Cathepsin B and Plasma Kallikrein Are Reliable Biomarkers to Discriminate Clinically Significant Hepatic Fibrosis in Patients with Chronic Hepatitis-C Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval of the Study Protocol
2.2. Exclusion Criteria
2.3. Recruitment
2.4. Cohorts
2.5. Blood Sampling
2.6. Laboratory Assays
2.7. Statistical Analyses
3. Results
3.1. Baseline Characteristics of CHC Patients
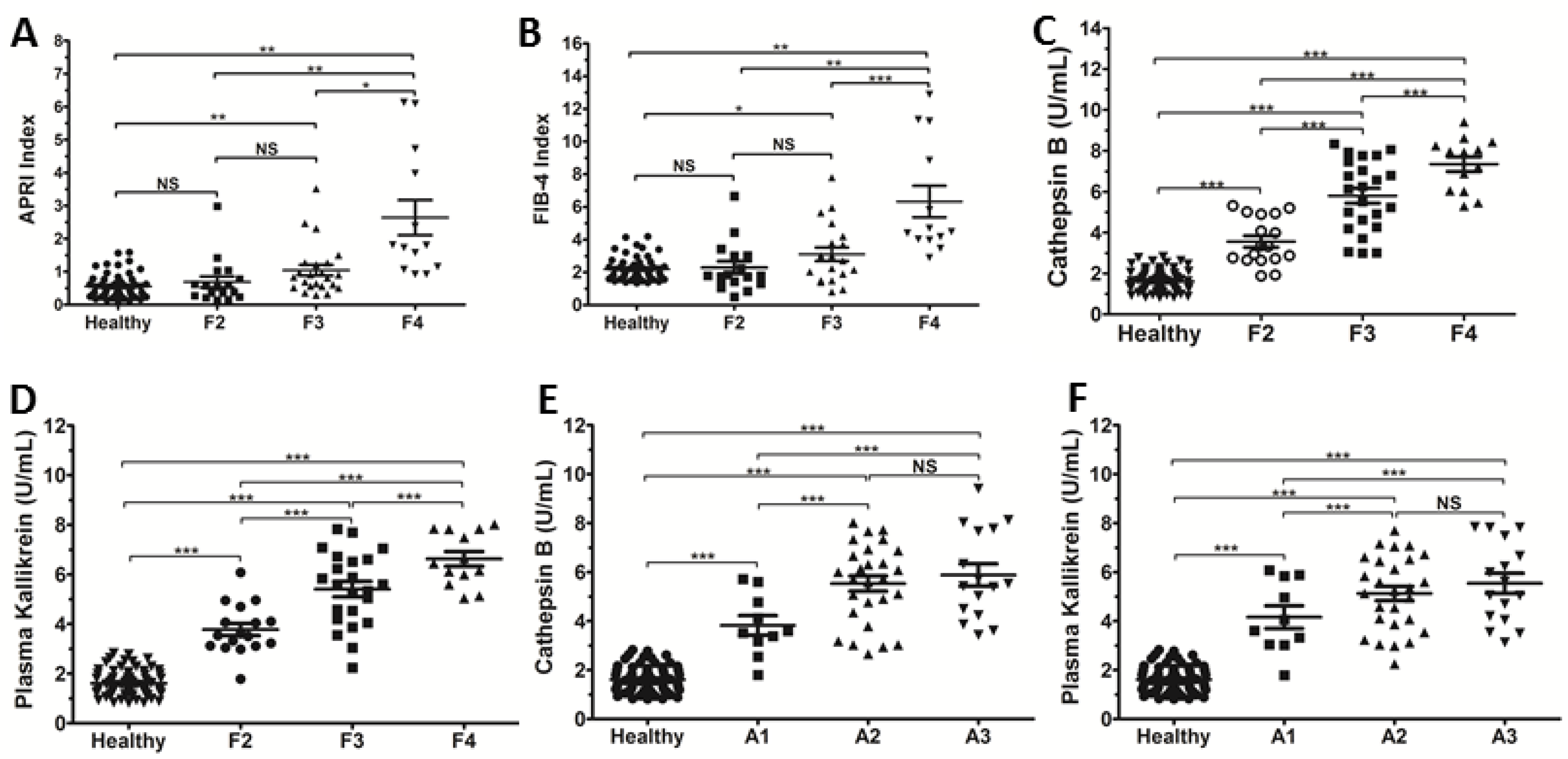
3.2. Serum Levels of Cat B and PKa Increased with the METAVIR Stages in HCV Patients
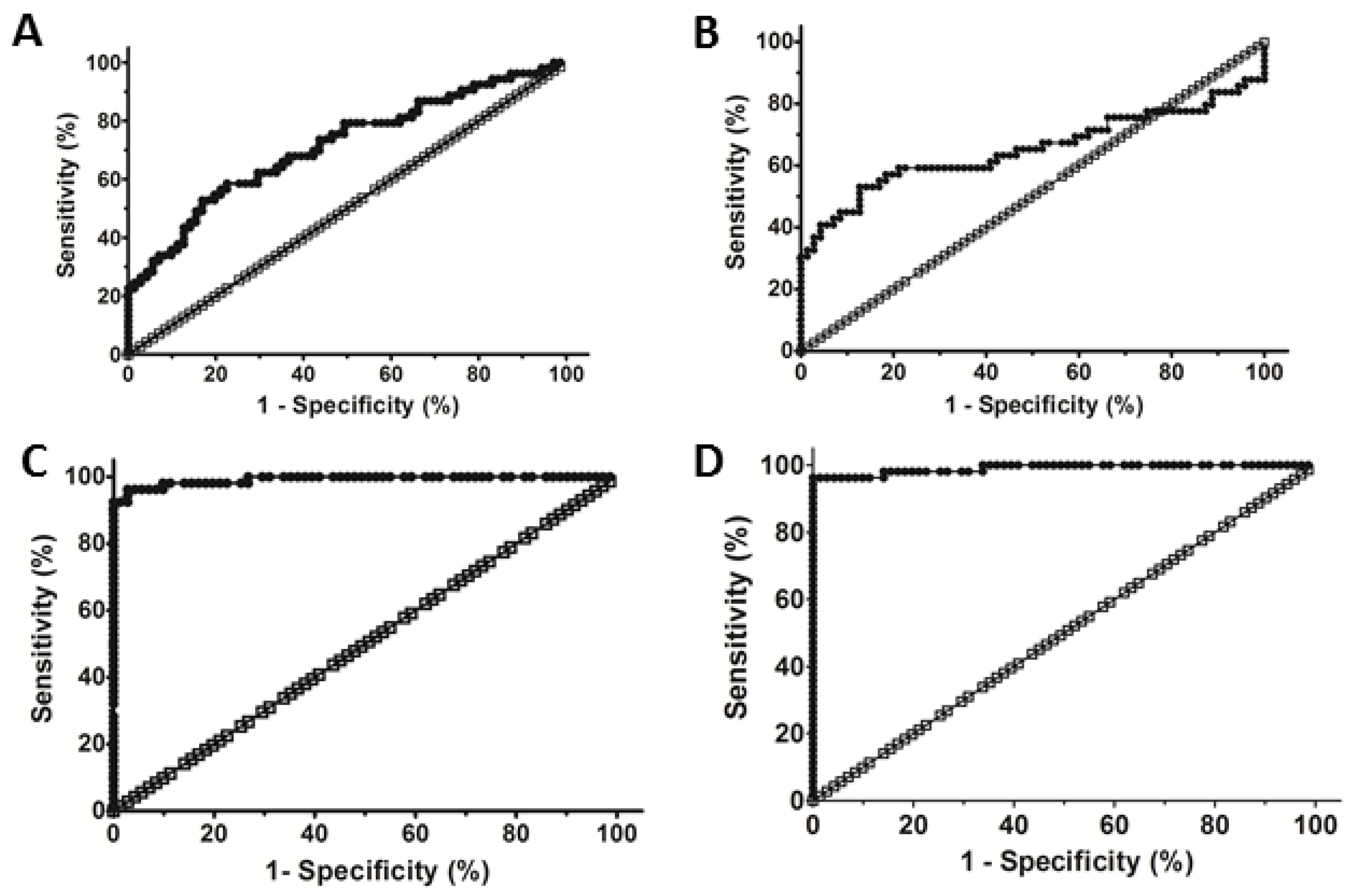
3.3. Evaluation of the Diagnostic Performance of Cat B and PKa to Detect Liver Fibrosis
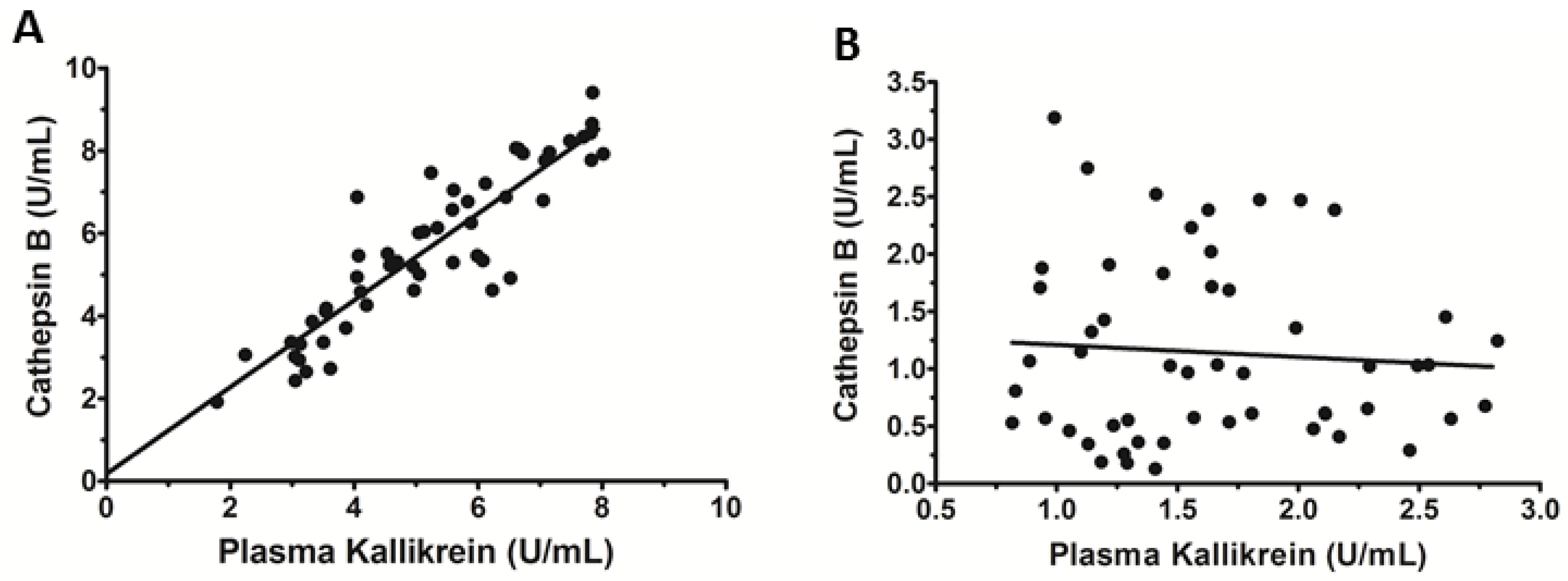
3.4. Serum Levels of Cat B and PKa Are Correlated in CHC Patients
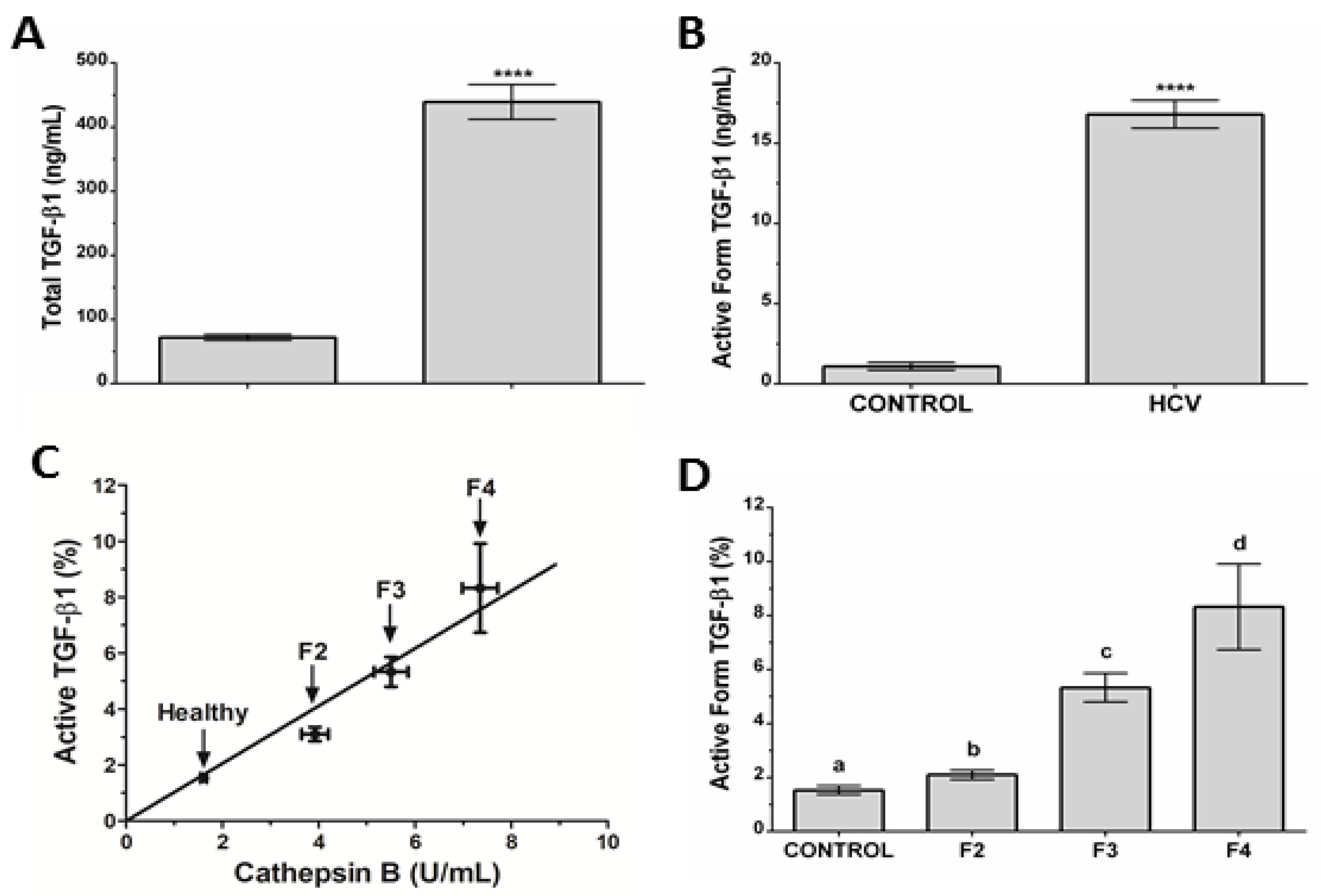
3.5. Association between Cat B and the Active Form of TGF-β1 in CHC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Hepatitis Report 2018; World Health Organization: Geneva, Switzerland, 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf?ua=1 (accessed on 29 January 2020).
- Ministry of Brazilian Health. Clinical Protocol and Therapeutic Guidance for Hepatitis C and Coinfections; Conitec: Brasilia, Brazil, 2017. Available online: http://conitec.gov.br/images/Protocolos/PCDT_HCV_2017.pdf (accessed on 29 January 2020).
- Ministry of Brazilian Health. Clinical Protocol and Therapeutic Guidance for Hepatitis C and Coinfections; Conitec: Brasilia, Brazil, 2019. Available online: http://www.aids.gov.br/pt-br/tags/publicacoes/protocolo-clinico-e-diretrizes-terapeuticas (accessed on 29 January 2020).
- Aizawa, Y.; Seki, N.; Nagano, T.; Abe, H. Chronic hepatitis C virus infection and lipoprotein metabolism. World J. Gastroenterol. 2015, 21, 10299–10313. [Google Scholar] [CrossRef]
- Fukuhara, T.; Ono, C.; Puig-Basagoiti, F.; Matsuura, Y. Roles of lipoproteins and apolipoproteins in particle formation of hepatitis C virus. Trends Microbiol. 2015, 23, 618–629. [Google Scholar] [CrossRef]
- Crouchet, E.; Baumert, T.F.; Schuster, C. Hepatitis C virus–apolipoprotein interactions: Molecular mechanisms and clinical impact. Expert Rev. Proteom. 2017, 4, 593–606. [Google Scholar] [CrossRef]
- Syed, G.H.; Amako, Y.; Siddiqui, A. Hepatitis C Virus Hijacks Host Lipid Metabolism. Trends Endocrinol. Metab. 2010, 21, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Scheel, T.H.; Rice, C.M. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med. 2013, 19, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakeem, M.S.; Bédard, N.; Murphy, D.; Bruneau, J.; Shoukry, N.H. Signatures of Protective Memory Immune Responses During HCV Reinfection. Gastroenterology 2014, 147, 870–881.e8. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of liver fibrosis: A translational success story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Massimo, P. Liver fibrosis: Pathophysiology, pathogenic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, M.; Das, P.; Gahlot, G.P.S.; Singh, R.; Roeb, E.; Roderfeld, M.; Gupta, S.D.; Saraya, A.; Pandey, R.M.; Chauhan, S.S. Cathepsin L and B as Potential Markers for Liver Fibrosis: Insights From Patients and Experimental Models. Clin. Transl. Gastroenterol. 2017, 8, e99. [Google Scholar] [CrossRef]
- Knight, V.; Tchongue, J.; Lourensz, D.; Tipping, P.; Sievert, W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology 2012, 55, 879–887. [Google Scholar] [CrossRef]
- Pinzani, M.; Rombouts, K.; Colagrande, S. Fibrosis in chronic liver diseases: Diagnosis and management. J. Hepatol. 2005, 42, S22–S36. [Google Scholar] [CrossRef]
- Seo, Y.S.; Kim, M.Y.; Kim, S.U.; Hyun, B.S.; Jan, J.Y.; Lee, J.W.; Lee, J.I.; Suh, S.J.; Park, S.Y.; Park, H.; et al. Accuracy of transient elastography in assessing liver fibrosis in chronic viral hepatitis: A multicentre, retrospective study. Liver Int. 2015, 35, 2246–2255. [Google Scholar] [CrossRef]
- Gutiérrez, V.M.; Romero, J.A.R. Elastografía hepática: ¿qué es, cómo se hace y cómo se interpreta? Radiología 2017, 60, 183–189. [Google Scholar]
- Nassef, Y.E.; Abu Shady, M.M.; Galal, E.M.; Hamed, M.A. Performance of diagnostic biomarkers in predicting liver fibrosis among hepatitis C virus-infected Egyptian children. Mem. Inst. Oswaldo Cruz 2013, 108, 887–893. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, L.D.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; de Lédinghen, V. Prospective comparison of transient elastography, fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- Murata, M.; Miyashita, S.; Yokoo, C.; Tamai, M.; Hanada, K.; Hatayama, K.; Towatari, T.; Nikawa, T.; Katunuma, N. Novel epoxysuccinyl peptides: Selective inhibitors of cathepsin B., in vitro. FEBS Lett. 1991, 280, 307–310. [Google Scholar] [CrossRef]
- Okamoto, S.; Okamoto, U.; Wanaka, K.; Hijikata-Okunomiya, A.; Bohgaki, M.; Naito, T.; Horie, N.; Okada, Y. Highly selective synthetic inhibitors with regard to plasma-kallikrein activities. Adv. Exp. Med. Biol. 1989, 247, 29–34. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, E. Quality of Life of Chronic Hepatitis C Patients and Its Associated factors. Osong Public Health Res. Perspect. 2017, 8, 124–129. [Google Scholar] [CrossRef]
- Guo, M.; Mathieu, P.A.; Linebaugh, B.; Sloane, B.F.; Reiners, J.J., Jr. Phorbol Ester Activation of a Proteolytic Cascade Capable of Activating Latent Transforming Growth Factor β. J. Biol. Chem. 2002, 277, 14829–14837. [Google Scholar] [CrossRef] [PubMed]
- Peek, M.; Moran, P.; Mendoza, N.; Wickramasinghe, D.; Kirchhofer, D. Unusual Proteolytic Activation of Pro-hepatocyte Growth Factor by Plasma Kallikrein and Coagulation Factor XIa. J. Biol. Chem. 2002, 277, 47804–47809. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Okuno, M.; Enya, M.; Imai, S.; Moriwaki, H.; Kawada, N.; Suzuki, Y.; Kojima, S. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alphakallikrein-mediated activation of latent TGF-beta. Gastroenterology 2002, 123, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Canbay, A.; Guicciardi, M.E.; Higuchi, H.; Feldstein, A.; Bronk, S.F.; Rydzewski, R.; Taniai, M.; Gores, G.J. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J. Clin. Investig. 2003, 112, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Dooley, S.; ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Mehta, S.H.; Lau, B.; Afdhal, N.H.; Thomas, D.L. Exceeding the limits of liver histology markers. J. Hepatol. 2009, 50, 36–41. [Google Scholar] [CrossRef]
- Lupsor, M.; Stefanescu, H.; Feier, D.; Badea, R. Non-Invasive Evaluation of Liver Steatosis, Fibrosis and Cirrhosis in Hepatitis C Virus Infected Patients Using Unidimensional Transient Elastography (FibroscanR). In Liver Biopsy: Indications, Procedures, Results; Tagaya, N., Ed.; IntechOpen: London, UK, 2012; pp. 209–234. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Pol, S. FIB-4: A simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology 2006, 44, 769–770. [Google Scholar] [CrossRef]
- Degos, F.; Perez, P.; Roche, B.; Mahmoud, A.; Asselineau, J.; Voitot, H.; Bedossa, P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: A multicenter prospective study (the FIBROSTIC study). J. Hepatol. 2010, 53, 1013–1021. [Google Scholar] [CrossRef]
- Fallatah, H.I. Noninvasive Biomarkers of Liver Fibrosis: An Overview. Adv. Hepatol. 2014, 2014, 357287. [Google Scholar] [CrossRef]
- Xu, H.; Kong, W.; Liu, L.; Chi, X.; Wang, X.; Wu, R.; Gao, X.; Wang, H.; Qu, L.; Qi, Y.; et al. Accuracy of M2BPGi, compared with Fibro Scan®, in analysis of liver fibrosis in patients with hepatitis C. BMC Gastroenterol. 2017, 17, 62. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of Liver Fibrosis. Annu. Rev. Pathol. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Lee, U.E.; Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Gonzalez-Peralta, R.P.; Qian, K.; Xu, Y.; Marousis, C.G.; Davis, G.L.; Lau, J.Y.N. Transforming growth factor-ß1 in chronic hepatitis C. J. Viral Hepat. 1997, 4, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Campden, R.I.; Zhang, I. The role of lysosomal cysteine cathepsins in NLRP3 inflammasome activation. Arch. Biochem. Biophys. 2019, 670, 32–42. [Google Scholar] [CrossRef]
- Robertson, I.B.; Rifkin, D.B. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021907. [Google Scholar] [CrossRef]
- Hara, M.; Inoue, I.; Yamazaki, Y.; Kirita, A.; Matsuura, T.; Friedman, S.L.; Rifkin, D.B.; Kojima, S. L59 TGF-β LAP degradation products serve as a promising blood biomarker for liver fibrogenesis in mice. Fibrogenesis Tissue Repair 2015, 8, 17. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Qiu, Q.; Mahdi, F.; Shariat-Madar, Z.; Røjkjaer, R.; Schmaier, A.H. Assembly and activation of HK-PK complex on endothelial cells results in bradykinin liberation and NO formation. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1821–H1829. [Google Scholar] [CrossRef]
- Simões, P.S.R.; Zanelatto, A.O.; Assis, M.C.; Varella, P.P.V.; Yacubian, E.M.; Carrete, H.; Centeno, R.; Araujo, M.S.; Cavalheiro, E.A.; Tersariol, I.L.S.; et al. Plasma kallikrein-kinin system contributes to peripheral inflammation in temporal lobe epilepsy. J. Neurochem. 2019, 150, 296–311. [Google Scholar] [CrossRef]
- Barros, N.M.T.; Tersariol, I.L.S.; Oliva, M.L.V.; Araújo, M.S.; Sampaio, C.A.; Juliano, L.; da Motta, G. High Molecular Weight Kininogen as Substrate for Cathepsin B. Biol. Chem. 2005, 385, 551–555. [Google Scholar] [CrossRef]
- Sebastiani, G. Non-invasive assessment of liver fibrosis in chronic liver diseases: Implementation in clinical practice and decisional algorithms. World J. Gastroenterol. 2009, 15, 2190–2203. [Google Scholar] [CrossRef]
- Castera, L.; Pinzani, M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: Does it take two to tango? Gut 2010, 59, 861–866. [Google Scholar] [CrossRef]
- Manns, M.P.; Czaja, A.J.; Gorham, J.D.; Krawitt, E.L.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M. Diagnosis and management of autoimmune hepatitis. Hepatology 2010, 51, 2193–2213. [Google Scholar] [CrossRef]
| CHC (N = 53) | References Value | |
|---|---|---|
| Age (years) | 57.24 ± 12.12 | - |
| Male/Female | 22/31 | - |
| Viral Genotype | ||
| 1 | 84.9% (45) | - |
| 3 | 15.1% (8) | - |
| Laboratory Date | ||
| TB (mg/dL) | 0.72 ± 0.41 | <1.0 |
| IB (mg/dL) | 0.49 ± 0.28 | <0.7 |
| DB (mg/dL) | 0.23 ± 0.18 | <0.3 |
| ALT (U/L) | 56.64 ± 40.45 | 14–59 (woman); 16–63 (men) |
| AST (U/L) | 76.98 ± 62.39 | 15–37 |
| ALP (U/L) | 88.64 ± 31.34 | 46–116 |
| GGT (U/L) | 120.89 ± 134.44 | 5–55 (women); 15–85 (men) |
| PT-activity (%) | 76 ± 15 | 79–122 |
| PTT (s) | 30.9 ± 5.1 | 24.5–32.8 |
| INR | 1.21 ± 0.39 | 1.00–1.40 |
| Leukocytes (103/mm) | 5.63 ± 1.92 | 4.5–10.5 |
| Erythrocytes (103/mm) | 4.53 ± 0.55 | 4.205.40 |
| Platelet count (103/mm) | 168.04 ± 84.08 | 150–400 |
| Stage of Liver Disease | ||
| F2 | 32.08% (17) | - |
| F3 | 43.40% (23) | - |
| F4 | 24.53% (13) | - |
| Grade of Inflammation | ||
| A1 | 18.87% (10) | - |
| A2 | 50.94% (27) | - |
| A3 | 30.19% (16) | - |
| APRI | 1.32 ± 1.36 | <0.5 can exclude cirrhosis |
| 0.7 can predict significant hepatic fibrosis | ||
| >1.0 can predict cirrhosis | ||
| >1.5 can include cirrhosis | ||
| FIB4 | 3.70 ± 2.79 | >3.25 suggests advanced fibrossis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanelatto, A.d.C.O.; Lacerda, G.d.S.; Accardo, C.d.M.; Rosário, N.F.d.; Silva, A.A.d.; Motta, G.; Tersariol, I.L.d.S.; Xavier, A.R. Cathepsin B and Plasma Kallikrein Are Reliable Biomarkers to Discriminate Clinically Significant Hepatic Fibrosis in Patients with Chronic Hepatitis-C Infection. Microorganisms 2022, 10, 1769. https://doi.org/10.3390/microorganisms10091769
Zanelatto AdCO, Lacerda GdS, Accardo CdM, Rosário NFd, Silva AAd, Motta G, Tersariol ILdS, Xavier AR. Cathepsin B and Plasma Kallikrein Are Reliable Biomarkers to Discriminate Clinically Significant Hepatic Fibrosis in Patients with Chronic Hepatitis-C Infection. Microorganisms. 2022; 10(9):1769. https://doi.org/10.3390/microorganisms10091769
Chicago/Turabian StyleZanelatto, Alexia de Cassia Oliveira, Gilmar de Souza Lacerda, Camila de Melo Accardo, Natalia Fonseca do Rosário, Andréa Alice da Silva, Guacyara Motta, Ivarne Luis dos Santos Tersariol, and Analucia Rampazzo Xavier. 2022. "Cathepsin B and Plasma Kallikrein Are Reliable Biomarkers to Discriminate Clinically Significant Hepatic Fibrosis in Patients with Chronic Hepatitis-C Infection" Microorganisms 10, no. 9: 1769. https://doi.org/10.3390/microorganisms10091769
APA StyleZanelatto, A. d. C. O., Lacerda, G. d. S., Accardo, C. d. M., Rosário, N. F. d., Silva, A. A. d., Motta, G., Tersariol, I. L. d. S., & Xavier, A. R. (2022). Cathepsin B and Plasma Kallikrein Are Reliable Biomarkers to Discriminate Clinically Significant Hepatic Fibrosis in Patients with Chronic Hepatitis-C Infection. Microorganisms, 10(9), 1769. https://doi.org/10.3390/microorganisms10091769






