Dose- and Sex-Dependent Bidirectional Relationship between Intravenous Fentanyl Self-Administration and Gut Microbiota
Abstract
:1. Introduction
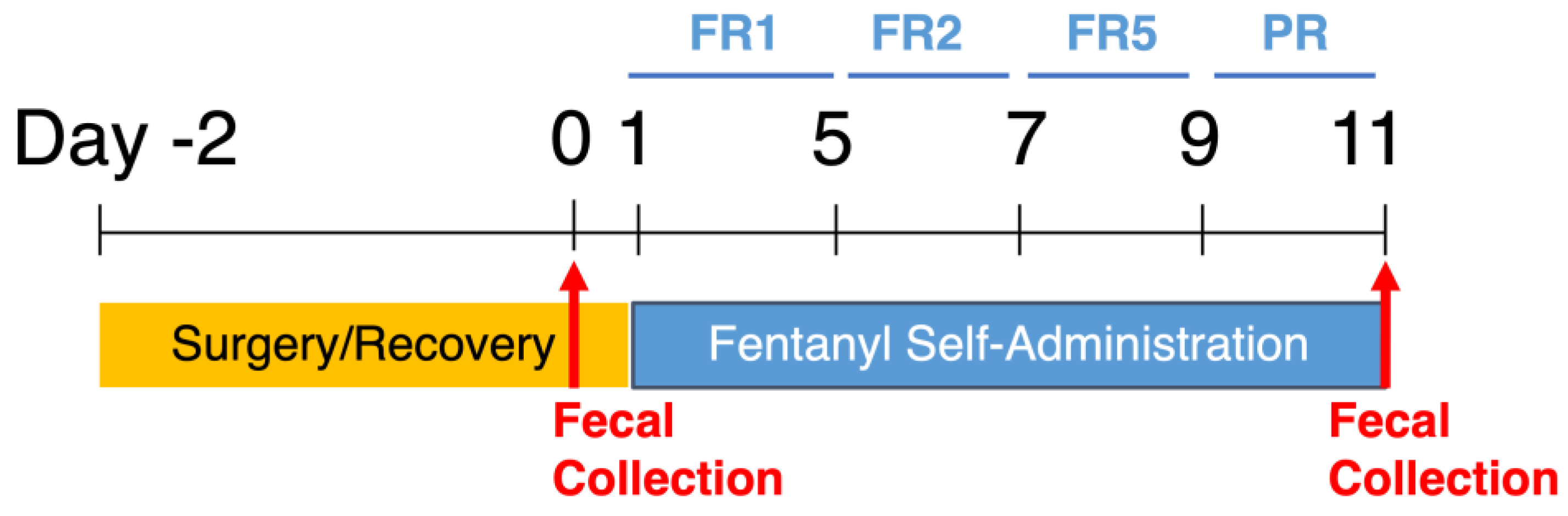
2. Materials and Methods
3. Results
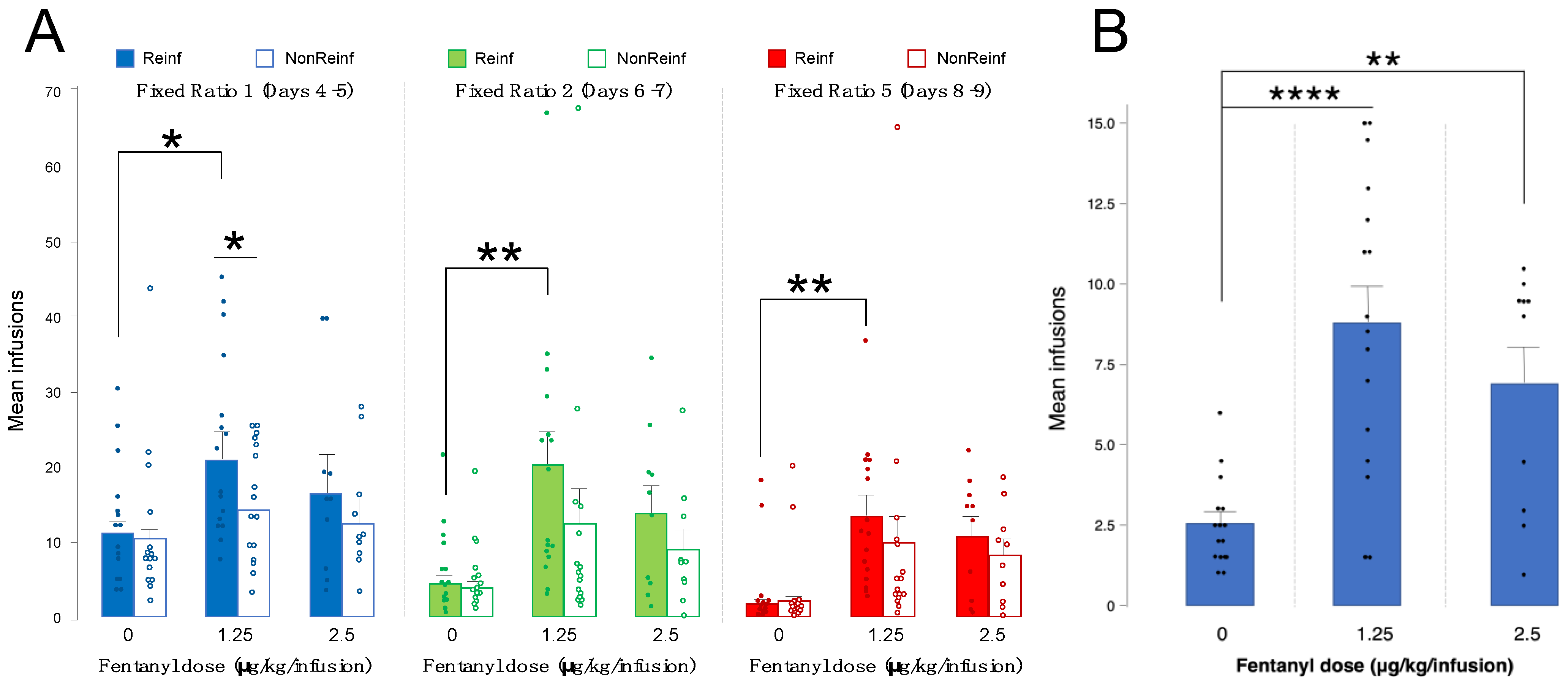
3.1. Intravenous Fentanyl Self-Administration Is Dependent on Fentanyl Dose
3.2. Motivation for Fentanyl Infusions Is Highest at 1.25 vs. 0 or 2.5 ug/kg/Infusion
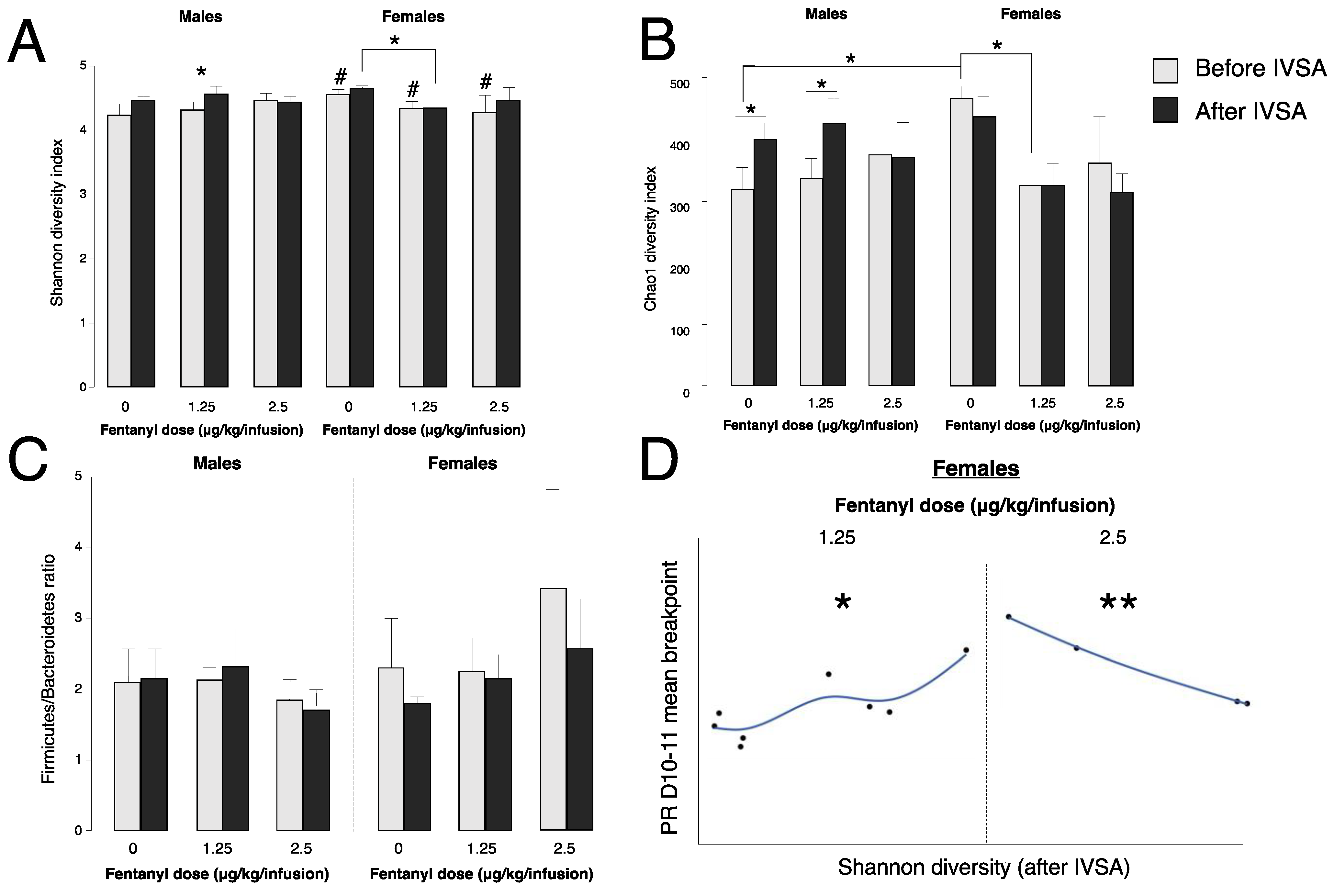
3.3. Fentanyl Self-Administration Changes Gut Bacteria Alpha Diversity
3.4. Baseline Gut Bacteria Alpha Diversity Does Not Predict Fentanyl Self-Administration
3.5. Gut Bacteria Alpha Diversity Predicts Progressive Ratio Responding in Females
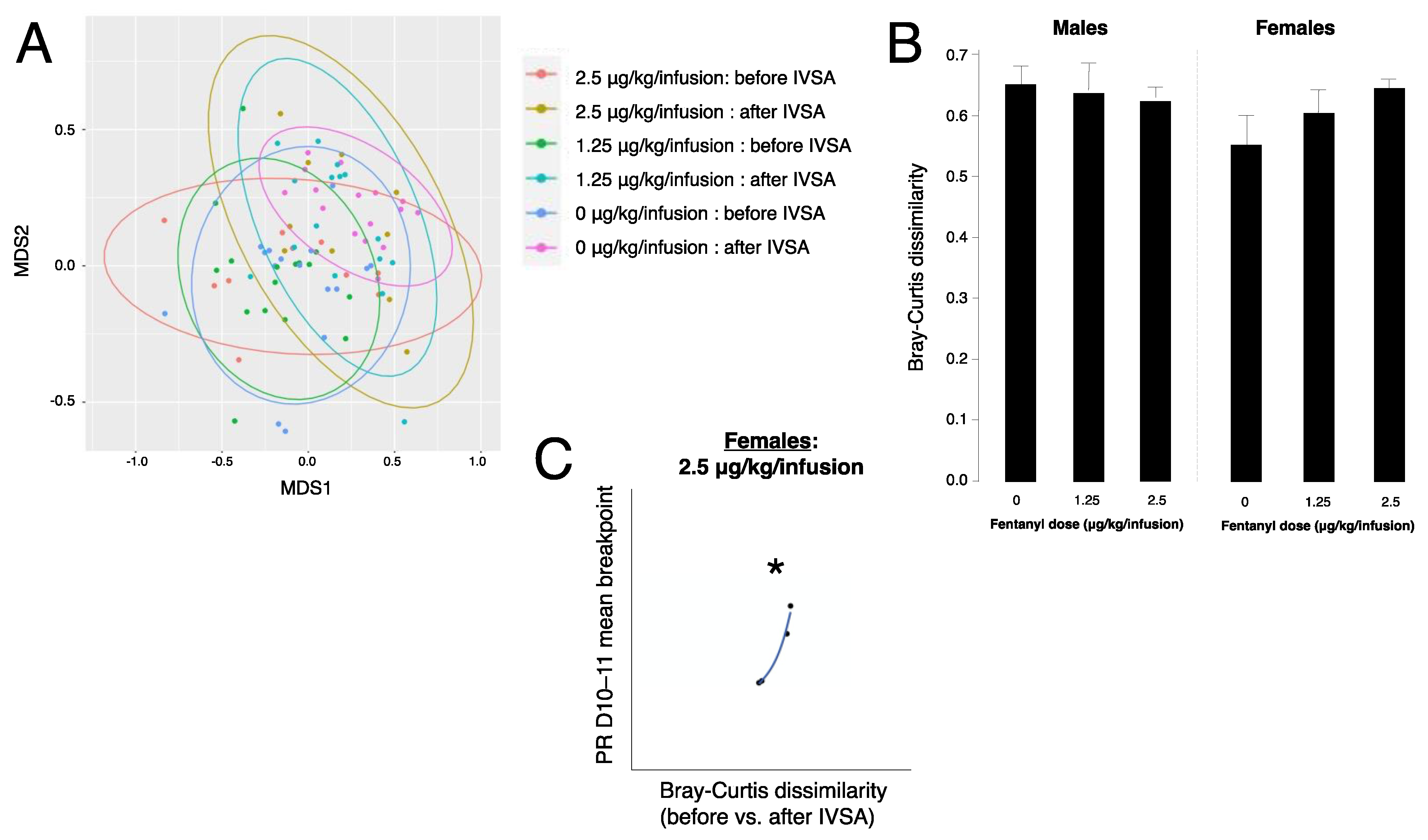
3.6. Fentanyl Self-Administration Does Not Alter Beta Diversity of Gut Bacteria
3.7. Gut Bacteria Beta Diversity Predicts Progressive Ratio Responding in Females
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, R.A.; Seth, P.; David, F.; Scholl, L. Increases in Drug and Opioid-Involved Overdose Deaths—United States, 2010–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1445–1452. [Google Scholar] [CrossRef] [Green Version]
- Vincent, C.; Miller, M.A.; Edens, T.J.; Mehrotra, S.; Dewar, K.; Manges, A.R. Bloom and Bust: Intestinal Microbiota Dynamics in Response to Hospital Exposures and Clostridium Difficile Colonization or Infection. Microbiome 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Roy, S. Gut Homeostasis, Microbial Dysbiosis, and Opioids. Toxicol. Pathol. 2016, 45, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, C.; Betrapally, N.; Gillevet, P.; Sterling, R.; Akbarali, H.; White, M.; Ganapathy, D.; Fagan, A.; Sikaroodi, M.; Bajaj, J. Chronic Opioid Use is Associated with Altered Gut Microbiota and Predicts Readmissions in Patients with Cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 319–331. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Z.; Wang, H.; Shen, Z.; Guo, Y.; Gao, Y.; Chen, X.; Wu, Q.; Li, X.; Wang, K. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S RRNA Gene Deep Sequencing. Sci. Rep. 2017, 7, 3628. [Google Scholar] [CrossRef]
- Barengolts, E.; Green, S.J.; Eisenberg, Y.; Akbar, A.; Reddivari, B.; Layden, B.T.; Dugas, L.; Chlipala, G. Gut Microbiota Varies by Opioid Use, Circulating Leptin and Oxytocin in African American Men with Diabetes and High Burden of Chronic Disease. PLoS ONE 2018, 13, e0194171. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Yu, H.; Ma, J.; Wang, J.; Banerjee, S.; Charboneau, R.; Barke, R.A.; Roy, S. Morphine Induces Bacterial Translocation in Mice by Compromising Intestinal Barrier Function in a TLR-Dependent Manner. PLoS ONE 2013, 8, e54040. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Sindberg, G.M.; Roy, S. Disruption of Gut Homeostasis by Opioids Accelerates HIV Disease Progression. Front. Microbiol. 2015, 6, 643. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Sindberg, G.; Wang, F.; Meng, J.; Sharma, U.; Zhang, L.; Dauer, P.; Chen, C.; Dalluge, J.; Johnson, T.; et al. Opioid-Induced Gut Microbial Disruption and Bile Dysregulation Leads to Gut Barrier Compromise and Sustained Systemic Inflammation. Mucosal Immunol. 2016, 9, 1418–1428. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Mischel, R.A.; Bhave, S.; Komla, E.; Cho, A.; Huang, C.; Dewey, W.L.; Akbarali, H.I. The Effect of Gut Microbiome on Tolerance to Morphine Mediated Antinociception in Mice. Sci. Rep. 2017, 7, 42658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Vuong, H.E.; Nusbaum, D.J.; Hsiao, E.Y.; Evans, C.J.; Taylor, A.M.W. The Gut Microbiota Mediates Reward and Sensory Responses Associated with Regimen-Selective Morphine Dependence. Neuropsychopharmacology 2018, 43, 2606–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine Induces Changes in the Gut Microbiome and Metabolome in a Morphine Dependence Model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofford, R.S.; Mervosh, N.L.; Euston, T.J.; Meckel, K.R.; Orr, A.T.; Kiraly, D.D. The Gut Microbiome and Its Metabolites are Necessary for Morphine Reward. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association with Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of Human Epithelial Tight Junction Proteins by Lactobacillus Plantarum in vivo and Protective Effects on the Epithelial Barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Kiraly, D.D.; Walker, D.M.; Calipari, E.S.; Labonte, B.; Issler, O.; Pena, C.J.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep. 2016, 6, 35455. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.-W.; Gao, X.-B. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Cabrerizo, R.; Carbia, C.; O’Riordan, K.J.; Schellekens, H.; Cryan, J.F. Microbiota-Gut-Brain Axis as a Regulator of Reward Processes. J. Neurochem. 2021, 157, 1495–1524. [Google Scholar] [CrossRef]
- Belluzzi, J.D.; Wang, R.; Leslie, F.M. Acetaldehyde Enhances Acquisition of Nicotine Self-Administration in Adolescent Rats. Neuropsychopharmacology 2005, 30, 705–712. [Google Scholar] [CrossRef]
- Roberts, D.; Loh, E.A.; Vickers, G. Self-Administration of Cocaine on a Progressive Ratio Schedule in Rats: Dose-Response Relationship and Effect of Haloperidol Pretreatment. Psychopharmacology 1989, 97, 535–538. [Google Scholar] [CrossRef]
- van Ree, J.M.; Slangen, J.L.; de Wied, D. Intravenous Self-Administration of Drugs in Rats. J. Pharmacol. Exp. Ther. 1978, 204, 547. [Google Scholar]
- Morgan, A.D.; Campbell, U.C.; Fons, R.D.; Carroll, M.E. Effects of Agmatine on the Escalation of Intravenous Cocaine and Fentanyl Self-Administration in Rats. Pharmacol. Biochem. Behav. 2002, 72, 873–880. [Google Scholar] [CrossRef]
- Wade, C.L.; Vendruscolo, L.F.; Schlosburg, J.E.; Hernandez, D.O.; Koob, G.F. Compulsive-like Responding for Opioid Analgesics in Rats with Extended Access. Neuropsychopharmacology 2015, 40, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S RRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2015, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.A.; Negus, S.S.; Caine, S.B.; Thomsen, M.; Banks, M.L. Sex Differences in Opioid Reinforcement under a Fentanyl vs. Food Choice Procedure in Rats. Neuropsychopharmacology 2019, 44, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Lacy, R.T.; Strickland, J.C.; Feinstein, M.A.; Robinson, A.M.; Smith, M.A. The Effects of Sex, Estrous Cycle, and Social Contact on Cocaine and Heroin Self-Administration in Rats. Psychopharmacology 2016, 233, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.C.; Popke, E.J.; Grunberg, N.E. Sex Differences in Effects of Predictable and Unpredictable Footshock on Fentanyl Self-Administration in Rats. Exp. Clin. Psychopharmacol. 1997, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.E.; Campbell, U.C.; Heideman, P. Ketoconazole Suppresses Food Restriction–Induced Increases in Heroin Self-Administration in Rats: Sex Differences. Exp. Clin. Psychopharmacol. 2001, 9, 307. [Google Scholar] [CrossRef]
- Cicero, T.J.; Aylward, S.C.; Meyer, E.R. Gender Differences in the Intravenous Self-Administration of Mu Opiate Agonists. Pharmacol. Biochem. Behav. 2003, 74, 541–549. [Google Scholar] [CrossRef]
- Kaplovitch, E.; Gomes, T.; Camacho, X.; Dhalla, I.A.; Mamdani, M.M.; Juurlink, D.N. Sex Differences in Dose Escalation and Overdose Death during Chronic Opioid Therapy: A Population-Based Cohort Study. PLoS ONE 2015, 10, e0134550. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. WJMH 2019, 38, 48–60. [Google Scholar] [CrossRef]
- Kovacs, A.; Ben-Jacob, N.; Tayem, H.; Halperin, E.; Iraqi, F.A.; Gophna, U. Genotype is a Stronger Determinant than Sex of the Mouse Gut Microbiota. Microb. Ecol. 2011, 61, 423–428. [Google Scholar] [CrossRef]
- Elderman, M.; Hugenholtz, F.; Belzer, C.; Boekschoten, M.; van Beek, A.; de Haan, B.; Savelkoul, H.; de Vos, P.; Faas, M. Sex and Strain Dependent Differences in Mucosal Immunology and Microbiota Composition in Mice. Biol. Sex Differ. 2018, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Van Nas, A.; GuhaThakurta, D.; Wang, S.S.; Yehya, N.; Horvath, S.; Zhang, B.; Ingram-Drake, L.; Chaudhuri, G.; Schadt, E.E.; Drake, T.A. Elucidating the Role of Gonadal Hormones in Sexually Dimorphic Gene Coexpression Networks. Endocrinology 2009, 150, 1235–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex Differences and Hormonal Effects on Gut Microbiota Composition in Mice. Null 2016, 7, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole Paul, W.; Ian, B.J. Gut Microbiota and Aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Hofford, R.S.; Mervosh, N.L.; Euston, T.J.; Meckel, K.R.; Orr, A.T.; Kiraly, D.D. Alterations in Microbiome Composition and Metabolic Byproducts Drive Behavioral and Transcriptional Responses to Morphine. Neuropsychopharmacology 2021, 46, 2062–2072. [Google Scholar] [CrossRef]
- Shade, A. Diversity is the Question, Not the Answer. ISME J. 2017, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Scotti, E.; Boué, S.; Sasso, G.L.; Zanetti, F.; Belcastro, V.; Poussin, C.; Sierro, N.; Battey, J.; Gimalac, A.; Ivanov, N.V. Exploring the Microbiome in Health and Disease: Implications for Toxicology. Toxicol. Res. Appl. 2017, 1, 2397847317741884. [Google Scholar] [CrossRef]
- Paul, B.E.; Elisabeth, M.B.; Charles, N.B.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Steven, R.G.; Karen, E.N.; David, A.R. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
| Fentanyl Dose | Number of Animals | Results |
|---|---|---|
| 0 μg/kg/infusion | 15 | Mean infusions: FR1 D4–5 Reinf: 11.80 FR1 D4–5 NonReinf: 10.60 FR2 D6–7 Reinf: 6.26 FR2 D6–7 NonReinf: 5.76 FR5 D8–9 Reinf: 4.0 FR5 D8–9 NonReinf: 4.26 PR: 2.56 |
| 1.25 μg/kg/infusion | 16 | Mean infusions: FR1 D4–5 Reinf: 20.90 FR1 D4–5 NonReinf: 14.65 FR2 D6–7 Reinf: 20.43 FR2 D6–7 NonReinf: 12.53 FR5 D8–9 Reinf: 13.25 FR5 D8–9 NonReinf: 9.53 PR: 8.81 |
| 2.5 μg/kg/infusion | 10 | Mean infusions: FR1 D4–5 Reinf: 16.45 FR1 D4–5 NonReinf: 12.40 FR2 D6–7 Reinf: 13.80 FR2 D6–7 NonReinf: 8.85 FR5 D8–9 Reinf: 10.70 FR5 D8–9 NonReinf: 8.15 PR: 6.90 |
| Bacterial Phylum or Genus | Results |
|---|---|
| Verrucomicrobia | Decreased after fentanyl IVSA (males, 1.25 μg/kg/infusion; p = 0.03) Increased in females vs. males before fentanyl IVSA (p = 0.01) |
| Prevotella | Increased after fentanyl IVSA (females, 1.25 μg/kg/infusion; p = 0.02) Decreased in females vs. males before fentanyl IVSA (p = 0.02) |
| Ruminococcus | Increased after fentanyl IVSA (males, 1.25 μg/kg/infusion; p = 0.03) |
| Akkermansia | Decreased after fentanyl IVSA (males, 1.25 μg/kg/infusion; p = 0.03) Increased in females vs. males before fentanyl IVSA (p = 0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Lotfipour, S. Dose- and Sex-Dependent Bidirectional Relationship between Intravenous Fentanyl Self-Administration and Gut Microbiota. Microorganisms 2022, 10, 1127. https://doi.org/10.3390/microorganisms10061127
Ren M, Lotfipour S. Dose- and Sex-Dependent Bidirectional Relationship between Intravenous Fentanyl Self-Administration and Gut Microbiota. Microorganisms. 2022; 10(6):1127. https://doi.org/10.3390/microorganisms10061127
Chicago/Turabian StyleRen, Michelle, and Shahrdad Lotfipour. 2022. "Dose- and Sex-Dependent Bidirectional Relationship between Intravenous Fentanyl Self-Administration and Gut Microbiota" Microorganisms 10, no. 6: 1127. https://doi.org/10.3390/microorganisms10061127
APA StyleRen, M., & Lotfipour, S. (2022). Dose- and Sex-Dependent Bidirectional Relationship between Intravenous Fentanyl Self-Administration and Gut Microbiota. Microorganisms, 10(6), 1127. https://doi.org/10.3390/microorganisms10061127






