Ultrasound Attenuation Improves Some Surface Properties of the Probiotic Strain Lacticaseibacillus casei ATCC 393
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Culture and Cell-Suspension Preparation
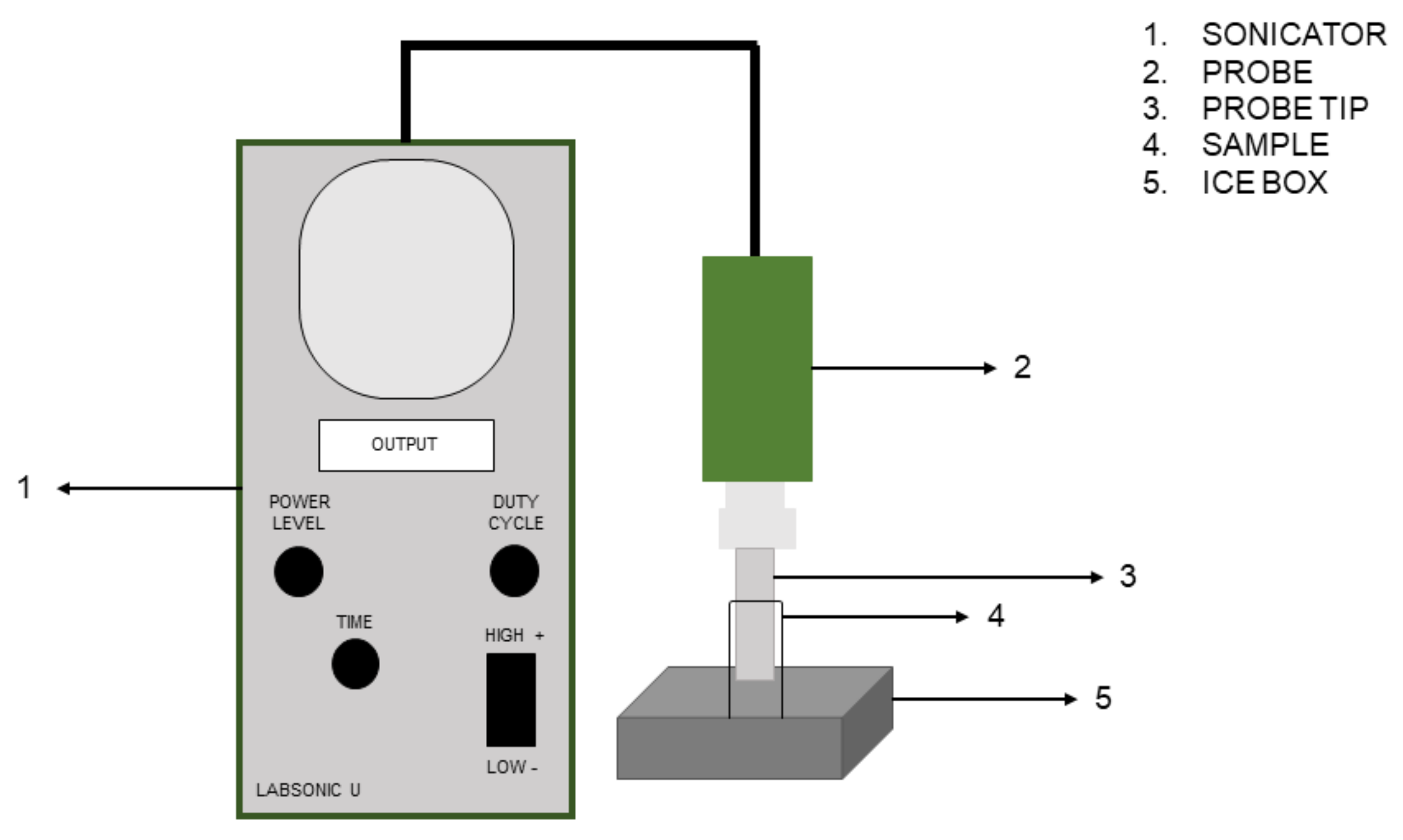
2.2. Experimental Design of Sonication Treatment
2.3. Acidifying Capabilities
2.4. Probiotic Cultivability
- − GI < 25% means complete inhibition;
- − 25% < GI < 75% means partial inhibition;
- − GI > 75% means no inhibition.
2.5. Characterization of Cell Surface Properties
2.5.1. Auto-Aggregation
2.5.2. Hydrophobicity
2.5.3. Biofilm Formation Assay
2.6. Membrane Permeability
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fermentative Metabolism Attenuation
3.2. Microbial Growth: Plate Count and Growth Index
3.3. Cell Surface Properties
3.4. Structural Alteration of Cell Membrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10 (Suppl. S1), S49–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Saxami, G.; Dimitrellou, D.; Santarmaki, V.; Galanis, A.; Kourkoutas, Y. Monitoring survival of Lactobacillus casei ATCC 393 in probiotic yogurts using an efficient molecular tool. J. Dairy Sci. 2013, 96, 3369–3377. [Google Scholar] [CrossRef] [Green Version]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem. 2014, 156, 264–270. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Petrović, T.; Dimitrijević-Branković, S.; Lević, S.; Nedović, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT-Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Sidira, M.; Galanis, A.; Ypsilantis, P.; Karapetsas, A.; Progaki, Z.; Simopoulos, C.; Kourkoutas, Y. Effect of probiotic-fermented milk administration ongastrointestinal survival of Lactobacillus casei ATCC 393 and modulation of intestinal microbial flora. Microb. Physiol. 2010, 19, 224–230. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, E.; Kim, S.H.; Whang, K.Y.; Oh, S.; Imm, J.Y. Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. Int. Dairy J. 2009, 19, 690–695. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Gamba, R.R.; Nagai, E.; Suzuki, T.; Koyanagi, T.; Enomoto, T. The biological activity of fermented milk produced by Lactobacillus casei ATCC 393 during cold storage. Int. Dairy J. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef] [PubMed]
- Tuorila, H.; Hartmann, C. Consumer responses to novel and unfamiliar foods. Curr. Opin. Food Sci. 2020, 33, 1–8. [Google Scholar] [CrossRef]
- Calasso, M.; Minervini, F.; De Filippis, F.; Ercolini, D.; De Angelis, M.; Gobbetti, M. Attenuated Lactococcus lactis and surface bacteria as tools for conditioning the microbiota and driving the ripening of semisoft Caciotta cheese. Appl. Environ. Microbiol. 2020, 86, e02165-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, A.; Casanova, F.P.; Petruzzi, L.; Sinigaglia, M.; Corbo, M.R. Using physical approaches for the attenuation of lactic acid bacteria in an organic rice beverage. Food Microbiol. 2016, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Racioppo, A.; Corbo, M.R.; Piccoli, C.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Ultrasound attenuation of lactobacilli and bifidobacteria: Effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017, 243, 78–83. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Speranza, B.; Gallo, M.; Corbo, M.R. A new frontier for starter cultures: Attenuation and modulation of metabolic and technological performance. In Starter Cultures in Food Production; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 148–161. [Google Scholar]
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; Freitas, M.K.; Cruz, A.G. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019, 57, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, M.; Pandur, Ž.; Perdih, T.S.; Stopar, D.; Petkovšek, M.; Dular, M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019, 57, 147–165. [Google Scholar] [CrossRef]
- Giordano, I.; Abuqwider, J.; Altamimi, M.; Di Monaco, R.; Puleo, S.; Mauriello, G. Application of ultrasound and microencapsulation on Limosilactobacillus reuteri DSM 17938 as a metabolic attenuation strategy for tomato juice probiotication. Heliyon 2022, 8, e10969. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Perricone, M.; Cannarsi, M.; Corbo, M.R.; Sinigaglia, M. Technological and spoiling characteristics of the yeast microflora isolated from Bella di Cerignola table olives. Int. J. Food Sci. Technol. 2009, 44, 2198–2207. [Google Scholar] [CrossRef]
- Fonseca, H.C.; de Sousa Melo, D.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Probiotic properties of lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Švabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, S.; Lou, X.; Cheng, S.; Liu, X.; Zheng, W.; Zheng, Z.; Wang, H. Biofilm formation and prevalence of adhesion genes among Staphylococcus aureus isolates from different food sources. MicrobiologyOpen 2020, 9, e00946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, A.; Racioppo, A.; Sinigaglia, M.; Speranza, B.; Campaniello, D.; Corbo, M.R. A low-power ultrasound attenuation improves the stability of biofilm and hydrophobicity of Propionibacterium freudenreichii subsp. freudenreichii DSM 20271 and Acidipropionibacterium jensenii DSM 20535. Food Microbiol. 2019, 78, 104–109. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Ultrasound-Attenuated Microorganisms Inoculated in Vegetable Beverages: Effect of Strains, Temperature, Ultrasound and Storage Conditions on the Performances of the Treatment. Microorganisms 2020, 8, 1219. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Multiple action sites of ultrasound on Escherichia coli and Staphylococcus aureus. Food Sci. Human Wellness 2018, 7, 102–109. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, A.R. Assessment of bacterial viability: A comprehensive review on recent advances and challenges. Microbiology 2019, 165, 593–610. [Google Scholar] [CrossRef]
- Wu, V.C.H. A review of microbial injury and recovery methods in food. Food Microbiol. 2008, 25, 735–744. [Google Scholar] [CrossRef]
- Brandão, L.R.; de Brito Alves, J.L.; da Costa, W.K.A.; Ferreira, G.D.A.H.; de Oliveira, M.P.; da Cruz, A.G.; de Andrade Braga, V.; de Souza Aquino, J.; Vidal, H.; Noronha, M.F.; et al. Live and ultrasound-inactivated Lacticaseibacillus casei modulate the intestinal microbiota and improve biochemical and cardiovascular parameters in male rats fed a high-fat diet. Food Funct. 2021, 12, 5287–5300. [Google Scholar] [CrossRef]
- Tabatabaie, F.; Mortazavi, A. Studying the effects of ultrasound shock on cell wall permeability and survival of some LAB in milk. World Appl. Sci. J. 2008, 3, 119–121. [Google Scholar]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109 (Suppl. S2), S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M. The hydrophobic barrier properties of gastrointestinal mucus. Annu. Rev. Physiol. 1995, 57, 565–583. [Google Scholar] [CrossRef] [PubMed]
| Sample | Time of Incubation (h) | |
|---|---|---|
| 6 | 24 | |
| LC_S0 | 0.38 ± 0.05 a | 2.16 ± 0.18 a |
| LC_S6 | 0.06 ± 0.02 b | 1.91 ± 0.12 a |
| LC_S8 | 0.02 ± 0.02 b | 0.97 ± 0.14 b |
| Sample | Log CFU/mL | GI |
|---|---|---|
| LC_S0 | 9.33 ± 0.06 a | |
| LC_S6 | 8.61 ± 0.16 b | >75% |
| LC_S8 | 6.47 ± 0.07 c | <25% |
| Sample | Auto-Aggregation (%) | Hydrophobicity (%) | Biofilm Production |
|---|---|---|---|
| LC_S0 | 23.98 ± 1.32 a | 6.29 ± 0.68 a | Weak |
| LC_S6 | 3.39 ± 0.78 b | 11.68 ± 2.65 b | Strong |
| LC_S8 | 0.65 ± 0.26 c | 15.01 ± 1.59 b | Strong |
| Sample | Wavelength (nm) | |
|---|---|---|
| 260 | 280 | |
| LC_S6 | 216 ± 8.16 a | 140 ± 10.00 a |
| LC_S8 | 256 ± 10.69 b | 165 ± 9.64 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, I.; Mauriello, G. Ultrasound Attenuation Improves Some Surface Properties of the Probiotic Strain Lacticaseibacillus casei ATCC 393. Microorganisms 2023, 11, 142. https://doi.org/10.3390/microorganisms11010142
Giordano I, Mauriello G. Ultrasound Attenuation Improves Some Surface Properties of the Probiotic Strain Lacticaseibacillus casei ATCC 393. Microorganisms. 2023; 11(1):142. https://doi.org/10.3390/microorganisms11010142
Chicago/Turabian StyleGiordano, Irene, and Gianluigi Mauriello. 2023. "Ultrasound Attenuation Improves Some Surface Properties of the Probiotic Strain Lacticaseibacillus casei ATCC 393" Microorganisms 11, no. 1: 142. https://doi.org/10.3390/microorganisms11010142
APA StyleGiordano, I., & Mauriello, G. (2023). Ultrasound Attenuation Improves Some Surface Properties of the Probiotic Strain Lacticaseibacillus casei ATCC 393. Microorganisms, 11(1), 142. https://doi.org/10.3390/microorganisms11010142








