The Role of Helminthophagous Fungi in the Biological Control of Human and Zoonotic Intestinal Helminths
Abstract
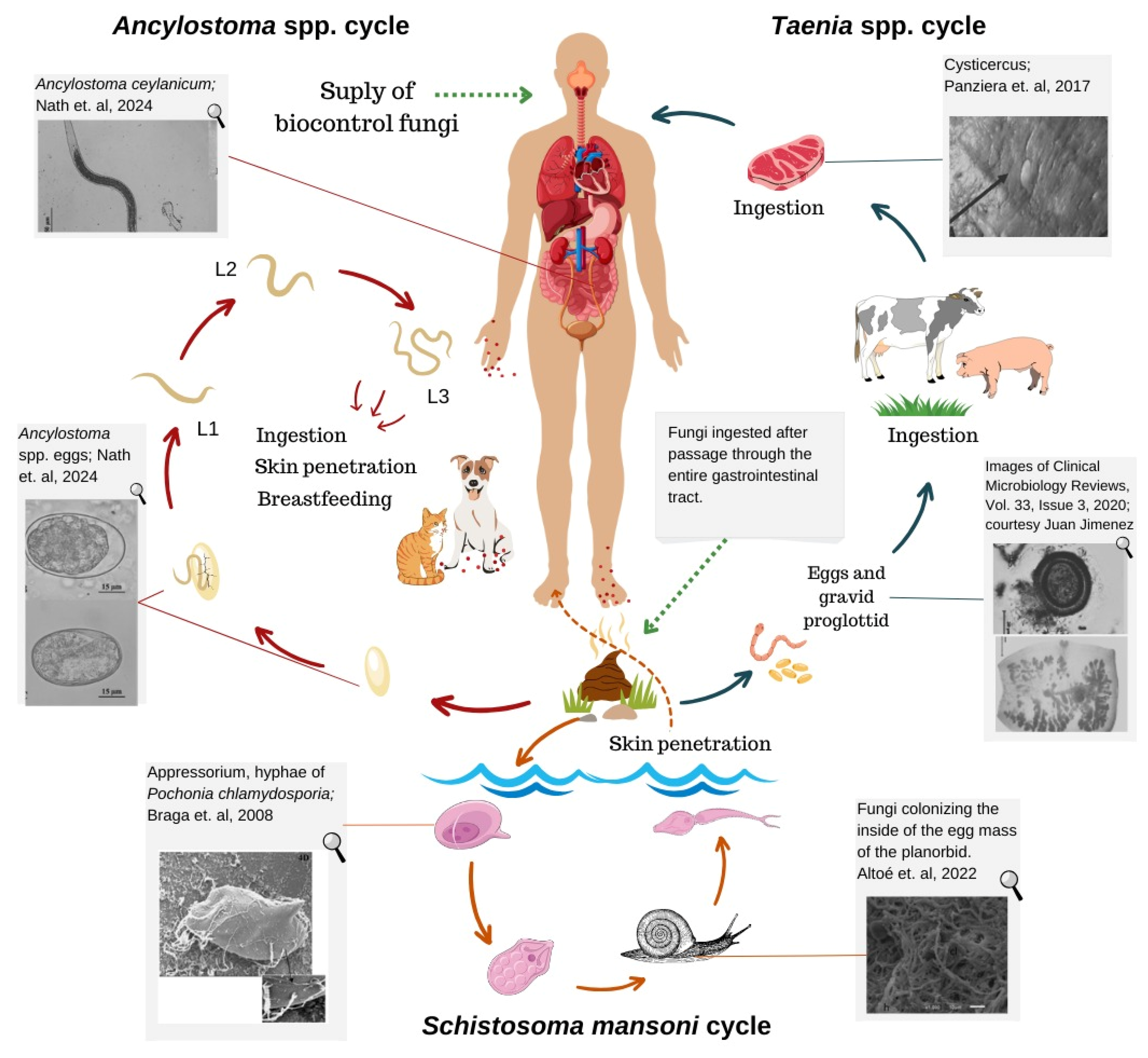
:1. Introduction
2. Helminthophagous Fungi—A Historical Report
3. Intestinal Parasites Transmitted between Animals and Humans
3.1. Dogs and Cats
3.2. Swine and Cattle: Teniasis–Cysticercosis Complex
4. Helminthophagous Fungi vs. Nematodes of Human Importance and Zoonotic Nematodes
5. Helminthophagous Fungi vs. Human-Associated Platyhelminths
6. Exploring the Potential of Helminthophagous Fungus
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Macpherson, C.N.L. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef]
- WHO. Centers for Disease Control and Prevention, Parasites. 2023. Available online: https://www.cdc.gov/sth/about/ (accessed on 15 May 2024).
- Pal, M.; Ayele, Y.; Hadush, A.; Kundu, P.; Jadhav, V.J.J. Public health significance of helminthiais: A systematic review. J. Exp. Food Chem. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Partridge, F.A.; Forman, R.; Bataille, C.J.R.; Wynne, G.M.; Nick, M.; Russell, A.J.; Else, K.J.; Sattelle, D.B.; Beilstein, J. Anthelmintic drug discovery: Target identification, screening. Org. Chem. 2020, 16, 1203–1224. [Google Scholar] [CrossRef]
- Marocco, C.; Bangert, M.; Joseph, S.A.; Fitzpatrick, C.; Montresor, A. Preventive chemotherapy in one year reduces by over 80% the number of individuals with soil-transmitted helminthiases causing morbidity: Results from meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 12–17. [Google Scholar] [CrossRef]
- Vokřál, I.; Podlipná, R.; Matoušková, P.; Skálová, L. Anthelmintics in the environment: Their occurrence, fate, and toxicity to non-target organisms. Chemosphere 2023, 345, 140446. [Google Scholar] [CrossRef]
- Braga, F.R.; Silva, A.R.; Araujo, J.M.; Carvalho, R.O.; Araújo, J.V.; Frassy, L.N. Atividade predatória dos fungos nematófagos Duddingtonia flagrans, Monacrosporium thaumasium e Artrobotrys robusta sobre larvas infectantes de Strongyloides stercoralis. Rev. Soc. Bras. Med. Trop. 2010, 43, 588–590. [Google Scholar] [CrossRef]
- Frassy, L.N.; Braga, F.R.; Silva, A.R.; Araújo, J.V.; Ferreira, S.R.; Freitas, L.G. Destruição de ovos de Toxocara canis pelo fungo nematófago Pochonia chlamydosporia. Rev. Soc. Bras. Med. Trop. 2010, 43, 102–104. [Google Scholar] [CrossRef]
- Carvalho, R.O.; Araújo, J.V.; Braga, F.R.; Araujo, J.M.; Alves, C.D.F. Ovicidal activity of Pochonia chlamydosporia and Paecilomyces lilacinus on Toxocara canis eggs. Vet. Parasitol. 2010, 169, 123–127. [Google Scholar] [CrossRef]
- Soares, F.E.F.; Queiroz, J.H.; Araújo, J.V.; Gouveia, A.; Queiroz, P.V.; Hiura, E.; Braga, F.R. Nematicidal action of chitinases produced by the fungus Monacrosporium thaumasium under laboratorial conditions. Biocontrol Sci. Techn. 2014, 25, 337–344. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Cavallero, S. Soil-transmitted helminth infections from a One Health perspective. Front. Med. 2023, 10, 1167812. [Google Scholar] [CrossRef]
- Mendes, L.Q.; Ferraz, C.M.; Ribeiro, N.R.C.; Ulfeldt, K.B.; Ribeiro, J.C.C.; Merizio, M.F.; Rossi, G.A.M.; Aguiar, A.A.R.M.; Araújo, J.V.; Soares, F.E.F.; et al. Efficacy of Duddingtonia flagrans (Bioverm®) on the biological control of buffalo gastrointestinal nematodes. Exp. Parasitol. 2023, 253, 108592. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.M.; Rodrigues, J.A.; Feitosa, T.F.; Braga, F.R.; Araújo, J.V.; Vilela, V.L.R. Evaluation of the fungus Duddingtonia flagrans (Bioverm®) on Ascaris suum eggs and infective larvae of Oesophagostomum spp. and Hyostrongylus rubidus from swine. Semin. Ciênc. Agrár. 2023, 44, 1587–1596. [Google Scholar] [CrossRef]
- Schwabe, C.W. Veterinary Medicine and Human Health; Williams and Wilkins: Baltimore, MD, USA, 1969; p. 713. [Google Scholar]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Tanner, M. From ‘one medicine’ to ‘one health’ and systemic approaches to health and well-being. Prev. Vet. Med. 2011, 101, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; Araújo, J.V.; Araujo, J.M.; Carvalho, R.O.; Silva, A.R. Efeito do fungo Paecilomyces lilacinus sobre ovos de Taenia saginata. Rev. Soc. Bras. Med. Trop. 2008, 41, 686–688. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Campos, A.K.; Siva, A.R.; Araujo, J.M.; Carvalho, R.O.; Correa, D.N.; Pereira, C.A.J. In vitro evaluation of the effect of the nematophagous fungi Duddingtonia flagrans, Monacrosporium sinense and Pochonia chlamydosporia on Schistosoma mansoni eggs. World J. Microbiol. Biotechnol. 2008, 24, 2713–2716. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Campos, A.K.; Araujo, J.M.; Silva, A.S.; Carvalho, R.O.; Tavela, A.O. In vitro evaluation of the action of the nematophagous fungi Duddingtonia flagrans, Monacrosporium sinense and Pochonia chlamydosporia on Fasciola hepatica eggs. World J. Microbiol. Biotechnol. 2008, 24, 1559–1564. [Google Scholar] [CrossRef]
- Lysek, H.; Sterba, J. Colonization of Ascaris lumbricoides eggs by the fungus Verticillium chlamydosporium Goddard. Folia Parasitol. 1991, 38, 255–259. [Google Scholar]
- Araujo, J.M.; Araújo, J.V.; Braga, F.R.; Carvalho, R.O.; SILVA, A.R.; Campos, A.K. Interaction and ovicidal activity of nematophagous fungus Pochonia chlamydosporia on Taenia saginata eggs. Exp. Parasitol. 2009, 121, 338–341. [Google Scholar] [CrossRef]
- Araujo, J.M.; Braga, F.R.; Araújo, J.V.; Benjamin, L.A. Comparison of the ovicidal activity of fungi Pochonia chlamydosporia and Paecilomyces lilacinus over eggs of Taenia saginata in lab conditions. RIAL 2010, 62, 1–11. [Google Scholar]
- Carvalho, R.O.; Araújo, J.V.; Braga, F.R.; Ferreira, S.R.; Araujo, J.M.; Silva, A.R.; Frassy, L.N.; Alves, C.D.F. Biological control of Ancylostomosis in dogs using the nematode-trapping fungus Monacrosporium thaumasium in southeastern Brazil. Vet. Parasitol. 2009, 165, 179–183. [Google Scholar] [CrossRef]
- De Souza Maia Filho, F.; Nunes, V.J.; Aires Berne, M.E.; Stoll, F.E.; Da Silva, N.P.; Pötter, L.; Brayer, P.D.I. Fungal ovicidal activity on Toxocara canis eggs. Rev. Iberoam. Micol. 2013, 30, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Deschiens, R. Capture et destruction de larves de Strongyloides du singe et du boeuf par des Hyphomycetes. Bull. Soc. Path. Exot. 1939, 32, 394–398. [Google Scholar]
- Deschiens, R. Conditions de capture des larves de Dictyocaulus par des Hyphomycétes prédateurs. Bull. Soc. Path. Exot. 1939, 32, 698–700. [Google Scholar]
- Descazeaux, J.; Capelle, R. Contribution à petude des champignons prédateurs de larves de nématodes parasites des animaux domestiques. Bull. Acad. Vet. 1939, 12, 284–288. [Google Scholar]
- Deschiens, R. Considérations relatives à la destruction des larves de nématodes parasites par des Hyphomycetes prédateurs. Bull. Soc. Path. Exot. 1939, 32, 459–464. [Google Scholar]
- Deschiens, R. Innocuité des Hyphomycétes prédateurs de nématodes pour la végétation des páturage et pour le bétail. C. R. Soc. Biol. 1941, 135, 830–832. [Google Scholar]
- Roubaud, E.; Descazeaux, J. Action de certains champignons prédateurs sur les larves de Strongylidés du cheval. Bull. Soc. Path. Exot. 1939, 32, 290–294. [Google Scholar]
- Roubaud, E.; Deschiens, R. Destruction de larves infectieuses d’ankylostomes et d’anguillules intestinales par Dactylella ellipsospora. Bull. Soc. Path. Exot. 1939, 32, 160–165. [Google Scholar]
- Roubaud, E.; Deschiens, R. Action des Hyphomycetes prédateurs sur les larves de synthétocaules et de Bunostomes. Bull. Soc. Path. Exot. 1941, 34, 127–130. [Google Scholar]
- Braga, F.R.; Araújo, J.V. Nematophagous fungi for biological control of gastrointestinal nematodes in domestic animals. Appl. Microbiol. Biotechnol. 2014, 98, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Soprunov, F.F.; Tendetnik, Y.Y. Results of an experiment in biological control of ancylostomiasis in coal mines. Med. Parasitol. 1957, 26, 602–612. [Google Scholar]
- Lysek, H. A scanning electron microscope study of the effects of ovicidal fungus on the eggs of Ascaris lumbricoides. Parasitology 1978, 77, 139–141. [Google Scholar] [CrossRef]
- Scheffer, G.K. O direito animal em tempos de pandemia. Rev. Bras. De Direito E Justiça 2020, 4, 118–153. [Google Scholar] [CrossRef]
- Parslow, R.A.; Jorm, A.F. Pet ownership and risk factors for cardiovascular disease: Another look. Med. J. Aust. 2003, 1799, 466–468. [Google Scholar] [CrossRef]
- Nath, T.C.; Tusher, P.C.; Siddiki, T.; Nyema, J.; Bhattacharjee, T.; Dey, N.; Mukutmoni, M.; Islam, K.M.; Bhuiyan, J.U. Rare case of human Ancylostoma ceylanicum infection in Bangladesh. IJID Reg. 2024, 11, 100376. [Google Scholar] [CrossRef] [PubMed]
- Panziera, W.; Vielmo, A.; Bianchi, R.M.; Andrade, C.P.; Pavarini, S.P.; Sonne, L.; Soares, J.F.; Driemeier, D. Aspectos macroscópicos e histológicos da cisticercose bovina. Pesq. Vet. Bras. 2017, 37, 1220–1228. [Google Scholar] [CrossRef]
- Altoé, L.S.C.; Martins, I.V.F.; Tunholi-Alves, V.M.; Amaral, L.S.; Pinheiro, J.; Araújo, J.V.; Monteiro, C.M.O.; Tunholi, V.M. Susceptibility of embryos of Biomphalaria tenagophila (Mollusca: Gastropoda) to infection by Pochonia chlamydosporia (Ascomycota: Sordariomycetes). Arch. Microbiol. 2022, 204, 271. [Google Scholar] [CrossRef]
- Fonseca, J.S.; Valverde, H.A.; Barbosa, B.B.; Santos, H.A.; Araújo, J.V. Assessing the applications and efficacy of using helminthophagous fungi to control canine gastrointestinal parasites. Acta Trop. 2024, 254, 107180. [Google Scholar] [CrossRef]
- Ayinmode, A.B.; Obebe, O.O.; Ghana, E.O. Prevalence of potentially zoonotic gastrointestinal parasites in canine faeces in Ibadan, Nigeria. Med. J. 2016, 50, 201–206. [Google Scholar] [CrossRef]
- Idrissi, H.; Khatat, S.E.H.; Duchateau, L.; Kachani, M.; Daminet, S.; Asatey, S.E.; Tazi, N.; Azrib, R.; Sahibi, H. Prevalence, risk factors and zoonotic potential of intestinal parasites in dogs from four locations in Morocco. Vet. Parasitol. 2022, 34, 100775. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Sáez, D.; Aravena, C. Determinación de parásitos intestinales en perros con dueño de la ciudad de Talca, Chile, y su asociación com variables epidemiológicas. Ver. Inv. Vet. 2023, 34, 23590. [Google Scholar] [CrossRef]
- Oliveira, D.G.; Coelho, F.A.S.; Gonçalves, M.D. Occurrence of zoonotic parasites in feces collected in dog parks (Parcão) in the municipality of Taubaté, São Paulo, Brazil. Rev. Biociências 2023, 29, 12–27. [Google Scholar]
- Staford, K.; Kollasch, T.M.; Duncan, K.T.; Horr, S.; Goddu, T.; Heinz-Loomer, C.; Rumschlag, A.J.; Ryan, W.G.; Sweet, S.; Little, S.E. Detection of gastrointestinal parasitismo at recreational canine sites in the USA: The DOGPARCS study. Parasit. Vectors 2020, 13, 275. [Google Scholar] [CrossRef]
- Trasviña-Muñoz, E.; López-Valencia, G.; Monge-Navarro, F.J.; Herrera-Ramírez, J.C.; Haro, P.; Gómez-Gómez, S.D.; Mercado-Rodríguez, J.A.; Flores-Dueñas, C.A.; Cueto-Gonzalez, S.A.; Burquez-Escobedo, M. Detection of Intestinal Parasites in Stray Dogs from a Farming and Cattle Region of Northwestern Mexico. Pathogens 2020, 9, 516. [Google Scholar] [CrossRef]
- Safaro, A.; Mihalca, A.D.; Park, G.; Akramova, F.; Ionică, A.M.; Abdinabiev, O.; Deak, G.; Azimov, D. A Survey of Helminths of Dogs in Rural and Urban Areas of Uzbekistan and the Zoonotic Risk to Human Population. Pathogens 2022, 11, 1085. [Google Scholar] [CrossRef]
- Daba, M.; Naramo, M.; Haile, G. Current Status of Ancylostoma Species in Domestic and Wild Animals and Their Zoonotic Implication: Review. Am. J. Anim. Vet. Sci. 2021, 9, 107–114. [Google Scholar] [CrossRef]
- Sweet, S.; Hegarty, E.; McCrann, D.J.; Coyne, M.; Kincaid, D.; Szlosek, D. Uma análise retrospectiva de 3 anos de parasitas intestinais caninos: Positividade de testes fecais por idade, região geográfica dos EUA e motivo da visita veterinária. Parasit. Vectors 2021, 4, 173. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.; Al-Araby, M.; Elmishmishy, B.; El-Alf, E. Gastrointestinal parasites of cats in Egypt: High prevalence high zoonotic risk. BMC Vet. Res. 2022, 18, 420. [Google Scholar] [CrossRef]
- Bayou, K.; Terefe, G.; Kumsa, B. Gastrointestinal parasites of owned cats in three districts of Central Ethiopia: Prevalence and risk factors. Vet. Parasitol. 2024, 52, 101053. [Google Scholar] [CrossRef]
- Arruda, I.F.; Ramos, R.C.F.; Barbosa, A.S.; Abboud, L.C.S.; Reis, I.C.; Millar, P.R.; Amendoeira, M.R.R. Intestinal parasites and risk factors in dogs and cats from Rio de Janeiro, Brazil. Vet. Parasitol. 2021, 24, 100552. [Google Scholar] [CrossRef]
- Souza, J.B.B.; Silva, Z.M.d.A.; Alves-Ribeiro, B.S.; Moraes, I.d.S.; Alves-Sobrinho, A.V.; Saturnino, K.C.; Ferraz, H.T.; Machado, M.R.F.; Braga, Í.A.; de Souza Ramos, D.G. Prevalence of IntestinalParasites, Risk Factors and Zoonotic Aspects in Dog and Cat Populations from Goiás, Brazil. Vet. Sci. 2023, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Taetzsch, S.J.; Gruszynski, K.R.; Bertke, A.S.; Dubey, J.P.; Monti, K.A.; Zajac, A.M.; Lindsay, D.S. Prevalence of zoonotic parasites in feral cats of Central Virginia, USA. Zoonoses Public Health 2018, 65, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Nagamoria, Y.; Payton, M.E.; Duncan-Decocq, R.; Johnsona, E.M. Fecal survey of parasites in free-roaming cats in northcentral Oklahoma, United States. Vet. Parasitol. 2018, 14, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.B.; Dhakal, M.A.; Ale, P.B.; Regmi, G.R.; Ghimire, T.R. Survey on the prevalence of intestinal parasites in domestic cats (Felis catus Linnaeus, 1758) in central Nepal. Vet Med. Sci. 2023, 9, 559–571. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, H. Prevalence and Risk Factors of Intestinal Parasites in Cats from China. Biomed Res. Int. 2015, 2015, 967238. [Google Scholar] [CrossRef]
- Sauda, F.; Malandrucco, L.; Liberato, C.; Perruccia, S. Gastrointestinal parasites in shelter cats of central Italy. Vet. Parasitol. 2019, 18, 100321. [Google Scholar] [CrossRef]
- Lorenzo-Rebenaque, L.; López-Fernández, S.; Marco-Jiménez, F.; Montoro-Dasi, L.; Marin, C.; Vega, S.; Martínez-Manzanares, E.; Fariñas, F. Zoonotic Parasites in Playgrounds in Southern Spain: A One Health Approach. Microorganisms 2023, 11, 721. [Google Scholar] [CrossRef]
- Mascarenhas, N.M.H. Complexo teníase-cisticercose e sua implicação para pecuária e saúde pública. Pesq. Agr. Amb. 2023, 11, 422–430. [Google Scholar] [CrossRef]
- Chieffi, P.P.; dos Santos, S.V. Teníase–cisticercose: Uma zoonose negligenciada. Arq. Med. Hosp. Fac. Cienc. Med. Santa Casa São Paulo 2020, 65, 48. [Google Scholar] [CrossRef]
- Fonseca, J.S.; Castro Altoé, L.S.; Carvalho, L.M.; Soares, F.E.F.; Braga, F.R.; Araújo, J.V. Nematophagous fungus Pochonia chlamydosporia to control parasitic diseases in animals. Appl. Microbiol. Biotechnol. 2023, 107, 3859–3868. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Campos, A.K.; Carvalho, R.O.; Silva, A.S.; Tavela, A.O.; Maciel, A.S. Observação in vitro dos isolados Duddingtonia flagrans, Monacrosporium thaumasium e Verticillium chlamydosporium sobre ovos de Ascaris lumbricoides (Lineu, 1758). Rev. Soc. Bras. Med. Trop. 2007, 40, 356–358. [Google Scholar] [CrossRef]
- Morgan-Jones, G.; White, J.F.; Rodríguez-Kábana, R. Phytonematode pathology: Ultrastuctural studies. Parasitism of Meloidogyne arenaria eggs by Verticillium chlamydosporium. Nematropica 1983, 13, 245–260. [Google Scholar]
- Braga, F.R.; Araújo, J.V.; Carvalho, R.O.; Silva, A.R.; Araujo, J.M.; Tavela, A.O.; Costa, P.R.S.; Campos, A.K. Ovicidal effect of nematophagous fungi on Taenia taeniaeformis eggs. World J. Microbiol. Biotechnol. 2009, 25, 533–535. [Google Scholar] [CrossRef]
- Braga, F.R.; Silva, A.R.; Carvalho, R.O.; Araújo, J.V.; Guimarães, P.H.G.; Fujiwara, R.T.; Frassy, L.N. In vitro predatory activity of the fungi Duddingtonia flagrans, Monacrosporium thaumasium, Monacrosporium sinense and Arthrobotrys robusta on Ancylostoma ceylanicum third stage larvae. Vet. Microbiol. 2010, 146, 183–186. [Google Scholar] [CrossRef]
- Paula, A.T.; Braga, F.R.; Carvalho, L.M.; Lelis, R.T.; Mello, I.N.K.; Tavela, A.O.; Soares, F.E.F.; Junior, A.; Garcia, J.S.; Araújo, J. First report of the activity of predatory fungi on Angiostrongylus cantonensis (Nematoda: Angiostrongylidae) first-stage larvae. Acta Trop. 2013, 127, 187–190. [Google Scholar] [CrossRef]
- Silva, L.P.C.; Oliveira, J.P.; Keojok, W.J.; Silva, A.R.; Aguira, A.R.; Guimarães, M.C.C.; Ferraz, C.M.; Araújo, J.V.; Tobias, F.L.; Braga, F.R. Extracellular biosynthesis of silver nanoparticles using the cell-free filtrate of nematophagous fungus Duddingtonia flagrans. Int. J. Nanomed. 2017, 12, 6373–6381. [Google Scholar] [CrossRef] [PubMed]
- Viña, C.; Salmo, R.; Pena, M.V.; Palomero, A.M.; Hernández, J.Á.; Cazapal-Monteiro, C.; Arias, M.S.; Sánchez-Andrade, R.; Paz-Silva, A. A New Comestible Formulation of Parasiticide Fungi to Reduce the Risk of Soil-Transmitted Helminth Infections in a Canine Shelter. Pathogens 2022, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.V.; Santos, M.A.; Ferraz, S. Efeito ovicida de fungos nematófagos sobre ovos embrionados de Toxocara canis. Arq. Bras. Med. Veterinária Zootec. 1995, 47, 37–42. [Google Scholar]
- Barbosa, A.C.M.S.; Silva, L.P.C.; Ferraz, C.M.; Braga, G.M.A.M.; Soares, F.E.F.; Araújo, J.V.; Rodrigues, F.B.; Loureiro, B.; Tobias, F.L.; Fronza, M.; et al. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int. J. Nanomed. 2019, 14, 2341–2348. [Google Scholar] [CrossRef]
- Bwalyaa, E.C.; Nalubambaa, K.S.; Hankangaa, C.; Namangala, B. Prevalence of canine gastrointestinal helminths in urban Lusaka and rural Katete Districts of Zambia. Prev. Vet. Med. 2011, 100, 252–255. [Google Scholar] [CrossRef]
- Jongwutiwes, S.; Putaporntip, C.; Chantachum, N.; Sampatanukul, P. Jejunal perforation caused by morphologically ab-normal Taenia Saginata infection. J. Infect. 2004, 49, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Costa, P.W.; Alvares, F.B.V.; Alves Bezerra, R.; Sarmento, W.F.; da Silva, F.F.; Rodrigues, J.A.; Ferreira, F.T.; Araújo, J.V.; Braga, F.R.; Vilela, V.L.R. Effect of refrigeration storage of nemathophagous fungi embedded in sodium alginate pellets on predatory activity against asinine gastrointestinal nematodes. Biocontrol. Sci. Techn. 2019, 29, 1106–1117. [Google Scholar] [CrossRef]
- Sobral, S.A.; Ferreira, B.S.; Senna, C.C.; Ferraz, C.M.; Moreira, T.F.; Fidelis Junior, O.L.; Hiura, E.; Tobias, F.L.; Machado, R.Z.; Araújo, J.V.; et al. Rhabditis spp., in the Espírito Santo, State of Brazil and evaluation of biological control. Rev. Bras. Parasitol. Vet. 2019, 28, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.A.C.; Ferraz, C.M.; Sobral, S.A.; Geniêr, H.L.A.; Soares, F.E.F.; Loureiro Junior, D.B.; Lima, M.R.; Araújo, J.V.; Tobias, F.L.; Vilela, V.L.R.; et al. Combined use of chemical and biological compounds to control hookworm. J. Helminthol. 2020, 94, e160. [Google Scholar] [CrossRef]
- Silva, B.F.; Ferraz, C.M.; Soares, F.E.F.; Tobias, F.L.; Hiura, E.; Lopes, A.C.G.; Horta, R.S.; Lima, J.A.C.; Sobral, S.A.; Araújo, J.V.; et al. Control of Toxocara canis with Nematophagous Fungus: Perspective to Public Health. Iran. J. Health Sci. 2022, 51, 958–960. [Google Scholar] [CrossRef]
- Hao, L.; Guo, Y.; Wang, X.; Gao, M.; Liu, T.; Ma, Y.; Zhang, Y.; Li, Q.; Wang, R.; You, X. Preparation and application of biocontrol formulation of nematode-trapping fungus Duddingtonia flagrans. Vet. Parasitol. 2024, 327, 110119. [Google Scholar] [CrossRef]
- Ferreira, T.S.; Ferraz, C.M.; Santos, P.H.D.; Soares, F.E.F.; Segantine, V.B.S.; Vilela, V.L.R.; Araújo, J.V.; Braga, F.R. Isolated and Associated Use of the Nematophagous Fungi Pochonia chlamydosporia and Duddingtonia flagrans to Control Taenia saginata Eggs. Parasitologia 2024, 4, 238–245. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef]
- Ferraz, C.M.; Costa Silva, L.P.; Soares, F.E.F.; Souza, R.L.O.; Tobias, F.L.; Araújo, J.V.; Veloso, F.B.R.; Laviola, F.P.; Endringer, D.C.; Mendoza de Gives, P.; et al. Effect of silver nanoparticles (AgNP’s) from Duddingtonia flagrans on cyathostomins larvae (subfamily: Cyathostominae). J. Invertebr. Pathol. 2020, 1, 107395. [Google Scholar] [CrossRef]
| References | Fungi | Nemathelminths |
|---|---|---|
| [33] | Arthrobotrys spp. | Ancylostoma duodenale |
| [19,34] | Pochonia chlamydosporia | Ascaris lumbricoides |
| [70] | Paecilomyces lilacinus | Toxocara canis |
| [65] | Pochonia chlamydosporia | Enterobius vermicularis |
| [22] | Monacrosporium thaumasium | Ancylostoma caninum |
| [66] | Duddingtonia flagrans, M. thaumasium, M. sinense and A. robusta | Ancylostoma ceylanicum |
| [7] | Duddingtonia flagrans, Monacrosporium thaumasium and Artrobotrys robusta | Strongyloides stercoralis |
| [9] | Pochonia chlamydosporia and Paecilomyces lilacinus | Toxocara canis |
| [8] | Pochonia chlamydosporia | Toxocara canis |
| [67] | Monacrosporium thaumasium, Monacrosporium sinense and Arthrobotrys robusta, Arthrobotrys cladodes and Arthrobotrys conoides | Angiostrongylus cantonensis |
| [23] | complex Trichoderma and Fusarium solani | Toxocara canis |
| [68,71] | Duddingtonia flagrans | Ancylostoma caninum |
| [69] | Mucor circinelloides and Duddingtonia flagrans | Toxocara canis, Toxascaris leonina, and Ancylostoma caninum |
| References | Fungi | Platyhelminths |
|---|---|---|
| [16] | Paecilomyces lilacinus | Taenia saginata |
| [17] | Duddingtonia flagrans, Monacrosporium sinense and Pochonia chlamydosporia | Schistosoma mansoni |
| [18] | Duddingtonia flagrans, Monacrosporium sinense and Pochonia chlamydosporia | Fasciola hepatica |
| [20] | Pochonia chlamydosporia, Duddingtonia flagrans and Monacrosporium thaumasium | Taenia saginata |
| [21] | Pochonia chlamydosporia and Paecilomyces lilacinus | Taenia saginata |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, J.V.d.; Fonseca, J.d.S.; Barbosa, B.B.; Valverde, H.A.; Santos, H.A.; Braga, F.R. The Role of Helminthophagous Fungi in the Biological Control of Human and Zoonotic Intestinal Helminths. Pathogens 2024, 13, 741. https://doi.org/10.3390/pathogens13090741
Araújo JVd, Fonseca JdS, Barbosa BB, Valverde HA, Santos HA, Braga FR. The Role of Helminthophagous Fungi in the Biological Control of Human and Zoonotic Intestinal Helminths. Pathogens. 2024; 13(9):741. https://doi.org/10.3390/pathogens13090741
Chicago/Turabian StyleAraújo, Jackson Victor de, Júlia dos Santos Fonseca, Beatriz Bacelar Barbosa, Helbert Ananias Valverde, Huarrisson Azevedo Santos, and Fabio Ribeiro Braga. 2024. "The Role of Helminthophagous Fungi in the Biological Control of Human and Zoonotic Intestinal Helminths" Pathogens 13, no. 9: 741. https://doi.org/10.3390/pathogens13090741






