data—Visualization of Geospatial Information about the Amazon. Available online: https://www.raisg.org/en/maps/ (accessed on 26 March 2024).
- de Souza, W.M.; Ribeiro, G.S.; de Lima, S.T.S.; de Jesus, R.; Moreira, F.R.R.; Whittaker, C.; Sallum, M.A.M.; Carrington, C.V.F.; Sabino, E.C.; Kitron, U.; et al. Chikungunya: A decade of burden in the Americas. Lancet Reg. Health-Am. 2024, 30, 100673. [Google Scholar] [CrossRef] [PubMed]
- Sahadeo, N.S.D.; Allicock, O.M.; De Salazar, P.M.; Auguste, A.J.; Widen, S.; Olowokure, B.; Gutierrez, C.; Valadere, A.M.; Polson-Edwards, K.; Weaver, S.C.; et al. Understanding the evolution and spread of chikungunya virus in the Americas using complete genome sequences. Virus Evol. 2017, 3, vex010. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.T.M. Emergent arboviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2007, 40, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.B.; Nunes, M.R.; Casseb, L.M.; Carvalho, V.L.; Pinto da Silva, E.V.; Silva, M.; Casseb, S.M.; Vasconcelos, P.F. Molecular epidemiology of Oropouche virus, Brazil. Emerg. Infect. Dis. 2011, 17, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.; Calisher, C.H. Emergence of Human Arboviral Diseases in the Americas, 2000–2016. Vector-Borne Zoonotic Dis. 2016, 16, 295–301. [Google Scholar] [CrossRef]
- Silva-Caso, W.; Aguilar-Luis, M.A.; Palomares-Reyes, C.; Mazulis, F.; Weilg, C.; Del Valle, L.J.; Espejo-Evaristo, J.; Soto-Febres, F.; Martins-Luna, J.; Del Valle-Mendoza, J. First outbreak of Oropouche Fever reported in a non-endemic western region of the Peruvian Amazon: Molecular diagnosis and clinical characteristics. Int. J. Infect. Dis. 2019, 83, 139–144. [Google Scholar] [CrossRef]
- Bastos, M.S.; Lessa, N.; Naveca, F.G.; Monte, R.L.; Braga, W.S.; Figueiredo, L.T.; Ramasawmy, R.; Mourão, M.P. Detection of Herpesvirus, Enterovirus, and Arbovirus infection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J. Med. Virol. 2014, 86, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Gibrail, M.M.; Fiaccadori, F.S.; Souza, M.; Almeida, T.N.; Chiang, J.O.; Martins, L.C.; Ferreira, M.S.; Cardoso, D. Detection of antibodies to Oropouche virus in non-human primates in Goiânia City, Goiás. Rev. Da Soc. Bras. De Med. Trop. 2016, 49, 357–360. [Google Scholar] [CrossRef] [PubMed]
- ProMED-PORT. Oropouche—Brasil (07) (BA), Atualização, Aumento do Número de Casos Confirmados, Surto. Available online: https://promedmail.org/promed-post/?id=8715990 (accessed on 17 April 2024).
- Pan American Health, O.; Pan American Health, O. Recommendations for Laboratory Detection and Diagnosis of Arbovirus Infections in the Region of the Americas; Pan American Health Organization: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos da Rosa, A.P.; Travassos da Rosa, J.F.; Ishak, R.; Freitas, R.B.; Gomes, M.L.; LeDuc, J.W.; Oliva, O.F. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am. J. Trop. Med. Hyg. 1981, 30, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.P.; Rosa, A.P.A.T.d.; Rosa, J.F.T.d.; Bensabath, G. An Outbreak of Oropouche Virus Disease in the Vicinity of Santarem, Para, Brazil. Tropenmed. Und Parasitol. 1976, 27, 213–223. [Google Scholar]
- Mourãão, M.P.; Bastos, M.S.; Gimaqu, J.B.; Mota, B.R.; Souza, G.S.; Grimmer, G.H.; Galusso, E.S.; Arruda, E.; Figueiredo, L.T. Oropouche fever outbreak, Manaus, Brazil, 2007–2008. Emerg. Infect. Dis. 2009, 15, 2063–2064. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.G.; Dos Santos, F.B.; Pauvolid-Corrêa, A. An Overview of Neglected Orthobunyaviruses in Brazil. Viruses 2022, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Zachary, I.G. The Utinga Virus: Antigenic Characterization and Serologic Epidemiology. Ph.D. Thesis, Yale University, New Haven, CT, USA, 1967. [Google Scholar]
- Shope, R.E.; Whitman, L. Nepuyo virus, a new group C agent isolated in Trinidad and Brazil. II. Serological studies. Am. J. Trop. Med. Hyg. 1966, 15, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Karabatsos, N.; Shope, R.E. Cross-reactive and type-specific complement-fixing structures of Oriboca virions. J. Med. Virol. 1979, 3, 167–176. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet. J. 2013, 195, 33–40. [Google Scholar] [CrossRef]
- Forthal, D.N. Functions of Antibodies. Microbiol Spectr 2014, 2, 1–17. [Google Scholar] [CrossRef]
- Mercier-Delarue, S.; Durier, C.; Colin de Verdière, N.; Poveda, J.D.; Meiffrédy, V.; Fernandez Garcia, M.D.; Lastère, S.; Césaire, R.; Manuggera, J.C.; Molina, J.M.; et al. Screening test for neutralizing antibodies against yellow fever virus, based on a flavivirus pseudotype. PLoS ONE 2017, 12, e0177882. [Google Scholar] [CrossRef] [PubMed]
- Dia, M.; Bob, N.S.; Talla, C.; Dupressoir, A.; Escadafal, C.; Thiam, M.S.; Diallo, A.; Ndiaye, O.; Heraud, J.M.; Faye, O.; et al. Performance assessment and validation of a plaque reduction neutralization test (PRNT) in support to yellow fever diagnostic and vaccine clinical trials. J. Med. Virol. 2023, 95, e28700. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Petar, P.; Dubois, D.; Rabin, B.S.; Shurin, M.R. Chapter 12—Immunoglobulin Titers and Immunoglobulin Subtypes. In Measuring Immunity; Lotze, M.T., Thomson, A.W., Eds.; Academic Press: London, UK, 2005; pp. 158–171. [Google Scholar] [CrossRef]
- Morens, D.M.; Halstead, S.B. Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J. Gen. Virol. 1990, 71 Pt 12, 2909–2914. [Google Scholar] [CrossRef]
- Linn, M.L.; Aaskov, J.G.; Suhrbier, A. Antibody-dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J. Gen. Virol. 1996, 77 Pt 3, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R. Antibody-Dependent Enhancement of Viral Infections; Springer Science and Business Media: Cham, Switzerland, 2020. [Google Scholar]
- Ribeiro Amorim, M.; Cornejo Pontelli, M.; Fabiano de Souza, G.; Primon Muraro, S.; de Toledo-Teixeira, D.A.; Forato, J.; Bispo-Dos-Santos, K.; Barbosa, N.S.; Cavalheiro Martini, M.; Lorencini Parise, P.; et al. Oropouche Virus Infects, Persists and Induces IFN Response in Human Peripheral Blood Mononuclear Cells as Identified by RNA PrimeFlow™ and qRT-PCR Assays. Viruses 2020, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Azevedo Rdo, S.; da Rosa, A.P.; Vasconcelos, P.F. Oropouche virus isolation, southeast Brazil. Emerg. Infect. Dis. 2005, 11, 1610–1613. [Google Scholar] [CrossRef]
- Lima-Camara, T.N. Emerging arboviruses and public health challenges in Brazil. Rev. Saude Publica 2016, 50, 36. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.M. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- Files, M.A.; Hansen, C.A.; Herrera, V.C.; Schindewolf, C.; Barrett, A.D.T.; Beasley, D.W.C.; Bourne, N.; Milligan, G.N. Baseline mapping of Oropouche virology, epidemiology, therapeutics, and vaccine research and development. NPJ Vaccines 2022, 7, 38. [Google Scholar] [CrossRef] [PubMed]
| CHIKV | Cases/Total | (%) |
|---|---|---|
| Positive RNA detection | 33/163 | 20.25 |
| Recent CHIKV infection (anti-CHIKV IgM) | 44/154 | 28.57 |
| CHIKV exposure (anti-CHIKV-IgG) | 19/151 | 12.58 |
| OROV | ||
| Positive RNA detection | 0/166 | |
| OROV exposure (NAb by PRNT90) | 17/166 | 10.24 |
| Patient ID | Gender/Years-Old | Days of Illness | Municipality | OROV PRNT90 Titer | CHIKV Diagnosis | ||
|---|---|---|---|---|---|---|---|
| Anti-CHIKV IgM | Anti-CHIKV IgG | CHIKV RT-qPCR (Ct) | |||||
| 7 AP | Female, 41 | NA | Santana | 80 | + | + | − |
| 13 AP | Male, 36 | 17 | Macapá | 80 | NT | NT | + (29,45) |
| 14 AP | Female, 56 | NA | Macapá | 80 | − | − | − |
| 26 AP | Male, 57 | 5 | Oiapoque | 20 | + | UND | − |
| 48 AP | Male, NA | 7 | Oiapoque | 160 | + | − | − |
| 55 AP | Female, 38 | 6 | Oiapoque | 160 | − | − | − |
| 87 AP | Male, 37 | NA | Santana | 80 | − | − | − |
| 97 AP | Male, 24 | NA | Macapá | 40 | + | − | − |
| 103 AP | Female, 50 | NA | Oiapoque | 320 | + | − | − |
| 111 AP | Male, 60 | NA | Santana | 160 | − | + | − |
| 115 AP | Female, 62 | NA | Macapá | ≥640 | + | + | − |
| 140 AP | Female, 59 | NA | Serra do Navio | 40 | + | − | − |
| 166 AP | Female, 88 | 2 | Oiapoque | 80 | − | UND | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, R.C.; Dias, H.G.; de Souza, T.M.A.; Familiar-Macedo, D.; Ribeiro, E.D.; Corrêa, V.C.e.; Pauvolid-Corrêa, A.; de Azeredo, E.L.; dos Santos, F.B. Oropouche Virus Exposure in Febrile Patients during Chikungunya Virus Introduction in the State of Amapá, Amazon Region, Brazil. Pathogens 2024, 13, 469. https://doi.org/10.3390/pathogens13060469
de Lima RC, Dias HG, de Souza TMA, Familiar-Macedo D, Ribeiro ED, Corrêa VCe, Pauvolid-Corrêa A, de Azeredo EL, dos Santos FB. Oropouche Virus Exposure in Febrile Patients during Chikungunya Virus Introduction in the State of Amapá, Amazon Region, Brazil. Pathogens. 2024; 13(6):469. https://doi.org/10.3390/pathogens13060469
Chicago/Turabian Stylede Lima, Raquel Curtinhas, Helver Gonçalves Dias, Thiara Manuele Alves de Souza, Débora Familiar-Macedo, Edcelha D’Athaide Ribeiro, Valmir Corrêa e Corrêa, Alex Pauvolid-Corrêa, Elzinandes Leal de Azeredo, and Flávia Barreto dos Santos. 2024. "Oropouche Virus Exposure in Febrile Patients during Chikungunya Virus Introduction in the State of Amapá, Amazon Region, Brazil" Pathogens 13, no. 6: 469. https://doi.org/10.3390/pathogens13060469









