Defense Inducers Mediated Mitigation of Bacterial Canker in Tomato through Alteration in Oxidative Stress Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Cmm and Planting Material
2.2. Preparation and Application of Defense Inducers
2.3. Bacterial Strain Inoculation
2.4. Sample Preparation for Enzymatic Activity Determination
2.4.1. Sample Collection
2.4.2. Biochemical Analysis
Polyphenol Oxidases (PPOs) Assay (EC 1.14.18.1)
Phenylalanine Ammonia Lyases (PALs) Assay (EC 4.1.3.5)
Peroxidases (POs) Assay (EC 1.11.1.7)
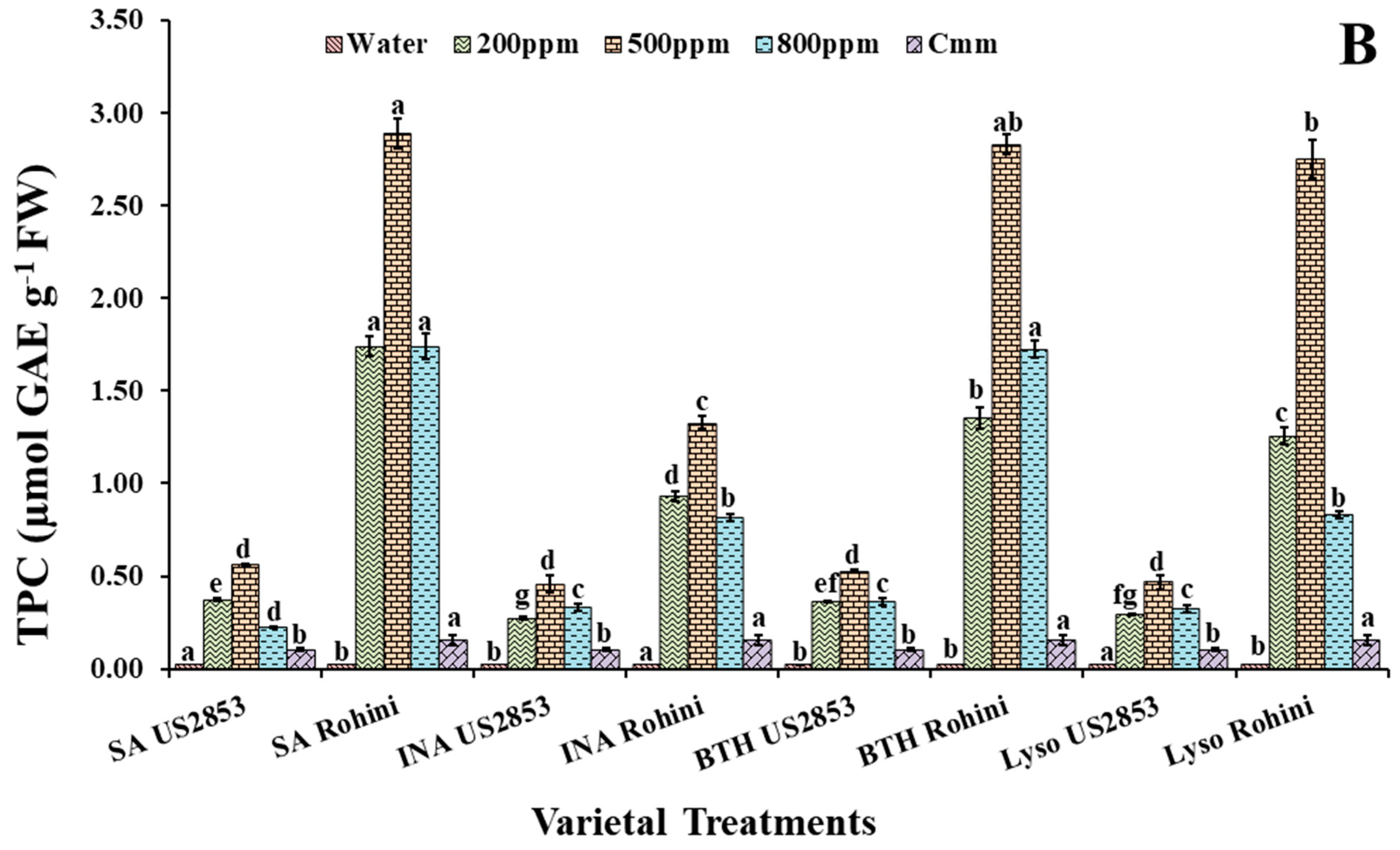
Total Phenol Content (TPC) Assay
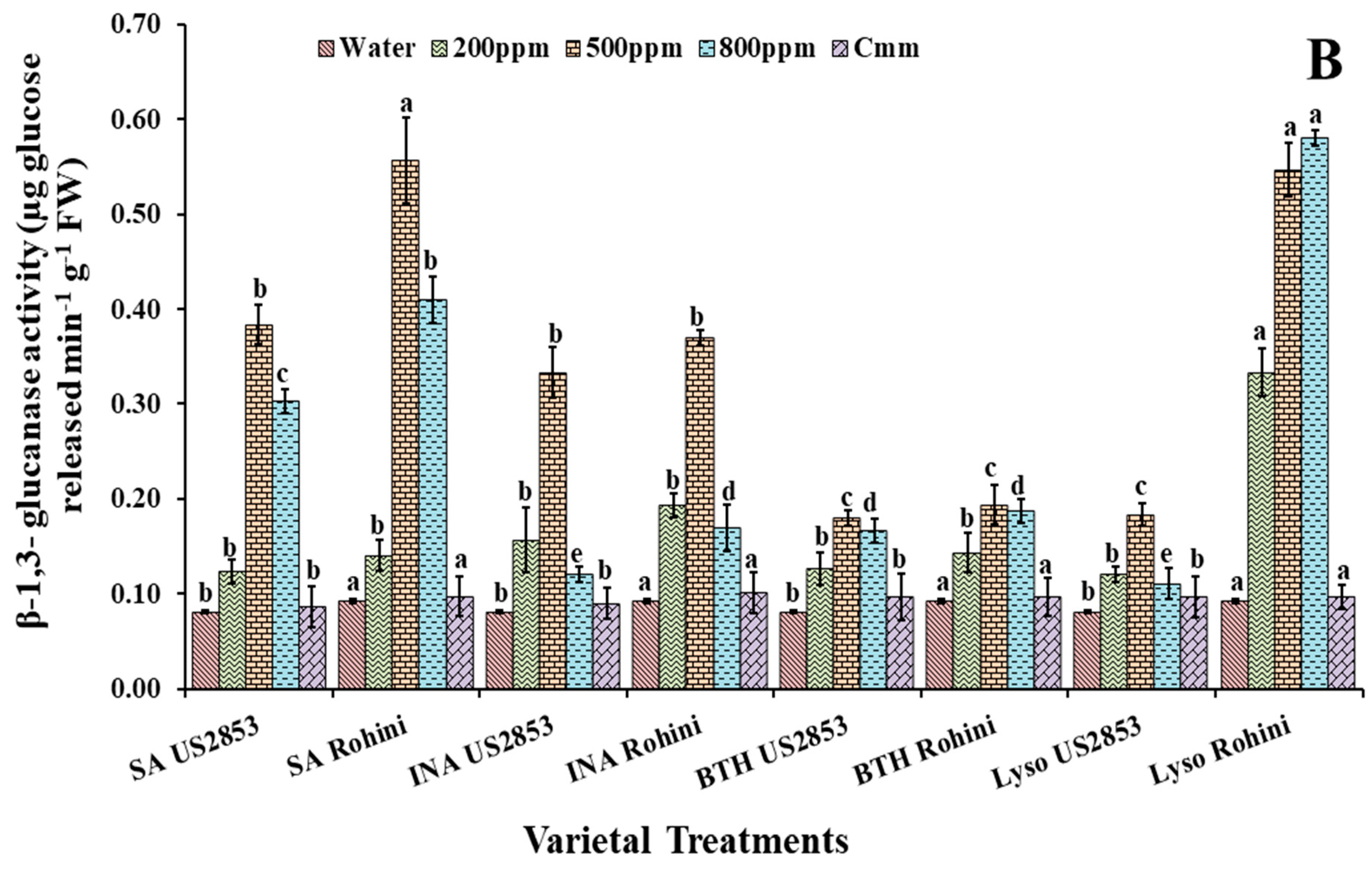
PR2 (β-1,3-Glucanase) Protein Assay
2.5. Histochemical Analysis
2.5.1. Hydrogen Peroxide Production
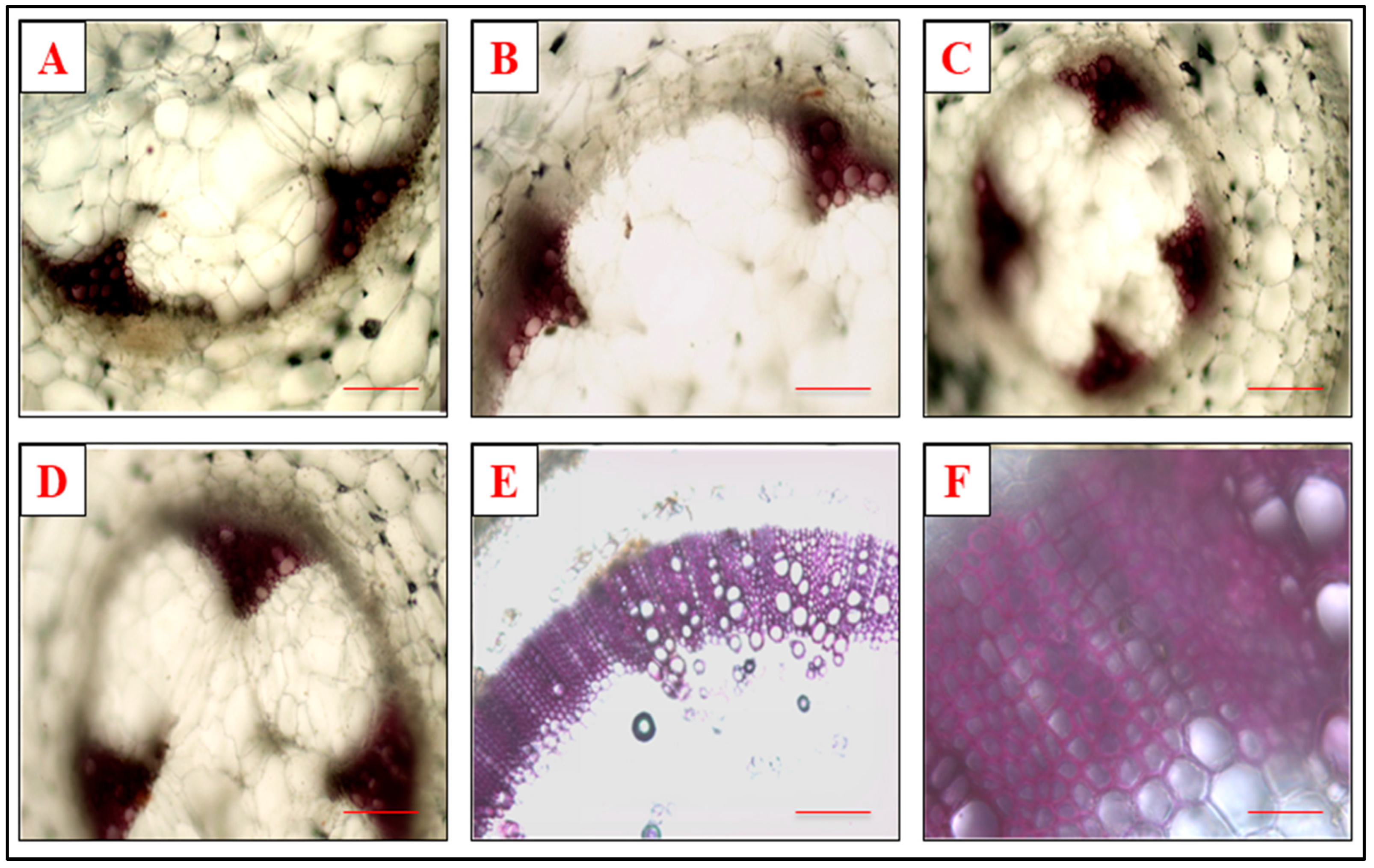
2.5.2. Lignification
2.6. Assessment of Defense Inducers Efficacy on Disease Incidence under Protected Conditions
2.7. Statistical Packages
3. Results
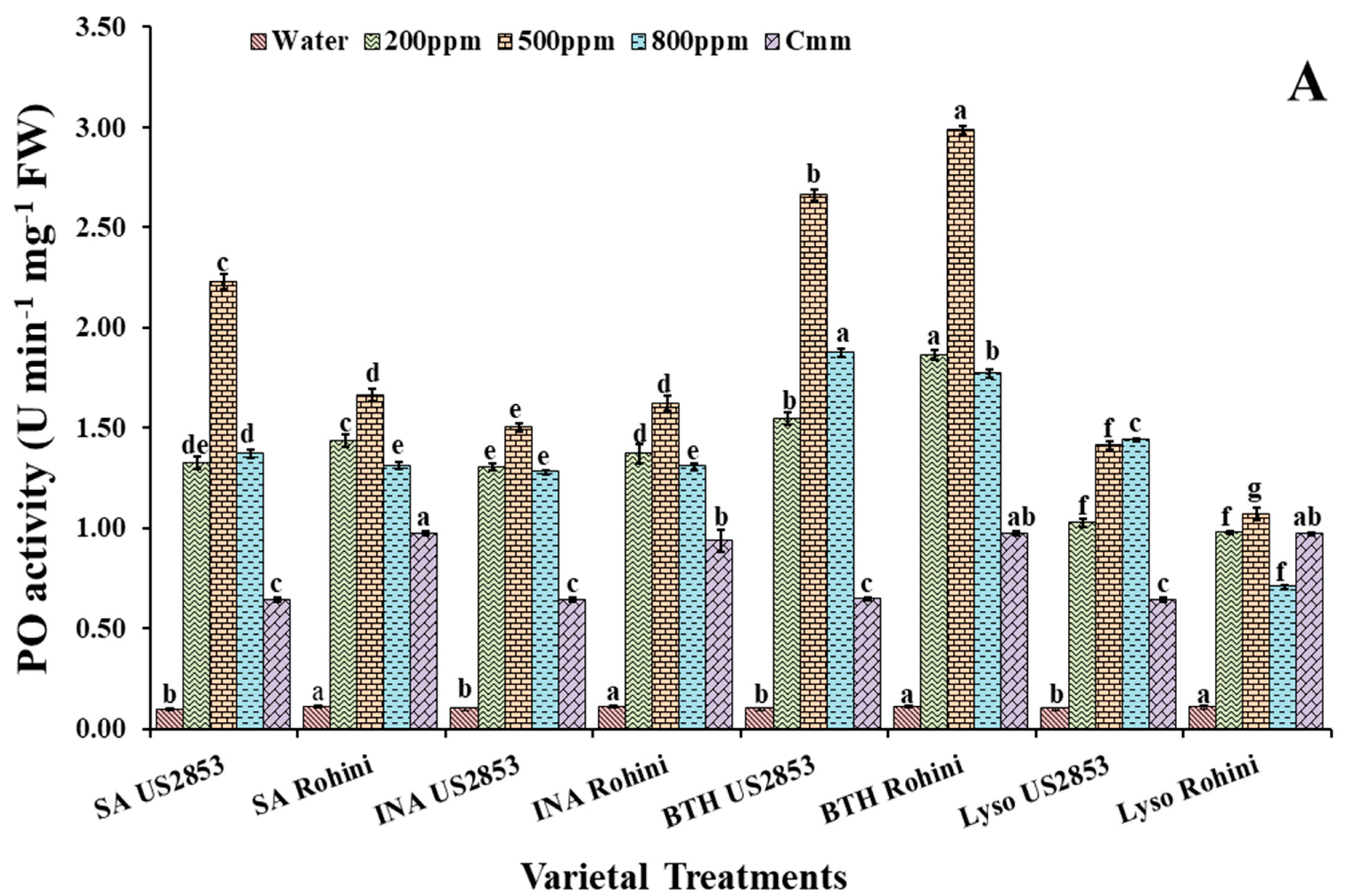
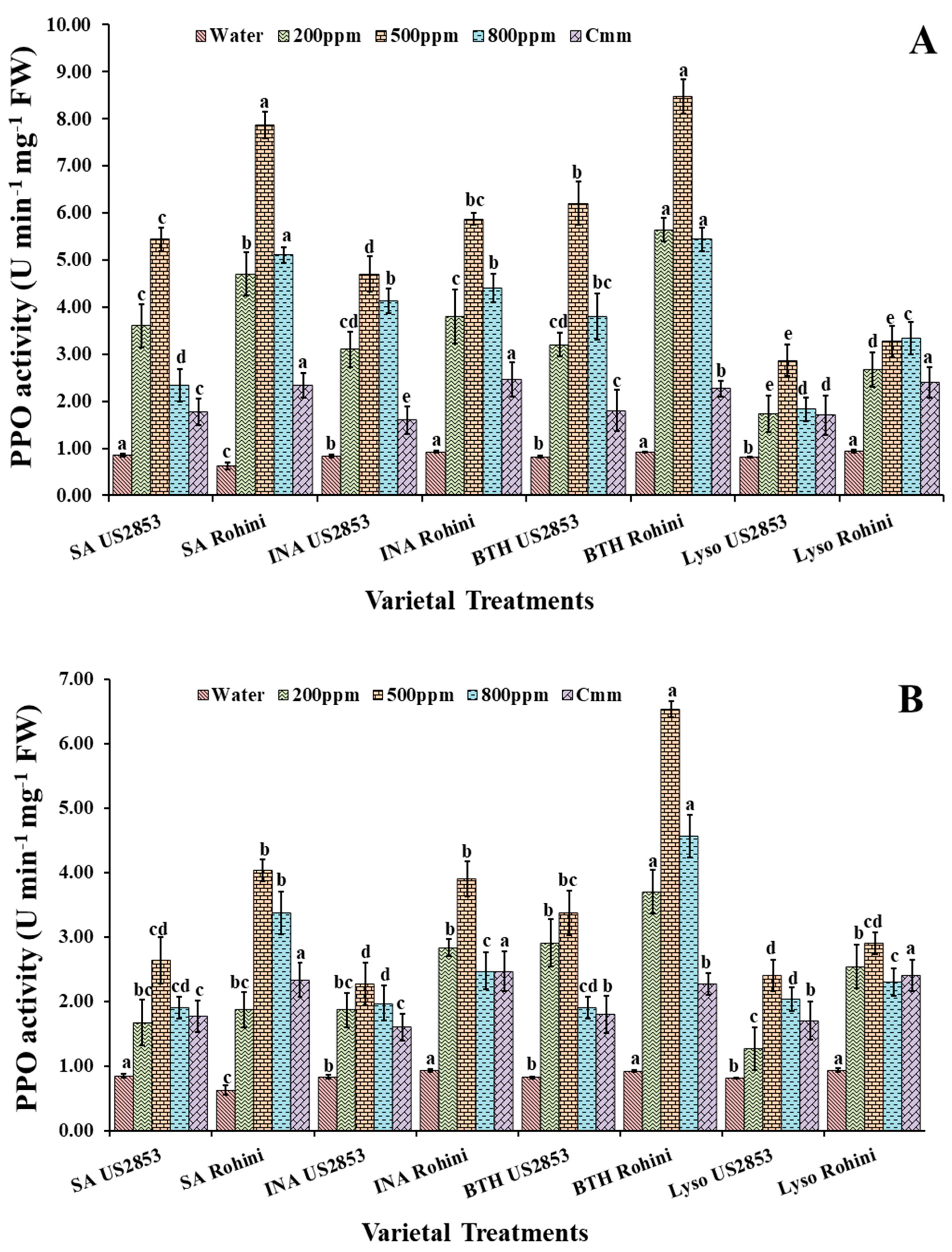
3.1. Effect on Peroxidase Activity
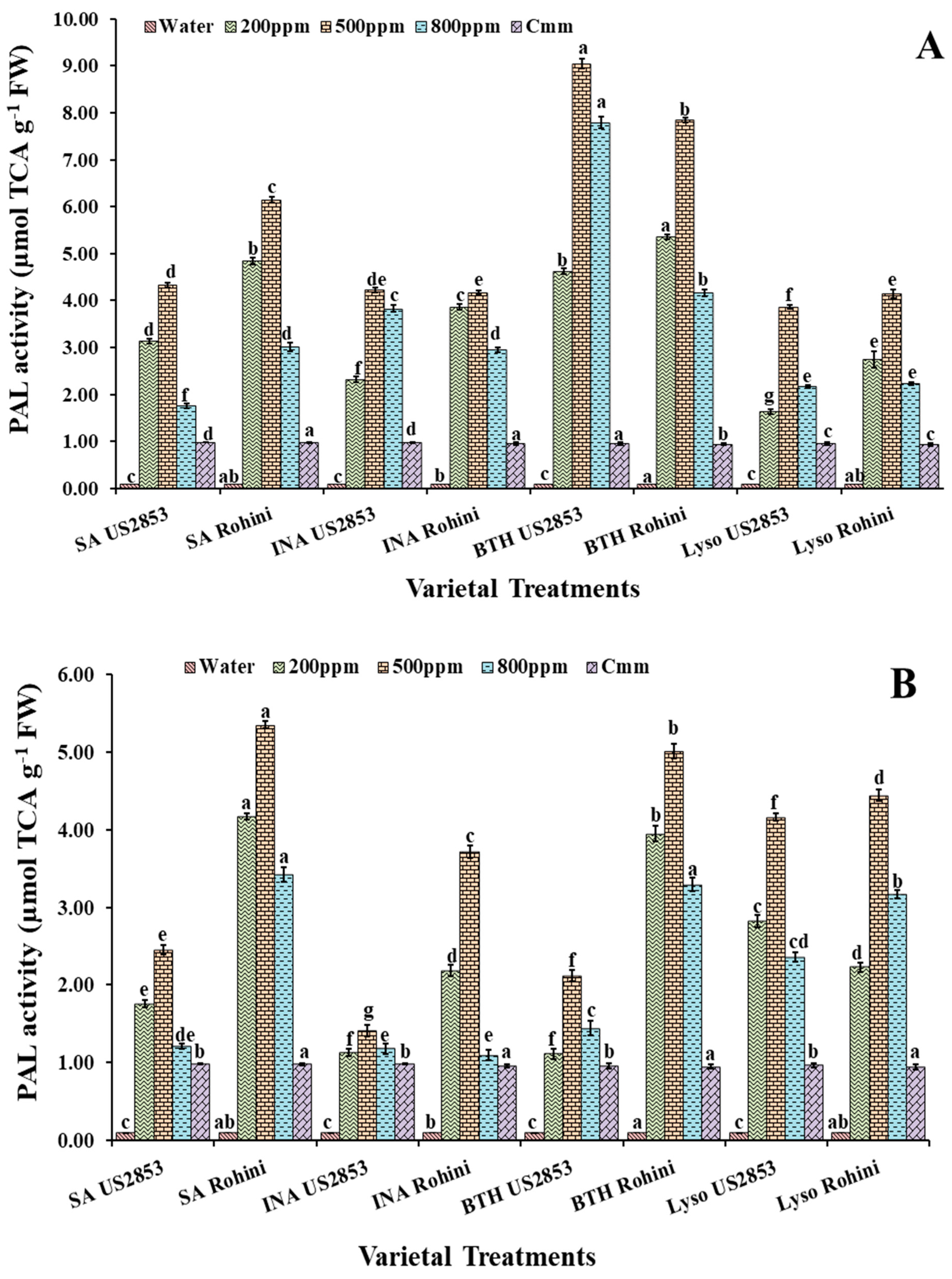
3.2. Effect of Defense Inducers on Phenylpropanoid Activity
3.2.1. PAL Activity
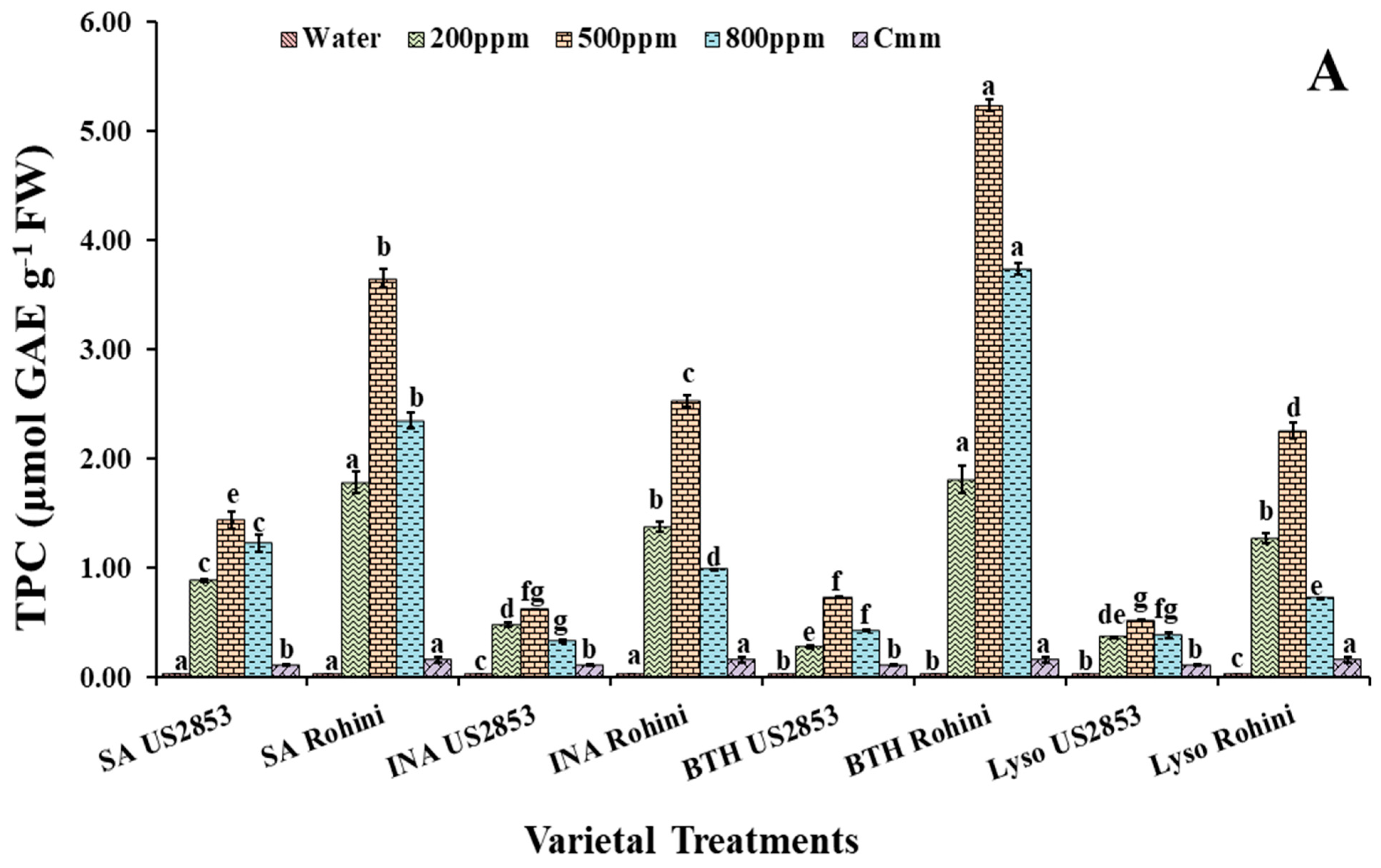
3.2.2. Total Phenolic Content
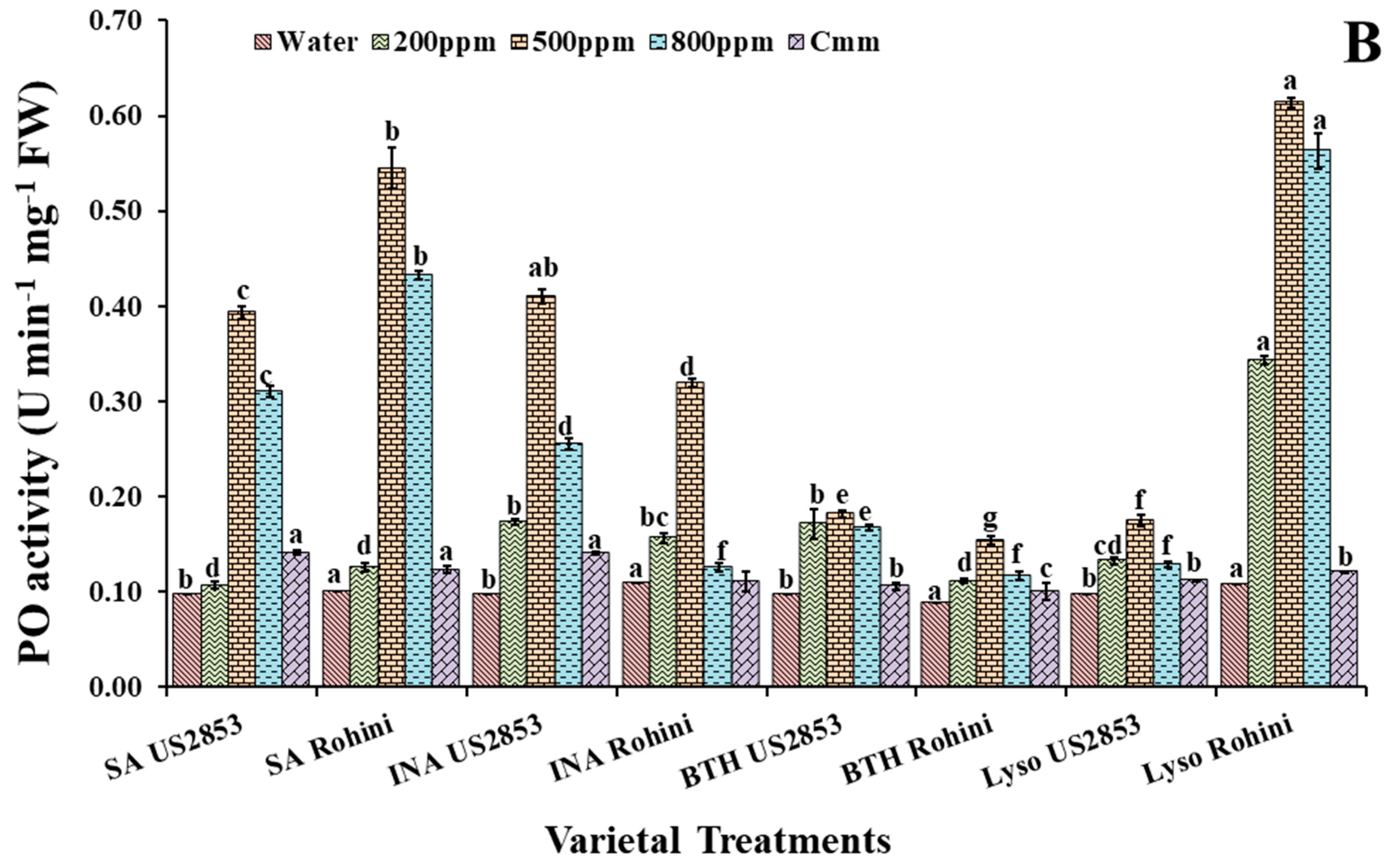
3.2.3. Polyphenol Oxidase Activity
3.3. Effect of Defense Inducers on β-1,3-Glucanases (PR-2 Protein) Activity
3.4. Effect of Defense Inducers on Hydrogen Peroxide Generation
3.5. Effect of Defense Inducers on Lignin Deposition
3.6. Assessment of Defense Inducers Efficacy on Disease Incidence Management and Disease Severity under Protected Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paduchuri, P.; Gohokar, S.; Thamke, B.; Subhas, M. Transgenic tomatoes. Int. J. Adv. Biotechnol. Res. 2010, 2, 69–72. [Google Scholar]
- Gupta, S.K.; Thind, T.S. Disease Problems in Vegetable Production; Scientific Publishers: Jodhpur, India, 2006; p. 576. [Google Scholar]
- de León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Clavibacter michiganesis subsp. michiganensis, a Seedborne Tomato Pathogen: Healthy Seeds Are Still the Goal. Plant Dis. 2011, 95, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.J.; Ries, S.M.; Pataky, J.K. Local sources of Clavibacter michiganensis ssp. michiganensis in the development of bacterial canker on tomatoes. Phytopathology 1992, 82, 553–560. [Google Scholar] [CrossRef]
- Gitaitis, R.D.; Beaver, R.W.; Voloudakis, A.E. Detection of Clavibacter michiganensis subsp. michiganensis in symptomless tomato transplants. Plant Dis. 1991, 75, 834–838. [Google Scholar] [CrossRef]
- Hadas, R.; Kritzman, G.; Klietman, F.; Gefen, T.; Manulis, S. Comparison of extraction procedures and determination of the detection threshold for Clavibacter michiganensis ssp. michiganensis in tomato seeds. Plant Pathol. 2005, 54, 643–649. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Tanina, K.; Inoue, K. Molecular typing and spread of Clavibacter michiganensis subsp. michiganensis in greenhouses in Japan. Plant Pathol. 2010, 59, 76–83. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Tanina, K.; Inoue, K. Spatiotemporal distribution of tomato plants naturally infected with bacterial canker in greenhouses. J. Gen. Plant Pathol. 2013, 79, 46–50. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Wiesel, L.; Newton, A.C.; Elliot, I.; Booty, D.; Gilory, E.M.; Birch, P.R.J.; Hein, I. Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front. Plant Sci. 2014, 5, 655. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, I.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A global perspective. Front Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Salazar, B.; Ortiz, A.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Singh, S.P.; Rekadwad, B.; Borriss, R.; Jain, A.; Singh, H.B.; et al. Bacillus spp. as Bio-factories for Antifungal Secondary Metabolites: Innovation Beyond Whole Organism Formulations. Microb. Ecol. 2022, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.S.; Hunt, M.D. Systemic Acquired Resistance. Plant Cell 1996, 8, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Mehdy, M.C.; Sharma, Y.K.; Sathasivan, K.; Bays, N.W. The role of activated oxygen species in plant disease resistance. Physiol. Plant. 1996, 98, 365–374. [Google Scholar] [CrossRef]
- Baysal, O.; Soylu, E.M.; Soylu, S. Induction of defense related enzymes and resistance by the plant activator acibenzolar S methyl in tomato seedlings against bacterial canker caused by Clavibacter michiganensis ssp michiganensis. Plant Pathol. J. 2003, 52, 747–753. [Google Scholar] [CrossRef]
- Soylu, S.; Baysal, O.; Soylu, E.M. Induction of disease resistance by the plant activator, acibencolar-Smethyl (ASM), against bacterial canker (Clavibacter michiganensis subsp. michiganensis) in tomato seedlings. Plant Sci. 2003, 165, 1069–1075. [Google Scholar] [CrossRef]
- Baysal, O.; Gursoy, Y.; Ornek, H.; Duru, A. Induction of oxidants in tomato leaves treated with DL-beta-amino butyric acid (BABA) and infected with Clavibacter michiganensis ssp. michiganensis. Eur. J. Plant. Pathol. 2005, 112, 361–369. [Google Scholar] [CrossRef]
- Van den Bulk, R.W.; Zevenhuizen, L.P.T.M.; Cordewener, J.H.G.; Dons, J.J.M. Characterization of the extracellular polysaccharide produced by Clavibacter michiganensis subsp. michiganensis. Phytopathology 1991, 81, 619–623. [Google Scholar] [CrossRef]
- Gauillard, F.; Richard-Forget, F.; Nicolas, J. New spectrophotometric assay for polyphenol oxidase activity. Ann. Biochem. 1993, 215, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Brueske, C.H. Phenylalanine ammonia-lyase activity in Lycopersicon esculentum roots infected and resistant to the root knot nematode, Meloidogyne incognita. Physiol. Plant Pathol. 1980, 16, 409–414. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Solid-state bioconversion of phenolics from cranberry pomace and role of Lentinus edodes β-glucosidase. J. Agric. Food Chem. 2000, 48, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.O.; Ye, X.S.; Kuc, J. A technique for detection of chitinase, β-1.3-glucanase, and protein patterns after a single separation using polyacrylamide gel electrophoresis or isoelectrofocusing. Phytopathology 1991, 81, 970–974. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Jensen, W. Botanical Histochemistry: Principles and Practice; W. H. Freeman: San Francisco, CA, USA, 1962. [Google Scholar]
- Keswani, C.; Dilnashin, H.; Birla, H.; Singh, S.P. Unravelling efficient applications of agriculturally important microorganisms for alleviation of induced inter-cellular oxidative stress in crops. Acta Agric. Slov. 2019, 114, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Montagu, M.V.; Inze, D.; Breusegem, F.V. Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Alscher, R.G.; Donahue, J.L.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant 1997, 100, 224–233. [Google Scholar] [CrossRef]
- Kuzniak, E.; Sklodowska, M. Ascorbate, glutathione andrelated enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant Sci. 2001, 160, 723–731. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Differential defensive response of groundnut germplasms to Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant Interact. 2011, 7, 45–55. [Google Scholar] [CrossRef]
- Hu, X.; Li, W.; Chen, Q.; Yang, Y. Early signal transduction linking the synthesis of jasmonic acid in plant. Plant Signal. Behav. 2009, 4, 696–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H. Dissection of salicylic acid-mediated defense signaling networks. Plant Signal. Behav. 2009, 4, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Xue, L.; Chintamanani, S.; Germain, H.; Lin, H.; Cui, C.; Cai, R.; Zuo, J.; Tang, X.; Li, X.; et al. Ethylene insensitive3 and ethylene insensitive3-like1 repress salicylic acid induction deficient2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 2009, 21, 2527–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.Y.; Chen, J.L.; Cheng, D.F.; Sun, J.R.; Liu, Y.; Tian, Z. Biochemical and molecular characterizations of Sitobion avenae–induced wheat defense responses. Crop Prot. 2009, 28, 435–442. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2009, 68, 14–25. [Google Scholar] [CrossRef]
- Rajjou, L.; Belghazi, M.; Huguet, R.; Robin, C.; Moreau, A.; Job, C.; Job, D. Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol. 2006, 141, 910–923. [Google Scholar] [CrossRef] [Green Version]
- Pallas, J.A.; Paiva, N.L.; Lamb, C.; Dixon, R.A. Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 1996, 10, 281–293. [Google Scholar] [CrossRef]
- Yu, Z.; Fu, C.; Han, Y.; Li, Y.; Zhao, D. Salicylic acid enhances jaceosidin and syringin production in cell cultures of Saussurea medusa. Biotechnol. Lett. 2006, 28, 1027–1031. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, P.; Mandal, P.; Saha, D.; Saha, A. Phenylalanine ammonia-lyase gene induction with benzothiadiazole elevates defence against Lasiodiplodia theobromae in tea in India. J. Phytopathol. 2017, 165, 755–761. [Google Scholar] [CrossRef]
- Sharma, H.C.; Sujana, G.; Manohar Rao, D. Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of Pigeonpea. Arthropod-Plant Interact. 2009, 3, 152–161. [Google Scholar] [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant Mineral Nutrition and Disease Resistance: A Significant Linkage for Sustainable Crop Protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- Usha Rani, P.; Jyothsana, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–701. [Google Scholar] [CrossRef]
- Kawano, T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003, 21, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithofer, A.; Boland, W. Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 2007, 68, 2946–2959. [Google Scholar] [CrossRef]
- Noreen, Z.; Ashraf, M. Change in antioxidant enzymes and some key metabolites in some genetically diverse cultivars of radish (Raphanus sativus L.). Environ. Exp. Bot. 2009, 67, 395–402. [Google Scholar] [CrossRef]
- Solano-Alvarez, N.; Valencia-Hernández, J.A.; Rico-García, E.; Torres-Pacheco, I.; Ocampo-Velázquez, R.V.; Escamilla-Silva, E.M.; Romero-García, A.L.; Alpuche-Solís, Á.G.; Guevara-González, R.G. Novel Isolate of Bacillus cereus Promotes Growth in Tomato and Inhibits Clavibacter michiganensis Infection under Greenhouse Conditions. Plants 2021, 10, 506. [Google Scholar] [CrossRef]
- Van Loon, L. Induced resistance in plants and the role of pathogenensis-related proteins. Eur. J. Plant Pathol. 1997, 103, 753–765. [Google Scholar] [CrossRef]
- Boller, T.; Gehri, A.; Mauch, F.; Vogeli, U. Chitinase in bean leaves: Induction by ethylene, purification, properties, and possible function. Planta 1983, 157, 22–31. [Google Scholar] [CrossRef]
- Heitz, T.; Segond, S.; Kauffmann, S.; Geoffroy, P.; Prasad, V.; Brunner, F.; Fritig, B.; Legrand, M. Molecular characterization of a novel tobacco PR protein: A new plant chitinase/lysozyme. Mol. Genet. Genom. 1994, 245, 246–254. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The Oxidative Burst in Plant Disease Resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death don’t have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 1996, 8, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.A.; Campbel, M.M. The genetic control of lignin deposition during plant growth and development. New Phytol. 2004, 164, 17–30. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose deposition: A multifaceted plant defense response. Mol. Plant-Microbe Interact. 2011, 24, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, P.; Ikeda, K.-I. Ultrastructural analysis of responses of host and fungal cells during plant infection. J. Gen. Plant Pathol. 2008, 74, 2–14. [Google Scholar] [CrossRef]
- Conrath, U.; Thulke, O.; Katz, V.; Schwindling, S.; Kohler, A. Priming as a mechanism in induced systemic resistance of plants. Eur. J. Plant Pathol. 2001, 107, 113–119. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Lajayer, B.A.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, R.; Vishunavat, K.; Tewari, R.; Kumar, S.; Minkina, T.; De Corato, U.; Keswani, C. Defense Inducers Mediated Mitigation of Bacterial Canker in Tomato through Alteration in Oxidative Stress Markers. Microorganisms 2022, 10, 2160. https://doi.org/10.3390/microorganisms10112160
Tripathi R, Vishunavat K, Tewari R, Kumar S, Minkina T, De Corato U, Keswani C. Defense Inducers Mediated Mitigation of Bacterial Canker in Tomato through Alteration in Oxidative Stress Markers. Microorganisms. 2022; 10(11):2160. https://doi.org/10.3390/microorganisms10112160
Chicago/Turabian StyleTripathi, Ruchi, Karuna Vishunavat, Rashmi Tewari, Sumit Kumar, Tatiana Minkina, Ugo De Corato, and Chetan Keswani. 2022. "Defense Inducers Mediated Mitigation of Bacterial Canker in Tomato through Alteration in Oxidative Stress Markers" Microorganisms 10, no. 11: 2160. https://doi.org/10.3390/microorganisms10112160






