Group A Rotavirus Detection and Genotype Distribution before and after Introduction of a National Immunisation Programme in Ireland: 2015–2019
Abstract
1. Introduction
2. Results
2.1. Sample Demographics
2.2. Detection of Viral Pathogens
2.3. Detection of Wild-Type Rotavirus
2.4. Seasonal Variation of Wild-Type Rotavirus
2.5. Detection of Vaccine-Derived Rotavirus (Rotarix)
2.6. Distribution of Genotypes in Ireland
2.7. Comparison of the Genotype Diversity Pre- and Post-Vaccine
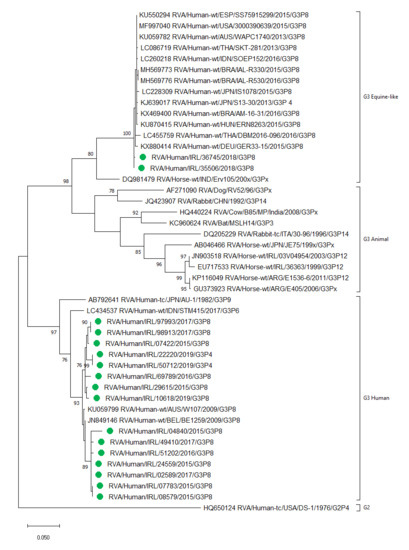
2.8. Detection of Human and Equine-Like Rotavirus G3
2.9. Genotypes Detected in Rotavirus Positive Samples from Those of Vaccine-Eligible Age
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Annual Birth Cohort in Ireland
4.3. Data Analysis
4.4. Sampling Strategy for Genotyping and Sequencing of Samples
4.5. Seasonality
4.6. Vaccine Eligibility
4.7. Laboratory Methods
4.8. GenBank Accession Numbers
4.9. Statistical Analysis
4.10. Ethical Statement
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hungerford, D.; Smith, K.; Tucker, A.; Iturriza-Gomara, M.; Vivancos, R.; McLeonard, C.; N, A.C.; French, N. Population effectiveness of the pentavalent and monovalent rotavirus vaccines: A systematic review and meta-analysis of observational studies. BMC Infect. Dis. 2017, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Blacker, B.; Khalil, I. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children < 5 years old: 2006–2019. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. ECDC Report: Expert Opinion on Rotavirus Vaccination in Infancy; ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- HSE Health Protection Surveillance Centre. Rotavirus 2018 Annual Epidemiological Report; HSE HPSC: Dublin, Ireland, 2019. [Google Scholar]
- Lynch, M.; O’Halloran, F.; Whyte, D.; Fanning, S.; Cryan, B.; Glass, R.I. Rotavirus in Ireland: National estimates of disease burden, 1997 to 1998. Pediatric Infect. Dis. J. 2001, 20, 693–698. [Google Scholar] [CrossRef]
- World Health Organization. Global Advisory Committee on Vaccine Safety, report of meeting held 17–18 June 2009. Wkly. Epidemiol. Rec. 2009, 84, 213–236. [Google Scholar]
- Poelaert, D.; Pereira, P.; Gardner, R.; Standaert, B.; Benninghoff, B. A review of recommendations for rotavirus vaccination in Europe: Arguments for change. Vaccine 2018, 36, 2243–2253. [Google Scholar] [CrossRef]
- Health Protection Surveillance Centre. Available online: https://www.hpsc.ie/az/vaccinepreventable/vaccination/immunisationuptakestatistics/immunisationuptakestatisticsat12and24monthsofage/ (accessed on 10 March 2020).
- Walker, J.L.; Andrews, N.J.; Atchison, C.J.; Collins, S.; Allen, D.J.; Ramsay, M.E.; Ladhani, S.N.; Thomas, S.L. Effectiveness of oral rotavirus vaccination in England against rotavirus-confirmed and all-cause acute gastroenteritis. Vaccine: X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Pietsch, C.; Liebert, U.G. Rotavirus vaccine effectiveness in preventing hospitalizations due to gastroenteritis: A descriptive epidemiological study from Germany. Clin. Microbiol. Infect. 2019, 25, 102–106. [Google Scholar] [CrossRef]
- Braeckman, T.; Van Herck, K.; Meyer, N.; Pircon, J.Y.; Soriano-Gabarro, M.; Heylen, E.; Zeller, M.; Azou, M.; Capiau, H.; De Koster, J.; et al. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: Case-control study. BMJ 2012, 345, e4752. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Otto, P.H.; Ciarlet, M.; Desselberger, U.; Van Ranst, M.; Johne, R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012, 157, 1177–1182. [Google Scholar] [CrossRef]
- Mihalov-Kovács, E.; Gellért, Á.; Marton, S.; Farkas, S.L.; Fehér, E.; Oldal, M.; Jakab, F.; Martella, V.; Bányai, K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg. Infect. Dis. 2015, 21, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Kemenesi, G.; Budinski, I.; Földes, F.; Zana, B.; Marton, S.; Varga-Kugler, R.; Oldal, M.; Kurucz, K.; Jakab, F. Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect. Genet. Evol. 2017, 48, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, D.; Vivancos, R.; EuroRotaNet Network Members. In-season and out-of-season variation of rotavirus genotype distribution and age of infection across 12 European countries before the introduction of routine vaccination, 2007/08 to 2012/13. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Roczo-Farkas, S.; Kirkwood, C.D.; Cowley, D.; Barnes, G.L.; Bishop, R.F.; Bogdanovic-Sakran, N.; Boniface, K.; Donato, C.M.; Bines, J.E. The Impact of Rotavirus Vaccines on Genotype Diversity: A Comprehensive Analysis of 2 Decades of Australian Surveillance Data. J. Infect. Dis. 2018, 218, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, D.; Allen, D.J.; Nawaz, S.; Collins, S.; Ladhani, S.; Vivancos, R.; Iturriza-Gomara, M. Impact of rotavirus vaccination on rotavirus genotype distribution and diversity in England, September 2006 to August 2016. Eurosurveillance 2019, 24, 1700774. [Google Scholar] [CrossRef]
- Zeller, M.; Rahman, M.; Heylen, E.; De Coster, S.; De Vos, S.; Arijs, I.; Novo, L.; Verstappen, N.; Van Ranst, M.; Matthijnssens, J. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 2010, 28, 7507–7513. [Google Scholar] [CrossRef]
- Markkula, J.; Hemming-Harlo, M.; Salminen, M.T.; Savolainen-Kopra, C.; Pirhonen, J.; Al-Hello, H.; Vesikari, T. Rotavirus epidemiology 5–6 years after universal rotavirus vaccination: Persistent rotavirus activity in older children and elderly. Infect. Dis. 2017, 49, 388–395. [Google Scholar] [CrossRef]
- Kaplon, J.; Grangier, N.; Pillet, S.; Minoui-Tran, A.; Vabret, A.; Wilhelm, N.; Prieur, N.; Lazrek, M.; Alain, S.; Mekki, Y.; et al. Predominance of G9P[8] rotavirus strains throughout France, 2014–2017. Clin. Microbiol. Infect. 2018, 24, 660.e661–660.e664. [Google Scholar] [CrossRef]
- Verberk, J.D.M.; Bruijning-Verhagen, P.; Melker, H.E. Rotavirus in the Netherlands: Background information for the Health Council. RIVM 2017. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Nakagomi, O.; Kirkwood, C.D.; Ciarlet, M.; Desselberger, U.; Ranst, M.V. Group A rotavirus universal mass vaccination: How and to what extent will selective pressure influence prevalence of rotavirus genotypes? Expert Rev. Vaccines 2012, 11, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Bilcke, J.; Ciarlet, M.; Martella, V.; Bányai, K.; Rahman, M.; Zeller, M.; Beutels, P.; Van Damme, P.; Van Ranst, M. Rotavirus disease and vaccination: Impact on genotype diversity. Future Microbiol. 2009, 4, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/Rota_virus_Q5_mortality_estimates_external_review_report_2006_may.pdf?ua=1 (accessed on 20 January 2020).
- Iturriza-Gomara, M.; Dallman, T.; Banyai, K.; Bottiger, B.; Buesa, J.; Diedrich, S.; Fiore, L.; Johansen, K.; Koopmans, M.; Korsun, N.; et al. Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol. Infect. 2011, 139, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Central Statistics Office. Available online: https://www.cso.ie/en/statistics/population (accessed on 8 May 2020).
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Coveney, J.; Barrett, M.; Fitzpatrick, P.; Kandamany, N.; McNamara, R.; Koe, S.; Okafor, I. National rotavirus vaccination programme implementation and gastroenteritis presentations: The paediatric emergency medicine perspective. Ir. J. Med Sci. 2019, 189, 327–332. [Google Scholar] [CrossRef]
- Burns, H.E.; Collins, A.M.; Fallon, U.B.; Marsden, P.V.; Ni Shuilleabhain, C.M. Rotavirus vaccination impact, Ireland, implications for vaccine confidence and screening. Eur. J. Public Health 2020, 281–285. [Google Scholar] [CrossRef]
- Shim, J.O.; Chang, J.Y.; Shin, S.; Moon, J.S.; Ko, J.S. Changing distribution of age, clinical severity, and genotypes of rotavirus gastroenteritis in hospitalized children after the introduction of vaccination: A single center study in Seoul between 2011 and 2014. BMC Infect. Dis. 2016, 16, 287. [Google Scholar] [CrossRef]
- Tate, J.E.; Panozzo, C.A.; Payne, D.C.; Patel, M.M.; Cortese, M.M.; Fowlkes, A.L.; Parashar, U.D. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics 2009, 124, 465–471. [Google Scholar] [CrossRef]
- Yandle, Z.; Coughlan, S.; Drew, R.J.; Cleary, J.; De Gascun, C. Diagnosis of rotavirus infection in a vaccinated population: Is a less sensitive immunochromatographic method more suitable for detecting wild-type rotavirus than real-time RT-PCR? J. Clin. Virol. 2018, 109, 19–21. [Google Scholar] [CrossRef]
- Gower, C.M.; Dunning, J.; Nawaz, S.; Allen, D.; Ramsay, M.E.; Ladhani, S. Vaccine-derived rotavirus strains in infants in England. Arch. Dis. Child. 2019, 105, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Whiley, D.M.; Ye, S.; Tozer, S.; Clark, J.E.; Bletchly, C.; Lambert, S.B.; Grimwood, K.; Nimmo, G.R. Over-diagnosis of rotavirus infection in infants due to detection of vaccine virus. Clin. Infect. Dis. 2019, ciz1196. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, G.N.; Taylor, S.L.; Moore, S.; Hewitt, J.; Upton, A.; Howe, A.S.; Best, E.J. Suboptimal performance of rotavirus testing in a vaccinated community population should prompt laboratories to review their rotavirus testing algorithms in response to changes in disease prevalence. Diagn. Microbiol. Infect. Dis. 2019, 93, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.; Räsänen, S.; Huhti, L.; Paloniemi, M.; Salminen, M.; Vesikari, T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur. J. Pediatr. 2013, 172, 739–746. [Google Scholar] [CrossRef]
- Bucardo, F.; Reyes, Y.; Svensson, L.; Nordgren, J. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS ONE 2014, 9, e98201. [Google Scholar] [CrossRef]
- Koo, H.L.; Neill, F.H.; Estes, M.K.; Munoz, F.M.; Cameron, A.; DuPont, H.L.; Atmar, R.L. Noroviruses: The Most Common Pediatric Viral Enteric Pathogen at a Large University Hospital After Introduction of Rotavirus Vaccination. J. Pediatric Infect. Dis. Soc. 2013, 2, 57–60. [Google Scholar] [CrossRef]
- Steyer, A.; Jevšnik, M.; Petrovec, M.; Pokorn, M.; Grosek, Š.; Fratnik Steyer, A.; Šoba, B.; Uršič, T.; Cerar Kišek, T.; Kolenc, M.; et al. Narrowing of the Diagnostic Gap of Acute Gastroenteritis in Children 0–6 Years of Age Using a Combination of Classical and Molecular Techniques, Delivers Challenges in Syndromic Approach Diagnostics. Pediatric Infect. Dis. J. 2016, 35, e262–e270. [Google Scholar] [CrossRef]
- Donaldson, A.L.; Clough, H.E.; O’Brien, S.J.; Harris, J.P. Symptom profiling for infectious intestinal disease (IID): A secondary data analysis of the IID2 study. Epidemiol. Infect. 2019, 147, e229. [Google Scholar] [CrossRef]
- O’Mahony, J.; Foley, B.; Morgan, S.; Morgan, J.G.; Hill, C. VP4 and VP7 genotyping of rotavirus samples recovered from infected children in Ireland over a 3-year period. J. Clin. Microbiol. 1999, 37, 1699–1703. [Google Scholar] [CrossRef]
- O’Halloran, F.; Lynch, M.; Cryan, B.; O’Shea, H.; Fanning, S. Molecular characterization of rotavirus in Ireland: Detection of novel strains circulating in the population. J. Clin. Microbiol. 2000, 38, 3370–3374. [Google Scholar] [CrossRef]
- Reidy, N.; O’Halloran, F.; Fanning, S.; Cryan, B.; O’Shea, H. Emergence of G3 and G9 rotavirus and increased incidence of mixed infections in the southern region of Ireland 2001–2004. J. Med Virol. 2005, 77, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Lennon, G.; Reidy, N.; Cryan, B.; Fanning, S.; O’Shea, H. Changing profile of rotavirus in Ireland: Predominance of P[8] and emergence of P[6] and P[9] in mixed infections. J. Med Virol. 2008, 80, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Mulherin, E.; O’Shea, H.; Cashman, O.; Lennon, G.; Pidgeon, E.; Coughlan, S.; Hall, W.; Fanning, S. Changing patterns of rotavirus strains circulating in Ireland: Re-emergence of G2P[4] and identification of novel genotypes in Ireland. J. Med Virol. 2015, 87, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Cashman, O.; Collins, P.J.; Lennon, G.; Cryan, B.; Martella, V.; Fanning, S.; Staines, A.; O’Shea, H. Molecular characterization of group A rotaviruses detected in children with gastroenteritis in Ireland in 2006-2009. Epidemiol. Infect. 2012, 140, 247–259. [Google Scholar] [CrossRef]
- Bernstein, D.I. Rotavirus Vaccines: Mind Your Ps and Gs. J. Infect. Dis. 2018, 218, 519–521. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Zeller, M.; Heylen, E.; De Coster, S.; Vercauteren, J.; Braeckman, T.; Van Herck, K.; Meyer, N.; PirÇon, J.Y.; Soriano-Gabarro, M.; et al. Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin. Microbiol. Infect. 2014, 20, O702–O710. [Google Scholar] [CrossRef]
- Pitzer, V.E.; Bilcke, J.; Heylen, E.; Crawford, F.W.; Callens, M.; De Smet, F.; Van Ranst, M.; Zeller, M.; Matthijnssens, J. Did Large-Scale Vaccination Drive Changes in the Circulating Rotavirus Population in Belgium? Sci. Rep. 2015, 5, 18585. [Google Scholar] [CrossRef]
- Zeller, M.; Donato, C.; Trovão, N.S.; Cowley, D.; Heylen, E.; Donker, N.C.; McAllen, J.K.; Akopov, A.; Kirkness, E.F.; Lemey, P.; et al. Genome-Wide Evolutionary Analyses of G1P[8] Strains Isolated Before and After Rotavirus Vaccine Introduction. Genome Biol. Evol. 2015, 7, 2473–2483. [Google Scholar] [CrossRef]
- Vesikari, T.; Karvonen, A.; Prymula, R.; Schuster, V.; Tejedor, J.C.; Cohen, R.; Meurice, F.; Han, H.H.; Damaso, S.; Bouckenooghe, A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: Randomised, double-blind controlled study. Lancet 2007, 370, 1757–1763. [Google Scholar] [CrossRef]
- Clarke, E.; Desselberger, U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015, 8, 1–17. [Google Scholar] [CrossRef]
- Ogden, K.M.; Tan, Y.; Akopov, A.; Stewart, L.S.; McHenry, R.; Fonnesbeck, C.J.; Piya, B.; Carter, M.H.; Fedorova, N.B.; Halpin, R.A.; et al. Multiple introductions and antigenic mismatch with vaccines may contribute to increased predominance of G12P[8] rotaviruses in the United States. J. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Malasao, R.; Saito, M.; Suzuki, A.; Imagawa, T.; Nukiwa-Soma, N.; Tohma, K.; Liu, X.; Okamoto, M.; Chaimongkol, N.; Dapat, C.; et al. Human G3P[4] rotavirus obtained in Japan, 2013, possibly emerged through a human-equine rotavirus reassortment event. Virus Genes 2015, 50, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Camilloni, B.; Bianchini, S.; Ianiro, G.; Polinori, I.; Farinelli, E.; Monini, M.; Principi, N. First detection of a reassortant G3P[8] rotavirus A strain in Italy: A case report in an 8-year-old child. Virol. J. 2019, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Tacharoenmuang, R.; Komoto, S.; Guntapong, R.; Upachai, S.; Singchai, P.; Ide, T.; Fukuda, S.; Ruchusatsawast, K.; Sriwantana, B.; Tatsumi, M.; et al. High prevalence of equine-like G3P[8] rotavirus in children and adults with acute gastroenteritis in Thailand. J. Med Virol. 2020, 92, 174–186. [Google Scholar] [CrossRef]
- Pietsch, C.; Liebert, U.G. Molecular characterization of different equine-like G3 rotavirus strains from Germany. Infect. Genet. Evol. 2018, 57, 46–50. [Google Scholar] [CrossRef]
- Cowley, D.; Donato, C.M.; Roczo-Farkas, S.; Kirkwood, C.D. Emergence of a novel equine-like G3P[8] inter-genogroup reassortant rotavirus strain associated with gastroenteritis in Australian children. J. Gen. Virol. 2016, 97, 403–410. [Google Scholar] [CrossRef]
- Kumar, N.; Malik, Y.S.; Sharma, K.; Dhama, K.; Ghosh, S.; Banyai, K.; Kobayashi, N.; Singh, R.K. Molecular characterization of unusual bovine rotavirus A strains having high genetic relatedness with human rotavirus: Evidence for zooanthroponotic transmission. Zoonoses Public Health 2018, 65, 431–442. [Google Scholar] [CrossRef]
- Tamim, S.; Matthijnssens, J.; Heylen, E.; Zeller, M.; Van Ranst, M.; Salman, M.; Hasan, F. Evidence of zoonotic transmission of VP6 and NSP4 genes into human species A rotaviruses isolated in Pakistan in 2010. Arch. Virol. 2019, 164, 1781–1791. [Google Scholar] [CrossRef]
- Central Statistics Office. Available online: https://www.cso.ie/en/statistics/birthsdeathsandmarriages (accessed on 30 May 2020).
- Gautam, R.; Esona, M.D.; Mijatovic-Rustempasic, S.; Ian Tam, K.; Gentsch, J.R.; Bowen, M.D. Real-time RT-PCR assays to differentiate wild-type group A rotavirus strains from Rotarix(®) and RotaTeq(®) vaccine strains in stool samples. Hum. Vaccines Immunother. 2014, 10, 767–777. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef]
- Tiemessen, C.T.; Nel, M.J. Detection and typing of subgroup F adenoviruses using the polymerase chain reaction. J. Virol. Methods 1996, 59, 73–82. [Google Scholar] [CrossRef]
- Oka, T.; Katayama, K.; Hansman, G.S.; Kageyama, T.; Ogawa, S.; Wu, F.T.; White, P.A.; Takeda, N. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol. 2006, 78, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.; O’Leary, J.J.; O’Sullivan, N. Real-time reverse transcription PCR detection of norovirus, sapovirus and astrovirus as causative agents of acute viral gastroenteritis. J. Virol. Methods 2007, 146, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.M.; Kerin, T.; Hull, J.; McCaustland, K.; Gentsch, J. Enhancement of detection and quantification of rotavirus in stool using a modified real-time RT-PCR assay. J. Med Virol. 2008, 80, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Mijatovic-Rustempasic, S.; Esona, M.D.; Tam, K.I.; Quaye, O.; Bowen, M.D. One-step multiplex real-time RT-PCR assay for detecting and genotyping wild-type group A rotavirus strains and vaccine strains (Rotarix(R) and RotaTeq(R)) in stool samples. PeerJ 2016, 4, e1560. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Lindh, M. Rotavirus genotype shifts among Swedish children and adults-Application of a real-time PCR genotyping. J. Clin. Virol. 2017, 96, 1–6. [Google Scholar] [CrossRef]
- World Health Organization. Method 16: G and P genotyping. In Manual of Rotavirus Detection and Characterization Methods; WHO/IVB/08.17; WHO: Geneva, Switzerland, 2009; pp. 91–98. [Google Scholar]
- Maes, P.; Matthijnssens, J.; Rahman, M.; Van Ranst, M. RotaC: A web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 2009, 9, 238. [Google Scholar] [CrossRef]



| Results (%) by Year | Total | |||||
|---|---|---|---|---|---|---|
| Virus Detected | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Rotavirus-wild-type | 662 (15.03) | 519 (13.09) | 250 (15.49) | 53 (4.42) | 70 (5.93) | 1554 |
| Rotavirus-Rotarix | 0 (0.00) | 1 (0.03) | 61 (3.78) | 49 (4.08) | 69 (5.84) | 180 |
| Norovirus | 482 (10.94) | 492 (12.41) | 210 (13.01) | 158 (13.17) | 141 (11.94) | 1483 |
| Adenovirus F | 156 (3.54) | 155 (3.91) | 101 (6.26) | 64 (5.33) | 47 (3.98) | 523 |
| Sapovirus | 202 (4.59) | 167 (4.21) | 85 (5.27) | 72 (6.00) | 33 (2.79) | 559 |
| Astrovirus | 197 (4.47) | 121 (3.05) | 77 (4.77) | 68 (5.67) | 53 (4.49) | 516 |
| No virus detected | 2705 (61.42) | 2511 (63.31) | 830 (51.43) | 736 (61.33) | 768 (65.03) | 7550 |
| Total samples tested | 4199 | 3787 | 1499 | 1159 | 1156 | |
| Total results | 4404 a | 3966 b | 1614 c | 1200 d | 1181 e | |
| Year | Number of Wild-Type Rotavirus Positive Samples/Total Number of Samples Tested (%) | Median Age (IQR) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1 Year | 1–2 Years | 2–3 Years | 3–4 Years | 4–5 Years | 5–6 Years | Total | |||
| Pre-vaccine | 2015 | 285/2280 (12.5) | 227/899 (25.3) | 90/413 (21.8) | 35/213 (16.4) | 17/213 (8.0) | 8/181 (4.4) | 662/4199 (15.8) | 1.17 (0.5-1.9) |
| 2016 | 200/2065 (9.7) | 217/792 (27.4) | 55/427 (12.9) | 24/224 (10.7) | 11/136 (8.1) | 12/143 (8.4) | 519/3787 (13.7) | 1.22 (0.8-1.8) | |
| Post-vaccine | 2017 | 57/760 (7.5) | 110/374 (29.4) | 49/144 (34.0) | 19/105 (18.1) | 6/55 (10.9) | 9/61 (14.8) | 250/1499 (16.7) | 1.59 (1.0–2.4) |
| 2018 | 8/587 (1.4) | 12/272 (4.4) | 23/125 (18.4) | 2/62 (3.2) | 3/59 (5.1) | 5/54 (9.2) | 53/1159 (4.6) | 2.24 (1.6–2.9) | |
| 2019 | 4/608 (0.7) | 16/233 (6.9) | 16/129 (12.4) | 21/86 (24.4) | 6/58 (10.3) | 7/42 (16.7) | 70/1156 (6.1) | 2.90 (1.9–3.5) | |
| Age | ||||||
|---|---|---|---|---|---|---|
| 2 mts | 3 mts | 4 mts | 5 mts | 6 mts | 7 mts | |
| Rotarix detected/total number Rotarix detected (%) | 99/180 (55.0) | 41/180 (22.8) | 26/180 (14.4) | 11/180 (6.1) | 2/180 (1.1) | 1/180 (0.6) |
| Pre-Vaccinen (%) | Post-Vaccinen (%) | Pre-Vaccine Data Combined | Post-Vaccine Data Combined | Pre vs. Post | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | 2015 | 2016 | 2017 | 2018 | 2019 | n (%) | CI 95% | n (%) | CI 95% | p = |
| G1P[8] | 125 (42.7) | 172 (71.1) | 70 (51.9) | 7 (14.6) | 3 (4.4) | 297 (55.5) | 51.3–59.7 | 80 (31.9) | 26.4–37.9 | <0.0001 |
| G2P[4] | 6 (2.0) | 16 (6.6) | 15 (11.1) | 7 (14.6) | 27 (39.7) | 22 (4.1) | 2.7–6.2 | 49 (19.5) | 15.1–24.9 | <0.0001 |
| G3P[8] | 10 (3.4) | 9 (3.7) | 17 (12.6) | 7 (14.6) | 16 (23.5) | 19 (3.6) | 2.3–5.5 | 40 (15.9) | 11.9–21.0 | <0.0001 |
| G4P[8] | 64 (21.8) | 0 (0.0) | 6 (4.4) | 11 (23.0) | 0 (0.0) | 64 (12.0) | 9.5–15.0 | 17 (6.8) | 4.3–10.6 | 0.0257 |
| G9P[8] | 69 (23.5) | 34 (14.1) | 20 (14.8) | 10 (20.9) | 4 (5.9) | 103 (19.3) | 16.1–22.8 | 34 (13.6) | 9.9–18.3 | 0.0493 |
| G12P[8] | 3 (1.0) | 4 (1.7) | 0 (0.0) | 1 (2.1) | 5 (7.4) | 7 (1.3) | 0.1–2.7 | 6 (2.4) | 1.1–5.1 | 0.2675 |
| Mixed | 7 (2.4) | 3 (1.2) | 1 (0.7) | 1 (2.1) | 4 (5.9) | 10 (1.9) | 1–3.4 | 6 (2.4) | 1.1–5.1 | 0.0014 |
| Uncommon | 2 (0.7) | 2 (0.8) | 1 (0.7) | 1 (2.1) | 8 (11.8) | 4 (0.8) | 0.03–1.9 | 10 (4.0) | 2.2–7.2 | 0.6295 |
| Untypable | 7 (2.4) | 2 (0.8) | 5 (3.7) | 3 (6.3) | 1 (1.5) | 9 (1.7) | 0.9–3.2 | 9 (3.6) | 1.9–6.7 | n/a |
| Total | 293 (100) | 242 (100) | 135 (100) | 48 (100) | 68 (100) | 535 (100) | n/a | 251 (100) | n/a | n/a |
| Genotype | Classification of Genotype | Number of Samples (%) |
|---|---|---|
| G1P[8] | Common | 5 (13.5) |
| G2P[4] | Common | 11 (29.7) |
| G3P[8] | Common | 6 (16.2) |
| G4P[8] | Common | 1 (2.7) |
| G9P[8] | Common | 4 (10.8) |
| G12P[8] | Common | 3 (8.1) |
| G3P[4] | Uncommon | 3 (8.1) |
| G9P[4] | Uncommon | 1 (2.7) |
| G1/3 P[8] | Mixed | 1 (2.7) |
| G9/12 P[8] | Mixed | 1 (2.7) |
| G9 P untypable | Untypable | 1 (2.7) |
| Total | 37 (100) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yandle, Z.; Coughlan, S.; Dean, J.; Tuite, G.; Conroy, A.; De Gascun, C.F. Group A Rotavirus Detection and Genotype Distribution before and after Introduction of a National Immunisation Programme in Ireland: 2015–2019. Pathogens 2020, 9, 449. https://doi.org/10.3390/pathogens9060449
Yandle Z, Coughlan S, Dean J, Tuite G, Conroy A, De Gascun CF. Group A Rotavirus Detection and Genotype Distribution before and after Introduction of a National Immunisation Programme in Ireland: 2015–2019. Pathogens. 2020; 9(6):449. https://doi.org/10.3390/pathogens9060449
Chicago/Turabian StyleYandle, Zoe, Suzie Coughlan, Jonathan Dean, Gráinne Tuite, Anne Conroy, and Cillian F. De Gascun. 2020. "Group A Rotavirus Detection and Genotype Distribution before and after Introduction of a National Immunisation Programme in Ireland: 2015–2019" Pathogens 9, no. 6: 449. https://doi.org/10.3390/pathogens9060449
APA StyleYandle, Z., Coughlan, S., Dean, J., Tuite, G., Conroy, A., & De Gascun, C. F. (2020). Group A Rotavirus Detection and Genotype Distribution before and after Introduction of a National Immunisation Programme in Ireland: 2015–2019. Pathogens, 9(6), 449. https://doi.org/10.3390/pathogens9060449