Lactobacillus salivarius UCC118™ Dampens Inflammation and Promotes Microbiota Recovery to Provide Therapeutic Benefit in a DSS-Induced Colitis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Culture and Administration of Lactobacillus salivarius UCC118™
2.3. DSS-Induced Colitis
2.4. DSS-Induced Colitis Recovery Model
2.5. Histological Analysis of Colon Sections
2.6. Colonic RNA Extraction and qRT-PCR
2.7. Colonic Myeloperoxidase (MPO) Assay
2.8. Caco-2 Cell Culture and Measurement of Transepithelial Electrical Resistance
2.9. Immunoblot
2.10. LPS-Stimulation of Bone Marrow-Derived Macrophages
2.11. FITC–Dextran Assay for Barrier Permeability
2.12. Enzyme Linked Immunosorbent Assay (ELISA)
2.13. 16s rRNA Sequencing for Microbiota Analysis
2.14. Statistical Analysis
3. Results
3.1. Lactobacillus Salivarius UCC118™ Reduces the Severity of DSS-Induced Colitis in Mice
3.2. Lactobacillus salivarius UCC118™ Does Not Affect Barrier Integrity In Vivo
3.3. Lactobacillus salivarius UCC118™ Promotes IL-10 in a DSS-Colitis Model
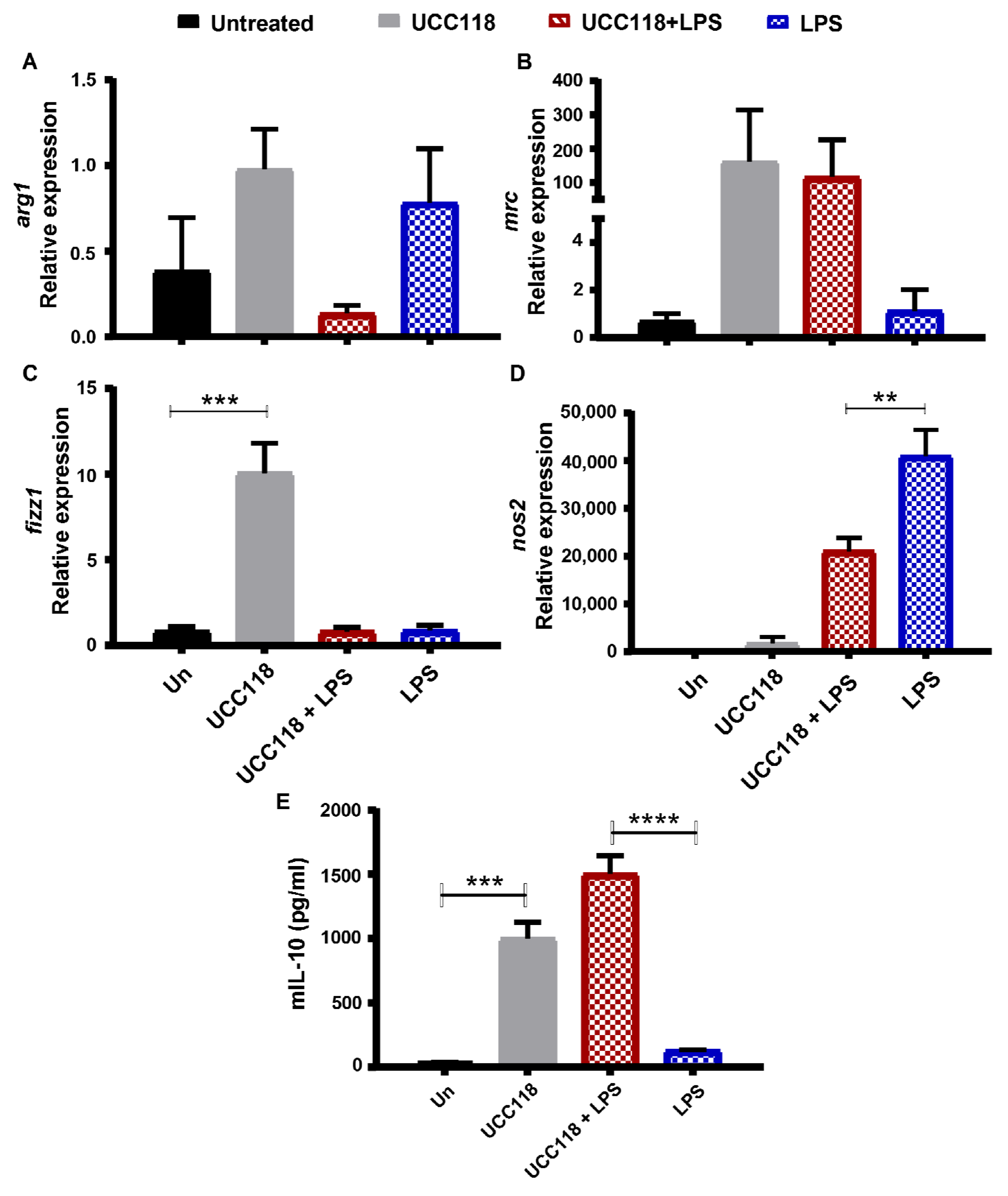
3.4. Lactobacillus salivarius UCC118™ Promotes M2 Phenotype in Bone Marrow-Derived Macrophages
3.5. Lactobacillus salivarius UCC118™ Promotes Recovery from DSS-Induced Colitis
3.6. Lactobacillus salivarius UCC118™ following Induction of DSS-Colitis Does Not Induce IL-10
3.7. Administration of Lactobacillus salivarius UCC118™ following Induction of DSS-Colitis Produces a Distinct Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Sands, B.E. From symptom to diagnosis: Clinical distinctions among various forms of intestinal inflammation. Gastroenterology 2004, 126, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Ladhoff, A.; Pernthaler, A.; Swidsinski, S.; Loening-Baucke, V.; Ortner, M.; Weber, J.; Hoffmann, U.; Schreiber, S.; Dietel, M.; et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002, 122, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annuk, H.; Shchepetova, J.; Kullisaar, T.; Songisepp, E.; Zilmer, M.; Mikelsaar, M. Characterization of intestinal lactobacilli as putative probiotic candidates. J. Appl. Microbiol. 2003, 94, 403–412. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Kalliomaki, M.; Isolauri, E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J. Allergy Clin. Immunol. 2002, 109, 119–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, L.; Dunne, C.; Kiely, B.; Shanahan, F.; O’Sullivan, G.C.; Collins, J.K. In vivo assessment of potential probiotic Lactobacillus salivarius strains: Evaluation of their establishment, persistence, and localisation in the murine gastrointestinal tract. Microb. Ecol. Health Dis. 1999, 11, 149–157. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, L.; Feeney, M.; O’Halloran, S.; Murphy, L.; Kiely, B.; FitzGibbon, J.; Lee, G.; O’Sullivan, G.; Shanahan, F.; Collins, J.K. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment. Pharmcol. Ther. 2001, 15, 1219–1225. [Google Scholar] [CrossRef]
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef] [Green Version]
- Feighery, L.M.; Smith, P.; O’Mahony, L.; Fallon, P.G.; Brayden, D.J. Effects of Lactobacillus salivarius 433118 on intestinal inflammation, immunity status and in vitro colon function in two mouse models of inflammatory bowel disease. Dig. Dis. Sci. 2008, 53, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; O’Callaghan, J.; Butto, L.F.; Hurley, G.; Melgar, S.; Tanabe, S.; Shanahan, F.; Nally, K.; O’Toole, P.W. Mechanism of protection of transepithelial barrier function by Lactobacillus salivarius: Strain dependence and attenuation by bacteriocin production. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1029–G1041. [Google Scholar] [CrossRef] [Green Version]
- Baltimore, D.; Boldin, M.P.; O’connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New regulators of immune cell development and function. Nat. Immunol. 2008, 9, 839–845. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; O’Mahony, L.; O’Callaghan, L.; Sheil, B.; Vaughan, E.E.; Fitzsimons, N.; FitzGibbon, J.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 2003, 52, 975–980. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Zou, Q.; Zhong, B.; Wang, H.; Mou, F.; Wu, L.; Fang, Y. Lactobacillus salivarius Isolated from Patients with Rheumatoid Arthritis Suppresses Collagen-Induced Arthritis and Increases Treg Frequency in Mice. J. Interferon. Cytokine Res. 2016, 36, 706–712. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; O’Callaghan, L.; McCarthy, J.; Shilling, D.; Scully, P.; Sibartie, S.; Kavanagh, E.; Kirwan, W.O.; Redmond, H.P.; Collins, J.K.; et al. Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G839–G845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riboulet-Bisson, E.; Sturme, M.H.; Jeffery, I.B.; O’Donnell, M.M.; Neville, B.A.; Forde, B.M.; Claesson, M.J.; Harris, H.; Gardiner, G.E.; Casey, P.G.; et al. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS ONE 2012, 7, e31113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Neville, B.A.; O’Toole, P.W. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Future Microbiol. 2010, 5, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Lomasney, K.W.; Cryan, J.F.; Hyland, N.P. Converging effects of a Bifidobacterium and Lactobacillus probiotic strain on mouse intestinal physiology. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G241–G247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayan, S.; Butto, L.F.; Rossini, V.; Velmurugan, J.; Martinez-Lopez, M.; Sancho, D.; Melgar, S.; O’Toole, P.W.; Nally, K. Macrophage cytokine responses to commensal Gram-positive Lactobacillus salivarius strains are TLR2-independent and Myd88-dependent. Sci. Rep. 2021, 11, 5896. [Google Scholar] [CrossRef] [PubMed]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Faas, M.M.; de Vos, P.L. plantarum, L. salivarius, and L. lactis attenuate Th2 responses and increase Treg frequencies in healthy mice in a strain dependent manner. PLoS ONE 2012, 7, e47244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef]
- Alican, I.; Kubes, P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am. J. Physiol. 1996, 270, G225–G237. [Google Scholar] [CrossRef]
- Brown, J.F.; Keates, A.C.; Hanson, P.J.; Whittle, B.J. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 1993, 265, G418–G422. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, O.; Petersson, J.; Walden, T.; Ahl, D.; Sandler, S.; Phillipson, M.; Holm, L. iNOS-dependent increase in colonic mucus thickness in DSS-colitic rats. PLoS ONE 2013, 8, e71843. [Google Scholar] [CrossRef] [Green Version]
- McCafferty, D.M.; Sihota, E.; Muscara, M.; Wallace, J.L.; Sharkey, K.A.; Kubes, P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G90–G99. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, C.F.; Cerwinka, W.H.; Laroux, F.S.; Salter, J.W.; Russell, J.M.; Schuermann, G.; Grisham, M.B.; Ross, C.R.; Granger, D.N. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: Divergent roles of superoxide and nitric oxide. J. Exp. Med. 2001, 194, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, F.; Kojouharoff, G.; Hans, W.; Scholmerich, J.; Gross, V.; Falk, W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. 1999, 116, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Faas, M.M.; De Vos, P. Probiotics can generate FoxP3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS ONE 2013, 8, e68952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative In silico Analysis of Butyrate Production Pathways in Gut Commensals and Pathogens. Front. Microbiol. 2016, 7, 1945. [Google Scholar] [CrossRef] [Green Version]
- Tedjo, D.I.; Smolinska, A.; Savelkoul, P.H.; Masclee, A.A.; van Schooten, F.J.; Pierik, M.J.; Penders, J.; Jonkers, D.M.A.E. The fecal microbiota as a biomarker for disease activity in Crohn’s disease. Sci. Rep. 2016, 6, 35216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]







| Gene | Primer Sequence |
|---|---|
| ZO-2 | FP: GCACCCTGACATCTATGCG |
| RP: CACTGCCGTAGCTTCCTCTG | |
| Claudin 1 | FP: ATGACCCCTTGACCCCCATC |
| RP: GGAGCAGGAAAGTAGGACACC | |
| Claudin 2 | FP: CCTGGGATTGTGCTTGAGGT |
| RP: TGACCCCCATCTACCACAGA | |
| Claudin 3 | FP: CCGCAAGGACTACGTCTGAG |
| RP: CAAGCAGACTGTGTGTCGTCT | |
| Claudin 4 | FP: GTAGAGTGGATGGACGGGTT |
| RP: CATTAGCAAGACAGTGCGGA | |
| Claudin 15 | FP: GGACCCTCCACATACTTGCT |
| RP: GCACTCCAGCCCAAGTAGAG | |
| ZO-1 | FP: CAAAGCCCACCAAGGTCAC |
| RP: TCTCTTTCCGAGGCATTAGCA | |
| Occludin | FP: CAGGGCTCTTTGGAGGAA |
| RP: TACACGATCGTGGCAATAAAC | |
| IL-10 | FP: TTGAATTCCCTGGGTGAGAAG |
| RP: TCCACTGCCTTGCTCTTATTT | |
| IFNgamma | FP: CAGGCTGTCCCTGAAAGAAA |
| RP: CATTCGGGTGTAGTCACAGTT | |
| IL-1beta | FP: TTCAGGCAGGCAGTATCACTC |
| RP: GAAGGTCCACGGGAAAGACAC | |
| Arg1 | FP: TTTTAGGGTTACGGCCGGTG |
| RP: CCTCGAGGCTGTCCTTTTGA | |
| Fizz1 | FP: ACTGCCTGTGCTTACTCGTTGACT |
| RP: AAAGCTGGGTTCTCCACCTCTTCA | |
| MRC | FP: GGCGAGCATCAAGAGTAAAGA |
| RP: CATAGGTCGGTCCCAACCAAA | |
| Nos2 | FP: CTTGTTCAGCTACGCCTTCAACA |
| RP: AGAGATTTCTTCAGAGTCTGCCCAT | |
| Rps13 | FP: TGCTCCCACCTAATT |
| RP: CTTGTGCACACAACAGCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, N.; Williams, M.A.; O’Callaghan, A.A.; Dempsey, E.; Cabrera-Rubio, R.; Raverdeau, M.; Crispie, F.; Cotter, P.D.; Corr, S.C. Lactobacillus salivarius UCC118™ Dampens Inflammation and Promotes Microbiota Recovery to Provide Therapeutic Benefit in a DSS-Induced Colitis Model. Microorganisms 2022, 10, 1383. https://doi.org/10.3390/microorganisms10071383
Iyer N, Williams MA, O’Callaghan AA, Dempsey E, Cabrera-Rubio R, Raverdeau M, Crispie F, Cotter PD, Corr SC. Lactobacillus salivarius UCC118™ Dampens Inflammation and Promotes Microbiota Recovery to Provide Therapeutic Benefit in a DSS-Induced Colitis Model. Microorganisms. 2022; 10(7):1383. https://doi.org/10.3390/microorganisms10071383
Chicago/Turabian StyleIyer, Namrata, Michelle A. Williams, Amy A. O’Callaghan, Elaine Dempsey, Raul Cabrera-Rubio, Mathilde Raverdeau, Fiona Crispie, Paul D. Cotter, and Sinéad C. Corr. 2022. "Lactobacillus salivarius UCC118™ Dampens Inflammation and Promotes Microbiota Recovery to Provide Therapeutic Benefit in a DSS-Induced Colitis Model" Microorganisms 10, no. 7: 1383. https://doi.org/10.3390/microorganisms10071383
APA StyleIyer, N., Williams, M. A., O’Callaghan, A. A., Dempsey, E., Cabrera-Rubio, R., Raverdeau, M., Crispie, F., Cotter, P. D., & Corr, S. C. (2022). Lactobacillus salivarius UCC118™ Dampens Inflammation and Promotes Microbiota Recovery to Provide Therapeutic Benefit in a DSS-Induced Colitis Model. Microorganisms, 10(7), 1383. https://doi.org/10.3390/microorganisms10071383






