A Plant Endophytic Bacterium Priestia megaterium StrainBP-R2 Isolated from the Halophyte Bolboschoenus planiculmis Enhances Plant Growth under Salt and Drought Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of the Endophytic Bacteria from the Bolboschoenus planiculmis Plants
2.2. Physiological and Biochemical Characterization of the Endophytic Bacteria Strain BP-R2
2.3. Analysis of Indole Acetic Acid (IAA) Production by the Colorimetric Assay
2.4. Inoculation of Plants with the Endophytic Bacteria Strain BP-R2 and Measurement of Plant Growth Parameters
2.5. Hydrogen Peroxide (H2O2) and Proline Content Determination
2.6. Electrolyte Leakage (EL) and Malondialdehyde (MDA) Content Determination
2.7. Statistical Analysis
3. Results
3.1. Isolation and Characterization of the Endophytic Bacteria Strain BP-R2 from the Roots of Bolboschoenus planiculmis Plants
3.2. Effects of Temperature, pH, NaCl Concentrations, and Carbon and Nitrogen Sources on the Synthesis of IAA by Bacteria Strain BP-R2
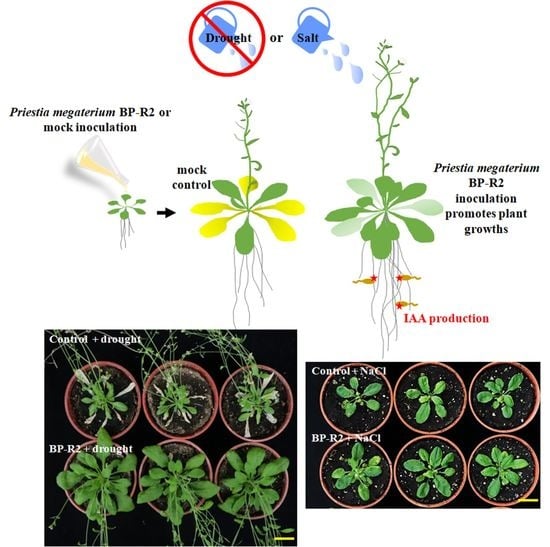
3.3. Inoculation of Arabidopsis with Bacteria Strain BP-R2 Promoted Plant Growth under Salt and Drought Stresses
3.4. The Endophytic Bacteria Strain BP-R2 Enhanced the Growth of Pak Choi Plants under Salt and Drought Stresses
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fita, A.; Rodriguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ye, W.; Wang, M.; Yan, X. Climate change and drought: A risk assessment of crop-yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Skz, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 2020, 36, 26. [Google Scholar] [CrossRef]
- Mohammadipanah, F.; Zamanzadeh, M. Bacterial mechanisms promoting the tolerance to drought stress in plants. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H., Keswani, C., Reddy, M., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 185–224. [Google Scholar]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic bacteria in plant salt stress tolerance: Current and future prospects. J. Plant Growth Regul. 2019, 38, 650–668. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Aydi Ben Abdallah, R.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Exploring the beneficial endophytic microorganisms for plant growth promotion and crop protection: Elucidation of some bioactive secondary metabolites involved in both effects. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H., Keswani, C., Reddy, M., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 319–352. [Google Scholar]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Compant, S.; Nowak, J.; Coenye, T.; Clément, C.; Ait Barka, E. Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol. Rev. 2008, 32, 607–626. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miliute, I.; Buzaite, O.; Baniulis, D.; Stanys, V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirb. Agric. 2015, 102, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, B.; Gontia, I.; Hartmann, A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Shurigin, V.; Gopalakrishnan, S.; Ram, S. Microbial strategies for the improvement of legume production in hostile environments. In Legumes Under Environmental Stress: Yield, Improvement and Adaptations; Azooz, M.M., Ahmad, P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; pp. 133–144. [Google Scholar]
- He, A.L.; Niu, S.Q.; Zhao, Q.; Li, Y.S.; Gou, J.Y.; Gao, H.J.; Suo, S.Z.; Zhang, J.L. Induced salt tolerance of perennial ryegrass by a novel bacterium strain from the rhizosphere of a desert shrub Haloxylon ammodendron. Int. J. Mol. Sci. 2018, 19, 469. [Google Scholar] [CrossRef] [PubMed]
- Fernando, T.C.; Cruz, J.A. Profiling and biochemical identification of potential plant growth-promoting endophytic bacteria from Nypa fruticans. Philipp. J. Crop Sci. 2019, 44, 77–85. [Google Scholar]
- El-Tarabily, K.A.; AlKhajeh, A.S.; Ayyash, M.M.; Alnuaimi, L.H.; Sham, A.; ElBaghdady, K.Z.; Tariq, S.; AbuQamar, S.F. Growth promotion of Salicornia bigelovii by Micromonospora chalcea UAE1, an endophytic 1-aminocyclopropane-1-carboxylic acid deaminase-producing actinobacterial isolate. Front. Microbiol. 2019, 10, 1694. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Shurigin, V.; Alaylar, B.; Wirth, S.; Bellingrath-Kimura, S.D. Bacterial endophytes from horseradish (Armoracia rusticana G. Gaertn., B. Mey. & Scherb.) with antimicrobial efficacy against pathogens. Plant Soil Environ. 2020, 66, 309–316. [Google Scholar]
- Shurigin, V.; Egamberdieva, D.; Li, L.; Davranov, K.; Panosyan, H.; Birkeland, N.K.; Wirth, S.; Bellingrath-Kimura, S.D. Endophytic bacteria associated with halophyte Seidlitzia rosmarinus Ehrenb. ex Boiss. from saline soil of Uzbekistan and their plant beneficial traits. J. Arid Land 2020, 12, 730–740. [Google Scholar] [CrossRef]
- Shurigin, V.; Alaylar, B.; Davranov, K.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. Diversity and biological activity of culturable endophytic bacteria associated with marigold (Calendula officinalis L.). AIMS Microbiol. 2021, 7, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Alikulov, B.; Shurigin, V.; Davranov, K.; Ismailov, Z. Plant growth-promoting endophytic bacteria associated with Halocnemum strobilaceum (Pall.) M. Bieb and their plant beneficial traits. Plant Sci. Today 2022, 8, 44–50. [Google Scholar] [CrossRef]
- Shurigin, V.; Alikulov, B.; Davranov, K.; Ismailov, Z. Bacterial endophytes from halophyte black saxaul (Haloxylon aphyllum Minkw.) and their plant growth-promoting properties. J. Appl. Biol. Biotech. 2021, 10, 45–53. [Google Scholar]
- Egamberdieva, D.; Alimov, J.; Shurigin, V.; Alaylar, B.; Wirth, S.; Bellingrath-Kimura, S.D. Diversity and plant growth-promoting ability of endophytic, halotolerant bacteria associated with Tetragonia tetragonioides (Pall.) Kuntze. Plants 2022, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Hroudov, Z.; Zakravsky, P.; Duchek, M.; Marhold, K. Taxonomy, distribution and ecology of Bolboschoenus in Europe. Ann. Bot. Fennici. 2007, 44, 81–102. [Google Scholar]
- Hroudova, Z.; Hrivnak, R.; Chytr, M. Classification of inland Bolboschoenus-dominated vegetation in Central Europe. Phytocoenologia 2009, 39, 205–215. [Google Scholar] [CrossRef]
- Ljevnaic-Masic, B.; Dzigurski, D.; Nikolic, L.; Brdar-Jokanovic, M.; Cabilovski, R.; Ciric, V.; Petrovic, A. Assessment of the habitat conditions of a rare and endangered inland saline wetland community with Bolboschoenus maritimus (L.) Palla dominance in Southeastern Europe: The effects of physical-chemical water and soil properties. Wetl. Ecol. Manag. 2020, 28, 421–438. [Google Scholar] [CrossRef]
- Chiang, Y.J.; Fan, H.Y.; Chiang, M.Y. Emergence, growth, and reproduction of Bolboschoenus planiculmis in two seasons. Weed Sci. Bull. 2009, 30, 119–128. [Google Scholar]
- An, Y.; Gao, Y.; Tong, S.; Liu, B. Morphological and physiological traits related to the response and adaption of Bolboschoenus planiculmis seedlings grown under salt-alkaline stress conditions. Front. Plant Sci. 2021, 12, 567782. [Google Scholar] [CrossRef]
- Huang, L.; Peng, Y.K.; Li, H.L.; Zhang, M.X.; Luo, F.L. Effects of soil moisture regimes on growth and photosynthesis of the riparian plant Bolboschoenus planiculmis. Forest Sci. Pract. 2013, 15, 105–113. [Google Scholar] [CrossRef]
- Ho, Y.N.; Chiang, H.M.; Chao, C.P.; Su, C.C.; Hsu, H.F.; Guo, C.T.; Hsieh, J.L.; Huang, C.C. In planta biocontrol of soilborne Fusarium wilt of banana through a plant endophytic bacterium, Burkholderia cenocepacia 869T2. Plant Soil 2015, 387, 295–306. [Google Scholar] [CrossRef]
- Hwang, H.H.; Chien, P.R.; Huang, F.C.; Hung, S.H.; Kuo, C.H.; Deng, W.L.; Chiang, E.P.I.; Huang, C.C. A plant endophytic bacterium, Burkholderia seminalis strain 869T2, promotes plant growth in Arabidopsis, pak choi, Chinese amaranth, lettuces, and other vegetables. Microorganisms 2021, 9, 1703. [Google Scholar] [CrossRef]
- Hwang, H.H.; Wang, M.H.; Lee, Y.L.; Tsai, Y.L.; Li, Y.H.; Yang, F.J.; Liao, Y.C.; Lin, S.K.; Lai, E.M. Agrobacterium-produced and exogenous cytokinin-modulated Agrobacterium-mediated plant transformation. Mol. Plant Pathol. 2010, 11, 677–690. [Google Scholar] [CrossRef]
- Hwang, H.H.; Wu, E.T.; Liu, S.Y.; Chang, S.C.; Tzeng, K.C.; Kado, C.I. Characterization and host range of five tumorigenic Agrobacterium tumefaciens strains and possible application in plant transient transformation assays. Plant Pathol. 2013, 62, 1384–1397. [Google Scholar] [CrossRef]
- Huang, F.C.; Hwang, H.H. Arabidopsis RETICULON-LIKE4 (RTNLB4) protein participates in Agrobacterium infection and VirB2 peptide-induced plant defense response. Int. J. Mol. Sci. 2020, 21, 1722. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016, 18, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Hosford, R.M. White blotch incited in wheat by Bacillus megaterium pv. cerealis. Phytopathology 1982, 72, 1453–1459. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar]
- Shwed, P.S.; Crosthwait, J.; Weedmark, K.; Hoover, E.; Dussault, F. Complete genome sequences of Priestia megaterium type and clinical strains feature complex plasmid arrays. Microbiol. Resour. Announc. 2021, 10, e00403-21. [Google Scholar] [CrossRef]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J. Gen. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
- Suliasih; Widawati, S. Isolation of Indole acetic acid (IAA) producing Bacillus siamensis from peat and optimization of the culture conditions for maximum IAA production. IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012025. [Google Scholar] [CrossRef]
- Bunk, B.; Schulz, A.; Stammen, S.; Munch, R.; Warren, M.J.; Rohde, M.; Jahn, D.; Biedendick, R. A short story about a big magic bug. Bioeng. Bugs 2010, 1, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through biomolecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [Green Version]
- Vary, P.S. Prime time for Bacillus megaterium. Microbiology 1994, 140, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Vary, P.S.; Biedendieck, R.; Fürch, T.; Meinhardt, F.; Rohde, M.; Deckwer, W.D.; Jahn, D. Bacillus megaterium—From simple soil bacterium to industrial protein production host. Appl. Microbiol. Biotechnol. 2007, 76, 957–967. [Google Scholar] [CrossRef]
- De Bary, A. Vergleichende Morphologie und Biologie der Pilze, Mycetozoen und Bacterien; Wilhelm Engelmann: Leipzig, Germany, 1884. [Google Scholar]
- Biedendieck, R.; Knuuti, T.; Moore, S.J.; Jahn, D. The “beauty in the beast”-the multiple uses of Priestia megaterium in biotechnology. Appl. Microbiol. Biotechnol. 2021, 105, 5719–5737. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Gerayeli, N.; Baghaee-Ravari, S.; Tarighi, S. Evaluation of the antagonistic potential of Bacillus strains against Pectobacterium carotovorum subsp. carotovorum and their role in the induction of resistance to potato soft rot infection. Eur. J. Plant Pathol. 2018, 150, 1049–1063. [Google Scholar]
- Karadeniz, A.; Topcuoglu, F.; Inan, S. Auxin, gibberellin, cytokinin and abscisic acid production in some bacteria. World J. Microbiol. Biotechnol. 2006, 22, 1061–1064. [Google Scholar] [CrossRef]
- Poveda, J.; González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef]
- Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 2018, 69, 417–435. [Google Scholar] [CrossRef]
- Bhatt, K.; Maheshwari, D.K. Bacillus megaterium strain CDK25, a novel plant growth promoting bacterium enhances proximate chemical and nutritional composition of Capsicum annuum L. Front. Plant Sci. 2020, 11, 1147. [Google Scholar] [CrossRef]
- Bhatt, K.; Maheshwari, D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech 2020, 10, 36. [Google Scholar] [CrossRef]
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid Pennisetum. Biotechnol. Rep. 2019, 24, e00374. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, M.A.; Desrut, A.; Moumen, B.; Verdon, J.; Mermouri, L.; Kacem, M.; Coutos-Thevenot, P.; Kaid-Harche, M.; Berges, T.; Vriet, C. Unearthing the plant growth-promoting traits of Bacillus megaterium RmBm31, an endophytic bacterium isolated from root nodules of Retama monosperma. Front. Plant Sci. 2020, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Bhutani, N.; Maheshwari, R.; Negi, M.; Suneja, P. Optimization of IAA production by endophytic Bacillus spp. from Vigna radiata for their potential use as plant growth promoters. Israel J. Plant Sci. 2018, 65, 83–96. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef] [Green Version]
- Apine, O.A.; Jadhav, J.P. Optimization of medium for indole-3-acetic acid production using Pantoea agglomerans strain PVM. J. Appl. Microbiol. 2011, 110, 1235–1244. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.D.; Singh, P.K.; Pandey, K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 2016, 6, 60. [Google Scholar] [CrossRef]
- Vilchez, J.I.; Tang, Q.; Kaushal, R.; Wang, W.; Lv, S.; He, D.; Chu, Z.; Zhang, H.; Liu, R.; Zhang, H. Genome sequence of Bacillus megaterium strain YC4-R4, a plant growth promoting rhizobacterium isolated from a high-salinity environment. Genome Announc. 2018, 6, e00527-18. [Google Scholar] [CrossRef] [Green Version]
- Vilchez, J.I.; Tang, Q.; Kaushal, R.; Wang, W.; Lv, S.; He, D.; Chu, Z.; Zhang, H.; Liu, R.; Zhang, H. Complete genome sequence of Bacillus megaterium strain TG1-E1, a plant drought tolerance-enhancing bacterium. Microbiol. Resour. Announc. 2018, 7, e00842-18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Ma, Z.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Wang, J. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Li, J.; Xia, Y.; Wang, W.; Luo, X.; Yin, J.; Zhong, J. Transcriptional response of Bacillus megaterium FDU301 to PEG200-mediated arid stress. BMC Microbiol. 2020, 20, 351. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-H.; Chien, P.-R.; Huang, F.-C.; Yeh, P.-H.; Hung, S.-H.W.; Deng, W.-L.; Huang, C.-C. A Plant Endophytic Bacterium Priestia megaterium StrainBP-R2 Isolated from the Halophyte Bolboschoenus planiculmis Enhances Plant Growth under Salt and Drought Stresses. Microorganisms 2022, 10, 2047. https://doi.org/10.3390/microorganisms10102047
Hwang H-H, Chien P-R, Huang F-C, Yeh P-H, Hung S-HW, Deng W-L, Huang C-C. A Plant Endophytic Bacterium Priestia megaterium StrainBP-R2 Isolated from the Halophyte Bolboschoenus planiculmis Enhances Plant Growth under Salt and Drought Stresses. Microorganisms. 2022; 10(10):2047. https://doi.org/10.3390/microorganisms10102047
Chicago/Turabian StyleHwang, Hau-Hsuan, Pei-Ru Chien, Fan-Chen Huang, Pin-Hsien Yeh, Shih-Hsun Walter Hung, Wen-Ling Deng, and Chieh-Chen Huang. 2022. "A Plant Endophytic Bacterium Priestia megaterium StrainBP-R2 Isolated from the Halophyte Bolboschoenus planiculmis Enhances Plant Growth under Salt and Drought Stresses" Microorganisms 10, no. 10: 2047. https://doi.org/10.3390/microorganisms10102047
APA StyleHwang, H.-H., Chien, P.-R., Huang, F.-C., Yeh, P.-H., Hung, S.-H. W., Deng, W.-L., & Huang, C.-C. (2022). A Plant Endophytic Bacterium Priestia megaterium StrainBP-R2 Isolated from the Halophyte Bolboschoenus planiculmis Enhances Plant Growth under Salt and Drought Stresses. Microorganisms, 10(10), 2047. https://doi.org/10.3390/microorganisms10102047