Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development
Abstract
:1. Introduction
2. How mVOCs Can Have Versatile Benefits in Sustainable Agriculture?
3. Roles of mVOCs in Sustainable Agriculture
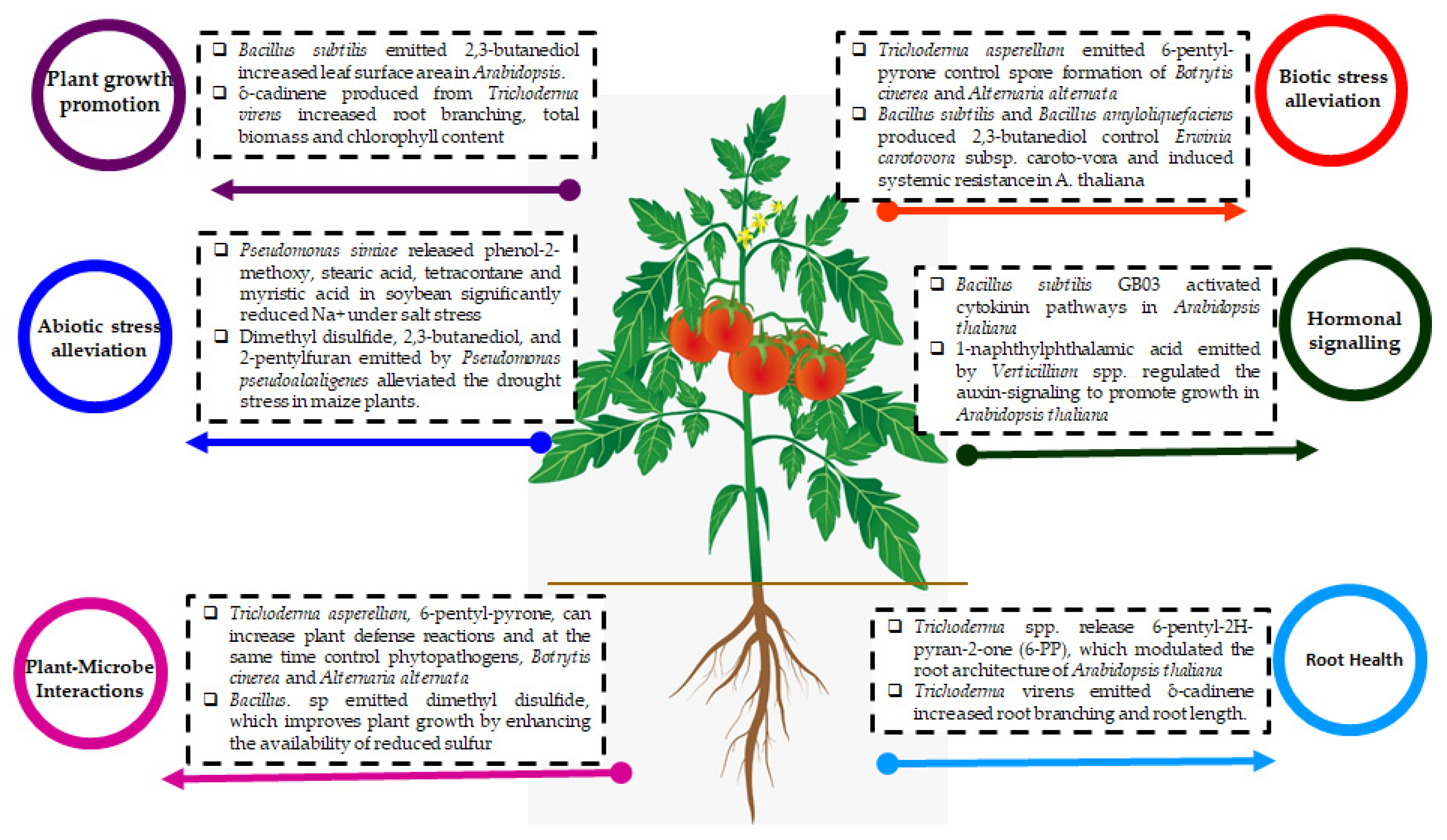
3.1. The mVOCs as Plant Growth Promoters
3.2. The mVOCs as a Biocontrol Agent and Plant Defense Mechanism
3.3. mVOCs as an Abiotic Stress Ameliorator
3.4. mVOCs Modulate Plant Hormonal Signaling
4. mVOCs on Intra and Inter-Species Interactions
5. Recent Research on mVOCs
| S.No. | Microbial Volatile Organic Compounds Produce Microorganisms | Biological Roles | References |
|---|---|---|---|
| 1. | Acinetobacter, Arthrobacter, Bacillus, Microbacterium, Pantoea, Pseudomonas, and Stenotrophomonas sp. | Antifungal activity against Alternaria alternata and Corynespora cassiicola | [108] |
| 2. | Funneliformis mosseae(AMF)–Ensifer meliloti (Rhizobacterium) interaction | Sustainable vineyard management | [39] |
| 3. | Bacillus sp. JC03 | Plant growth promotion in Arabidopsis thaliana, | [36] |
| 4. | Aureobasidium pullulansi L1 and L8 | Monilinia fructigena, and Monilinia fructicola yeast Antagonism and post-harvest brown rot control | [116] |
| 5. | Starmerella bacillaris | Apple gray mold disease control and the rich aroma of cider through benzyl alcohol | [115] |
| 6. | Endophytic fungus in Trichoderma asperellum T1 | Antifungal activity against Corynespora cassiicola and Curvularia aeria, plant growth promotion and defense mechanisms | [112] |
| 7. | Bacillus pumilus TM-R | Wide antifungal activity | [117] |
| 8. | Endophytic Trichoderma spp- Sclerotinia sclerotiorum-TSS, Sclerotium rolfsii-CSR, and Fusarium oxysporum-CFO interaction | Mycoparasitic activity | [113] |
| 9. | Endophytic Pseudomonas putida BP25 | Broad spectrum activity against oomycete pathogens (Phytophthora capsici and Pythium myriotylum), fungal pathogens (Rhizoctonia solani, Colletotrichum gloeosporioides, Athelia rolfsii, Gibberella moniliformis and Magnaporthe oryzae), bacterial pathogens (Ralstonia pseudosolanacearum), and plant parasitic nematodes (Radopholus similis) | [19] |
| 10. | Rhizospheric Rhizoctonia solani (pathogenic) | Beneficial soil and plant health | [118] |
| 11. | Non-pathogenic Fusarium oxysporum FO12 | Verticillium wilt abatement | [114] |
| 12. | Streptomyces sp. strain S97 | Botrytis cinerea control in strawberry | [123] |
| 13. | Bacillus spp. in avocado rhizosphere | Dieback disease due to Fusarium sp. | [119] |
| 15. | Bacillus subtilis CF-3 | Antifungal activity against Colletotrichum gloeosporioides and Monilinia fructicola | [120] |
| 16. | Streptomyces yanglinensis 3–10 | Control of Aspergillus flavus and Aspergillus parasiticus in peanut kernel storage | [73] |
| 17. | Entomoptahogenic fungi Beauveria bassiana (Bb1TS11) and Metarhizium robertsii (Mr4TS04) | The arrest of repelling banana weevil pests, Cosmopolites sordidus | [126] |
| 18. | Pseudomonas sp. ST–TJ4 | Wide spectrum phytopathogenic activity in agroforestry | [19] |
| 19. | Wickerhamomyces anomalus, Metschnikowia pulcherrima, Aureobasidium pullulans, and Saccharomyces cerevisiae (Biocontrol yeasts) | Biocontrol agents and carbon dioxide synergy for prevention of post-harvest loss | [124] |
| 20. | Bacillus velezensis CT32 | Biofumigation activities against Verticillium dahliae and Fusarium oxysporum, vascular wilt pathogens | [122] |
| 21. | Insect–microbe symbiosis in Spruce bark beetle, Ips typographus | Forest pest management | [128] |
6. Environmental Friendliness and Limitations of mVOCs
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci. 2015, 20, 206–211. [Google Scholar] [CrossRef]
- Schalchli, H.; Tortella, G.R.; Rubilar, O.; Parra, L.; Hormazabal, E.; Quiroz, A. Fungal volatiles: An environmentally friendly tool to control pathogenic microorganisms in plants. Crit. Rev. Biotechnol. 2016, 36, 144–152. [Google Scholar] [CrossRef]
- Schenkel, D.; Lemfack, M.C.; Piechulla, B.; Splivallo, R. A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front. Plant Sci. 2015, 6, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra-and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veselova, A.; Plyuta, V.A.; Khmel, I.A. Volatile compounds of bacterial origin: Structure, biosynthesis, and biological activity. Microbiology 2019, 88, 261–274. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Nickel, J.; Dunkel, M.; Preissner, R.; Piechulla, B. mVOC: A database of microbial volatiles. Nucleic Acids Res. 2014, 42, D744–D748. [Google Scholar] [CrossRef] [Green Version]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.; Withall, D.; Birkett, M. Harnessing microbial volatiles to replace pesticides and fertilizers. Microb. Biotechnol. 2020, 13, 1366–1376. [Google Scholar] [CrossRef]
- Li, F.; Tang, M.; Tang, X.; Sun, W.; Gong, J.; Yi, Y. Bacillus subtilis–Arabidopsis thaliana: A model interaction system for studying the role of volatile organic compounds in the interchange between plants and bacteria. Botany 2019, 97, 661–669. [Google Scholar] [CrossRef]
- Jalali, F.; Zafari, D.; Salari, H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol. 2017, 29, 67–75. [Google Scholar] [CrossRef]
- Li, N.; Kang, S. Do volatile compounds produced by Fusarium oxysporum and Verticillium dahliae affect stress tolerance in plants? Mycology 2018, 9, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Del Rosario-Cappellari, L.; Banchio, E. Microbial volatile organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission. J. Plant Growth Regul. 2019, 39, 764–775. [Google Scholar] [CrossRef]
- Zheng, L.; Situ, J.J.; Zhu, Q.F.; Xi, P.G.; Zheng, Y.; Liu, H.X.; Zhou, X. Identification of volatile organic compounds for the biocontrol of postharvest litchi fruit pathogen Peronophythora litchii. Postharvest Biol. Technol. 2019, 155, 37–46. [Google Scholar] [CrossRef]
- Usseglio, V.L.; Pizzolitto, R.P.; Rodriguez, C.; Zunino, M.P.; Zygadlo, J.A.; Areco, V.A.; Dambolena, J.S. Volatile organic compounds from the interaction between Fusarium verticillioides and maize kernels as a natural repellents of Sitophilus zeamais. J. Stor. Prod. Res. 2017, 73, 109–114. [Google Scholar] [CrossRef]
- Rajer, F.U.; Wu, H.; Xie, Y.; Xie, S.; Raza, W.; Tahir, H.A.S.; Gao, X. Volatile organic compounds produced by a soil-isolate, Bacillus subtilis FA26 induce adverse ultra-structural changes to the cells of Clavibacter michiganensis ssp. Sepedonicus, the causal agent of bacterial ring rot of potato. Microbiology 2017, 163, 523–530. [Google Scholar]
- Wang, Z.; Zhong, T.; Chen, X.; Yang, B.; Du, M.; Wang, K.; Zalán, Z.; Kan, J. Potential of volatile organic compounds emitted by Pseudomonas fluorescens ZX as biological fumigants to control Citrus green mold decay at postharvest. J. Agric. Food. Chem. 2021, 69, 2087–2098. [Google Scholar] [CrossRef]
- Giorgio, A.; de Stradis, A.; Cantore, P.; Iacobellis, N.S. Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 2015, 6, 1056. [Google Scholar] [CrossRef] [Green Version]
- Agisha, V.N.; Kumar, A.; Eapen, S.J.; Sheoran, N.; Suseelabhai, R. Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic Pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Sci. Tech. 2019, 29, 1069–1089. [Google Scholar] [CrossRef]
- Ossowicki, A.; Jafra, S.; Garbeva, P. The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 2017, 12, e0174362. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Zheng, L.; Deng, Y.; Xu, D.; Xi, P.; Li, M.; Kong, G.; Jiang, Z. Antifungal activity of natural volatile organic compounds against litchi downy blight pathogen Peronophythora litchii. Molecules 2018, 23, 358. [Google Scholar] [CrossRef] [PubMed]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. The impact of green revolution on indigenous crops of India. J. Ethn. Foods 2019, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, R.; Ryu, C.M. Revisiting bacterial volatile-mediated plant growth promotion: Lessons from the past and objectives for the future. Ann. Bot. 2018, 122, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Mulla, S.I.; Lee, K.J.; Chae, J.C.; Shukla, P. VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit. Rev. Biotechnol. 2018, 38, 1277–1296. [Google Scholar] [CrossRef]
- Sharifi, R.; Jeon, J.S.; Ryu, C.M. Belowground plant–microbe communications via volatile compounds. J. Exp. Bot. 2022, 73, 463–486. [Google Scholar] [CrossRef]
- Ryu, C.M.; Faragt, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Pare, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [Green Version]
- Asari, S.; Matzen, S.; Petersen, M.A.; Bejai, S.; Meijer, J. Multiple effects of Bacillus amyloliquefaciens volatile compounds: Plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol. Ecol. 2016, 92, fiw07. [Google Scholar] [CrossRef] [Green Version]
- Rath, M.; Mitchell, T.R.; Gold, S.E. Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture-dependent. Microbiol. Res. 2018, 208, 76–84. [Google Scholar] [CrossRef]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Zou, C.; Li, Z.; Yu, D. Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J. Microbiol. 2010, 48, 460–466. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, F.M.; Lopez-Bucio, J.; Altamirano-Hernandez, J.; Valencia-Cantero, E.; De La Cruz, H.R.; Macías-Rodríguez, L. Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 2010, 51, 75–83. [Google Scholar] [CrossRef]
- Blom, D.; Fabbri, C.; Eberl, L.; Weisskopf, L. Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl. Environ. Microbiol. 2011, 77, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.H.; Xie, Y.S.; Zhu, K.; Wang, N.; Li, Z.J.; Yu, G.J.; Guo, J.H. Volatile organic compounds emitted by Bacillus sp. JC03 promote plant growth through the action of auxin and strigolactone. Plant Growth Regul. 2019, 87, 317–328. [Google Scholar] [CrossRef]
- Fincheira, P.; Venthur, H.; Mutis, A.; Parada, M.; Quiroz, A. Growth promotion of Lactuca sativa in response to volatile organic compounds emitted from diverse bacterial species. Microbiol. Res. 2016, 193, 39–47. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; Santoyo, G.; Farías-Rodríguez, R.; Valencia-Cantero, E. Medicago truncatula increases its iron-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiol. 2013, 58, 579–585. [Google Scholar] [CrossRef]
- Velásquez, A.; Vega-Celedón, P.; Fiaschi, G.; Agnolucci, M.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. Responses of Vitis vinifera cv. Cabernet Sauvignon roots to the arbuscular mycorrhizal fungus Funneliformis mosseae and the plant growth-promoting rhizobacterium Ensifer meliloti include changes in volatile organic compounds. Mycorrhiza 2020, 30, 161–170. [Google Scholar] [CrossRef]
- Santoro, M.V.; Zygadlo, J.; Giordano, W.; Banchio, E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol. Biochem. 2011, 49, 1177–1182. [Google Scholar] [CrossRef]
- Park, Y.S.; Dutta, S.; Ann, M.; Raaijmakers, J.M.; Park, K. Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 2015, 461, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Herrera-Estrella, A.; Lopez-Bucio, J. The 4-phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant Soil 2014, 379, 261–274. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecol. 2013, 6, 19–26. [Google Scholar] [CrossRef]
- Lee, S.; Hung, R.; Yap, M.; Bennett, J.W. Age matters: The effects of volatile organic compounds emitted by Trichoderma atroviride on plant growth. Arch. Microbiol. 2015, 197, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ezquer, I.; Bahaji, A.; Montero, M.; Ovecka, M.; Baroja-Fernandez, E.; Muñoz, F.J.; Merida, A.; Almagro, G.; Hidalgo, M.; et al. Microbial volatile-induced accumulation of exceptionally high levels of starch in Arabidopsis leaves is a process involving NTRC and starch synthase classes III and IV. Mol. Plant-Microbe Interact. 2011, 24, 1165–1178. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Lopez, A.M.; Baslam, M.; De Diego, N.; Munoz, F.J.; Bahaji, A.; Almagro, G.; García-Gómez, P.; Li, J.; Humplík, J.F.; Novák, O.; et al. Volatile compounds emitted by diverse phytopathogenic microorganisms promote plant growth and flowering through cytokinin action. Plant Cell Environ. 2016, 39, 2592–2608. [Google Scholar] [CrossRef] [Green Version]
- Schenkel, D.; Maciá-Vicente, J.G.; Bissell, A.; Splivallo, R. Fungi indirectly affect plant root architecture by modulating soil volatile organic compounds. Front. Microbiol. 2018, 9, 1847. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, W.; Bitas, V.; Subbarao, K.; Liu, X.; Kang, S. Volatile compounds emitted by diverse Verticillium species enhance plant growth by manipulating auxin signaling. Mol. Plant-Microbe Interact. 2018, 31, 1021–1031. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.H.; Song, G.C.; Ryu, C.M. Sweet scents from good bacteria: Case studies on bacterial volatile compounds for plant growth and immunity. Plant Mol. Biol. 2016, 90, 677–687. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Gu, Q.; Tahir, H.A.S.; Wu, H.; Gao, X. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef]
- Kai, M.; Effmert, U.; Berg, G.; Piechulla, B. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 2007, 187, 351–360. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef] [Green Version]
- Raza, W.; Wang, J.; Wu, Y.; Ling, N.; Wei, Z.; Huang, Q.; Shen, Q. Effects of volatile organic compounds produced by Bacillus amyloliquefaciens on the growth and virulence traits of tomato bacterial wilt pathogen Ralstonia solanacearum. Appl. Microbiol. Biotechnol. 2016, 100, 7639–7650. [Google Scholar] [CrossRef]
- Xie, S.; Zang, H.; Jun Wu, H.; Uddin Rajer, F.; Gao, X. Antibacterial effects of volatiles produced by Bacillus strain D13 against Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 2018, 19, 49–58. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Strobel, G.A.; Moore, E.; Robison, R.; Sears, J. Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microbiology 2010, 156, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Syed-Ab-Rahman, S.F.; Xiao, Y.; Carvalhais, L.C.; Ferguson, B.J.; Schenk, P.M. Suppression of Phytophthora capsici infection and promotion of tomato growth by soil bacteria. Rhizosphere 2019, 9, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Raza, W.; Ling, N.; Liu, D.; Wei, Z.; Huang, Q.; Shen, Q. Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol. Res. 2016, 192, 103–113. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 35–37. [Google Scholar] [CrossRef]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma volatiles effecting Arabidopsis: From inhibition to protection against phytopathogenic fungi. Front. Microbiol. 2015, 6, 995. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Leon, R.; Rojas-Solís, D.; Contreras-Perez, M.; del Carmen Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmon, E.; Contreras-Perez, M.; Rocha-Granados, M.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonasmaltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Fialho, M.B.; Toffano, L.; Pedroso, M.P.; Augusto, F.; Pascholati, S.F. Volatile organic compounds produced by Saccharomyces cerevisiae inhibit the in vitro development of Guignardia citricarpa, the causal agent of citrus black spot. World J. Microbiol. Biotechnol. 2010, 26, 925–932. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Teixid o, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Pare, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Farag, M.A.; Park, H.B.; Kloepper, J.W.; Lee, S.H.; Ryu, C.M. Induced resistance by a long-chain bacterial volatile: Elicitation of plant systemic defense by a C13 Volatile produced by Paenibacillus polymyxa. PLoS ONE 2012, 7, e48744. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J.; Turlings, T.C.J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef] [Green Version]
- Rudrappa, T.; Biedrzycki, M.L.; Kunjeti, S.G.; Donofrio, N.M.; Czymmek, K.J.; Paul, W.P.; Bais, H.P. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun. Integr. Biol. 2010, 3, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, D.; Garladinne, M.; Lee, Y.H. Volatile indole produced by rhizobacterium Proteus vulgaris JBLS202 stimulates growth of Arabidopsis thaliana through auxin, cytokinin, and brassinosteroid pathways. J. Plant Growth Regul. 2015, 34, 158–168. [Google Scholar] [CrossRef]
- Choi, H.K.; Song, G.C.; Yi, H.S.; Ryu, C.M. Field evaluation of the bacterial volatile derivative 3-pentanol in priming for induced resistance in pepper. J. Chem. Ecol. 2014, 40, 882–892. [Google Scholar] [CrossRef]
- Yang, M.; Lu, L.; Pang, J.; Hu, Y.; Guo, Q.; Li, Z.; Wu, S.; Liu, H.; Wang, C. Biocontrol activity of volatile organic compounds from Streptomyces alboflavus TD-1 against Aspergillus flavus growth and aflatoxin production. J. Microbiol. 2019, 57, 396–404. [Google Scholar] [CrossRef]
- Lyu, A.; Yang, L.; Wu, M.; Zhang, J.; Li, G. High efficacy of the volatile organic compounds of Streptomyces yanglinensis 3-10 in suppression of Aspergillus contamination on peanut kernels. Front. Microbiol. 2020, 11, 142. [Google Scholar] [CrossRef] [Green Version]
- Ojaghian, S.; Wang, L.; Xie, G.L.; Zhang, J.Z. Effect of volatiles produced by Trichoderma spp. On expression of glutathione transferase genes in Sclerotinia sclerotiorum. Biol. Control 2019, 136, 103999. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.; Pare, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Kim, Y.H.; Anderson, A.J.; Kim, Y.C. Nitric oxide and hydrogen peroxide production are involved in systemic drought tolerance induced by 2R,3R-butanediol in Arabidopsis thaliana. Plant Pathol. J. 2013, 29, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Murzello, C.; Kim, M.S.; Xie, X.; Jeter, R.M.; Zak, J.C.; Paré, P.W.; Dowd, S.E. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol. Plant Microbe. Interact. 2010, 23, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Choudhary, D.K. Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J. Appl. Microbiol. 2015, 119, 539–551. [Google Scholar] [CrossRef]
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 2016, 56, 1274–1288. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kannaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 18388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Li, P.S.; Kong, W.L.; Wu, X.Q.; Zhang, Y. Volatile Organic Compounds of the Plant Growth-Promoting Rhizobacteria JZ-GX1 Enhanced the Tolerance of Robinia pseudoacacia to Salt Stress. Front. Plant Sci. 2021, 12, 753332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.; Groenhagen, U.; Schulz, S.; Geisler, M.; Eberl, L.; Weisskopf, L. The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J. 2014, 80, 758–771. [Google Scholar] [CrossRef] [Green Version]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Munoz-Parra, E.; Raya-Gonzalez, J.; Mendez-Bravo, A.; Macıas-Rodrıguez, L.; López- Luiz-Herrera, L.F.; Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Flores, P.; Valencia-Cantero, E.; Altamirano-Hernández, J.; Pelagio-Flores, R.; López-Bucio, J.; García-Juárez, P.; Macías-Rodríguez, L. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 2017, 254, 2201–2213. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, L.; Ma, Z.; Wang, J. Bacillus amyloliquefaciens SAY09 increases cadmium resistance in plants by activation of auxin-mediated signaling pathways. Genes 2017, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Cordovez, V.; Mommer, L.; Moisan, K.; Lucas-Barbosa, D.; Pierik, R.; Mumm, R.; Carrion, V.J.; Raaijmakers, J.M. Plant phenotypic and transcriptional changes induced by volatiles from the fungal root pathogen Rhizoctonia solani. Front. Plant Sci. 2017, 8, 1262. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Cao, M.; Liu, F.; Wang, Y.; Wan, J.; Wang, R.; Zhou, H.; Wang, W.; Xu, J. The volatile organic compounds of Floccularia luteovirens modulate plant growth and metabolism in Arabidopsis thaliana. Plant Soil 2020, 456, 207–221. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]
- Song, G.C.; Jeon, J.S.; Sim, H.J.; Lee, S.; Jung, J.; Kim, S.G.; Moon, S.Y.; Ryu, C.M. Dual functionality of natural mixtures of bacterial volatile compounds on plant growth. J. Exp. Bot. 2022, 73, 571–583. [Google Scholar] [CrossRef]
- Martínez-Cámara, R.; Montejano-Ramírez, V.; Moreno-Hagelsieb, G.; Santoyo, G.; Valencia-Cantero, E. The volatile organic compound dimethyl hexadecylamine affects bacterial growth and swarming motility of bacteria. Folia Microbiol. 2020, 65, 523–532. [Google Scholar] [CrossRef]
- Jones, S.E.; Pham, C.A.; Zambri, M.P.; McKillip, J.; Carlson, E.E.; Elliot, M.A. Streptomyces volatile compounds influence exploration and microbial community dynamics by altering iron availability. mBio 2019, 10, e00171-19. [Google Scholar] [CrossRef] [Green Version]
- Chernin, L.; Toklikishvili, N.; Ovadis, M.; Kim, S.; Ben-Ari, J.; Khmel, I.; Vainstein, A. Quorum-sensing quenching by rhizobacterial volatiles. Environ. Microbiol. Rep. 2011, 3, 698–704. [Google Scholar] [CrossRef]
- Sieber, S.; Mathew, A.; Jenul, C.; Kohler, T.; Bär, M.; Carrión, V.J.; Cazorla, F.M.; Stalder, U.; Hsieh, Y.C.; Bigler, L.; et al. Mitigation of Pseudomonas syringae virulence by signal inactivation. Sci. Adv. 2021, 7, eabg2293. [Google Scholar] [CrossRef]
- Becher, P.G.; Verschut, V.; Bibb, M.J.; Bush, M.J.; Molnár, B.P.; Barane, E.; Al-Bassam, M.M.; Chandra, G.; Song, L.; Challis, G.L.; et al. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020, 5, 821–829. [Google Scholar] [CrossRef]
- Ampt, E.A.; Bush, D.S.; Siegel, J.P.; Berenbaum, M.R. Larval preference and performance of Amyelois transitella (navel orangeworm, Lepidoptera: Pyralidae) in relation to the fungus Aspergillus flavus. Environ. Entomol. 2016, 45, 155–162. [Google Scholar] [CrossRef]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Gaines, A.; Ludovice, M.; Xu, J.; Zanghi, M.; Meinersmann, R.J.; Berrang, M.; Daley, W.; Britton, D. The dialogue between protozoa and bacteria in a microfluidic device. PLoS ONE 2019, 14, e0222484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matz, C.; Kjelleberg, S. Off the hook–how bacteria survive protozoan grazing. Trends Microbiol. 2005, 13, 302–327. [Google Scholar] [CrossRef]
- Rybakova, D.; Rack-Wetzlinger, U.; Cernava, T.; Schaefer, A.; Schmuck, M.; Berg, G. Aerial warfare: A volatile dialogue between the plant pathogen Verticillium longisporum and its antagonist Paenibacillus polymyxa. Front. Plant Sci. 2017, 8, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spraker, J.E.; Jewell, K.; Roze, L.V.; Scherf, J.; Ndagano, D.; Beaudry, R.; Linz, J.E.; Allen, C.; Keller, N.P. A volatile relationship: Profiling an inter-kingdom dialogue between two plant pathogens, Ralstonia solanacearum and Aspergillus flavus. J. Chem. Ecol. 2014, 40, 502–513. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Bejarano-Bolívar, A.A.; Kiel-Martínez, A.L.; Ramírez-Vázquez, M.; Méndez-Bravo, A.; von Wobeser, E.A.; Sánchez-Rangel, D.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with Kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol. Res. 2019, 219, 74–83. [Google Scholar] [CrossRef]
- Schmidt, R.; Jager, V.; Zuhlke, D.; Wolff, C.; Bernhardt, J.; Cankar, K.; Beekwilder, J.; Ijcken, W.V.; Sleutels, F.; Boer, W.; et al. Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 2017, 7, 862. [Google Scholar] [CrossRef]
- López, S.M.; Pastorino, G.N.; Balatti, P.A. Volatile organic compounds profile synthesized and released by endophytes of tomato (Solanum lycopersici L.) and their antagonistic role. Arch. Microbiol. 2021, 203, 1383–1397. [Google Scholar] [CrossRef]
- Pescador, L.; Fernandez, I.; Pozo, M.J.; Romero-Puertas, M.C.; Pieterse, C.M.; Martínez-Medina, A. Nitric oxide signalling in roots is required for MYB72-dependent systemic resistance induced by Trichoderma volatile compounds in Arabidopsis. J. Exp. Bot. 2022, 73, 584–595. [Google Scholar] [CrossRef]
- Guo, Y.; Jud, W.; Weikl, F.; Ghirardo, A.; Junker, R.R.; Polle, A.; Benz, J.P.; Pritsch, K.; Schnitzler, J.P.; Rosenkranz, M. Volatile organic compound patterns predict fungal trophic mode and lifestyle. Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef]
- Pennerman, K.K.; Yin, G.; Bennett, J.W. Eight-carbon volatiles: Prominent fungal and plant interaction compounds. J. Exp. Bot. 2022, 73, 487–497. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.I.; Sunpapao, A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Shaanker, R.U. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Cernava, T.; Turrà, D.; Schaefer, A.; Di Pietro, A.; López-Escudero, F.J.; Trapero, A.; Berg, G. The role of volatile organic compounds and rhizosphere competence in mode of action of the non-pathogenic Fusarium oxysporum FO12 toward Verticillium wilt. Front. Microbiol. 2019, 10, 1808. [Google Scholar] [CrossRef] [Green Version]
- Junior, W.J.L.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile organic compounds from Starmerella bacillaris to control gray mold on apples and modulate cider aroma profile. Food Microbiol. 2020, 89, 103446. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Foggia, M.; Baraldi, E. Aureobasidium pullulans volatile organic compounds as alternative postharvest method to control brown rot of stone fruits. Food Microbiol. 2020, 87, 103395. [Google Scholar] [CrossRef]
- Morita, T.; Tanaka, I.; Ryuda, N.; Ikari, M.; Ueno, D.; Someya, T. Antifungal spectrum characterization and identification of strong volatile organic compounds produced by Bacillus pumilus TM-R. Heliyon 2019, 5, e01817. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Liu, X.; Si, Z.; Li, X.; Bi, J.; Dong, W.; Chen, M.; Wang, S.; Zhang, J.; Song, A.; et al. Volatile organic compounds from rice rhizosphere bacteria inhibit growth of the pathogen Rhizoctonia solani. Agriculture 2021, 11, 368. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Bravo-Castillo, K.R.; Monribot-Villanueva, J.L.; Kiel-Martínez, A.L.; Ramírez-Vázquez, M.; Guerrero-Analco, J.A.; Reverchon, F. Diffusible and volatile organic compounds produced by avocado rhizobacteria exhibit antifungal effects against Fusarium kuroshium. Braz. J Microbiol. 2020, 51, 861–873. [Google Scholar] [CrossRef]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on volatile organic compounds from Bacillus subtilis CF-3: Biocontrol effects on fruit fungal pathogens and dynamic changes during fermentation. Front. Microbiol. 2018, 9, 456. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Li, P.; Wu, S.; Zhao, P.; Gao, H. Bacillus subtilis CF-3 volatile organic compounds inhibit Monilinia fructicola growth in peach fruit. Front. Microbiol. 2019, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Shi, X.; Wang, B.; Li, M.; Wang, Q.; Zhang, S. Antifungal effect of volatile organic compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. [Google Scholar] [CrossRef]
- Ayed, A.; Kalai-Grami, L.; Ben Slimene, I.; Chaouachi, M.; Mankai, H.; Karkouch, I.; Djebali, N.; Elkahoui, S.; Tabbene, O.; Limam, F. Antifungal activity of volatile organic compounds from Streptomyces sp. strain S97 against Botrytis cinerea. Biocontrol Sci. Technol. 2021, 31, 1330–1348. [Google Scholar] [CrossRef]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, A.; Taffner, J.; Guimarães, R.A.; Coyne, D.; Berg, G. Novel strategies for soil-borne diseases: Exploiting the microbiome and volatile-based mechanisms toward controlling Meloidogyne-based disease complexes. Front. Microbiol. 2019, 10, 1296. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). Insects 2020, 11, 509. [Google Scholar] [CrossRef]
- Kong, W.L.; Li, P.S.; Wu, X.Q.; Wu, T.Y.; Sun, X.R. Forest tree associated bacterial diffusible and volatile organic compounds against various phytopathogenic fungi. Microorganisms 2020, 8, 590. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, D.; Gershenzon, J.; Andersson, M.N.; Hammerbacher, A. Volatile organic compounds influence the interaction of the Eurasian spruce bark beetle (Ips typographus) with its fungal symbionts. ISME J. 2019, 13, 1788–1800. [Google Scholar] [CrossRef] [Green Version]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [Green Version]
- Giagnoni, L.; Maienza, A.; Baronti, S.; Vaccari, F.P.; Genesio, L.; Taiti, C.; Martellini, T.; Scodellini, R.; Cincinelli, A.; Costa, C.; et al. Long-term soil biological fertility, volatile organic compounds and chemical properties in a vineyard soil after biochar amendment. Geoderma 2019, 344, 127–136. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef]
- Raza, W.; Shen, Q. Volatile organic compounds mediated plant-microbe interactions in soil. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 209–219. [Google Scholar]
- Lee, H.H.; Molla, M.N.; Cantor, C.R.; Collins, J.J. Bacterial charity work leads to population-wide resistance. Nature 2010, 467, U82–U113. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Dickschat, J.S.; Kunze, B.; Wagner-Dobler, I.; Diestel, R.; Sasse, F. Biological activity of volatiles from marine and terrestrial bacteria. Mar. Drugs 2010, 8, 2976. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wu, L.; Hao, J.; Luo, L.; Cao, Y.; Li, J. Biofumigation on post- harvest diseases of fruits using a new volatile- producing fungus of Ceratocystis fimbriata. PLoS ONE 2015, 10, e0132009. [Google Scholar] [CrossRef] [Green Version]
- Wan, M.; Li, G.; Zhang, J.; Jiang, D.; Huang, H.C. Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol. Control 2008, 46, 552–559. [Google Scholar] [CrossRef]
- Li, Q.L.; Ning, P.; Zheng, L.; Huang, J.B.; Li, G.Q.; Hsiang, T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]
- Huang, R.; Li, G.Q.; Zhang, J.; Yang, L.; Che, H.J.; Jiang, D.H.; Huang, H.C. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869. [Google Scholar] [CrossRef]

| Microorganism | Microbial Volatile Compounds | Controlled Plant Pathogen | References |
|---|---|---|---|
| Pseudomonas fluorescens Pseudomonas corrugate Pseudomonas chlororaphis Pseudomonas aurantiaca | Benzothiazole Cyclohexanol n-Decanal Dimethyl trisulfide 2-Ethyl 1-hexanol Nonanal | Sclerotinia sclerotiorum | [50] |
| Bacillus velezensis | Benzothiazole | Sclerotinia sclerotiorum | [51] |
| Pseudomonas fluorescens Pseudomonas trivialis Serratia plymuthica Serratia odorifera Stenotrophomonas maltophilia Stenotrophomonas rhizophila | β-Phenylethanol Dimethyl trisulfide | Rhizoctonia solani | [52] |
| Bacillus subtilis | Benzaldehyde Nonanal Benzothiazole Acetophenone | Clavibacter michiganensis sp. sepedonicus | [16] |
| Bacillus amyloliquefaciens | 2-Undecanone 2-Tridecanone Heptadecane | Ralstonia solanacearum | [53,54] |
| Bacillus strain D13 | Decyl alcohol 3,5,5-Trimethylhexanol | Xanthomonas oryzae | [55] |
| Muscodor crispans | Propanoic acid 2-Methyl- compounds | Pythium ultimum Phytophthora cinnamom Sclerotinia sclerotiorum Mycosphaerella fijiensis Xanthomonas axonopodis pv. citri | [56] |
| Bacillus and Acinetobacter | 3-Methyl-1-Butanol Isovaleraldehyde Isovaleric acid 2-Ethylhexanol 2-Heptanone | Phytophthora capsici | [57] |
| Pseudomonas fluorescens WR-1 | Toluene, Ethyl benzene, m-Xylene Benzothiazole | Ralstonia solanacearum | [58] |
| Penicillium glabrum | 1-Octen-3-ol | Botrytis cinerea | [59] |
| Trichoderma asperellum | 6-Pentyl-pyrone | Botrytis cinerea Alternaria alternata | [60] |
| Pseudomonas fluorescens Pseudomonas stutzeri Stenotrophomonas maltophilia | Dimethyldisulfide | Botrytis cinerea | [61,62] |
| Saccharomyces cerevisiae | Phenyl Ethanol Ethyl acetate Methylbutanol | Guignardia citricarpa | [63] |
| Bacillus amyloliquefaciens | 1-(2-Aminophenyl) Ethanone Benzothiazole | Peronophythora litchii | [14] |
| Bacillus amyloliquefaciens | 1,3 Pentadiene Acetoin Thiophene | Monilinia laxa Monilinia fructicola | [64] |
| Bacillus subtilis Bacillus amyloliquefaciens | 2,3-Butanediol | Erwinia carotovora subsp. carotovora | [65] |
| Paenibacillus polymyxa | Tridecane | Pseudomonas syringae pv. maculicola | [66] |
| Enterobacter aerogenes | Acetoin | Setosphaeria turcica | [67] |
| Bacillus subtilis | acetoin (3-hydroxy-2-butanone) | Pseudomonas syringae pv. tomato DC3000 | [68] |
| Ampelomyces sp. and Cladosporium sp. | m-cresol and methyl benzoate | Pseudomonas syringae pv. tomato DC3000 | [69] |
| Proteus vulgaris JBLS202 | Indole | Plant hormone signaling pathway | [70] |
| Bacillus amyloliquefaciens | 3-Pentanol | Xanthomonas axonopodis pv. vesicatoria | [71] |
| Streptomyces alboflavus TD-1 | Dimethyl trisulfide Benzenamine | Aspergillus flavus | [72] |
| Streptomyces yanglinensis 3–10 | 2-Methylbutyrate 2-Phenylethanol β-Caryophyllene | Aspergillus flavus Aspergillus parasiticus | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms 2023, 11, 42. https://doi.org/10.3390/microorganisms11010042
Chandrasekaran M, Paramasivan M, Sahayarayan JJ. Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms. 2023; 11(1):42. https://doi.org/10.3390/microorganisms11010042
Chicago/Turabian StyleChandrasekaran, Murugesan, Manivannan Paramasivan, and Jesudass Joseph Sahayarayan. 2023. "Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development" Microorganisms 11, no. 1: 42. https://doi.org/10.3390/microorganisms11010042
APA StyleChandrasekaran, M., Paramasivan, M., & Sahayarayan, J. J. (2023). Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms, 11(1), 42. https://doi.org/10.3390/microorganisms11010042







