Ultrasonic Activated Biochar and Its Removal of Harmful Substances in Environment
Abstract
:1. Introduction
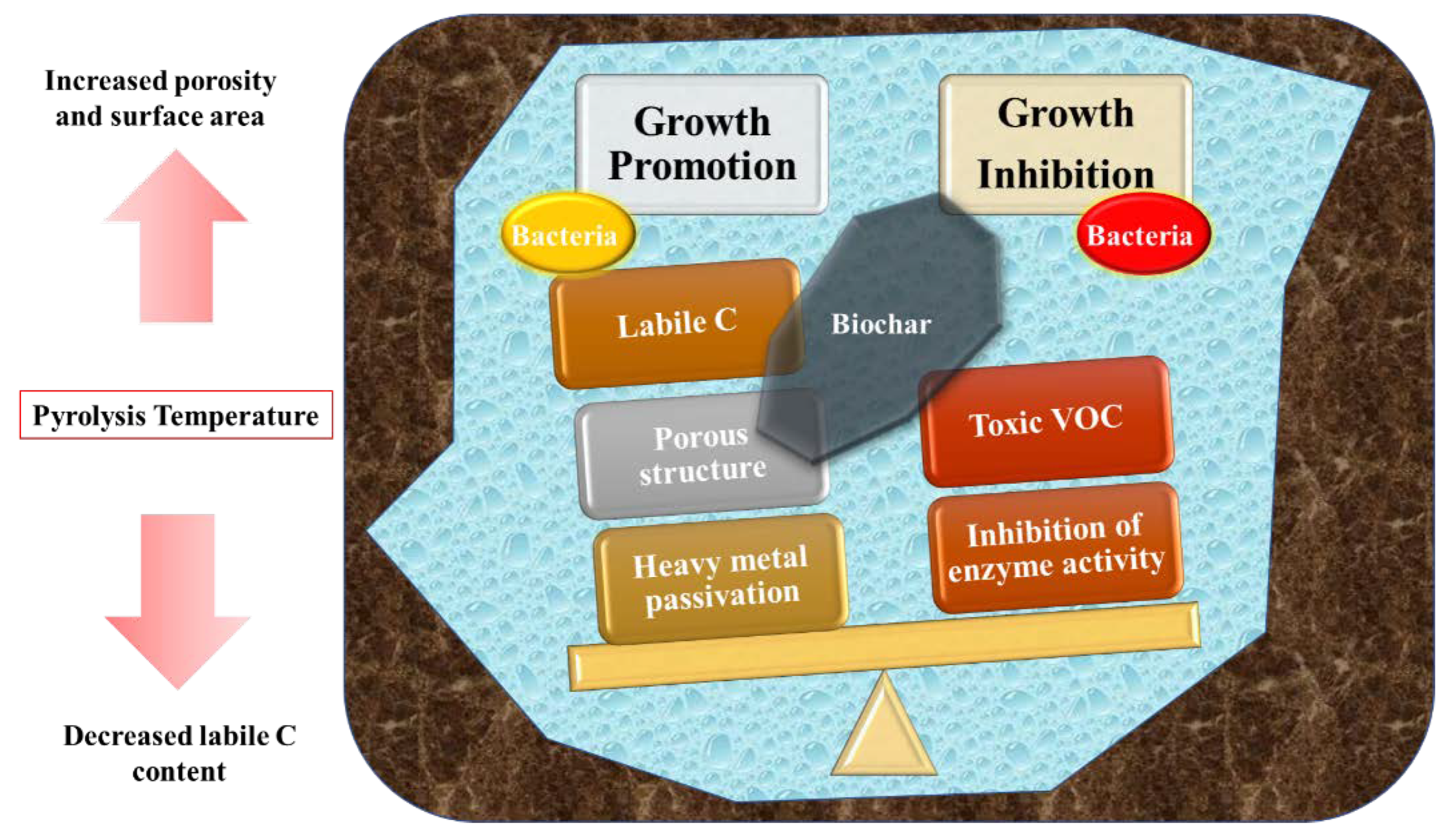
2. Effects of Biochar on Bacteria in Soil
2.1. Main Physical and Chemical Properties of Biochar
2.2. Bacterial Response
2.3. The Effect of Biochar on the Nutrient Cycling of Soil by Acting on Bacteria
3. Ultrasonic Modification of Biochar
3.1. Ultrasound Modification
3.2. Ultrasonic-Chemical Modification
3.3. Metal Oxide-Biochar Composites
3.4. Microwave-Ultrasound Fabrication of Biochar
3.5. Ultrasonic Regeneration
4. Pollutant Removal Using Ultrasound with Biochar
4.1. Heterogeneous Reactions
4.2. Ultrasound-Assisted Adsorption
5. Discussion
6. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Zhang, H.; Zeng, G.; Liu, J.; Ye, J.; Fang, W.; Gou, X. Possibility of sludge conditioning and dewatering with rice husk biochar modified by ferric chloride. Bioresour. Technol. 2016, 250, 258–263. [Google Scholar] [CrossRef]
- Nguyen, T.; Chen, H.; To, T.; Chang, Y.; Tsai, C.; Chen, K.; Tsai, Y. Development of biochars derived from water bamboo (Zizania latifolia) shoot husks using pyrolysis and ultrasound-assisted pyrolysis for the treatment of Reactive Black 5 (RB5) in wastewater. Water 2021, 13, 1615. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Li, P.; Yang, L.; Wu, L.; Gao, F.; Qi, X.; Zhang, Z. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour. Technol. 2021, 319, 124199. [Google Scholar] [CrossRef] [PubMed]
- Gholami, P.; Khataee, A.; Soltani, R.D.C.; Bhatnagar, A. A review on carbon-based materials for heterogeneous sonocatalysis: Fundamentals, properties and applications. Ultrason. Sonochem. 2019, 58, 104681. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility: A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Patwardhan, S.B.; Pandit, S.; Kumar Gupta, P.; Kumar Jha, N.; Rawat, J.; Joshi, H.C.; Priya, K.; Gupta, M.; Lahiri, D.; Nag, M.; et al. Recent advances in the application of biochar in microbial electrochemical cells. Fuel 2022, 311, 122501. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Feng, W.; Wang, Y.; Liu, S.; Dong, Z.; Li, X. A critical review on metal complexes removal from water using methods based on Fenton-like reactions: Analysis and comparison of methods and mechanisms. J. Hazard. Mater. 2021, 414, 125517. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Vochozka, M.; Maroušková, A.; Váchal, J.; Straková, J. Biochar pricing hampers biochar farming. Clean Technol. Environ. Policy 2016, 18, 1225–1231. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Y.; Deng, Y.; Ren, F.; Tan, S.; Wang, Z. Ultrasonic-assisted fabrication of porous carbon materials derived from agricultural waste for solid-state supercapacitors. J. Mater. Sci. 2020, 55, 11512–11523. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 174, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, Z.; Feng, X.; Zhou, F.; Li, Y. Effects of biochar additions on the soil chemical properties, bacterial community structure and rape growth in an acid purple soil. Plant Soil Environ. 2021, 3. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 1, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dai, Q.; Jin, X.; Dong, X.; Peng, J.; Wu, M.; Liang, N.; Pan, B.; Xing, B. Negative impacts of biochars on urease activity: High pH, heavy metals, polycyclic aromatic hydrocarbons, or free radicals? Environ. Sci. Technol. 2018, 21, 12740–12747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Ghidotti, M.; Fabbri, D.; Hornung, A. profiles of volatile organic compounds in biochar: Insights into process conditions and quality assessment. Acs. Sustain. Chem. Eng. 2017, 5, 510–517. [Google Scholar] [CrossRef]
- Zhu, K.; Ye, X.; Ran, H.; Zhang, P.; Wang, G. Contrasting effects of straw and biochar on microscale heterogeneity of soil O2 and pH: Implication for N2O emissions. Soil Biol. Biochem. 2022, 166, 108564. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, G.; Hu, C.; Zhou, S.; Clough, T.J.; Monning, N.W.; Luo, J.; Qin, S. Electron shuttle potential of biochar promotes dissimilatory nitrate reduction to ammonium in paddy soil. Soil Biol. Biochem. 2022, 172, 108760. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2018, 9, 9338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Tan, X.; Liu, Y.; Liu, S. Biomass-derived porous graphitic carbon materials for energy and environmental applications. J. Mater. Chem. 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Peter, A.; Chabot, B.; Loranger, E. Pre- and post-pyrolysis effects on iron impregnation of ultrasound pre-treated softwood biochar for potential catalysis applications. SN Appl. Sci. 2021, 3, 643. [Google Scholar] [CrossRef]
- Peter, A.; Chabot, B.; Loranger, E. Enhanced activation of ultrasonic pre-treated softwood biochar for efficient heavy metal removal from water. J. Environ. Manag. 2021, 290, 112569. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Chabot, B.; Loranger, E. The influence of ultrasonic pre-treatments on metal adsorption properties of softwood-derived biochar. Bioresour. Technol. Rep. 2020, 11, 100445. [Google Scholar] [CrossRef]
- Sajjadi, B.; Shrestha, R.M.; Chen, W.Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Double-layer magnetized/functionalized biochar composite: Role of microporous structure for heavy metal removals. J. Water Process Eng. 2021, 39, 101677. [Google Scholar] [CrossRef]
- Hazrati, S.; Farahbakhsh, M.; Cerda, A.; Heydarpoor, G. Functionalization of ultrasound enhanced sewage sludge-derived biochar: Physicochemical improvement and its effects on soil enzyme activities and heavy metals availability. Chemosphere 2021, 269, 128767. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Sajjadi, B.; Mattern, D.L.; Chen, W.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Egiebor, N.O.; Hammer, N. Ultrasound cavitation intensified amine functionalization: A feasible strategy for enhancing CO2 capture capacity of biochar. Fuel 2018, 225, 287–298. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.; Mattern, D.L.; Hammer, N.; Dorris, A. Low-temperature acoustic-based activation of biochar for enhanced removal of heavy metals. J. Water Process Eng. 2020, 34, 101166. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chen, W.; Mattern, D.L.; Egiebor, N.O.; Hammer, N.; Raman, V. Low frequency ultrasound enhanced dual amination of biochar: A nitrogen-enriched sorbent for CO2 capture. Energy Fuels 2019, 33, 2366–2380. [Google Scholar] [CrossRef]
- Wang, T.; Li, G.; Yang, K.; Zhang, X.; Wang, K.; Cai, J.; Zheng, J. Enhanced ammonium removal on biochar from a new forestry waste by ultrasonic activation: Characteristics, mechanisms and evaluation. Sci. Total Environ. 2021, 778, 146295. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, D.; Zhang, M. Effects of different pretreatment methods on biochar properties from pyrolysis of corn stover. J. Energy Inst. 2021, 98, 294–302. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, W.; Lisovytskiy, D.; Kamińska, A.; Łomot, D. Dual functionality of TiO2/biochar hybrid materials: Photocatalytic phenol degradation in the liquid phase and selective oxidation of methanol in the gas phase. ACS Sustain. Chem. Eng. 2017, 5, 6274–6287. [Google Scholar] [CrossRef] [Green Version]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochar from spent malt rootlets for the degradation of trimethoprim in the presence of inorganic ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene oxide and graphene quantum dots from miscanthus via ultrasound-assisted mechano-chemical cracking method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sajjadi, B.; Chen, W.; Chatterjee, R. Ultrasound-assisted amine functionalized graphene oxide for enhanced CO2 adsorption. Fuel 2019, 247, 10–18. [Google Scholar] [CrossRef]
- Sajjadi, B.; Broome, J.W.; Chen, W.Y.; Mattern, D.L.; Egiebor, N.O.; Hammer, N.; Smith, C.L. Urea functionalization of ultrasound-treated biochar: A feasible strategy for enhancing heavy metal adsorption capacity. Ultrason. Sonochem. 2019, 51, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chen, W.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Impact of biomass sources on acoustic-based chemical functionalization of biochars for improved CO2 adsorption. Energy Fuels 2020, 34, 8608–8627. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.; Adeniyi, A.; Mattern, D.L.; Mobley, J.; Huang, C.; Fan, R. Variables governing the initial stages of the synergisms of ultrasonic treatment of biochar in water with dissolved CO2. Fuel 2019, 235, 1131–1145. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Cao, Y. Pb(II) sorption by biochar derived from Cinnamomum camphora and its improvement with ultrasound-assisted alkali activation. Colloids Sur. A 2018, 556, 177–184. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.; Qi, Y.; Yang, L.; Wu, L.; He, L.; Li, P.; Qi, X.; Gao, F.; Ding, Y.; et al. An efficient, green and sustainable potassium hydroxide activated magnetic corn cob biochar for imidacloprid removal. Chemosphere 2022, 291, 132707. [Google Scholar] [CrossRef]
- Aswani, M.T.; Yadav, M.; Vinod Kumar, A.; Tiwari, S.; Kumar, T.; Pavan Kumar, M.V. Ultrasound-acid modified Merremia vitifolia biomass for the biosorption of herbicide 2,4-D from aqueous solution. Water Sci. Technol. 2020, 82, 468–480. [Google Scholar] [CrossRef]
- Bispo, D.; Santos, R.; Granja, H.; Freitas, L. Ultrasonic Pretreatment of cowpea bean pod with K3PO4: Effect on bio-oil yield and phenolic compounds content. J. Braz. Chem. Soc. 2021, 32, 1752–1761. [Google Scholar] [CrossRef]
- Altaf, A.R.; Teng, H.; Gang, L.; Adewuyi, Y.G.; Zheng, M. Effect of sonochemical treatment on thermal stability, elemental mercury (Hg(0)) removal, and regenerable performance of magnetic tea biochar. ACS Omega 2021, 6, 23913–23923. [Google Scholar] [CrossRef]
- Vidal, J.L.; Gallant, S.M.V.; Connors, E.P.; Richards, D.D.; MacQuarrie, S.L.; Kerton, F.M. Green solvents for the liquid-phase exfoliation of biochars. ACS Sustain. Chem. Eng. 2021, 9, 9114–9125. [Google Scholar] [CrossRef]
- Almeida, L.C.; Barbosa, A.S.; Fricks, A.T.; Freitas, L.S.; Lima, Á.S.; Soares, C.M.F. Use of conventional or non-conventional treatments of biochar for lipase immobilization. Process Biochem. 2017, 61, 24–129. [Google Scholar] [CrossRef]
- Lima, D.R.; Lima, E.C.; Thue, P.S.; Dias, S.L.P.; Machado, F.M.; Seliem, M.K.; Sher, F.; dos Reis, G.S.; Saeb, M.R.; Rinklebe, J. Comparison of acidic leaching using a conventional and ultrasound-assisted method for preparation of magnetic-activated biochar. J. Environ. Chem. Eng. 2021, 9, 105865. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Zhang, M.; Wang, Y.; Tan, Z.; Li, J.; Xi, B. Ultrasound-assisted room-temperature in situ precipitation synthesis of BC doped Bi4O5Br2 for enhanced photocatalytic activity in pollutants degradation under visible light. J. Alloys Compd. 2021, 889, 161609. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Renewable bionanohydrogels based on tragacanth gum for the adsorption of Pb2+: Study of isotherm, kinetic models, and phenomenology. Env. Technol. Innov. 2021, 23, 101723. [Google Scholar] [CrossRef]
- Luo, Q.; Li, H. Antibiotics in livestock wastewater treatment by using biomass-derived activated carbon supported ZnS nanomaterials. Water Sci. Technol. 2019, 80, 1367–1373. [Google Scholar] [CrossRef]
- Jafari, K.; Heidari, M.; Rahmanian, O. Wastewater treatment for Amoxicillin removal using magnetic adsorbent synthesized by ultrasound process. Ultrason. Sonochem. 2018, 45, 248–256. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, W.; Lisovytskiy, D.; Grzonka, J.; Kurzydłowski, K. Design and fabrication of TiO2/lignocellulosic carbon materials: Relevance of low-temperature sonocrystallization to photocatalysts performance. ChemCatChem 2018, 10, 3469–3480. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, W.; Wu, X. Microcystis aeruginosa removal by the combination of ultrasound and TiO2/biochar. RSC Adv. 2021, 11, 24985–24990. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Yang, W.; Li, Y.; Liu, Y.; Pan, J. Preparation of microwave-activated magnetic bio-char adsorbent and study on removal of elemental mercury from flue gas. Sci. Total Environ. 2019, 697, 134049. [Google Scholar] [CrossRef]
- Ateş, A. The effect of microwave and ultrasound activation on the characteristics of biochar produced from tea waste in the presence of H3PO4 and KOH. Biomass Convers Bior. 2021, 1–20. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, Y.; Yang, L.; Wu, L.; Li, P.; Gao, F.; Qi, X.; Zhang, Z. Adsorptive removal of imidacloprid by potassium hydroxide activated magnetic sugarcane bagasse biochar: Adsorption efficiency, mechanism and regeneration. J. Clean. Prod. 2021, 292, 126005. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, Y.; Lu, T.; Yang, L.; Wu, L.; Cui, S.; Ding, Y.; Zhang, Z. Highly efficient removal of imidacloprid using potassium hydroxide activated magnetic microporous loofah sponge biochar. Sci. Total Environ. 2021, 765, 144253. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, F.; Li, H.; Cui, J.; Ren, Y.; Yu, X. Recent Progress in Biochar-Based Photocatalysts for Wastewater Treatment: Synthesis, Mechanisms, and Applications. Appl. Sci. 2020, 10, 1019. [Google Scholar] [CrossRef] [Green Version]
- Zou, R.; Qian, M.; Wang, C.; Mateo, W.; Wang, Y.; Dai, L.; Lin, X.; Zhao, Y.; Huo, E.; Wang, L.; et al. Biochar: From by-products of agro-industrial lignocellulosic waste to tailored carbon-based catalysts for biomass thermochemical conversions. Chem. Eng. J. 2022, 441, 135972. [Google Scholar] [CrossRef]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Biochar as a catalyst in the production of syngas and biodiesel from peanut waste. Int. J. Energy Res. 2022, 1–13. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Singh, A.; Saha, P.; Choi, Y.; Maharana, M.; Patil, S.V.; Kim, D.D. Nano-Biochar as a Sustainable Catalyst for Anaerobic Digestion: A Synergetic Closed-Loop Approach. Catalysts 2022, 12, 186. [Google Scholar] [CrossRef]
- Diao, Z.; Dong, F.; Yan, L.; Chen, Z.; Guo, P.; Xia, X.; Chu, W. A new insight on enhanced Pb(II) removal by sludge biochar catalyst coupling with ultrasound irradiation and its synergism with phenol removal. Chemosphere 2021, 263, 128287. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Nikolaou, S.; Vakros, J.; Diamadopoulos, E.; Mantzavinos, D. Sonochemical degradation of propylparaben in the presence of agro-industrial biochar. J. Environ. Chem. Eng. 2020, 8, 104010. [Google Scholar] [CrossRef]
- Dong, F.X.; Yan, L.; Huang, S.T.; Liang, J.Y.; Zhang, W.X.; Yao, X.W.; Chen, X.; Qian, W.; Guo, P.R.; Kong, L.J.; et al. Removal of antibiotics sulfadiazine by a biochar based material activated persulfate oxidation system: Performance, products and mechanism. Process Saf. Eniron. 2022, 157, 411–419. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, S.; Li, X.; Zhang, S.; Thang Nguyen, T.; Guo, M.; Gao, X. Ultrasound-assisted heterogeneous Fenton-like process for methylene blue removal using magnetic MnFe2O4/biochar nanocomposite. Appl. Surf. Sci. 2021, 556, 150654. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Lee, Y.J.; Choi, J.W. Ultrasound-assisted heterogeneous Fenton-like process for bisphenol A removal at neutral pH using hierarchically structured manganese dioxide/biochar nanocomposites as catalysts. Ultrason. Sonochem. 2019, 57, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. J. Water Process Eng. 2020, 378, 101455. [Google Scholar] [CrossRef]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Enhanced sonocatalytic degradation of bisphenol A with a magnetically recoverable biochar composite using rice husk and rice bran as substrate. J. Environ. Chem. Eng. 2021, 9, 105284. [Google Scholar] [CrossRef]
- Yu, D.; Pei, Y. Removal of ibuprofen by sodium alginate-coated iron-carbon granules combined with the ultrasound and Fenton technologies: Influencing factors and degradation intermediates. Environ. Sci. Pollut. Res. Int. 2021, 28, 21183–21192. [Google Scholar] [CrossRef]
- Sadeghi Rad, T.; Khataee, A.; Sadeghi Rad, S.; Arefi-Oskoui, S.; Gengec, E.; Kobya, M.; Yoon, Y. Zinc-chromium layered double hydroxides anchored on carbon nanotube and biochar for ultrasound-assisted photocatalysis of rifampicin. Ultrason. Sonochem. 2022, 82, 105875. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Gholami, P.; Kalderis, D.; Pachatouridou, E.; Konsolakis, M. Preparation of novel CeO2-biochar nanocomposite for sonocatalytic degradation of a textile dye. Ultrason. Sonochem. 2018, 41, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Razmi, R.; Ardjmand, M.; Ramavandi, B.; Heydarinasab, A. Optimization of phenol removal from wastewater by activation of persulfate and ultrasonic waves in the presence of biochar catalyst modified by lanthanum chloride. Water Environ. J. 2018, 33, 499–507. [Google Scholar] [CrossRef]
- Pan, X.; Bai, L.; Wang, H.; Wu, Q.; Wang, H.; Liu, S.; Xu, B.; Shi, X.; Liu, H. Metal-organic-framework-derived carbon nanostructure augmented sonodynamic cancer therapy. Adv. Mater. 2018, 30, 1800180. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, W.; Huang, Z.; Liu, S.; Guo, J.; Zhang, F.; Yuan, H.; Li, X.; Liu, F.; Liu, H. MOF-Derived double-layer hollow nanoparticles with oxygen generation ability for multimodal imaging-guided sonodynamic therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 13557–13561. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Wang, X.; Bai, S.; Gong, F.; Yang, N.; Gong, Y.; Hou, L.; Cao, M.; Liu, Z.; Cheng, L. Biodegradable Fe-doped vanadium disulfide theranostic nanosheets for enhanced sonodynamic/chemodynamic therapy. ACS Appl. Mater. Interfaces 2020, 12, 52370–52382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Bai, L.; Xu, J.; Gong, F.; Dong, Z.; Yang, Z.; Zeng, Z.; Liu, Z.; Cheng, L. Ultrafine titanium monoxide (TiO1+x) nanorods for enhanced sonodynamic therapy. J. Am. Chem. Soc. 2020, 142, 6527–6537. [Google Scholar] [CrossRef]
- Bai, S.; Yang, N.; Wang, X.; Gong, F.; Dong, Z.; Gong, Y.; Liu, Z.; Cheng, L. Ultrasmall iron-doped titanium oxide nanodots for enhanced sonodynamic and chemodynamic cancer therapy. ACS Nano 2020, 14, 15119–15130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Gao, X.; Qian, J.; Pan, B. Structural evolution of lanthanum hydroxides during long-term phosphate mitigation: Effect of nanoconfinement. Environ. Sci. Technol. 2021, 55, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, G.; Liu, H.; Qu, J. Confining free radicals in close vicinity to contaminants enables ultrafast Fenton-like processes in the interspacing of MoS2 membranes. Angew. Chem. Int. Ed. Engl. 2019, 58, 8134–8138. [Google Scholar] [CrossRef]
- Amin, M.; Chetpattananondh, P.; Khan, M.N. Ultrasound assisted adsorption of reactive dye-145 by biochars from marine Chlorella sp. extracted solid waste pyrolyzed at various temperatures. J. Environ. Chem. Eng. 2020, 8, 104403. [Google Scholar] [CrossRef]
- Anfar, Z.; Zbair, M.; Ait Ahsiane, H.; Jada, A.; El Alem, N. Microwave assisted green synthesis of Fe2O3/biochar for ultrasonic removal of nonsteroidal anti-inflammatory pharmaceuticals. RSC Adv. 2020, 10, 11371–11380. [Google Scholar] [CrossRef] [PubMed] [Green Version]

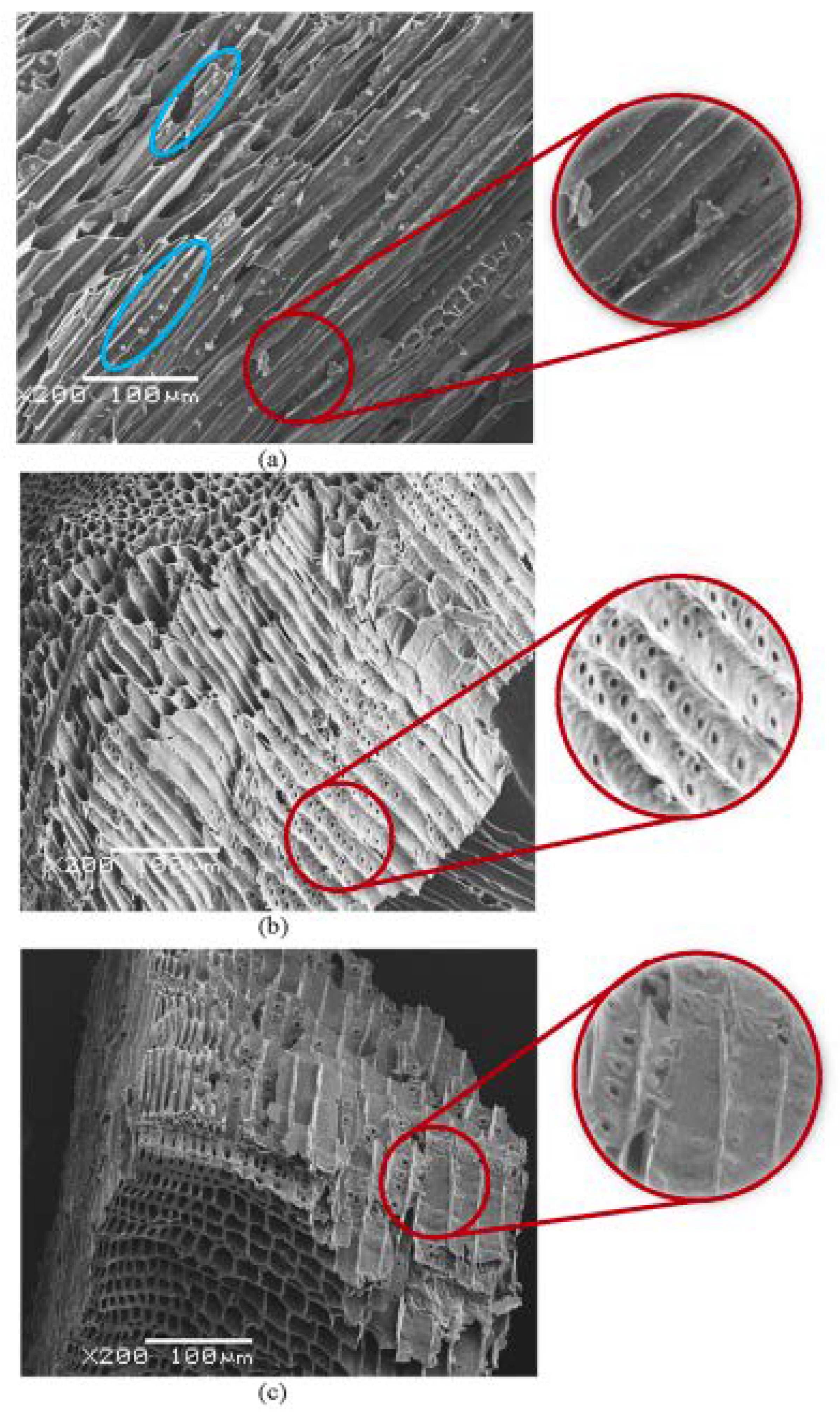

| Material | Ultrasonic Conditions | Biochar Properties after Ultrasound Modification | Ref. | |||
|---|---|---|---|---|---|---|
| Device | Frequency | Intensity | Time (s) | |||
| Biomass from mixed softwoods | Bath | 40 kHz 170 kHz | 250 W 1000 W | 3600 3600 | Obtained a porous structure and increased heterogeneity of the surface | [27] |
| Woodchips | Bath | 40 kHz 170 kHz | 250 W 1000 W | 3600 7200 | A better surface morphology | [28] |
| Woodchips | Bath | 40 kHz 170 kHz | 250 W 1000 W | 3600 7200 s | Enhanced surface area | [29] |
| Pine wood | Probe | 20 kHz | 700 W | 30,60 | Enhanced porosity | [30] |
| Sludge-derived biochar | Probe | 24 kHz | 400 W | 30 | Enhanced pore volume and surface area | [31] |
| Pine wood | Probe | 20 kHz | 475 W 700 W | 30,60,180 | Creating empty pores | [32] |
| Pine wood | Probe | 20 kHz | 700 W | 30 | A smooth surface with new circular pores | [33] |
| Pine wood-based biochar | Probe | 20 kHz | 700 W | 30 | Elevated adsorption capacity | [34] |
| Caragana korshinskii | Bath | 45, 80, 100 kHz | 300 W and 700 W | 1800−14,640 | Removed the ash content from the biochar and increased the specific surface area | [35] |
| Corn stover | Probe | 20 kHz | 500 W | 60 s | Obtained multilayered and porous structures | [36] |
| Water bamboo husks | Probe | 20 kHz | 65 W | 30−480 | Improved the surface properties | [3] |
| Biochar | Bath | 35 kHz | 560 W | 3600 | Enhanced BET surface area | [37] |
| Biochar prepared from spent malt rootlets | Probe | 20 kHz | 4.32 W | n.a. | Surface activation | [38] |
| Milled miscanthus particles | Bath | 40 kHz | 300 W | 3600 | Synthesis of graphene oxide | [39] |
| Biochar | n.a. | 20 kHz | 475 W | 300− 21,600 | Exfoliation and enhanced reactivity of the surface functional groups | [40] |
| Biochar-Based Material | Contaminants | Ultrasonic Conditions | Results | Ref. | |||
|---|---|---|---|---|---|---|---|
| Device | Frequency | Intensity | Time | ||||
| 1.5 g/L biochar from sludge | 80 mL of 40 mg/L Pb (II) and/or 5 mg/L phenol solution | Probe | 20 kHz | 50 W | 60 min | 98.9% of Pb (II) and 94.45% of phenol was removed. | [67] |
| 90 mg/L pine wood-based biochar | 250 μg/L sulfamethoxazole | n.a. | 20 kHz | n.a. | 30 min | 100% of sulfamethoxazole was degraded (250 mg/L persulfate). | [68] |
| 125 mg/L agroindustrial biochar | 200 mL of 1 mg/L propylparaben solution | Probe | 20 kHz | 20–60 W/L | 45 min | 80% of propylparaben was degraded. | [69] |
| 2 g/L Fe0 and Al0@sludge biochar | 60 mL of 20 mg/L bisphenol A solution | Probe | n.a. | 60 W | 80 min | 98.6% of bisphenol A was degraded [PS]0=3 mM | [70] |
| 90 mg/L biochar | 500 µg/L | Probe | 20 kHz | 36 W/L | 120 min | 90% trimethoprim (500 mg/L persulfate). | [38] |
| 0.7 g/L MnFe2O4 and biochar derived from polar wood powder | 200 mL of 20.0 mg/L methylene blue solution | n.a. | 40 kHz | 665 W | 20 min | 95% of methylene blue was degraded (pH=5, 15 mol/L H2O2). | [71] |
| 0.5 g/L MnO2 with rice husk biochar | 200 mL of 100 µM bisphenol A solution | Probe | 20 kHz | 130 W at 40% amplitude | 120 min | 100% of bisphenol A was degraded. [H2O2]0 = 10 mM | [72] |
| 2 g/L magnetic biochar derived from food waste | 10 mL of 50 mg/L methylene blue solution 10 mL of 50 mg/L methyl orange solution | Bath | 37 kHz | 35.3 W/L | 60 min, 180 min | methylene blue and methyl orange 100% degraded (200 mM H2O2). | [73] |
| 1 g/L magnetic biochar from rice bran | 200 mL of 0.1 mM bisphenol A | Probe | 20 kHz | 51.95 W/L | 40 min | 94.25% of bisphenol A was degraded (10 mM H2O2). | [74] |
| sodium alginate-coated iron granules with biochar | 100 mL of 100 mg/L ibuprofen | Bath | 40 kHz | 250 W | 8 h | 74.72% of ibuprofen was degraded. | [75] |
| 50 mg/L TiO2 loaded on biochar | 20 mL of 1.3 × 107 cells per mL Microcystis aeruginosa cells | Bath | 600 kHz | 0.3 W/mL | 90 s | the number of cyanobacteria cells decreased to 0.8 × 105 cells per mL. | [58] |
| 0.6 g/L ZnCr and LDH biochar | 15 mg/L rifampicin | Bath | 36 kHz | 150 W | 40min | 100% of rifampicin was degraded with ultrasound and visible light irradiation. | [76] |
| 1 g/L CeO2 on biochar | 100 mL of 10 mg/L Reactive Red 84 | n.a. | n.a. | 450 W | 60 min | 98.5% of Reactive Red 84 was degraded. | [77] |
| 43 mg/L Tamarix hispida biochar modified by lanthanum chloride | 50 mL of 86 mg/L phenol | Bath | 370 kHz | n.a. | 63 min | 99.43 % of phenol was degraded. (86 mg/L persulfate) | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, W.; Zhao, Z.; Musoke, F.S.N.; Wu, X. Ultrasonic Activated Biochar and Its Removal of Harmful Substances in Environment. Microorganisms 2022, 10, 1593. https://doi.org/10.3390/microorganisms10081593
Wang J, Li W, Zhao Z, Musoke FSN, Wu X. Ultrasonic Activated Biochar and Its Removal of Harmful Substances in Environment. Microorganisms. 2022; 10(8):1593. https://doi.org/10.3390/microorganisms10081593
Chicago/Turabian StyleWang, Juanjuan, Wenshu Li, Zhirui Zhao, Florence Sharon Nabukalu Musoke, and Xiaoge Wu. 2022. "Ultrasonic Activated Biochar and Its Removal of Harmful Substances in Environment" Microorganisms 10, no. 8: 1593. https://doi.org/10.3390/microorganisms10081593
APA StyleWang, J., Li, W., Zhao, Z., Musoke, F. S. N., & Wu, X. (2022). Ultrasonic Activated Biochar and Its Removal of Harmful Substances in Environment. Microorganisms, 10(8), 1593. https://doi.org/10.3390/microorganisms10081593





