Abstract
Campylobacter is one of the most common bacterial pathogens of food safety concern. Campylobacter jejuni infects chickens by 2–3 weeks of age and colonized chickens carry a high C. jejuni load in their gut without developing clinical disease. Contamination of meat products by gut contents is difficult to prevent because of the high numbers of C. jejuni in the gut, and the large percentage of birds infected. Therefore, effective intervention strategies to limit human infections of C. jejuni should prioritize the control of pathogen transmission along the food supply chain. To this end, there have been ongoing efforts to develop innovative ways to control foodborne pathogens in poultry to meet the growing customers’ demand for poultry meat that is free of foodborne pathogens. In this review, we discuss various approaches that are being undertaken to reduce Campylobacter load in live chickens (pre-harvest) and in carcasses (post-harvest). We also provide some insights into optimization of these approaches, which could potentially help improve the pre- and post-harvest practices for better control of Campylobacter.
1. Introduction
Campylobacter spp. are the leading bacterial cause of food-borne illness globally [1]. Campylobacter jejuni and C. coli are closely related species [2], both involved in food-borne illness, with C. jejuni being the most commonly isolated species [3]. The incidence of campylobacteriosis is increasing in many countries, and it is hyperendemic in children under 5 years of age in developing tropical regions [4,5]. C. jejuni infections are often associated with gastroenteritis and approximately 30% of acute enteritis cases develop a severely debilitating irritable bowel syndrome with a disease fatality rate that is estimated to be about 5 deaths per 100,000 cases [4,6,7]. Furthermore, some strains of Campylobacter have been associated with several autoimmune disorders, most notably Guillain-Barré syndrome [8,9]. It is estimated that approximately 40% of the reported Guillain-Barré syndrome cases are attributed to this bacterium [10]. While severe complications are not as prevalent in developed countries, C. jejuni remains one of the most reportable bacterial pathogens of food safety concern in Europe and North America [11,12]. According to the latest USDA-economic research service report, the annual healthcare costs incurred by foodborne diseases in the US are estimated to be about $15 billion, of which $1.6 billion caused by Campylobacter species [13]. Data from the Centers for Disease Control and Prevention (CDC) indicates “there are about 1.3 million cases of Campylobacter infection each year in the US alone” [14].
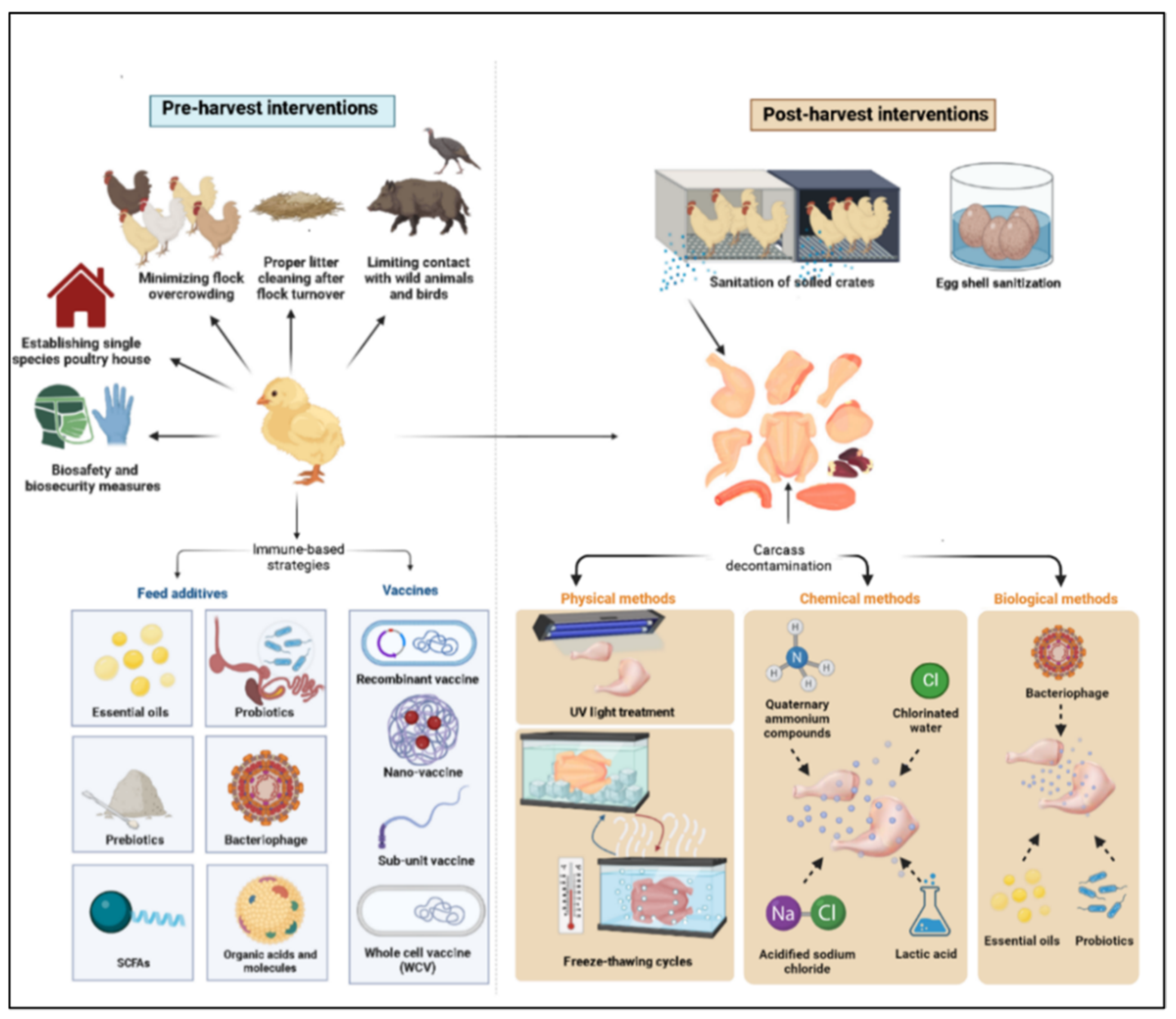
Although domestic mammals and environmental contamination may be sources of infection, chickens are the main source of infection in humans, with 50–80% of the reported cases of campylobacteriosis in Europe being attributed to consumption of poultry products contaminated with this microbe [15,16,17]. Chickens are considered a natural reservoir for C. jejuni, as their intestinal tract offers an optimal biological niche for the survival and proliferation of this bacterium [18]. Chicks often become colonized by 2–3 weeks of age; however, they are largely asymptomatic post-colonization [19,20,21]. Contamination of chicken carcasses with the gut content of Campylobacter-infected chickens during processing at slaughter plants poses a threat to human health [22]. Therefore, three general disease control strategies have been put in place to reduce Campylobacter burden in poultry encompassing pre- and post-harvest intervention checkpoints. The pre-harvest checkpoints are (a) reducing environmental exposure to Campylobacter via biosecurity measures, (b) increasing avian host defense through vaccination, and (c) using antibiotic alternative products to reduce or clear infection load in chickens. The post-harvest checkpoints include (a) slaughter plants cleaning and sanitation, (b) carcass decontamination, and (c) eggshell sanitation. In this context, several approaches have been investigated for control of C. jejuni colonization and transmission in poultry, including on-farm biosecurity measures and the use of immune-based strategies, such as vaccines and feed additives (prebiotics, probiotics, essential oils, bacteriophages, etc.) [23,24,25,26]. However, none of these strategies, by themselves, can eliminate the microbial carriage and the risk of shedding and transmission. Therefore, other complementary control measures are needed to reduce Campylobacter load on chicken carcasses. This article aims to shed light on various control measures being investigated to reduce the risk of Campylobacter transmission from poultry products to humans, including pre- and post-harvest control programs (Figure 1).
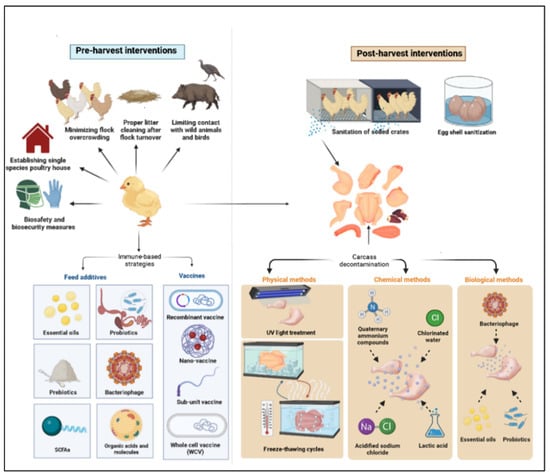
Figure 1.
Pre- and post-harvest control measures.
2. Pre-Harvest Control Measures (On-Farm Control)
Biosecurity measures and intervention strategies that have been applied to reduce Campylobacter burden in poultry flocks.
2.1. On-Farm Biosecurity Measures
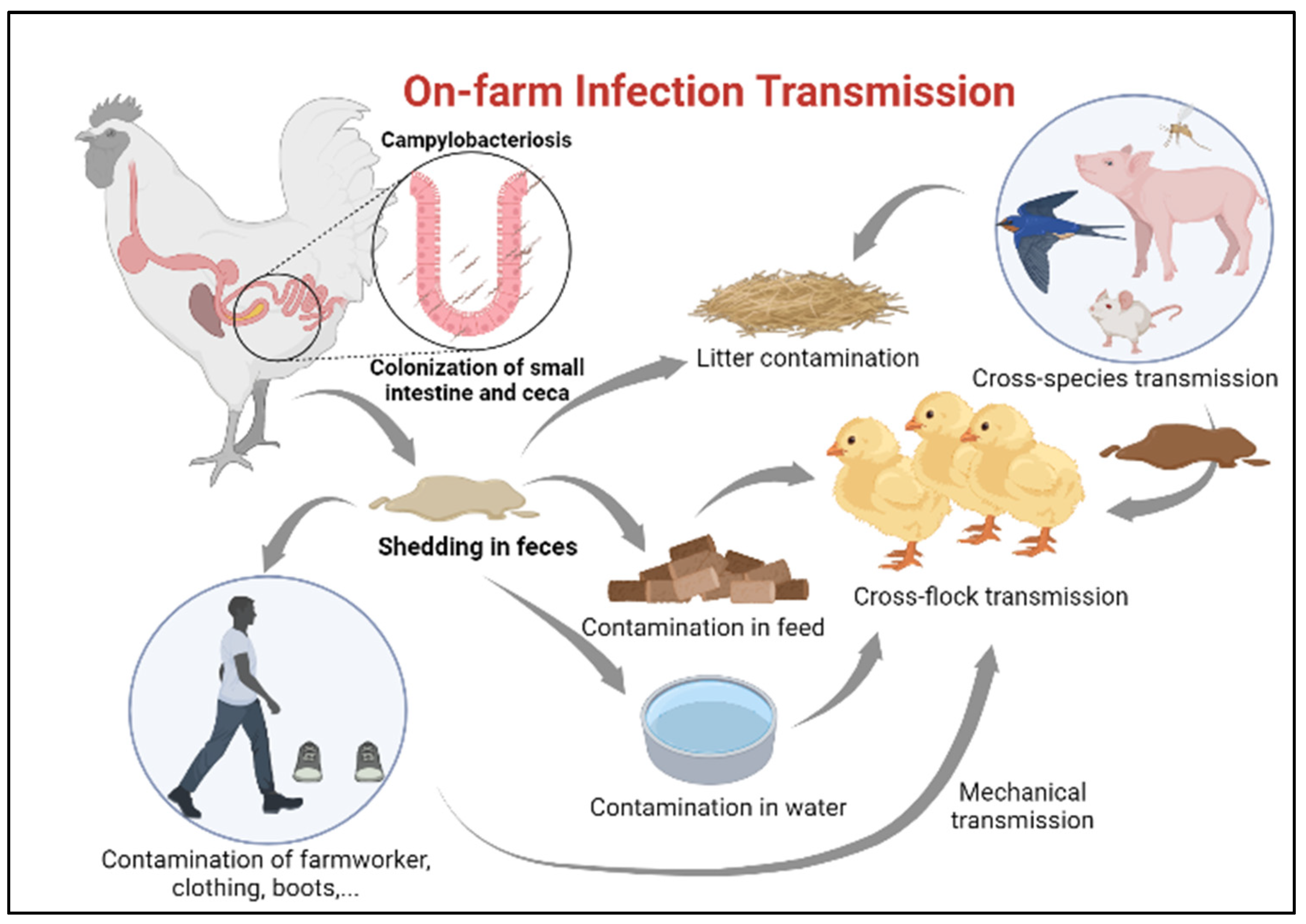
Ensuring newly hatched chicks are protected from infection with Campylobacter requires identification of potential sources for transmission [27]. Horizontal transmission from older flocks is considered the most common route of Campylobacter infection in juvenile chickens [28], especially in farms lacking sophisticated isolation between poultry houses (Figure 2). A minimum infectious dose as little as 50 organisms can colonize the chicken gut [29]. Once Campylobacter reaches the intestinal tract of chickens, it colonizes the small intestine and cecum in high numbers [20]. Infected chickens carry and shed this bacterium in their feces and subsequently spread the infection to the entire flock without developing clinical disease [20] The presence of many poultry houses and older flocks on site is, indeed, amongst the greatest risk factors for flock colonization by Campylobacter [30].
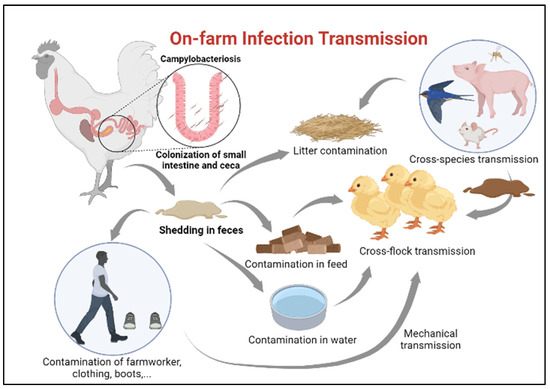
Figure 2.
On-farm infection transmission.
Contamination of the surrounding environment with fecal droppings and contaminated water from the adjacent farm, rodents, insects, and wild animals also considered potential sources of C. jejuni infection in broilers [31,32,33,34,35]. Nonetheless, even with establishing single species farms with secured access points, traffic control and a minimum of 200 M distance between the poultry houses, these measures are still insufficient to prevent the transmission of C. jejuni into the poultry houses [19].
In addition to horizontal transmission from neighboring flocks, one of the other significant sources of infection in production facilities can be from the lack of proper litter treatment between flock turnover or recycling old litters from a previous flock which is quite common in some countries [36].
While the horizontal route plays a major role in Campylobacter transmission, vertical transmission is unlikely [34,37]. A number of studies have reported infrequent detection of C. jejuni in chicken eggs [38,39]; however, whether it is due to internal eggshell contamination from the hen’s reproductive tract or external eggshell contamination with feces of infected chicken remains controversial. In an experimental trial, C. jejuni was detected in 20% of Specific Pathogen Free eggs (SPF) 3 h following the exposure to C. jejuni-contaminated wood shavings. In another study, inoculation of C. jejuni into the air space of embryonated SPF eggs resulted in high embryonic mortality, with 87% of embryos died on the second day post incubation [40]. Nonetheless, there is as yet no evidence whether heavy contamination of eggshell with Campylobacter from environmental sources would lead to similar outcomes.
Campylobacter transmission can also be attributed to human activity on poultry farms; contaminated clothes, skin, and boots of farmworkers and transport crates, either due to biosecurity breaches during the process of partial flock depopulation (thinning) or insufficient implementation of strict biosecurity measures, may contribute to the transmission of Campylobacter from the external environment into poultry houses [20,33,41]. Partial flock depopulation is a common practice in many European countries where a portion of a flock is removed and sent for slaughtering before the final slaughter age. This process was found to be associated with an increased risk of Campylobacter introduction into the broiler house [42]. In a recent survey in Ireland, an increase in prevalence of Campylobacter infection was observed due to the thinning process, where Campylobacter was detected in 38% of the neck skin samples of chickens removed early for slaughtering compared to 67% in the remainder flock [43].
It is widely believed that raising chickens in modern state-of-the-art production systems and full implementation of biosecurity measures would significantly tackle Campylobacter infections. Yet, Campylobacter remains a challenge despite the fact that such measures are being undertaken in European countries, such as the UK, and New Zealand [41,44]. As a result, novel on-farm mitigation strategies and complementary approaches should be applied.
2.2. Immune-Based Strategies to Control Campylobacter Colonization in Poultry Flocks
2.2.1. Vaccines
Vaccine development for poultry has long been pursued with the hopes of reducing Campylobacter colonization and ultimately the incidence of human disease by reducing Campylobacter load on chicken carcasses [45,46,47]. Campylobacter colonization in young chickens is generally not seen until 2–3 weeks post-hatching, probably due to the availability of maternally derived antibodies to this bacterium [48]. Indeed, serum IgY antibodies against C. jejuni are routinely found in breeder hens, and maternal IgY antibodies are detected in serum of their progenies up to about 14 days post-hatch. However, following this period of passive immunity, chicks become receptive to colonization, especially in cases where Campylobacter is present in older flocks and the production facility environment [48]. The immune responses following infection do not appear to limit Campylobacter colonization by the age when broiler birds are slaughtered [20].
Due to the asymptomatic nature of Campylobacter colonization in poultry, with no associated reduction in growth or production efficiency, the poultry industry has not significantly pushed for the development of Campylobacter vaccines. However, the burden of disease in humans is substantial with billions of dollars lost annually due to medical care attributed to the disease [1]. Despite over two decades of attempts to develop a successful Campylobacter vaccine in broilers, successful commercial vaccines are not currently available. Many vaccination trials have been carried out to date, employing a variety of methodologies, such as whole-cell or subunit vaccinations, microorganism-vectored vaccines as well as nanoparticles vaccines (Table 1).

Table 1.
Vaccination trials for C. jejuni infection in poultry (modified from [49]).
Whole Cell Vaccines (WCV)
Vaccines made up of killed whole-cell or attenuated bacteria have been trialed for the control of Campylobacter infections in chickens, however, they have demonstrated minimal effectiveness. The use of killed bacteria, which lack the ability to replicate and colonize chicken’s gut, has been tested against Campylobacter colonization in poultry. In three vaccine trials [46], repeated oral administration of formalin-inactivated Campylobacter cells to chickens, followed by exposure to Campylobacter, was reported to result in a significant elevation in serum and bile C. jejuni-specific IgA titers in the vaccinated birds and a variable reduction in Campylobacter colonization ranging from 16 to 93% compared to the non-vaccinated control chickens. Intraabdominal injection of attenuated Campylobacter cells paired with flagellin protein and 3 other Campylobacter antigens resulted in a 2- log10 reduction in cecal Campylobacter loads; however, when administered orally, no effect was observed. Similarly, Ziprin et al. [50] have attempted to use viable non-colonizing Campylobacter cells, manufactured by mutating a highly infectious strain of Campylobacter, however, the vaccine failed to provide protection against colonization. Additionally, Noor et al. [63] found that chicks inoculated in ovo and boosted orally with WCV after hatching had a significant immunological response, with C. jejuni-specific IgY, IgA, and IgM antibodies detectable in serum and IgA in intestinal contents and bile. On the other hand, Glünder et al. [64] found that despite the presence of specific antibodies in chicken serum following subcutaneous immunization with formalin-inactivated C. jejuni and complete Freund’s adjuvant, a little reduction in colonization was observed after a homologous challenge, while no effect was observed after a heterologous inoculation.
Subunit Vaccines
The immunodominant antigen of Campylobacter, flagellin, was used to create the first subunit vaccination in chickens [45]. Flagellin is regarded as a major virulence factor of Campylobacter as it plays a crucial role in bacterial motility, adhesion and colonization [65]. It was reported that the administration of a C. jejuni flagellin subunit vaccine to 18-day-old chicken embryos resulted in a significant production of serum IgY and IgM antibodies, however, it failed to stimulate intestinal IgA production, and was also ineffective at protecting chickens against Campylobacter colonization [66]. Some researchers have proposed the use of purified native flagellin for subunit vaccination. For example, Neal-McKinney et al. [53] found that birds vaccinated with flagellin coupled with the Montanide adjuvant had a high specific IgY antibodies and a 3 log10 decrease in intestinal Campylobacter count. Despite the encouraging results, flagellin cannot be employed for large-scale chicken immunization due to: (1) variations in flagellin across Campylobacter strains could potentially lead to failure of vaccines to adequately confer cross protection against the variety of strains that can colonize broilers, (2) anti-flagellin antibodies may target non-surface-exposed epitopes, and thus fail to mediate Campylobacter clearance, and (3) anti-flagellin antibodies may recognize glycosylated residues distributed over the surface of flagellin, allowing Campylobacter to elude the host’s immune system [67,68,69].
The potential of Campylobacter capsular polysaccharide-diphtheria toxoid conjugated subunit vaccine to protect against Campylobacter has also been evaluated in both humans and animals [54,70]. Despite the ability of this vaccine to afford protection against Campylobacter in mice and Aotus monkey models, it resulted in minimal reduction (0.64 log10) of cecal colony forming units (CFUs) of C. jejuni in broiler chickens. Nonetheless, these results provide insightful information that successful Campylobacter vaccines for chickens and humans may differ in their antigenic targets.
Generally, several factors are associated with the failure of conventional vaccines to provide effective protection against Campylobacter infection, foremost of which is the high genetic diversity across Campylobacter serotypes [71], and the fact that chickens are exposed to several different strains of Campylobacter over their lifespans. Overall, although several studies have demonstrated the development of specific antibody immune responses with whole-cell and subunit vaccines, these vaccines have largely been inconsistent and unsuccessful in chickens [64,66,72], and the focus on conventional vaccine development has largely shifted towards the development of recombinant and nanoparticles vaccines.
Recombinant Vaccines
These vaccines are manufactured through recombinant DNA technology, whereby antigen-specific DNA is inserted into bacterial or mammalian cells which subsequently replicate producing high levels of antigen [73]. Several recombinant vaccines have been developed against Campylobacter colonization in broilers. Neal-McKinney et al. [53] have reported that amongst several recombinant Campylobacter antigens tested, FlaA, FlpA and a CaDF-FlaA-FlpA fused protein resulted in up to 2 log10 reduction in Campylobacter colonization. Similarly, Theoret and colleagues [74] reported a 2.5 log10 reduction in Campylobacter colonization in chickens when a recombinant attenuated Salmonella enterica was used to synthesize a Dsp antigen. Wyszynska et al. [59] reported that oral vaccination with an avirulent Salmonella vaccine expressing Campylobacter CjA resulted in increased production of serum IgY and intestinal IgA, associated with up to 6 log10 reductions in cecal Campylobacter counts on day 12 post challenge. On the other hand, Buckley et al. [58] demonstrated that vaccination of chickens with Salmonella serovar Typhimurium expressing CjaA resulted in serum IgY and biliary IgA production, but only observed a 1.4 log10 reduction in cecal Campylobacter. Similar results were obtained in chickens vaccinated with an attenuated Salmonella-vectored CjaA protein on the day of hatch and challenged with C. jejuni after three weeks of vaccination [67]. Despite these promising results, however the need for two doses of vaccine at a two-week interval, followed by a 28-day withdrawal time (required for live Salmonella vaccines) before slaughter raises questions about the practicality of this approach. Moreover, one other challenge with Salmonella vectors is that they poorly colonize the chicken intestines, thus failing to prime the immune system effectively [75].
Nanoparticles-Based Vaccines
In recent years, various groups have investigated the potential of oral and in-ovo vaccination with nanoparticles-based vaccines. Recently, we demonstrated that vaccination of broiler chickens with C. jejuni lysate and a TLR21 ligand CpG ODN 2007, encapsulated in the poly (lactic-co-glycolic acid) (PLGA) nanoparticles, induced mucosal innate responses in the intestine and cecal tonsils, increased serum anti-C. jejuni IgY antibody (Ab) titers, modulated the composition of cecal microbiota and reduced cecal C. jejuni count by 2.4 log10 in vaccinated broiler chickens [62,76]. Huang and colleagues [60] tested the effect of intranasal immunization of chickens with chitosan nanoparticles containing a recombinant plasmid pCAGGS-flaA (a gene encoding a flagellin protein), on intestinal and cecal colonization with Campylobacter. The results revealed an increase in the serum IgY and intestinal secretory IgA with up to 3 and 2 log10 CFU/g reduction in Campylobacter loads in the large intestine and cecum, respectively. Despite it seems promising, however this vaccine was tested against only one strain of Campylobacter (C. jejuni ALM-80) and thus, further studies are required to assess its efficacy in a heterologous challenge model. On the other hand, Kobierecka et al. [61] reported that in-ovo immunization of 18-day-old embryonated chicken eggs with Gram-positive Enhancer Matrix (GEM) particles containing two Campylobacter antigens (CjaA and CjaD) reduced cecal colonization with Campylobacter by only 1 log10 in 3- and 4-week-old broiler chickens following challenge with a heterologous C. jejuni strain. In the same study, a higher reduction in Campylobacter count by 2 log10 was observed, when these antigens were encapsulated in a liposome. Although showing only a moderate level of Campylobacter reduction, these results suggest in-ovo immunization as a successful strategy that could potentially be enhanced with booster vaccinations post-hatching. Along similar lines, subcutaneous administration of a crude mixture of C. jejuni outer membrane proteins (OMPs)-loaded PLGA nanoparticles has been shown to induce systemic protective antibody responses and to reduce C. jejuni colonization below the limit of detection in broiler chickens [55]. However, the subcutaneous route is not deemed feasible for mass administration in poultry production.
Despite extensive research over the past few decades, none of the vaccines developed by different groups of researchers conferred “full protection” against infection with this bacterium in chickens [77]. Recent advancements in molecular approaches have opened new avenues for the identification of novel vaccine antigens, through strategies such as reverse vaccinology [78], which could be a steppingstone for vaccine preparation and optimization in the future.
2.2.2. Feed Additives
Prebiotics
With efforts to minimize the use of antibiotics as growth promotants in poultry production, alternative strategies are urgently needed to compensate for their effects. Prebiotics are broadly defined as “indigestible fibers that beneficially influence the gut microbiome when used as feed additives”. Among these prebiotics, mannan-oligosaccharides, β-glucans, and fructans are the most used prebiotics in commercial poultry farming [79,80]. Mannan oligosaccharides (MOS) are largely derived from outer cell membranes of Saccharomyces cerevisiae yeast. These prebiotics are resistant to hydrolysis by digestive enzymes and are widely used in poultry feed to reduce pathogenic organisms in the gut and to enhance productivity. MOS is rich in mannoproteins, mannan, and glucan, and can inhibit gastrointestinal colonization of pathogens by binding to their type-1 fimbriae appendages and inhibiting lectin [81,82]. In general, supplementation of prebiotics alone does not appear to offer the best protection against Campylobacter in chickens. For instance, a recent study demonstrated that dietary supplementation of Saccharomyces-derived prebiotic reduced Campylobacter count by up to 1 log10 CFU per gram of cecal contents [83]. Similarly, Baurhoo and colleagues [84] have reported that dietary inclusion of 0.2% or 0.5% of MOS resulted in a minimal reduction in Campylobacter colonization by approximately 0.25 log. In addition to MOS, fermentation of long-chain fructans, which is extracted from plants, by gut microbes, results in the production of short-chain fatty acids (SCFAs) and lactic acid [85]. The abundance of these acids in the chicken gut promotes the growth and metabolic activity of beneficial microorganisms in the chicken gut and lowers luminal pH, thereby contributing to the prevention of pathogen colonization [85]. In this context, supplementation with chicory fructans, such as inulin or chicory oligofructose, significantly lowered cecum Campylobacter load by a 1.6 log10 [86]. Although several studies showed that dietary supplementation with single prebiotic results in a reduction in Campylobacter colonization, the simultaneous use of multiple prebiotics did not confer additional protection against Campylobacter infection. For instance, while a significant reduction in Campylobacter colonization was observed in chickens received fructans [86], co-administration of plum fibers, fructooligosaccharides, and galactooligosaccharides showed no such effect [87].
Probiotics and Their Products
Probiotics: Over the past few years, probiotics have received considerable attention as antimicrobial alternatives for in-feed antibiotics in the poultry diet [88,89]. Probiotics are defined as “beneficial live microorganisms that confer various health benefits to the host when used as supplements” [90]. In addition to their role in competing with microbial pathogens for adhesion and colonization sites and in modulating intestinal immune responses and microbiome composition [90,91,92], probiotic bacteria produce anti-microbial substances, such as bacteriocins, lactic acid, and hydrogen peroxide that possess direct bactericidal activity against enteric pathogens [88,89,90,93,94,95]. We have recently assessed the immunomodulatory and anti-Campylobacter activities of different Lactobacillus species, including L. salivarius, L. johnsonii, L. reuteri, L. crispatus, and L. gasseri, in vitro [96]. The results revealed that Lactobacillus species exhibited differential anti-C. jejuni activities as demonstrated by inhibition of Campylobacter growth, abrogation of the quorum sensing signal, inhibition of Campylobacter invasion in cultured intestinal epithelial cells and a reduction in the expression of C. jejuni virulence genes (except L. reuteri), including genes involved in motility (flaA, flaB, and flhA), autoinducer production (luxS), and invasion (ciaB and iamA). Additionally, Lactobacillus species have shown potential to enhance the phagocytic activity of chicken macrophages and modulate their immune responses as demonstrated by an enhanced expression of cytokines, including interferon (IFN)-γ, interleukin (IL)-1β, IL-12p40, and IL-10, chemokine, including CXCL8, and the co-stimulatory surface molecules, including CD40, CD80, and CD86 [96]. Similarly, probiotic E. coli strain Nissle 1917 (EcN) (free or Chitosan-alginate microencapsulated) has been shown to modulate the immune responses in intestinal cell lines [97,98,99].
Whilst many studies support the role of probiotics in providing protection against Campylobacter infection, the outcomes of these studies are greatly heterogeneous. It is unclear whether the inconsistencies in the effectiveness of probiotics are due to strain-specific effects and/or related to the differences of the age and type of the bird, dosage and combinations of probiotics, route of administration, dosing frequency, duration of application, and other environmental and managemental factors including the type of the housing and the dietary regimen (Table 2).

Table 2.
Probiotics used to treat Campylobacter infections in chickens (modified from [100]).
Various lactic acid-producing bacteria have been studied for their ability to reduce Campylobacter colonization in broiler chickens [119]. More specifically, the members of the genera Lactobacillus and Bifidobacterium are amongst the most widely used probiotics in the poultry industry [90]. Administration of L. gasseri to newly hatched chicks was shown to reduce cecal C. jejuni counts by approximately 250-fold at day 14 post infection [105]. Repeated oral administration of L. salivarius, every 2–3 days starting from day one to day 35 of age, has been reported to reduce cecal Campylobacter loads by 0.8 log10 at 14 days and a higher reduction of 2.8 log10 was observed at 35 days of age [102]. Recently, Helmy et al. [101] showed that oral treatment of chickens with free or chitosan-alginate microencapsulated probiotic E. coli Nissle 1917, three times per week for two consecutive weeks, reduced cecal C. jejuni colonization by 2 and 2.5 log CFU/g, respectively, and enhanced the growth performance, intestinal morphology and immunity of the treated chickens without adversely affecting the gut microbiota. While single species probiotics have been found to be effective in reducing Campylobacter colonization, combinations of several different probiotic species appear to be more effective [120]. For instance, a substantial reduction of up to 5 log10 CFU/mL in cecal Campylobacter count has been reported with the use of probiotic mixtures containing L. paracasei J.R and L. rhamnosus 15b, L. lactis Y and L. lactis [120]. Ghareeb et al. [119] have also reported a reduction in the cecal load of C. jejuni by up to 6 log10 in broiler chickens received a mixture of probiotics containing Bifidobacterium animalis, Enterococcus faecium, L. salivarius, and L. reuteri, and Pediococcus acidilactici.
By-products of probiotic bacteria: Bacteriocins are ribosomally synthesized peptides that possess antimicrobial activity against other bacterial strains which may or may not be taxonomically related [123,124]. Indeed, several studies reported a substantial reduction in Campylobacter colonization following the administration of various bacteriocins. For example, Messaoudi and colleagues [125] reported a 2 log10 reduction in Campylobacter count following in vitro exposure to bacteriocins derived from L. salvaris SMZD51. Similarly, Stern et al. [126] have reported that administration of bacteriocin OR-7 derived from L. salivarus NRRL B-30514 can reduce Campylobacter colonization by up to 6 log10 units. Furthermore, Stern et al. have reported that administration of a class 2a bacteriocin, derived from Paenibacillus polymyxa NRRL B-30509, resulted in significant reductions in Campylobacter colonization, to the extent that at certain time points of the trial, Campylobacter was reduced to undetectable levels, while an average of 7.2 log10 CFU/g of Campylobacter was detected in the non-treated, infected birds. In another study conducted on turkey poults, co-administration of bacteriocin OR-7 and bacteriocin B602, derived from Paenibacillus polymyxa NRRL B-30509, reduced C. coli colonization to below detectable levels in the duodenum and cecum [127].
It is noteworthy that, while administration of live bacteriocin-producing bacteria (L. salivarius NRRL B-30514 and Paenibacillus polymyxa NRRL B-30509) failed to protect against Campylobacter, their bacteriocins were shown to reduce colonization by up to 6 log10 [128]. However, although various bacteriocins have shown potential to reduce the Campylobacter burden in chickens (Table 3), the development of bacteriocin resistance and their impact on the gut microbiota need to be investigated further.

Table 3.
Bacteriocins used for the treatment of Campylobacter colonization in poultry (modified from [49]).
Synbiotics
To increase the efficacy of probiotics, studies have found that co-administration of prebiotics provides an additive or synergistic protection against Campylobacter infection [133,134]. For example, although not observed in all clinical trials, administration of Bacillus spp., L. salivarius subsp. salivarius and L. salivarius subsp. salicinus alone has been shown to lower Campylobacter counts by 1–2 log10, while when combined with 0.4% MOS, L. salivarius subsp. salicinus resulted in a 3 log10 reduction in Campylobacter counts [116]. Lifelong dietary supplementation of synbiotic (probiotic strain Bifidobacterium longum subsp. longum PCB13 and prebiotic Xylooligosaccharides) to chickens challenged with C. jejuni strain M1 showed a better efficacy compared to short-term supplementation [135]. Together, these results indicate that concurrent administration of prebiotics with probiotics augments their potential to combat Campylobacter colonization.
Essential Oils
As the demand for antibiotic-free poultry products grows, several alternative strategies, such as essential oils (EOs), are being identified at pre- and post-harvest stages [136]. Essential oils are “volatile or ethereal oils with oily plant-based liquids that possess aromatic properties” [137]. They are extracted from plants using hydrodistillation, steam distillation, or solvent extraction to produce concentrate of aromatic and volatile compounds [137]. A series of studies conducted by Solis de los Santos et al. [138,139] have demonstrated that providing a 0.7% caprylic acid, extracted from coconut oil and palm kernel oil, in feed at concentrations below 1% significantly reduced Campylobacter colonization in the cecum of broilers at different ages. The effectiveness of plant extracts, containing natural essential oils, against Campylobacter colonization in chickens has also been assessed [140]. Although a minimal reduction in Campylobacter colonization was observed following dietary inclusion of thymol and carvacrol by 2% and 1%, respectively, their combination did not have additive effects as demonstrated by only 0.5% reduction in Campylobacter counts following their supplementation compared to either one alone. Furthermore, a mixture of garlic and cinnamon extract, which are rich sources of essential oils, reduced Campylobacter colonization in the cecum by 1 log10 CFU/g at 3 days post-infection (day 11 of age), whereas no significant effects were observed on day 35 or day 42 of age [140]. In the same study, no additive effects were observed when a combination of these plant extracts with other additives, including prebiotics and other herbs, were supplemented to chickens.
Recently, Szott et al. [141] showed that adding carvacrol in broilers feed (at a concentration of 120 mg/kg feed) for 4 days reduced C. jejuni colonization by up to 1.2 log10 in the cloacal swabs and colon between 1 and 28 days of age, while no significant effect was observed on cecal colonization at 33-day-old of age. In another study [142], feeding 0.3% trans-cinnamaldehyde-coated feed to C. jejuni-infected broilers exhibited no significant reduction in Campylobacter colonization in the cecum after 1 week. In an in vitro model, treatment of chicken cecal contents with different concentrations (10, 20, and 30 mM) of thymol, eugenol, and carvacrol and then spiking them with 105 CFU/mL C. jejuni was found to reduce C. jejuni to undetectable levels after 8 h of incubation, while treatment with trans-cinnamaldehyde reduced the levels to less than 1 log10 CFU/mL at the same incubation time [143]. In an ex vivo study, Kurekci et al. [144] used a fermentation assay to evaluate the anti-Campylobacter activity of three essential oils, including tea tree oil, lemon myrtle oil and Leptospermum oil. Addition of these oils to cecal content of 20-day old chicken spiked with 3 × 108 CFU/mL of C. jejuni reduced C. jejuni concentrations by 3.3 log10 CFU/mL, without altering the fermentation profile of the cecal microbiota. Collectively, these findings suggest the use of these essential oils to reduce Campylobacter burden in poultry; however, dose optimization and different treatment modalities may be required to attain more desirable outcomes.
Organic Acids
Organic acids (OAs) are naturally occurring organic compounds that retain acidic properties and are distinguished from other acids by the functional group -COOH [145]. OAs are mainly composed of SCFAs (≤C6), such as formic, propionic, acetic, lactic, butyric, and other medium-chain (C7 to C10), and long-chain fatty acids (LCFA; ≥C11) [145,146]. Previous studies suggested that OAs can be used as acidifiers in poultry drinking water and as antimicrobial feed additives [145]. In addition to their antimicrobial activities, dietary inclusion of OAs has been shown to increase feed conversion efficiency, nutrient digestibility, and to modulate anti-oxidative status of the gastrointestinal tract of chickens [145,146,147]. However, the exact mechanisms of action of these OAs remain unclear. Dittoe and colleagues [148] reported that since compounds comprising organic acids are acidic, dietary supplementation of these compounds to chickens alters the pH of the gastrointestinal tract and thus, shielding it from pH-sensitive pathogens. However, in another study, an association was observed between the concentration of dissociated organic acids, but not the pH, and inhibition of C. jejuni growth following exposure to various organic acids. Regardless of the discrepancies on the mechanisms of action of OAs, these findings suggest the on-farm use of these OAs to control bacterial pathogens [149].
In fact, the use of combinations of various organic acids was shown to produce additive or synergistic effects. For instance, Peh et al. [150] reported that a combination of caprylic acid, sorbic acid and caproic acid exhibited synergistic effects on six C. jejuni and four C. coli isolates and reduced the MIC90 values of these compounds using broth microdilution method. Despite evidence indicating that OAs possess potent bactericidal activities when tested in vitro, it should be noted that the OAs may not exhibit the same activity when used in live birds. This is consistent with the findings of Hermans et al. [151] who also demonstrated that low concentrations of caprylic or capric acids (4 mM) and caproic acid (16 mM) killed six C. jejuni strains within 24 h when tested in vitro; however, these marked bactericidal effects did not replicate when tested in vivo. Nonetheless, other studies have pointed out that a more desirable outcome could be achieved by optimization of organic acid concentration. For instance, while a combination of 1% formic acid and 0.1% sorbate did not reduce cecal Campylobacter counts, a higher concentration 1.5–2% of formic acid and 0.1% sorbate resulted in complete elimination of C. jejuni colonization in chickens [152]. In another study, a dose-dependent reduction in Campylobacter count was observed following dietary supplementation of propionic, sorbic acids and pure botanicals [153]. In addition to their synergistic effects when used in a combination, the microencapsulation of these acids was shown to enhance their bactericidal activity as demonstrated by a significant reduction in Campylobacter counts by up to 5.2 log10 at 42 days of age. Nonetheless, this study did not assess the effects of these organic acids on feed intake and growth performance of chickens. Although their undeniable beneficial effects, it is important to note that inclusion of these organic acids in poultry feed may affect its palatability [154], thereby reducing chicken feed intake. Regardless of these potential limitations, the effectiveness of organic acids appears to be largely dependent on the type, concentration, and combination of the organic acid used.
Small Molecule Inhibitors
Small molecule (SM) is defined as “a low molecular weight organic compound, involved in molecular pathways by targeting important proteins” [155]. SM inhibitors are promising alternatives to antibiotics that can be utilized for the control of Campylobacter infection in poultry. They possess the ability to target specific pathways in bacterial cellular processes and perform narrow-broad spectrum antimicrobial activity [156]. In addition, SMs have a long half-life which broadens their potential in the clinical applications of the drug. Although SMs can be effective alternatives to antibiotics, they have their own sets of pharmacological limitations: foremost among these limitations is that the small and compact structure required for the broader bioavailability of the drug decreases its specificity and sensitivity over time [157]. Johnson and his group have screened a pool of 147,000 SMs inhibitors, with several compounds have shown inhibitory activity against C. jejuni. These compounds have demonstrated remarkable ability to suppress motility and biofilm formation without exerting cytotoxic effects on eukaryotic cells. The inhibitory activities of these compounds were also tested in one-day-old chicken experimentally infected with C. jejuni and the results revealed that only campynexin A was able to decrease cecal colonization by 1 log [158].
In a similar study, Deblais et al. [159] screened a library of about 4200 SMs and identified SM based on thiophene sulfonamide with activity against C. jejuni 86–176 and C. coli ATCC33559. The results revealed a reduction in cecal Campylobacter load of three-week-old chicken by 1 log and 2 log following treatment with a benzyl thiophene sulfonamide based small molecule compounds (TH-4 and TH-8), respectively. Additionally, while no significant changes in the microbiota were observed in the TH-8-treated chickens, TH-4 treatment increased the abundance of Coprococcus by 2.57-fold, and a reduction in Peptostreptococcacae, Erypelotrichacae cc115, and Eubacterium abundance by 5.52-fold, 9.6-fold, and 3.0-fold, respectively.
Among 4182 bioactive SMs compounds screened against C. jejuni by Kumar et al. [160], only 478 had a bactericidal effect and 303 had a bacteriostatic effect. A further screening of 79 bactericidal compounds was conducted against different C. jejuni isolates, with only 12 compounds showed consistent bactericidal effect on C. jejuni. These 12 compounds had shown minimal cytotoxic effect on Caco-2 cells and hemolytic effect on sheep RBC. Following further screening, 10 compounds completely cleared C. jejuni populations even at the concentration of 25 µM under in vitro conditions.
Evidence indicates that plant-based compounds can also be used as anti-Campylobacter therapeutics. It was reported that phenolic compounds exhibit antimicrobial activity against both antibiotic-sensitive and -resistant strains of Campylobacter. Among 9 phenolic compounds used, epigallocatechin gallate (EGCG) and carnosic acid had shown a significant anti-Campylobacter effect with a MIC of 78 µg/mL and 19.5 µg/mL, respectively. Similarly, rosemarinic acid showed antagonistic activity against C. jejuni with a MIC of 158 µg/mL [161]. Notably, most of the studies evaluating the antimicrobial activity of various SMs have been carried out in vitro with only a few studies have reported their efficacy in vivo. Further in vivo research is strongly needed to validate the in vitro observations and determine whether SMs could substitute in-feed antibiotic and combat Campylobacter in poultry.
Short Chain Fatty Acids
Short chain fatty acids (SCFAs), including acetate, propionate, butyrate, iso-butyrate, valerate, iso-valerate, hexanoate, are microbial metabolites produced by fatty acid-producing bacteria, such as members of phyla Bacteroides and Firmicutes in the gut [162,163,164]. In addition to exhibiting immunostimulatory and bactericidal properties, the abundance of these metabolites lowers the pH of the gut, making the condition unfavorable for the growth of pathogenic bacteria that are sensitive to acidic pH [163]. For instance, van der Wielen et al. [165] have observed a significant reduction in Enterobacteriaceae growth following in vitro exposure to volatile fatty acids and when administered to chickens, undissociated acetate, propionate, and butyrate was shown to reduce Enterobacteriaceae levels in the gut. However, although butyrate was shown to exhibit bactericidal activity against Campylobacter in vitro, the use of butyrate-coated micro-beads as a feed additive did not reduce C. jejuni colonization in the cecum of two-week-old broiler chicks [107]. The authors attributed this to a rapid absorption of butyrate by the enterocytes and speculated that elevation of butyrate level could probably result in a decrease in C. jejuni colonization. It is therefore conceivable that administration of SCFAs-producing bacteria may potentially provide a continuous source of butyrate, which may, in turn, reduce Campylobacter colonization in broiler chickens.
Bacteriophages
Bacteriophages are “viruses that can infect and kill targeted bacterial cells” [166]. They have been extensively researched and used as antimicrobial agents worldwide for the treatment of several human diseases and recently as preventative and therapeutic agents to control Campylobacter colonization in poultry [167,168,169,170,171,172]. In a recent study, oral administration of two field bacteriophages to experimentally infected broiler chickens at 37 days of age significantly reduced Campylobacter counts by 2 log10 CFU/g at 40 days of age [168]. Fischer et al. [169] have also demonstrated a significant reduction in cecal Campylobacter count by up to 2.8 log10 CFU/g following administration of a single NCTC 12673 or multiple phage cocktail consisting of phages NCTC 12672 12673 12674 and 12678. Interestingly, while NCTC 12673 alone showed similar efficacy to the phage cocktail, administering the phage cocktail significantly reduced initial resistance levels, suggesting that multiple phages may result in delaying the onset of Campylobacter infection. Similar observations were made by Kittler et al. [170] who also found that administration of the same bacteriophage cocktail results in a significant reduction in fecal shedding of Campylobacter by approximately 3 log10 in chickens at the slaughter age. Additionally, in this trial Campylobacter count was reduced below the detection limit during the first 24 h of its administration. Together, these findings suggest that a combination of selected phages administered a few days prior to slaughter may substantially reduce Campylobacter levels in poultry meat.
In the context of their therapeutic efficacy against Campylobacter, a previous study showed that administration of NCTC12671 and 12669 at 1010 PFU to 39-day-old chickens after 7 days of challenge with Campylobacter resulted in a significant reduction in C. jejuni by up to 1.5 log10 [167]. A higher, but transient, reduction in Campylobacter colonization was observed when phage 71 was administered for ten consecutive days to younger birds (two-week-old chicks) by up to 3 log10, suggesting the need for a booster dose or continuous administration to maintain colonization resistance against Campylobacter. In view of this, previous studies have shown that administering phages during the early stages of chicken’s life, followed by a booster dose at the end of the production cycle results in a greater reduction in Campylobacter colonization. For example, oral administration of phage 71 to 7-day-old chicks, followed by Campylobacter challenge on day 10 of age, and phage treatment until day 16 of age, resulted in a delay in the onset of Campylobacter colonization as well as a reduction in cecal C. jejuni count by 1 log10 [167].
In addition to possible synergistic interactions, using multiple phages equipped with various defense mechanisms is thought to result in optimal efficacy due to the possible development of phage resistance when used alone, which is likely the reason for the transient reduction in Campylobacter levels observed immediately after phage administration followed by stabilization, and rebounding of Campylobacter levels. However, phage cocktails must be carefully designed based on the types of phages being administered to achieve maximal benefits as phages exhibit different mechanisms of infection. For example, group 2 phages isolated from C. jejuni RM1221 typically use flagella as a route of entry, while group 3 phages isolated from C. jejuni NCTC12662 target bacterial capsular polysaccharide receptors [171,173]. In this context, Hammer et al. [174] have demonstrated that administration of a group 3 phage CP14 alone reduced fecal counts by 1 log10, while when co-administered with CP81, which belongs to the same group, no further reduction in fecal Campylobacter count was observed. On the other hand, a greater reduction > 3 log10 of fecal Campylobacter counts was observed in three-week-old chickens when a group 2 CP61 phage was administered 24 h following administration of group 3 CP14 [174]. These findings indicate that concomitant administration of phages belonging to the same family may result in greater phage resistance and warrant proper selection of phages from different groups to achieve optimal efficacy.
Despite the number of studies reporting significant efficacy in experimental settings, the major shortcomings of commercial application of bacteriophages, include the high specificity of the selected phages, as such universal applicability and efficacy of phage strains are not achievable, in addition to the lack of evidence over their safety and stability [175]. For example, in a previous study, phage CP8 exhibited variable effectiveness against different serotypes of C. jejuni [172]. In chickens experimentally infected with either C. jejuni GIIC8 or HPC5 serotypes, the efficacy of orally administered CP8 to 25-day-old chickens was notably higher in GIIC8 infected birds with substantial reductions in Campylobacter in the cecum by up to 5.6 log10 within 24 h of phage administration, and final reductions of up to 2.1 log10, while CP8 administration in HPC5 infected birds resulted in no significant reduction. In the same study, phage CP34 exhibited an opposite pattern of efficacy between the two Campylobacter serotypes with significantly greater reductions seen in HPC5 infected chickens of up to 3.9 log10 within 24 h of phage administration [172]. Overall, although bacteriophages have shown remarkable reduction in Campylobacter colonization, previous findings highlight the critical shortcoming of bacteriophages being highly specific for their targets. As such, challenges will be faced in finding suitable phages, which can be effective against a large number of Campylobacter serotypes, to be widely used in the poultry industry.
2.2.3. Fecal Microbial Transplant and Microbial Consortia
The protective role of the gut microbiota against Campylobacter colonization has been recently investigated. Higher levels of Campylobacter were observed in the cecal contents, spleen, liver, and ileum of chickens experimentally infected with Campylobacter and raised under germ-free conditions and also in chickens with a compromised microbiome than in conventionally farmed chickens [176]. Furthermore, Campylobacter infection was shown to result in the development of intestinal lesions in these groups implying that C. jejuni may not simply be a commensal microbe in the poultry gut, but rather a pathogen that has symptomatic effects, notably in hosts with atypical gut microbiome [177].
Considering the integral role of the gut microbiome in the intestinal immune system development and defense against pathogens [178,179], there has been an increasing interest in manipulating the gut microbiome for prevention of enteric infections and colonization by food-borne pathogens, including C. jejuni. While the prophylactic use of antimicrobial alternatives has been studied for their potential to induce anti-Campylobacter mucosal immune responses, and although such strategies have independent mechanisms of action, their indirect effects on the microbiome may have significant contributions to the observed efficacy. Recently, we have reported changes in microbiome compositions in chickens administered with PLGA-encapsulated CpG oligodeoxynucleotides and C. jejuni lysates [62]. In addition to the lower numbers of C. jejuni observed in the treated chickens, significantly higher levels of microbial diversity, particularly members of phyla Firmicutes and Bacteroidetes, were also observed.
Manipulation of the gut microbiota via probiotics and fecal microbial transplants (FMT), particularly at early points in the production cycle, has shown significant potential to reduce Campylobacter colonization. At present, a very limited number of studies have investigated the ability of gut microbiome transplants to reduce Campylobacter colonization in broilers. Gilroy et al. [180] have recently reported undefined fecal transplants obtained from eight-weeks old chickens, which were free of Campylobacter, resulted in lower levels of colonization in chicks that were exposed to Campylobacter either through a direct challenge or a seeder bird model. Furthermore, changes in the microbiome were observed with FMT-treated birds having greater phylogenetic diversity amongst the species constituting the gut microbiome [181]. A significant increase in the levels of lactobacilli was observed in chicks received FMTs, with an average of a 4.5-fold increase in the abundance of Lactobacillales in FMT birds relative to controls. Conversely, a 1.78-fold decrease in the abundance of the order Clostridiales, which was relatively abundant in the gut microbiome of chickens colonized by Campylobacter [180]. However, it should be noted that FMT lacks batch-to-batch consistency and microbial identification is needed to avoid potential carryover of undesirable microorganisms from apparently healthy chickens to recipient chickens. Research efforts have been undertaken to overcome these challenges by developing a well-characterized competitive exclusion culture (microbial consortium) which constitutes a safer alternative to the FMT [182]. Besides their role in improving gut health, supplementation of a consortium of beneficial bacteria to newly hatched chicks allows early colonization of the gastrointestinal tract and may preclude pathogen’s attachment to the intestinal mucosal surface. We have recently demonstrated that addition of competitive exclusion cultures (Aviguard and CEL) to drinking water immediately post-hatch resulted in a significant reduction in Campylobacter colonization in broiler chickens relative to treatment with bacitracin and a lower, but non-significant, reduction relative to the untreated controls, albeit, these treatments resulted in an increase in the relative abundance of Bacteroidaceae and Rikenellaceae, with both playing a role in improving host immunity and metabolic functions [182]. Aside from their minimal role in reducing Campylobacter colonization, the ability of these consortia to modulate the microbiome composition, during the course of Campylobacter infection, suggest their potential use as a safer alternative to bacitracin in poultry feed to tackle the growing threat of antibiotic resistance.
3. Post-Harvest Control Measures (Production Chain Interventions)
On-farm control measures alone have not been sufficient to eliminate Campylobacter in poultry. Sanitation practices in poultry processing facilities should also be implemented to further reduce Campylobacter levels at later stages of the food supply chain. In a recent European Food Safety Authority (EFSA) report [183], the proportion of broiler flocks infected by Campylobacter varies widely (ranging from 2 to 100%), and strongly correlates with the prevalence of Campylobacter on broiler carcasses (4.9% to 100%). Chickens carry a high load of Campylobacter of approximately 8 log10 CFU/g in their caeca prior to slaughter. Contamination of chickens’ feathers with fecal material during transportation to the slaughterhouse can also be a significant external source of carcass contamination during the plucking/defeathering process [184,185].
Transportation crates are a particularly problematic source of cross-facility transmission of Campylobacter, as birds are kept in crates for extended periods of up to 3–12 h, such as before transportation or slaughter, during which the crates are soiled with fecal droppings and Campylobacter shedding can be especially high [25]. Even with measures taken for cleaning and disinfecting transportation crates, Campylobacter was detected in 57% of the swab samples collected from cleaned crates and a notable increase in the number of infected chickens by 9% was also observed in the cloacal swabs following transportation, but whether the increase in the number of infected birds enhanced the risk of carcass contamination during processing was not investigated in this study.
In addition to possible stress-induced impairment of the gastrointestinal tract, feed restrictions during these times may also result in increasingly neutral pH levels in the gastrointestinal tract, providing a microenvironment suitable for optimal Campylobacter growth [25,26]. Increased turnaround periods have also been reported to reduce the risk of new flocks becoming colonized. This is largely because Campylobacter is increasingly less effective at colonization with greater periods spent outside the host gastrointestinal tract. Lazaro et al. [186] have reported that Campylobacter can survive up to 7 months in a viable but not culturable state. As such, increasing down time of crates between flocks and effective cleaning could put in place to lower the risk of horizontal exposure. In addition to these measures, effective carcass decontamination practices should be considered to reduce Campylobacter concentration in the poultry meat. Contamination of meat products by gut contents is difficult to prevent during processing at slaughter plants because of the high numbers of C. jejuni in the gut, and the large percentage of birds infected. Data from surveillance studies in different countries indicated a high prevalence of Campylobacter on raw retail chicken meat. For example, Campylobacter was detected in 28.6%, 36.5%, 41.2% and 52.2%, 59.9% of samples from chicken meat from retail stores in the United Arab Emirates, Qatar, the United Kingdom, Saudi Arabia, and Canada, respectively [187,188,189,190,191]. It is estimated that a reduction in Campylobacter counts in the neck and breast skin to 103 CFU/g reduces the public health risk by 50% [192]. The following section summarizes the physical, chemical, and biological control measures that have been, or are being taken, to reduce Campylobacter load on poultry carcasses.
3.1. Slaughter Plants Cleaning and Sanitation
In addition to the risk of carcass contamination during evisceration, cross contamination from contaminated processing equipment surfaces, due to insufficient cleaning and disinfection, should also be considered as another source of carcass contamination during the slaughter process. Sounmet and Sanders were the first to report the survival of Campylobacter on cleaned and disinfected surfaces of four French slaughterhouses [193]. It is, however, unclear, whether the transportation crates or the previously slaughter flock was responsible for the observed contamination, since the same Campylobacter strain was isolated from the crates and chicken carcasses. These data highlight the importance of carcass treatment at the end of the process line.
3.2. Carcass Decontamination
Several interventions used at the slaughterhouse level have been assessed for their degree of impact on reducing human campylobacteriosis and have extensively been reported elsewhere [194]. Amongst some of the greatest potential risk reductions, the use of carcass decontamination methods, including physical, chemical, and biological methods, is of notable potential because they can be fully implemented with the introduction of mandatory government food safety regulations at the later stage of the food chain.
3.2.1. Physical and Chemical Methods
Poultry carcass treatment with 2 % lactic acid is estimated to reduce the risk of Campylobacter infection in humans between 37–56 %, whereas treatment with acidified sodium chlorite (1200 mg/L) and trisodium phosphate (10–12 %, pH 12) was found to reduce the risk by 75–96%, and 67–84%, respectively [194]. In the US, organic acids and quaternary ammonium compounds have also been used for decontamination at the slaughterhouses [195]. Slaughterhouse stage is also an optimal point in the production cycle for decontamination with irradiation and/or cooking. U.V light and high temperatures have long been used to reduce meat contamination and have the potential for very high levels of efficacy if they are successfully implemented in high volume slaughter lines [196]. Overall, despite the large degree of efficacy for these various carcass decontamination methods, a major drawback of the currently available methods is the effect they have on the sensory attributes of the meat. In particular, freeze–thaw cycles, irradiation and precooked meats are amongst the most unfavorable qualities for consumers concerning sensory attributes and overall perception of the food [197].
In addition to different decontamination methods, poultry meat is often frozen during some point in the farm to fork continuum. Although not offering a significant reduction in Campylobacter counts to be regarded as a primary solution to reduce Campylobacter at the production level, freezing can result in moderate reductions in the pathogen on poultry meat. Despite this, it was reported that freeze cycles have low efficacy in reducing Campylobacter levels on poultry carcasses in contrast to some of the previously mentioned compounds, such as sodium triphosphate sprays [26]. Nonetheless, a variety of factors such as duration of freeze periods, numbers of freeze–thaw cycles as well as methods of freezing may contribute to the discrepancies within the literature [198]. Thermophilic Campylobacter growth occurs between 37–42 °C, while retardation of growth occurs below 30 degrees [199]. Although Campylobacter is unable to grow below 30 degrees, it can survive in temperatures as low as 4 °C under moist conditions for periods of up to 7 months [199,200,201]. Bhaduri et al. [199] have reported reductions in Campylobacter counts between 0.31 to 0.81 log10 CFU/g after refrigeration at 4 °C for 3–7 days. While freezing Campylobacter is known to kill the bacteria to some extent, each freeze cycle is only able to eliminate a portion of the total bacterial load. As such, Sampers et al. [201] have reported that freezing poultry to −22 °C is effective at reducing the pathogen by about 1 log10 units, over the initial 24 h period, however beyond that the bacterial load remains relatively stable. Similarly, Bhaduri et al. [199] have reported that freezing to −20 °C results in Campylobacter reductions between 0.56–3.39 CFU/g over two weeks.
Various models have been applied to estimate the translational efficacy of reducing Campylobacter levels in live chickens and poultry meats and the downstream impact these reductions have on the incidence of human campylobacteriosis. Estimated risk reductions for human cases of campylobacteriosis from freezing broiler carcasses vary based on the length of the freeze cycle, with short time freezes of 2–3 days resulting in an estimated 62–93% risk reduction, while longer freeze cycles of three weeks are estimated to result in an 87–98% risk reduction [201]. Similar degrees of risk reduction are seen with hot water immersion and irradiation/cooking, up to 75–89% and 100%, respectively [192]. Furthermore, at the poultry farm level, disease risk reduction models have been used to estimate to what extent certain levels of reductions in chicken cecum Campylobacter colonization can contribute to reducing human campylobacteriosis [192]. In a most recent modelling approach, cecum concentration reductions of up to 2 or 3 log10 units are estimated to reduce human disease by 42% or 58%, respectively [183].
Although ultrasonication technology has been proven safe and effective for water purification and decontamination of carcasses and meat surfaces, numerous studies indicated that its application to Campylobacter-contaminated chicken meat and skin did not significantly reduce Campylobacter numbers. Detailed information on the effectiveness of other physical and chemical methods to reduce the concentration of Campylobacter on chicken carcasses during the slaughter process have been reviewed elsewhere [192,202].
3.2.2. Biological Methods
As consumer demands for the availability of high quality and safe food products increase, C. jejuni decontamination and preservation using natural and chemical-free mechanisms has received greater attention by the industry. Some of the biological intervention technologies (BITs) that have shown promise in reducing food-borne pathogen load post-harvest include EOs, bacteriophages, bacteriocins, and probiotics. The effects of these strategies on pre-harvest control of C. jejuni have been described above. While research on the effect of BITs to reduce C. jejuni load pos-harvest is sparce, findings on other pathogens of food safety concern, such as Salmonella spp. and Listeria spp. indicates their potential for C. jejuni control post-harvest [203,204].
Probiotics and bacteriocins: two lactobacilli (L. salivarius and L. hamsteri) and 1% and 2% caprylic acid, alone or in combination with 2% chitosan solution, resulted in a consistent reduction in the number of C. jejuni on chicken wingettes that lasted for at least 5 days post treatment [205]. In another experiment, B. longum ssp. longum PCB133 resulted in 1.16 log CFU/g reduction in C. jejuni from chicken legs inoculated with 5.30 log CFU/g C. jejuni and packaged under a modified atmosphere (50% CO2/10% O2/40%N2) [206]. Previously, treatment of chicken meat and skin with 500 IU/gm nisin, a bacteriocin produced by L. lactis, in combination with 2% (w/w) sodium lactate, resulted in a significant reduction in Arcobacter butzlerei, a common Campylobacter-like organism with clinical and microbial features similar to C. jejuni, by up to 1 log [207].
Bacteriophages: In addition to their role in reducing C. jejuni counts in poultry pre-harvest, bacteriophages have also been tested with effective reduction in C. jejuni post-harvest. Application of a single 107 PFU of group III phage φ2 (NCTC 12674, ACTC 35922-B2) therapy to chicken skin, inoculated with 106 CFU of C. jejuni and stored at 4 °C for 10 days, resulted in 1 log CFU/cm2 reduction in this bacterium, and with further reduction of 2.5 log CFU/cm2 during an additional storage of poultry skin at −20 °C [208]. A recent study also showed the potential of bacteriophages against C. jejuni on chicken skin with a 0.73 log 10 reduction in the bacterial counts [209]. Another study by Zmapara et al. [210] showed that the application of Innolysins, which combine the enzymatic activity of endolysins with the binding capacity of phage receptor binding proteins to enhance endolysins activity against Gram-negative bacteria, on chicken skin refrigerated to 5 °C and contaminated with C. jejuni, resulted in 1.18 to 1.63 log reduction in this bacterium.
Essential oils: In addition to their potential application for pre-harvest control of Campylobacter, EOs have also shown a promising effect on the control of C. jejuni post-harvest. Djenane et al. [211] showed a 5 log CFU/g reduction in C. jejuni in skinless chicken breasts stored in microaerobic condition at 3 ± 2 °C, treated with Inula graveolens (MIC of 0.2%), Laurus nobilis (MIC of 0.6%), Pistacia lentiscus (MIC of 0.6%) and Atureja gontana (MIC of 0.6%) and experimentally contaminated with 5 × 105 CFU/g of C. jejuni compared to untreated but contaminated control, which reached about 8 log 10 CFU/g after 1 week. Shrestha and colleagues [212] have also recently demonstrated that washing chicken carcasses with carvacrol significantly reduced C. jejuni on chicken skin by approximately 2.4 to 4 log10 CFU/sample. In addition to the effect on poultry products, essential oils can also play a role in reducing biofilm formation at the processing plant, which is challenging to control using commonly used antimicrobials and sanitizers, such as chlorine and peracetic acid. The biofilm formation of C. jejuni and C. coli were reduced by 70 to 80% with the treatment of coriander and linalool at concentrations of 2 μg/mL, and a lower concentration of up to 0.025 μg/mL resulted in reduction in biofilm by 10 to 20% compared to untreated biofilm controls [213]. While the above BITs show potential in reducing C. jejuni load post-harvest, more research is needed to develop effective formulations that can replace chemical intervention strategies.
Altogether, although these treatments appear to be promising in treatment of chicken skin and meat; however, their use in slaughter and processing plants has not yet been implemented, probably due to regulatory hurdles.
3.3. Eggshell Sanitation
Transmission of Campylobacter from fertile eggs to commercial flocks: Some of the initial studies conducted in the 1980s to investigate the ability of Campylobacter for shell penetration, reported the phenomenon to rarely occur and in a temperature-dependent manner [214,215]. Campylobacter can penetrate eggshell at 4 °C, with the viability once inside the eggs generally reported to be less than 72 h [214,215]. Although vertical transmission from laying hens to the progeny has rarely been reported to occur [37], it is unclear to what degree Campylobacter presence in the reproductive tract of hens may be contributing to infertility and inviable offspring. To date, there is no clear evidence that Campylobacter can be vertically transmitted from parent breeders to fertile eggs. It is also remains unclear whether trans-shell penetration by Campylobacter from the external environment poses a significant risk factor to commercial flocks [183]. Overall, the literature suggests that strategies that control Campylobacter transmission to flocks need to focus on routes other than vertical transmission through eggs.
Transmission of Campylobacter from table eggs to humans: Campylobacter was reported to be viable in the egg yolk stored at 18 °C for up to 14 days, and in the albumen and air sac for up to 8 days [216]. However, the authors also reported that in realistic settings although between 4–6% of newly laid eggs were Campylobacter positive, after storage of the eggs at 18 °C for 7 days, no viable Campylobacter remained. This raises the possibility that consumption of freshly laid eggs in a non-intensive production system could pose a significant threat to human health.
Despite the ability to penetrate the shell and survive in the yolk, numerous studies have shown that Campylobacter is extremely sensitive to atmospheric conditions. For example, Neill et al. [215] reported that these bacteria were unable to survive for more than 6 h when present in eggs that were incubated at 37 °C and exposed to a ventilated atmosphere. Yet, previous studies have reported a small number of viable organisms are recovered from the contents of chicken eggs. While most of the literature has focused on strategies in reducing Campylobacter presence on poultry meats, relatively little has been done to reduce Campylobacter transmission from eggs.
Altogether, the literature suggests that the combination of industry setting exposures such as fumigation, storage at cool temperatures and chemical sanitation such as quaternary ammonium, sodium hydroxide, phenol formaldehyde and hydrogen peroxide, together can significantly prevent Campylobacter survival. However, the concerns over the potential presence of chemical residues on the eggshell suggests the need for safer alternatives to improve microbiological safety and reduce the presence of chemical hazards in eggs. Physical sanitation of eggshell, such as ultraviolet light, infrared and ozone, has been proposed in some studies but have not yet been implemented [217,218].
4. Conclusions and Future Prospects
The continued increase in the incidence of human campylobacteriosis, which is estimated to be increased by 70% in 2018 from 2006 data, and associated healthcare costs necessitate an urgent need for effective ways to combat Campylobacter infection in poultry and prevent its transmission to humans through contaminated poultry products. Although there is no effective intervention measure available to “completely” eliminate Campylobacter in poultry, there is a considerable amount of promise for the future, with continued identification of novel bacteriophages, bacteriocins, prebiotics and probiotics, and anti-Campylobacter vaccine antigens. While the potentiality of these strategies to combat Campylobacter has been extensively investigated, their commercialization remains murky. In fact, several questions should be asked to determine the suitability of these approach for commercialization: For pre-harvest strategies: (a) can they provide consistent heterotypic protection? preferably reducing intestinal colony counts by at least 3 log10, (b) are they cost effective? (c) are they suitable for mass administration? (d) are they safe for chickens?. For post-harvest strategies: (a) can they consistently reduce Campylobacter load on poultry carcasses and eggshell? (b) do they have residual effects for humans?
More research is, indeed, needed to improve existing strategies or perhaps identify a novel strategy that meets the aforementioned criteria and more importantly, it should be industrially scalable and suitable for different commercial poultry systems in different countries. Future research should be directed at (a) identifying a novel and highly conserved immunogenic proteins that can induce cross-protective immunity against different strains of C. jejuni. Perhaps the innovative use of new technologies, such as reverse vaccinology, for prediction of novel antigenic targets could lead to development of multi-epitope vaccine capable of inducing cross-protection against different Campylobacter strains; (b) developing delivery systems for targeted delivery of vaccine formulations to the sites of Campylobacter colonization in chickens. The use of nanoparticles-based technologies for development of Campylobacter vaccines was found to be a promising replacement for older vaccine delivery methods. The uniquely tunable properties of nanoparticles enable them to be fine-tuned to be released in accordance with pathologic stimuli (pH, temperature, etc.) and thus can be specifically designed and engineered for targeted delivery of antigens to the immune inductive sites of the intestine (the sites of Campylobacter colonization; (c) developing a targeted competitive exclusion microbial consortium that possesses antagonistic ability against Campylobacter. Computational models can be employed to identify stable microbial communities with diverse immunomodulatory and anti-Campylobacter capabilities to be given to newly hatched chicks to establish a healthy and stable gut microbiome and prevent Campylobacter infection; and (d) developing a multifaceted approach that combines the previously mentioned strategies.
Numerous studies have examined the combined effects of prebiotics and probiotics, however, to our knowledge, no study has been undertaken to investigate the combined effects of different feed additives and Campylobacter vaccines and feed additives. Implementing a combination strategy will likely result in synergistic effects, which may successfully reduce Campylobacter colonization of broilers to a degree where significant reductions in contamination of poultry products can be achieved. In addition to these measures, there is a need to increase public health awareness about proper handling of raw poultry meat and kitchen hygiene to minimize the risk of infection.
Author Contributions
Conceptualization, K.T.-A.; methodology and literature data collection, K.T.-A., M.S., Y.A.H. and S.S. (Shreeya Sharma); writing—original draft preparation, K.T.-A., S.S. (Shreeya Sharma), M.S., S.S. (Shayan Sharif), Y.A.H., R.R.K., M.A. and A.Y.; writing—review and editing, K.T.-A., S.S. (Shayan Sharif), A.Y., R.R.K., Y.A.H. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef] [PubMed]
- Fernández, H.; Lobos, M.; Concha, M. Inducing enterotoxigenic properties in Campylobacter jejuni and Campylobacter coli by serial intraperitoneal passage in mice. Memórias Do Inst. Oswaldo Cruz 1999, 94, 101–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79. [Google Scholar]
- Acheson, D.; Allos, B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Terefe, Y.; Deblais, L.; Ghanem, M.; Helmy, Y.A.; Mummed, B.; Chen, D.; Singh, N.; Ahyong, V.; Kalantar, K.; Yimer, G.; et al. Co-occurrence of Campylobacter Species in Children From Eastern Ethiopia, and Their Association With Environmental Enteric Dysfunction, Diarrhea, and Host Microbiome. Front. Public Health 2020, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Blaser, M.J. Fatalities associated with Campylobacter jejuni infections. JAMA 1985, 253, 2873–2875. [Google Scholar] [CrossRef]
- Peters, S.; Pascoe, B.; Wu, Z.; Bayliss, S.C.; Zeng, X.; Edwinson, A.; Veerabadhran-Gurunathan, S.; Jawahir, S.; Calland, J.K.; Mourkas, E. Campylobacter jejuni genotypes are associated with post-infection irritable bowel syndrome in humans. Commun. Biol. 2021, 4, 1015. [Google Scholar] [CrossRef]
- Malik, A.; Brudvig, J.M.; Gadsden, B.J.; Ethridge, A.D.; Mansfield, L.S. Campylobacter jejuni induces autoimmune peripheral neuropathy via Sialoadhesin and Interleukin-4 axes. Gut Microbes 2022, 14, 2064706. [Google Scholar] [CrossRef]
- Kassem, I.; Helmy, Y.A.; Kashoma, I.P.; Rajashekara, G. The emergence of antibiotic resistance on poultry farms. In AchievingSustainable Production of Poultry Meat: Safety, Quality and Sustainability; Ricke, S., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2016; Volume 1, ISBN 978-1-78676-064-7. [Google Scholar]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter species and Guillain-Barre syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef]
- Epps, S.V.; Harvey, R.B.; Hume, M.E.; Phillips, T.D.; Anderson, R.C.; Nisbet, D.J. Foodborne Campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health 2013, 10, 6292–6304. [Google Scholar] [CrossRef]
- Kassem, I.I.; Kehinde, O.O.; Helmy, Y.A.; Kumar, A.; Chandrashekhar, K.; Pina-Mimbela, R.; Rajashekara, G. Campylobacter in poultry: The conundrums of highly adaptable and ubiquitous foodborne pathogens. In Foodborne Diseases: Case Studies of Outbreaks in the Agri-Food Industries; Mei Soon, J., Manning, L., Wallace, C.A., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Buzby, J.C.; Allos, B.M.; Roberts, T. The economic burden of Campylobacter-associated Guillain-Barre syndrome. J. Infect. Dis. 1997, 176, S192–S197. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.; Chatham-Stephens, K.; Geissler, A. Chapter 4: Travel-Related Infectious Disease: Campylobacteriosis. In Centers for Disease Control and Prevention. CDC Yellow Book 2020: Health Information for International Travel; Oxford University Press: Oxford, UK, 2017; pp. 180–181. [Google Scholar]
- Wilson, D.J.; Gabriel, E.; Leatherbarrow, A.J.; Cheesbrough, J.; Gee, S.; Bolton, E.; Fox, A.; Fearnhead, P.; Hart, C.A.; Diggle, P.J. Tracing the source of campylobacteriosis. PLoS Genet. 2008, 4, e1000203. [Google Scholar] [CrossRef]
- van Gerwe, T. Poultry meat as a source of human campylobacteriosis. Tijdschr. Voor Diergeneeskd. 2012, 137, 172–176. [Google Scholar]
- Hailu, W.; Helmy, Y.A.; Carney-Knisely, G.; Kauffman, M.; Fraga, D.; Rajashekara, G. Prevalence and Antimicrobial Resistance Profiles of Foodborne Pathogens Isolated from Dairy Cattle and Poultry Manure Amended Farms in Northeastern Ohio, the United States. Antibiotics 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; Van Deun, K.; Martel, A.; Van Immerseel, F.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011, 42, 82. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Molnár, A.; Aschenbach, J.R.; Ghareeb, K.; Khayal, B.; Hess, C.; Liebhart, D.; Dublecz, K.; Hess, M. Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immun. 2015, 21, 151–160. [Google Scholar] [CrossRef]
- Newell, D.; Fearnley, C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef] [PubMed]
- Connerton, P.L.; Richards, P.J.; Lafontaine, G.M.; O’Kane, P.M.; Ghaffar, N.; Cummings, N.J.; Smith, D.L.; Fish, N.M.; Connerton, I.F. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.; Elvers, K.; Dopfer, D.; Hansson, I.; Jones, P.; James, S.; Gittins, J.; Stern, N.; Davies, R.; Connerton, I. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 2011, 77, 8605–8614. [Google Scholar] [CrossRef]
- Meunier, M.; Guyard-Nicodème, M.; Dory, D.; Chemaly, M. Control strategies against C ampylobacter at the poultry production level: Biosecurity measures, feed additives and vaccination. J. Appl. Microbiol. 2016, 120, 1139–1173. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Marmion, M.; Ferone, M.; Wall, P.; Scannell, A. On farm interventions to minimise Campylobacter spp. contamination in chicken. Br. Poult. Sci. 2021, 62, 53–67. [Google Scholar] [CrossRef]
- Hakeem, M.J.; Lu, X. Survival and control of Campylobacter in poultry production environment. Front. Cell. Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef]
- Barrios, P.R.; Reiersen, J.; Lowman, R.; Bisaillon, J.-R.; Michel, P.; Fridriksdóttir, V.; Gunnarsson, E.; Stern, N.; Berke, O.; McEwen, S. Risk factors for Campylobacter spp. colonization in broiler flocks in Iceland. Prev. Vet. Med. 2006, 74, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.G.; Bueschkens, D.H. Horizontal spread of human and poultry-derived strains of Campylobacter jejuni among broiler chicks held in incubators and shipping boxes. J. Food Prot. 1988, 51, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Shivaprasad, H.; Schaberg, D.; Wier, F.; Weber, S.; Bandli, D. Campylobacter jejuni infection in broiler chickens. Avian Dis. 2006, 50, 55–58. [Google Scholar] [CrossRef]
- Guerin, M.T.; Martin, W.; Reiersen, J.; Berke, O.; McEwen, S.A.; Bisaillon, J.-R.; Lowman, R. A farm-level study of risk factors associated with the colonization of broiler flocks with Campylobacter spp. in Iceland, 2001–2004. Acta Vet. Scand. 2007, 49, 18. [Google Scholar] [CrossRef]
- Ellis-Iversen, J.; Ridley, A.; Morris, V.; Sowa, A.; Harris, J.; Atterbury, R.; Sparks, N.; Allen, V. Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiol. Infect. 2012, 140, 916–924. [Google Scholar] [CrossRef]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound. Emerg. Dis. 2018, 65, 30–48. [Google Scholar] [CrossRef]
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A review of the effect of management practices on Campylobacter prevalence in poultry farms. Front. Microbiol. 2018, 9, 2002. [Google Scholar] [CrossRef]
- Battersby, T.; Whyte, P.; Bolton, D. The pattern of Campylobacter contamination on broiler farms; external and internal sources. J. Appl. Microbiol. 2016, 120, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Kassem, I.I.; Helmy, Y.A.; Kathayat, D.; Candelero-Rueda, R.A.; Kumar, A.; Deblais, L.; Huang, H.C.; Sahin, O.; Zhang, Q.; Rajashekara, G. Nonculturability Might Underestimate the Occurrence of Campylobacter in Broiler Litter. Foodborne Pathog. Dis. 2017, 14, 472–477. [Google Scholar] [CrossRef]
- Bailey, M.A.; Bourassa, D.V.; Krehling, J.T.; Munoz, L.; Chasteen, K.S.; Escobar, C.; Macklin, K.S. Effects of Common Litter Management Practices on the Prevalence of Campylobacter jejuni in Broilers. Animals 2022, 12, 858. [Google Scholar] [CrossRef]
- Callicott, K.A.; Friðriksdóttir, V.; Reiersen, J.; Lowman, R.; Bisaillon, J.-R.; Gunnarsson, E.; Berndtson, E.; Hiett, K.L.; Needleman, D.S.; Stern, N.J. Lack of evidence for vertical transmission of Campylobacter spp. in chickens. Appl. Environ. Microbiol. 2006, 72, 5794–5798. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Béjaoui, A.; Ben Hamda, C.; Alaya, N.; Hamrouni, S.; Bessoussa, G.; Ghram, A.; Maaroufi, A. Campylobacter spp. in Eggs and Laying Hens in the North-East of Tunisia: High Prevalence and Multidrug-Resistance Phenotypes. Vet. Sci. 2022, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.A.; Fonseca, B.B.; Melo, R.T.d.; Felipe, G.d.S.; Silva, P.L.d.; Mendonça, E.P.; Filgueiras, A.L.L.; Beletti, M.E. Transmission of Campylobacter coli in chicken embryos. Braz. J. Microbiol. 2012, 43, 535–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fonseca, B.; Beletti, M.; Melo, R.; Mendonca, E.; Vieira, C.; Levenhagen, M.; Rossi, D. Transfer, viability and colonisation of Campylobacter jejuni in the chicken vitellus and in embryos. Br. Poult. Sci. 2011, 52, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Royden, A.; Christley, R.; Prendiville, A.; Williams, N.J. The Role of Biosecurity in the Control of Campylobacter: A Qualitative Study of the Attitudes and Perceptions of UK Broiler Farm Workers. Front. Vet. Sci. 2021, 8, 1552. [Google Scholar] [CrossRef]
- Hertogs, K.; Heyndrickx, M.; Gelaude, P.; De Zutter, L.; Dewulf, J.; Rasschaert, G. The effect of partial depopulation on Campylobacter introduction in broiler houses. Poult. Sci. 2021, 100, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.; Franklin-Hayes, P.; Koolman, L.; Egan, J.; Gutierrez, M.; Byrne, W.; Golden, O.; Bolton, D.; Reid, P.; Coffey, A. Prevalence and levels of Campylobacter in broiler chicken batches and carcasses in Ireland in 2017–2018. Int. J. Food Microbiol. 2022, 372, 109693. [Google Scholar] [CrossRef]
- Greening, S.S.; Zhang, J.; Midwinter, A.C.; Wilkinson, D.A.; Fayaz, A.; Williamson, D.A.; Anderson, M.J.; Gates, M.C.; French, N.P. Transmission dynamics of an antimicrobial resistant Campylobacter jejuni lineage in New Zealand’s commercial poultry network. Epidemics 2021, 37, 100521. [Google Scholar] [CrossRef] [PubMed]
- Widders, P.; Thomas, L.; Long, K.; Tokhi, M.; Panaccio, M.; Apos, E. The specificity of antibody in chickens immunised to reduce intestinal colonisation with Campylobacter jejuni. Vet. Microbiol. 1998, 64, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.E.; Rollins, D.M.; Mallinson, E.T.; Carr, L.; Joseph, S.W. Campylobacter jejuni in broiler chickens: Colonization and humoral immunity following oral vaccination and experimental infection. Vaccine 1997, 15, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Guyard-Nicodeme, M.; Vigouroux, E.; Poezevara, T.; Beven, V.; Quesne, S.; Bigault, L.; Amelot, M.; Dory, D.; Chemaly, M. Promising new vaccine candidates against Campylobacter in broilers. PLoS ONE 2017, 12, e0188472. [Google Scholar] [CrossRef] [PubMed]
- Cawthraw, S.; Newell, D. Investigation of the presence and protective effects of maternal antibodies against Campylobacter jejuni in chickens. Avian Dis. 2010, 54, 86–93. [Google Scholar] [CrossRef]
- Kashoma, I.P.; Srivastava, V.; Rajashekara, G. Advances in vaccines for controlling Campylobacter in poultry. In Food Safety in Poultry Meat Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 191–210. [Google Scholar]
- Ziprin, R.L.; Young, C.R.; Byrd, J.A.; Stanker, L.H.; Hume, M.E.; Gray, S.A.; Kim, B.J.; Konkel, M.E. Role of Campylobacter jejuni potential virulence genes in cecal colonization. Avian Dis. 2001, 45, 549–557. [Google Scholar] [CrossRef]
- Jeon, B.; Saisom, T.; Sasipreeyajan, J.; Luangtongkum, T. Live-Attenuated Oral Vaccines to Reduce Campylobacter Colonization in Poultry. Vaccines 2022, 10, 685. [Google Scholar] [CrossRef]
- Nothaft, H.; Davis, B.; Lock, Y.Y.; Perez-Munoz, M.E.; Vinogradov, E.; Walter, J.; Coros, C.; Szymanski, C.M. Engineering the Campylobacter jejuni N-glycan to create an effective chicken vaccine. Sci. Rep. 2016, 6, 26511. [Google Scholar] [CrossRef]
- Neal-McKinney, J.M.; Samuelson, D.R.; Eucker, T.P.; Nissen, M.S.; Crespo, R.; Konkel, M.E. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS ONE 2014, 9, e114254. [Google Scholar] [CrossRef]
- Hodgins, D.C.; Barjesteh, N.; St Paul, M.; Ma, Z.; Monteiro, M.A.; Sharif, S. Evaluation of a polysaccharide conjugate vaccine to reduce colonization by Campylobacter jejuni in broiler chickens. BMC Res. Notes 2015, 8, 204. [Google Scholar] [CrossRef]
- Annamalai, T.; Pina-Mimbela, R.; Kumar, A.; Binjawadagi, B.; Liu, Z.; Renukaradhya, G.; Rajashekara, G. Evaluation of nanoparticle-encapsulated outer membrane proteins for the control of Campylobacter jejuni colonization in chickens. Poult. Sci. 2013, 92, 2201–2211. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, F.; Guo, J.; Cao, X.; Wang, H.; Yang, B.; Zhou, H.; Su, X.; Zeng, X.; Lin, J.; et al. Immunization of Chickens with the Enterobactin Conjugate Vaccine Reduced Campylobacter jejuni Colonization in the Intestine. Vaccines 2020, 8, 747. [Google Scholar] [CrossRef]
- Nothaft, H.; Perez-Muñoz, M.; Gouveia, G.; Duar, R.; Wanford, J.; Lango-Scholey, L.; Panagos, C.; Srithayakumar, V.; Plastow, G.; Coros, C.J.A.; et al. Coadministration of the Campylobacter jejuni N-glycan-based vaccine with probiotics improves vaccine performance in broiler chickens. Appl. Environ. Microbiol. 2017, 83, e01523-17. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.M.; Wang, J.; Hudson, D.L.; Grant, A.J.; Jones, M.A.; Maskell, D.J.; Stevens, M.P. Evaluation of live-attenuated Salmonella vaccines expressing Campylobacter antigens for control of C. jejuni in poultry. Vaccine 2010, 28, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Wyszyńska, A.; Raczko, A.; Lis, M.; Jagusztyn-Krynicka, E.K. Oral immunization of chickens with avirulent Salmonella vaccine strain carrying C. jejuni 72Dz/92 cjaA gene elicits specific humoral immune response associated with protection against challenge with wild-type Campylobacter. Vaccine 2004, 22, 1379–1389. [Google Scholar] [CrossRef]
- Huang, J.-l.; Yin, Y.-X.; Pan, Z.-m.; Zhang, G.; Zhu, A.-p.; Liu, X.-f.; Jiao, X.-a. Intranasal immunization with chitosan/pCAGGS-flaA nanoparticles inhibits Campylobacter jejuni in a White Leghorn model. J. Biomed. Biotechnol. 2010, 2010, 589476. [Google Scholar] [CrossRef] [PubMed]
- Kobierecka, P.A.; Wyszyńska, A.K.; Gubernator, J.; Kuczkowski, M.; Wiśniewski, O.; Maruszewska, M.; Wojtania, A.; Derlatka, K.E.; Adamska, I.; Godlewska, R. Chicken anti-Campylobacter vaccine–comparison of various carriers and routes of immunization. Front. Microbiol. 2016, 7, 740. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Alkie, T.N.; Quinteiro-Filho, W.; Yitbarek, A.; Astill, J.; Sharif, S. Oral administration of PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate reduces cecal colonization by Campylobacter jejuni in chickens. Vaccine 2018, 36, 388–394. [Google Scholar] [CrossRef]
- Noor, S.M.; Husband, A.; Widders, P. In ovo oral vaccination with cmpylobacter jejuni establishes early development of intestinal immunity in chickens. Br. Poult. Sci. 1995, 36, 563–573. [Google Scholar] [CrossRef]
- Glünder, G.; Spiering, N.; Hinz, K. Investigations on parenteral immunization of chickens with a Campylobacter mineral oil vaccine. In Proceedings of the International Congress of the World Veterinary Poultry Association; Nagy, B., Mulder, R., Eds.; European Commission: Budapest, Hungary, 1997; pp. 247–253. [Google Scholar]
- Guerry, P. Campylobacter flagella: Not just for motility. Trends Microbiol. 2007, 15, 456–461. [Google Scholar] [CrossRef]
- Radomska, K.A.; Vaezirad, M.M.; Verstappen, K.M.; Wösten, M.M.; Wagenaar, J.A.; van Putten, J.P. Chicken immune response after in ovo immunization with chimeric TLR5 activating flagellin of Campylobacter jejuni. PLoS ONE 2016, 11, e0164837. [Google Scholar] [CrossRef] [PubMed]
- Layton, S.; Morgan, M.; Cole, K.; Kwon, Y.; Donoghue, D.; Hargis, B.; Pumford, N. Evaluation of Salmonella-vectored Campylobacter peptide epitopes for reduction of Campylobacter jejuni in broiler chickens. Clin. Vaccine Immunol. 2011, 18, 449–454. [Google Scholar] [CrossRef] [PubMed]
- de Zoete, M.R.; van Putten, J.P.; Wagenaar, J.A. Vaccination of chickens against Campylobacter. Vaccine 2007, 25, 5548–5557. [Google Scholar] [CrossRef] [PubMed]
- Hanuszkiewicz, A.; Pittock, P.; Humphries, F.; Moll, H.; Rosales, A.R.; Molinaro, A.; Moynagh, P.N.; Lajoie, G.A.; Valvano, M.A. Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses. J. Biol. Chem. 2014, 289, 19231–19244. [Google Scholar] [CrossRef]
- Monteiro, M.A.; Baqar, S.; Hall, E.R.; Chen, Y.-H.; Porter, C.K.; Bentzel, D.E.; Applebee, L.; Guerry, P. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect. Immun. 2009, 77, 1128–1136. [Google Scholar] [CrossRef]
- Würfel, S.; Da Silva, W.; de Oliveira, M.; Kleinubing, N.; Lopes, G.; Gandra, E.; Dellagostin, O. Genetic diversity of Campylobacter jejuni and Campylobacter coli isolated from poultry meat products sold on the retail market in Southern Brazil. Poult. Sci. 2019, 98, 932–939. [Google Scholar] [CrossRef]
- Vohra, P.; Chintoan-Uta, C.; Terra, V.S.; Bremner, A.; Cuccui, J.; Wren, B.W.; Vervelde, L.; Stevens, M.P. Evaluation of glycosylated FlpA and SodB as subunit vaccines against Campylobacter jejuni colonisation in chickens. Vaccines 2020, 8, 520. [Google Scholar] [CrossRef]
- Nascimento, I.; Leite, L. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef]
- Theoret, J.R.; Cooper, K.K.; Zekarias, B.; Roland, K.L.; Law, B.F.; Curtiss III, R.; Joens, L.A. The Campylobacter jejuni Dps homologue is important for in vitro biofilm formation and cecal colonization of poultry and may serve as a protective antigen for vaccination. Clin. Vaccine Immunol. 2012, 19, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Parreira, V.R.; Roland, K.L.; Curtiss, R.; Prescott, J.F. Assessment of attenuated Salmonella vaccine strains in controlling experimental Salmonella Typhimurium infection in chickens. Can. J. Vet. Res. 2014, 78, 23–30. [Google Scholar]
- Taha-Abdelaziz, K.; Yitbarek, A.; Alkie, T.N.; Hodgins, D.C.; Read, L.R.; Weese, J.S.; Sharif, S. PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate modulate cecal microbiota composition in broiler chickens experimentally challenged with C. jejuni. Sci. Rep. 2018, 8, 12076. [Google Scholar] [CrossRef]
- Pumtang-On, P.; Mahony, T.J.; Hill, R.A.; Vanniasinkam, T. A systematic review of Campylobacter jejuni vaccine candidates for chickens. Microorganisms 2021, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Rappuoli, R. Reverse vaccinology: Developing vaccines in the era of genomics. Immunity 2010, 33, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Naik, S.; Vakil, B. Probiotics, prebiotics and synbiotics-a review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.-Y.; Kim, W.K. Roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 2018, 5, 245. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Jang, M.J.; Kim, S.Y.; Yang, Y.; Pavlidis, H.O.; Ricke, S.C. Potential for prebiotics as feed additives to limit foodborne Campylobacter establishment in the poultry gastrointestinal tract. Front. Microbiol. 2019, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Duysburgh, C.; Rakebrandt, M.; Marzorati, M. Dried yeast cell walls high in beta-glucan and mannan-oligosaccharides positively affect microbial composition and activity in the canine gastrointestinal tract in vitro. J. Anim. Sci. 2020, 98, skaa173. [Google Scholar] [CrossRef]
- Froebel, L.; Jalukar, S.; Lavergne, T.; Lee, J.; Duong, T. Administration of dietary prebiotics improves growth performance and reduces pathogen colonization in broiler chickens. Poult. Sci. 2019, 98, 6668–6676. [Google Scholar] [CrossRef]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Hargis, B.M.; Tellez, G. Use of prebiotics as an alternative to antibiotic growth promoters in the poultry industry. In Prebiotics and Probiotics-Potential Benefits in Nutrition and Health; IntechOpen: London, UK, 2019. [Google Scholar]
- Yusrizal, Y.; Chen, T. Effect of adding chicory fructans in feed on fecal and intestinal microflora and excreta volatile ammonia. Int. J. Poult. Sci. 2003, 2, 188–194. [Google Scholar]
- Park, S.H.; Lee, S.I.; Kim, S.A.; Christensen, K.; Ricke, S.C. Comparison of antibiotic supplementation versus a yeast-based prebiotic on the cecal microbiome of commercial broilers. PLoS ONE 2017, 12, e0182805. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Sharif, S.; Taha-Abdelaziz, K. Probiotics as Alternatives to Antibiotics for the Prevention and Control of Necrotic Enteritis in Chickens. Pathogens 2022, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Shojadoost, B.; Boodhoo, N.; Astill, J.; Taha-Abdelaziz, K.; Hodgins, D.C.; Kulkarni, R.R.; Sharif, S. Necrotic enteritis in chickens: A review of pathogenesis, immune responses and prevention, focusing on probiotics and vaccination. Anim. Health Res. Rev. 2021, 22, 147–162. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Taha-Abdelaziz, K.; Shojadoost, B.; Astill, J.; Sharif, S. Gastrointestinal diseases of poultry: Causes and nutritional strategies for prevention and control. In Improving Gut Health in Poultry; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 205–236. [Google Scholar]
- Alizadeh, M.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Karimi, S.H.; Bavananthasivam, J.; Kulkarni, R.R.; Sharif, S. Effects of in ovo inoculation of multi-Strain Lactobacilli on cytokine gene expression and antibody-mediated Immune responses in chickens. Front. Vet. Sci. 2020, 7, 105. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Lammers, A.; Alkie, T.N.; Sharif, S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: A review. Vet. Immunol. Immunopathol. 2018, 201, 1–11. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bavananthasivam, J.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Alqazlan, N.; Boodhoo, N.; Shoja Doost, J.; Sharif, S. In ovo and oral administration of probiotic lactobacilli modulate cell-and antibody-mediated immune responses in newly hatched chicks. Front. Immunol. 2021, 12, 664387. [Google Scholar] [CrossRef]
- Kathayat, D.; Closs, G., Jr.; Helmy, Y.A.; Deblais, L.; Srivastava, V.; Rajashekara, G. In Vitro and In Vivo Evaluation of Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 Against Avian Pathogenic Escherichia coli and Identification of Novel Probiotic-Derived Bioactive Peptides. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef] [PubMed]
- Kathayat, D.; Closs, G., Jr.; Helmy, Y.A.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Peptides Affecting the Outer Membrane Lipid Asymmetry System (MlaA-OmpC/F) Reduce Avian Pathogenic Escherichia coli (APEC) Colonization in Chickens. Appl. Env. Microbiol. 2021, 87, e0056721. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Astill, J.; Kulkarni, R.R.; Read, L.R.; Najarian, A.; Farber, J.M.; Sharif, S. In vitro assessment of immunomodulatory and anti-Campylobacter activities of probiotic lactobacilli. Sci. Rep. 2019, 9, 17903. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kassem, I.I.; Rajashekara, G. Immuno-modulatory effect of probiotic E. coli Nissle 1917 in polarized human colonic cells against Campylobacter jejuni infection. Gut Microbes 2021, 13, 1857514. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kassem, I.; Kumar, A.; Rajashekara, G. In Vitro Evaluation of the Impact of the Probiotic E. coli Nissle 1917 on Campylobacter jejuni’s Invasion and Intracellular Survival in Human Colonic Cells. Front. Microbiol. 2017, 8, 1588. [Google Scholar] [CrossRef] [PubMed]
- Mawad, A.; Helmy, Y.A.; Shalkami, A.G.; Kathayat, D.; Rajashekara, G.E. coli Nissle microencapsulation in alginate-chitosan nanoparticles and its effect on Campylobacter jejuni in vitro. Appl Microbiol. Biotechnol. 2018, 102, 10675–10690. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Guyard-Nicodème, M.; Messaoudi, S.; Chemaly, M.; Cappelier, J.-M.; Dousset, X.; Haddad, N.J.F.i.m. Recent advances in screening of anti-Campylobacter activity in probiotics for use in poultry. Front. Microbiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Closs, J.G.; Jung, k.; Kathayat, D.; Vlasova, A.; Rajashekara, G. Effect of probiotic E. coli Nissle 1917 supplementation on the growth performance, immune responses, intestinal morphology, gut microbes, of Campylobacter jejuni infected chickens. Infect. Immun. 2022, 90, e0033722. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Haddad, N.; Taminiau, B.; Poezevara, T.; Quesne, S.; Amelot, M.; Daube, G.; Chemaly, M.; Dousset, X.; Guyard-Nicodème, M. Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. Int. J. Food Microbiol. 2017, 247, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kobierecka, P.A.; Wyszyńska, A.K.; Aleksandrzak-Piekarczyk, T.; Kuczkowski, M.; Tuzimek, A.; Piotrowska, W.; Górecki, A.; Adamska, I.; Wieliczko, A.; Bardowski, J. In vitro characteristics of Lactobacillus spp. strains isolated from the chicken digestive tract and their role in the inhibition of Campylobacter colonization. MicrobiologyOpen 2017, 6, e00512. [Google Scholar] [CrossRef]
- Nishiyama, K.; Nakazato, A.; Ueno, S.; Seto, Y.; Kakuda, T.; Takai, S.; Yamamoto, Y.; Mukai, T. Cell surface-associated aggregation-promoting factor from L actobacillus gasseri SBT 2055 facilitates host colonization and competitive exclusion of C ampylobacter jejuni. Mol. Microbiol. 2015, 98, 712–726. [Google Scholar] [CrossRef]
- Nishiyama, K.; Seto, Y.; Yoshioka, K.; Kakuda, T.; Takai, S.; Yamamoto, Y.; Mukai, T. Lactobacillus gasseri SBT2055 reduces infection by and colonization of Campylobacter jejuni. PLoS ONE 2014, 9, e108827. [Google Scholar] [CrossRef]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]
- Guyard-Nicodeme, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sánchez, J.; Vesseur, P.; Martín, Á.; Medel, P. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef]
- Gracia, M.; Millán, C.; Sanchez, J.; Guyard-Nicodeme, M.; Mayot, J.; Carre, Y.; Csorbai, A.; Chemaly, M.; Medel, P. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: Part B. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, V.F.; Donoghue, A.M.; Arsi, K.; Reyes-Herrera, I.; Metcalf, J.H.; de los Santos, F.S.; Blore, P.J.; Donoghue, D.J. Targeting motility properties of bacteria in the development of probiotic cultures against Campylobacter jejuni in broiler chickens. Foodborne Pathog. Dis. 2013, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Fritts, C.; Kersey, J.; Motl, M.; Kroger, E.; Yan, F.; Si, J.; Jiang, Q.; Campos, M.; Waldroup, A.; Waldroup, P. Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens. J. Appl. Poult. Res. 2000, 9, 149–155. [Google Scholar] [CrossRef]
- Robyn, J.; Rasschaert, G.; Hermans, D.; Pasmans, F.; Heyndrickx, M. In vivo broiler experiments to assess anti-Campylobacter jejuni activity of a live Enterococcus faecalis strain. Poult. Sci. 2013, 92, 265–271. [Google Scholar] [CrossRef]
- Netherwood, T.; Gilbert, H.; Parker, D.; O’donnell, A. Probiotics shown to change bacterial community structure in the avian gastrointestinal tract. Appl. Environ. Microbiol. 1999, 65, 5134–5138. [Google Scholar] [CrossRef] [PubMed]
- Baffoni, L.; Gaggìa, F.; Di Gioia, D.; Santini, C.; Mogna, L.; Biavati, B. A Bifidobacterium-based synbiotic product to reduce the transmission of C. jejuni along the poultry food chain. Int. J. Food Microbiol. 2012, 157, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Baffoni, L.; Gaggia, F.; Granata, M.; Gasbarri, R.; Di Gioia, D.; Biavati, B. Characterization of probiotic strains: An application as feed additives in poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010, 141, S98–S108. [Google Scholar] [CrossRef]
- Morishita, T.Y.; Aye, P.P.; Harr, B.S.; Cobb, C.W.; Clifford, J.R. Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis. 1997, 41, 850–855. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.; Woo-Ming, A.; Blore, P.; Donoghue, D. The efficacy of selected probiotic and prebiotic combinations in reducing Campylobacter colonization in broiler chickens. J. Appl. Poult. Res. 2015, 24, 327–334. [Google Scholar] [CrossRef]
- Willis, W.L.; Reid, L. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult. Sci. 2008, 87, 606–611. [Google Scholar] [CrossRef]
- Aho, M.; Nuotio, L.; Nurmi, E.; Kiiskinen, T. Competitive exclusion of campylobacters from poultry with K-bacteria and Broilact®. Int. J. Food Microbiol. 1992, 15, 265–275. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.; Mohnl, M.; Porta, R.; Biarnes, M.; Böhm, J.; Schatzmayr, G. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 2012, 91, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Cean, A.; Stef, L.; Simiz, E.; Julean, C.; Dumitrescu, G.; Vasile, A.; Pet, E.; Drinceanu, D.; Corcionivoschi, N. Effect of human isolated probiotic bacteria on preventing Campylobacter jejuni colonization of poultry. Foodborne Pathog. Dis. 2015, 12, 122–130. [Google Scholar] [CrossRef]
- Smialek, M.; Burchardt, S.; Koncicki, A. The influence of probiotic supplementation in broiler chickens on population and carcass contamination with Campylobacter spp.-Field study. Res. Vet. Sci. 2018, 118, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Schoeni, J.L.; Wong, A. Inhibition of Campylobacter jejuni colonization in chicks by defined competitive exclusion bacteria. Appl. Environ. Microbiol. 1994, 60, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [PubMed]
- Zhang, T.; Zhang, Y.; Li, L.; Jiang, X.; Chen, Z.; Zhao, F.; Yi, Y. Biosynthesis and Production of Class II Bacteriocins of Food-Associated Lactic Acid Bacteria. Fermentation 2022, 8, 217. [Google Scholar] [CrossRef]
- Messaoudi, S.; Kergourlay, G.; Dalgalarrondo, M.; Choiset, Y.; Ferchichi, M.; Prévost, H.; Pilet, M.-F.; Chobert, J.-M.; Manai, M.; Dousset, X. Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51. Food Microbiol. 2012, 32, 129–134. [Google Scholar] [CrossRef]
- Stern, N.; Svetoch, E.; Eruslanov, B.; Perelygin, V.; Mitsevich, E.; Mitsevich, I.; Pokhilenko, V.; Levchuk, V.; Svetoch, O.; Seal, B. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. 2006, 50, 3111–3116. [Google Scholar] [CrossRef]
- Cole, K.; Farnell, M.; Donoghue, A.; Stern, N.; Svetoch, E.; Eruslanov, B.; Volodina, L.; Kovalev, Y.; Perelygin, V.; Mitsevich, E. Bacteriocins reduce Campylobacter colonization and alter gut morphology in turkey poults. Poult. Sci. 2006, 85, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.J.; Svetoch, E.A.; Eruslanov, B.V.; Kovalev, Y.N.; Volodina, L.I.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Levchuk, V.P. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J. Food Prot. 2005, 68, 1450–1453. [Google Scholar] [CrossRef]
- Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Borzenkov, V.N.; Levchuk, V.P.; Svetoch, O.E.; Kovalev, Y.N.; Stepanshin, Y.G. Diverse antimicrobial killing by Enterococcus faecium E 50-52 bacteriocin. J. Agric. Food Chem. 2008, 56, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Line, J.; Svetoch, E.; Eruslanov, B.; Perelygin, V.; Mitsevich, E.; Mitsevich, I.; Levchuk, V.; Svetoch, O.; Seal, B.; Siragusa, G. Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 2008, 52, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.J.; Eruslanov, B.V.; Pokhilenko, V.D.; Kovalev, Y.N.; Volodina, L.L.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Borzenkov, V.N.; Levchuk, V.P.J.M.E.i.H.; et al. Bacteriocins reduce Campylobacter jejuni colonization while bacteria producing bacteriocins are ineffective. Microb. Ecol. Health Dis. 2008, 20, 74–79. [Google Scholar]
- Hoang, K.; Stern, N.J.; Lin, J. Development and stability of bacteriocin resistance in Campylobacter spp. J. Appl. Microbiol. 2011, 111, 1544–1550. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics and synbiotics: Concepts and nutritional properties. Br. J. Nutr. 1998, 80, S197–S202. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggìa, F.; Garofolo, G.; Di Serafino, G.; Buglione, E.; Di Giannatale, E.; Di Gioia, D. Evidence of Campylobacter jejuni reduction in broilers with early synbiotic administration. Int. J. Food Microbiol. 2017, 251, 41–47. [Google Scholar] [CrossRef]
- Micciche, A.; Rothrock Jr, M.J.; Yang, Y.; Ricke, S.C. Essential oils as an intervention strategy to reduce Campylobacter in poultry production: A review. Front. Microbiol. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Irshad, M.; Subhani, M.A.; Ali, S.; Hussain, A. Biological importance of essential oils. Essent. Oils-Oils Nat. 2020, 22, 70. [Google Scholar]
- de Los Santos, F.S.; Donoghue, A.; Venkitanarayanan, K.; Dirain, M.; Reyes-Herrera, I.; Blore, P.; Donoghue, D. Caprylic acid supplemented in feed reduces enteric Campylobacter jejuni colonization in ten-day-old broiler chickens. Poult. Sci. 2008, 87, 800–804. [Google Scholar] [CrossRef]
- De Los Santos, F.S.; Donoghue, A.; Venkitanarayanan, K.; Metcalf, J.; Reyes-Herrera, I.; Dirain, M.; Aguiar, V.; Blore, P.; Donoghue, D. The natural feed additive caprylic acid decreases Campylobacter jejuni colonization in market-aged broiler chickens. Poult. Sci. 2009, 88, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Arsi, K.; Donoghue, A.; Venkitanarayanan, K.; Kollanoor-Johny, A.; Fanatico, A.; Blore, P.; Donoghue, D. The efficacy of the natural plant extracts, thymol and carvacrol against Campylobacter colonization in broiler chickens. J. Food Saf. 2014, 34, 321–325. [Google Scholar] [CrossRef]
- Szott, V.; Reichelt, B.; Alter, T.; Friese, A.; Roesler, U. In vivo efficacy of carvacrol on Campylobacter jejuni prevalence in broiler chickens during an entire fattening period. Eur. J. Microbiol. Immunol. 2020, 10, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; Martel, A.; Van Deun, K.; Van Immerseel, F.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. The cinnamon-oil ingredient trans-cinnamaldehyde fails to target Campylobacter jejuni strain KC 40 in the broiler chicken cecum despite marked in vitro activity. J. Food Prot. 2011, 74, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Johny, A.K.; Darre, M.; Donoghue, A.; Donoghue, D.; Venkitanarayanan, K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010, 19, 237–244. [Google Scholar] [CrossRef]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Broom, L. Organic acids for improving intestinal health of poultry. World’s Poult. Sci. J. 2015, 71, 630–642. [Google Scholar] [CrossRef]
- Khan, R.U.; Chand, N.; Akbar, A. Effect of organic acids on the performance of Japanese quails. Pak. J. Zool. 2016, 48, 1799–1803. [Google Scholar]
- Giannenas, I.; Papaneophytou, C.; Tsalie, E.; Pappas, I.; Triantafillou, E.; Tontis, D.; Kontopidis, G. Dietary supplementation of benzoic acid and essential oil compounds affects buffering capacity of the feeds, performance of turkey poults and their antioxidant status, pH in the digestive tract, intestinal microbiota and morphology. Asian-Australas. J. Anim. Sci. 2014, 27, 225–236. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.C.; Byrd, J.A.; Caldwell, D.; Andrews, K.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Inhibition and interactions of Campylobacter jejuni from broiler chicken houses with organic acids. Microorganisms 2019, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Peh, E.; Kittler, S.; Reich, F.; Kehrenberg, C. Antimicrobial activity of organic acids against Campylobacter spp. and development of combinations—A synergistic effect? PLoS ONE 2020, 15, e0239312. [Google Scholar] [CrossRef]
- Hermans, D.; Martel, A.; Van Deun, K.; Verlinden, M.; Van Immerseel, F.; Garmyn, A.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult. Sci. 2010, 89, 1144–1155. [Google Scholar] [CrossRef]
- Skånseng, B.; Kaldhusdal, M.; Moen, B.; Gjevre, A.G.; Johannessen, G.; Sekelja, M.; Trosvik, P.; Rudi, K. Prevention of intestinal Campylobacter jejuni colonization in broilers by combinations of in-feed organic acids. J. Appl. Microbiol. 2010, 109, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Vitari, F.; Domeneghini, C.; Palmonari, A.; Tosi, G.; Fantinati, P.; Massi, P.; Piva, A. Development of a feed additive to reduce caecal C ampylobacter jejuni in broilers at slaughter age: From in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013, 114, 308–317. [Google Scholar] [CrossRef]
- Ramana, J.; Reddy, R.; Rao, D.; Shakila, S.; Suresh, J. Effect of organic acid supplementation on performance of poultry. J. Anim. Feed Sci. Technol 2017, 5, 15–23. [Google Scholar]
- Li, Q.; Kang, C. Mechanisms of action for small molecules revealed by structural biology in drug discovery. Int. J. Mol. Sci. 2020, 21, 5262. [Google Scholar] [CrossRef]
- Johnson, T.J.; Shank, J.M.; Johnson, J.G. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front. Microbiol. 2017, 8, 487. [Google Scholar] [CrossRef]
- Lewis, R.J.; Vetter, I.; Cardoso, F.C.; Inserra, M.; King, G. Does Nature Do Ion Channel Drug Discovery Better than Us; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Johnson, J.G.; Yuhas, C.; McQuade, T.J.; Larsen, M.J.; DiRita, V.J. Narrow-spectrum inhibitors of Campylobacter jejuni flagellar expression and growth. Antimicrob. Agents Chemother. 2015, 59, 3880–3886. [Google Scholar] [CrossRef] [PubMed]
- Deblais, L.; Helmy, Y.A.; Kumar, A.; Antwi, J.; Kathayat, D.; Acuna, U.M.; Huang, H.-c.; de Blanco, E.C.; Fuchs, J.R.; Rajashekara, G. Novel narrow spectrum benzyl thiophene sulfonamide derivatives to control Campylobacter. J. Antibiot. 2019, 72, 555–565. [Google Scholar] [CrossRef]
- Kumar, A.; Drozd, M.; Pina-Mimbela, R.; Xu, X.; Helmy, Y.A.; Antwi, J.; Fuchs, J.R.; Nislow, C.; Templeton, J.; Blackall, P.J. Novel anti-Campylobacter compounds identified using high throughput screening of a pre-selected enriched small molecules library. Front. Microbiol. 2016, 7, 405. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Možina, S.S.; Zhang, Q. Anti-Campylobacter activities and resistance mechanisms of natural phenolic compounds in Campylobacter. PLoS ONE 2012, 7, e51800. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, H.; Gao, Y.; An, N.; Li, X.; Pan, X.; Yang, X.; Tian, L.; Sun, J.; Xiong, X. Gut microbiota-derived short-chain fatty acids and hypertension: Mechanism and treatment. Biomed. Pharmacother. 2020, 130, 110503. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Lassen, B.; Helwigh, B.; Kahl Petersen, C.; Ellis-Iversen, J. Systematic review of products with potential application for use in the control of Campylobacter spp. in organic and free-range broilers. Acta Vet. Scand. 2022, 64, 24. [Google Scholar] [CrossRef]
- van der Wielen, P.W.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.; van Knapen, F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef]
- Kasman, L.M.; Porter, L.D. Bacteriophages. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Wagenaar, J.A.; Van Bergen, M.A.; Mueller, M.A.; Wassenaar, T.M.; Carlton, R.M. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 2005, 109, 275–283. [Google Scholar] [CrossRef]
- D’angelantonio, D.; Scattolini, S.; Boni, A.; Neri, D.; Di Serafino, G.; Connerton, P.; Connerton, I.; Pomilio, F.; Di Giannatale, E.; Migliorati, G. Bacteriophage therapy to reduce colonization of campylobacter jejuni in broiler chickens before slaughter. Viruses 2021, 13, 1428. [Google Scholar] [CrossRef]
- Fischer, S.; Kittler, S.; Klein, G.; Glünder, G. Impact of a single phage and a phage cocktail application in broilers on reduction of Campylobacter jejuni and development of resistance. PLoS ONE 2013, 8, e78543. [Google Scholar] [CrossRef]
- Kittler, S.; Fischer, S.; Abdulmawjood, A.; Glünder, G.; Klein, G. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl. Environ. Microbiol. 2013, 79, 7525–7533. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Wang, Y.; Song, Z.; Ying, H.; Kong, L.; Jiao, X.A.; Huang, J. Campylobacter jejuni Developed the Resistance to Bacteriophage CP39 by Phase Variable Expression of 06875 Encoding the CGPTase. Viruses 2022, 14, 485. [Google Scholar] [CrossRef]
- Loc Carrillo, C.; Atterbury, R.J.; El-Shibiny, A.; Connerton, P.L.; Dillon, E.; Scott, A.; Connerton, I.F. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 2005, 71, 6554–6563. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Hammerl, J.A.; Jäckel, C.; Alter, T.; Janzcyk, P.; Stingl, K.; Knüver, M.T.; Hertwig, S. Reduction of Campylobacter jejuni in broiler chicken by successive application of group II and group III phages. PLoS ONE 2014, 9, e114785. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Rahman, S.R. Use of Phages to Treat Antimicrobial-Resistant Salmonella Infections in Poultry. Vet. Sci. 2022, 9, 438. [Google Scholar] [CrossRef]
- Han, Z.; Willer, T.; Li, L.; Pielsticker, C.; Rychlik, I.; Velge, P.; Kaspers, B.; Rautenschlein, S. Influence of the gut microbiota composition on Campylobacter jejuni colonization in chickens. Infect. Immun. 2017, 85, e00380-17. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio 2014, 5, e01364-14. [Google Scholar] [CrossRef]
- Yitbarek, A.; Taha-Abdelaziz, K.; Hodgins, D.C.; Read, L.; Nagy, É.; Weese, J.S.; Caswell, J.L.; Parkinson, J.; Sharif, S. Gut microbiota-mediated protection against influenza virus subtype H9N2 in chickens is associated with modulation of the innate responses. Sci. Rep. 2018, 8, 13189. [Google Scholar]
- Bavananthasivam, J.; Astill, J.; Matsuyama-Kato, A.; Taha-Abdelaziz, K.; Shojadoost, B.; Sharif, S. Gut microbiota is associated with protection against Marek’s disease virus infection in chickens. Virology 2021, 553, 122–130. [Google Scholar] [CrossRef]
- Gilroy, R.; Chaloner, G.; Wedley, A.; Lacharme-Lora, L.; Jopson, S.; Wigley, P. Campylobacter jejuni transmission and colonisation in broiler chickens is inhibited by faecal microbiota transplantation. BioRxiv 2018, 476119. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Siegerstetter, S.-C.; Magowan, E.; Lawlor, P.G.; O′ Connell, N.E.; Zebeli, Q. Fecal microbiota transplant from highly feed efficient donors affects cecal physiology and microbiota in low-and high-feed efficient chickens. Front. Microbiol. 2019, 10, 1576. [Google Scholar] [CrossRef]
- Ty, M.; Taha-Abdelaziz, K.; Demey, V.; Castex, M.; Sharif, S.; Parkinson, J. Performance of distinct microbial based solutions in a Campylobacter infection challenge model in poultry. Anim. Microbiome 2022, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020, 18, e06090. [Google Scholar] [PubMed]
- Seliwiorstow, T.; Baré, J.; Van Damme, I.; Uyttendaele, M.; De Zutter, L. Campylobacter carcass contamination throughout the slaughter process of Campylobacter-positive broiler batches. Int. J. Food Microbiol. 2015, 194, 25–31. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Zhao, J.; Liu, N.; Li, Y.; Wang, L.; Gao, Y.; Wang, J.; Qu, Z.; Liu, J. Risk Prevention and Control Points Through Quantitative Evaluation of Campylobacter in a Large Broiler Slaughterhouse. Front. Vet. Sci. 2020, 7, 172. [Google Scholar] [CrossRef]
- Lázaro, B.; Cárcamo, J.; Audícana, A.; Perales, I.; Fernández-Astorga, A. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl. Environ. Microbiol. 1999, 65, 4677–4681. [Google Scholar] [CrossRef]
- Habib, I.; Mohamed, M.-Y.I.; Lakshmi, G.B.; Khan, M.; Li, D. Quantification of Campylobacter contamination on chicken carcasses sold in retail markets in the United Arab Emirates. Int. J. Food Contam. 2022, 9, 9. [Google Scholar] [CrossRef]
- Abu-Madi, M.; Behnke, J.M.; Sharma, A.; Bearden, R.; Al-Banna, N. Prevalence of virulence/stress genes in Campylobacter jejuni from chicken meat sold in Qatari retail outlets. PLoS ONE 2016, 11, e0156938. [Google Scholar] [CrossRef]
- Alarjani, K.M.; Elkhadragy, M.F.; Al-Masoud, A.H.; Yehia, H.M. Detection of Campylobacter jejuni and Salmonella typhimurium in chicken using PCR for virulence factor hipO and invA genes (Saudi Arabia). Biosci. Rep. 2021, 41, BSR20211790. [Google Scholar] [CrossRef]
- Royden, A.; Christley, R.; Jones, T.; Williams, A.; Awad, F.; Haldenby, S.; Wigley, P.; Rushton, S.P.; Williams, N.J. Campylobacter contamination at retail of Halal Chicken produced in the United Kingdom. J. Food Prot. 2021, 84, 1433–1445. [Google Scholar] [CrossRef]
- Deckert, A.; Valdivieso-Garcia, A.; Reid-Smith, R.; Tamblyn, S.; Seliske, P.; Irwin, R.; Dewey, C.; Boerlin, P.; McEwen, S.A. Prevalence and antimicrobial resistance in Campylobacter spp. isolated from retail chicken in two health units in Ontario. J. Food Prot. 2010, 73, 1317–1324. [Google Scholar] [CrossRef]
- The European Food Safety Authority. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Peyrat, M.; Soumet, C.; Maris, P.; Sanders, P. Recovery of Campylobacter jejuni from surfaces of poultry slaughterhouses after cleaning and disinfection procedures: Analysis of a potential source of carcass contamination. Int. J. Food Microbiol. 2008, 124, 188–194. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Bai, Y.; Ding, X.; Zhao, Q.; Sun, H.; Li, T.; Li, Z.; Wang, H.; Zhang, L.; Zhang, C.; Xu, S. Development of an organic acid compound disinfectant to control food-borne pathogens and its application in chicken slaughterhouses. Poult. Sci. 2022, 101, 101842. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Arshad, M.S.; Rahman, U.U. Postharvest intervention technologies for safety enhancement of meat and meat based products; a critical review. J. Food Sci. Technol. 2016, 53, 19–30. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hossain, M.M.; Rahman, S.M.E.; Hashem, M.A.; Oh, D.-H. Effect of repeated freeze-thaw cycles on beef quality and safety. Korean J. Food Sci. Anim. Resour. 2014, 34, 482. [Google Scholar] [CrossRef]
- Georgsson, F.; Þorkelsson, Á.E.; Geirsdóttir, M.; Reiersen, J.; Stern, N.J. The influence of freezing and duration of storage on Campylobacter and indicator bacteria in broiler carcasses. Food Microbiol. 2006, 23, 677–683. [Google Scholar] [CrossRef]
- Bhaduri, S.; Cottrell, B. Survival of cold-stressed Campylobacter jejuni on ground chicken and chicken skin during frozen storage. Appl. Environ. Microbiol. 2004, 70, 7103–7109. [Google Scholar] [CrossRef]
- Park, S.F. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 2002, 74, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sampers, I.; Habib, I.; De Zutter, L.; Dumoulin, A.; Uyttendaele, M. Survival of Campylobacter spp. in poultry meat preparations subjected to freezing, refrigeration, minor salt concentration, and heat treatment. Int. J. Food Microbiol. 2010, 137, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Albert, T.; Braun, P.G.; Saffaf, J.; Wiacek, C. Physical methods for the decontamination of meat surfaces. Curr. Clin. Microbiol. Rep. 2021, 8, 9–20. [Google Scholar] [CrossRef]
- Maragkoudakis, P.A.; Mountzouris, K.C.; Psyrras, D.; Cremonese, S.; Fischer, J.; Cantor, M.D.; Tsakalidou, E. Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. Int. J. Food Microbiol. 2009, 130, 219–226. [Google Scholar] [CrossRef]
- Sakaridis, I.; Soultos, N.; Batzios, C.; Ambrosiadis, I.; Koidis, P. Lactic acid bacteria isolated from chicken carcasses with inhibitory activity against Salmonella spp. and Listeria monocytogenes. Czech J. Food Sci. 2014, 32, 61–68. [Google Scholar] [CrossRef]
- Woo-Ming, A.N. Reduction of Campylobacter jejuni on Chicken Wingettes by Treatment with Caprylic Acid, Chitosan or Protective Cultures of Lactobacillus spp.; University of Arkansas: Fayetteville, AR, USA, 2015. [Google Scholar]
- Melero, B.; Vinuesa, R.; Diez, A.; Jaime, I.; Rovira, J. Application of protective cultures against Listeria monocytogenes and Campylobacter jejuni in chicken products packaged under modified atmosphere. Poult. Sci. 2013, 92, 1108–1116. [Google Scholar] [CrossRef]
- Long, C.; Phillips, C.A. The effect of sodium citrate, sodium lactate and nisin on the survival of Arcobacter butzleri NCTC 12481 on chicken. Food Microbiol. 2003, 20, 495–502. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Connerton, P.L.; Dodd, C.E.; Rees, C.E.; Connerton, I.F. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 2003, 69, 6302–6306. [Google Scholar] [CrossRef]
- Zampara, A.; Sørensen, M.C.H.; Elsser-Gravesen, A.; Brøndsted, L. Significance of phage-host interactions for biocontrol of Campylobacter jejuni in food. Food Control 2017, 73, 1169–1175. [Google Scholar] [CrossRef]
- Zampara, A.; Sørensen, M.C.H.; Gencay, Y.E.; Grimon, D.; Kristiansen, S.H.; Jørgensen, L.S.; Kristensen, J.R.; Briers, Y.; Elsser-Gravesen, A.; Brøndsted, L. Developing innolysins against campylobacter jejuni using a novel prophage receptor-binding protein. Front. Microbiol. 2021, 12, 619028. [Google Scholar] [CrossRef]
- Djenane, D.; Yangueela, J.; Gomez, D.; Roncales, P. perspectives on the use of essential oils as antimicrobials against Campylobacter jejuni CECT 7572 in retail chicken meats packaged in microaerobic atmosphere. J. Food Saf. 2012, 32, 37–47. [Google Scholar] [CrossRef]
- Shrestha, S.; Wagle, B.; Upadhyay, A.; Arsi, K.; Donoghue, D.; Donoghue, A. Carvacrol antimicrobial wash treatments reduce Campylobacter jejuni and aerobic bacteria on broiler chicken skin. Poult. Sci. 2019, 98, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Shanker, S.; Lee, A.; Sorrell, T. Campylobacter jejuni in broilers: The role of vertical transmission. Epidemiol. Infect. 1986, 96, 153–159. [Google Scholar]
- Neill, S.; Campbell, J.; O’brien, J. Egg penetration by Campylobacter jejuni. Avian Pathol. 1985, 14, 313–320. [Google Scholar] [CrossRef]
- Sahin, O.; Kobalka, P.; Zhang, Q. Detection and survival of Campylobacter in chicken eggs. J. Appl. Microbiol. 2003, 95, 1070–1079. [Google Scholar] [CrossRef]
- Alkaya, G.B.; Erdogdu, F.; Halkman, A.K.; Ekiz, H.I. Surface decontamination of whole-shell eggs using far-infrared radiation. Food Bioprod. Process. 2016, 98, 275–282. [Google Scholar] [CrossRef]
- Turtoi, M.; Borda, D. Decontamination of egg shells using ultraviolet light treatment. World’s Poult. Sci. J. 2014, 70, 265–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).