Drought and Competition Mediate Mycorrhizal Colonization, Growth Rate, and Nutrient Uptake in Three Artemisia Species
Abstract
:1. Introduction
2. Materials and Methods
3. Results
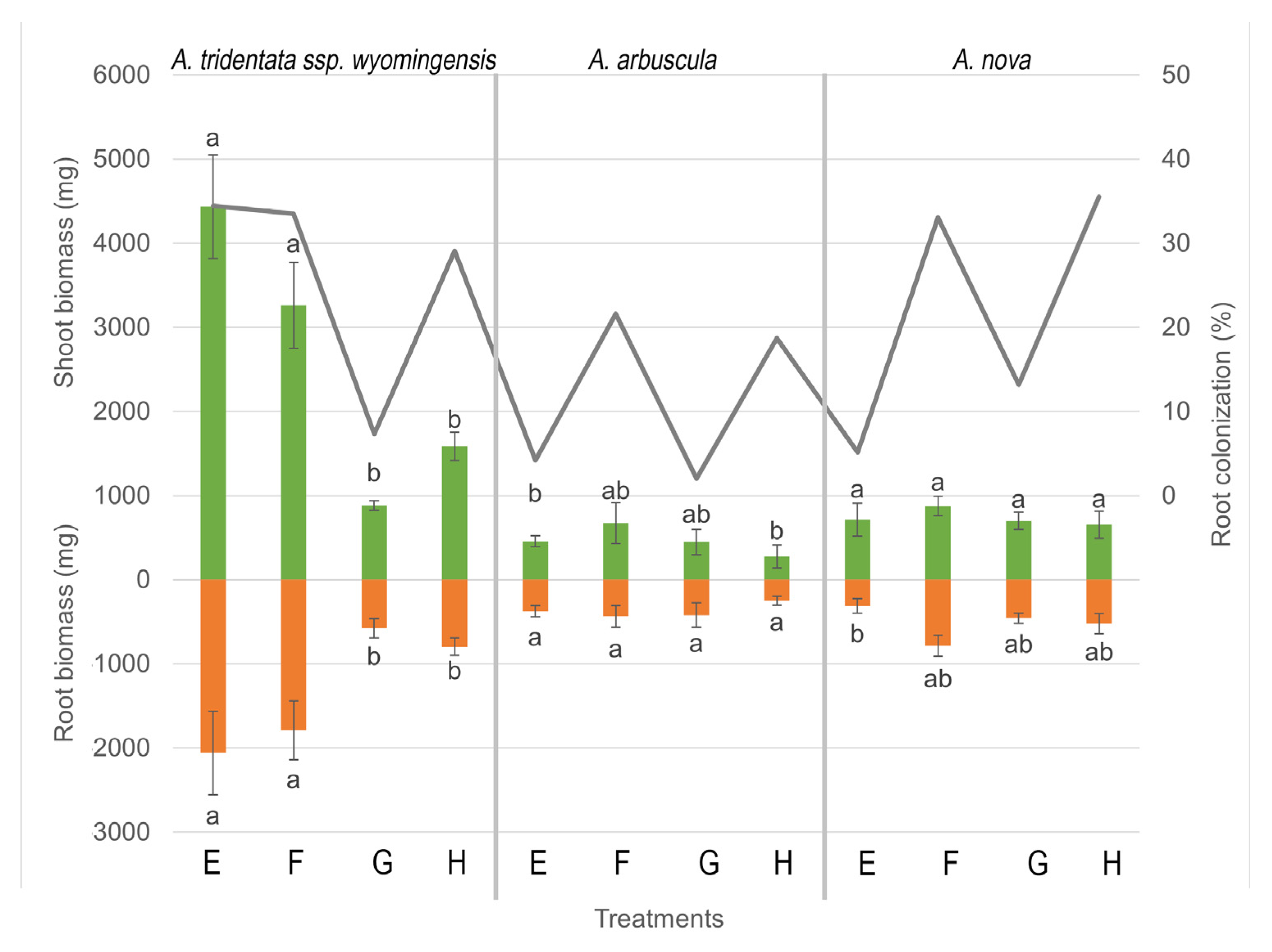
3.1. AMF Root Colonization of Artemisia Species
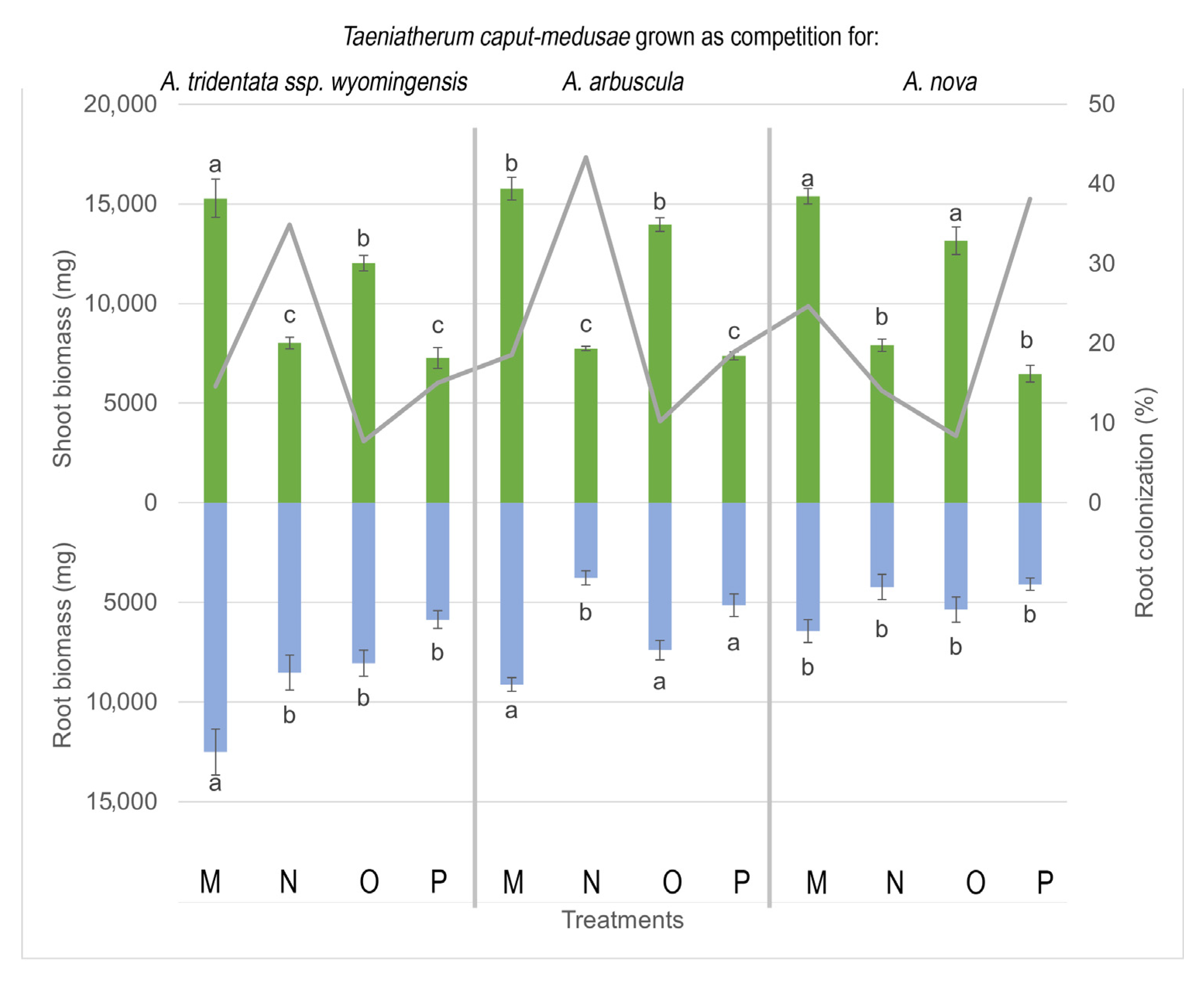
3.2. AMF Root Colonization of the Invasive T. caput medusae
3.3. Biomass Production of the 3 Artemisia Species
3.4. Biomass Production of T. caput medusae
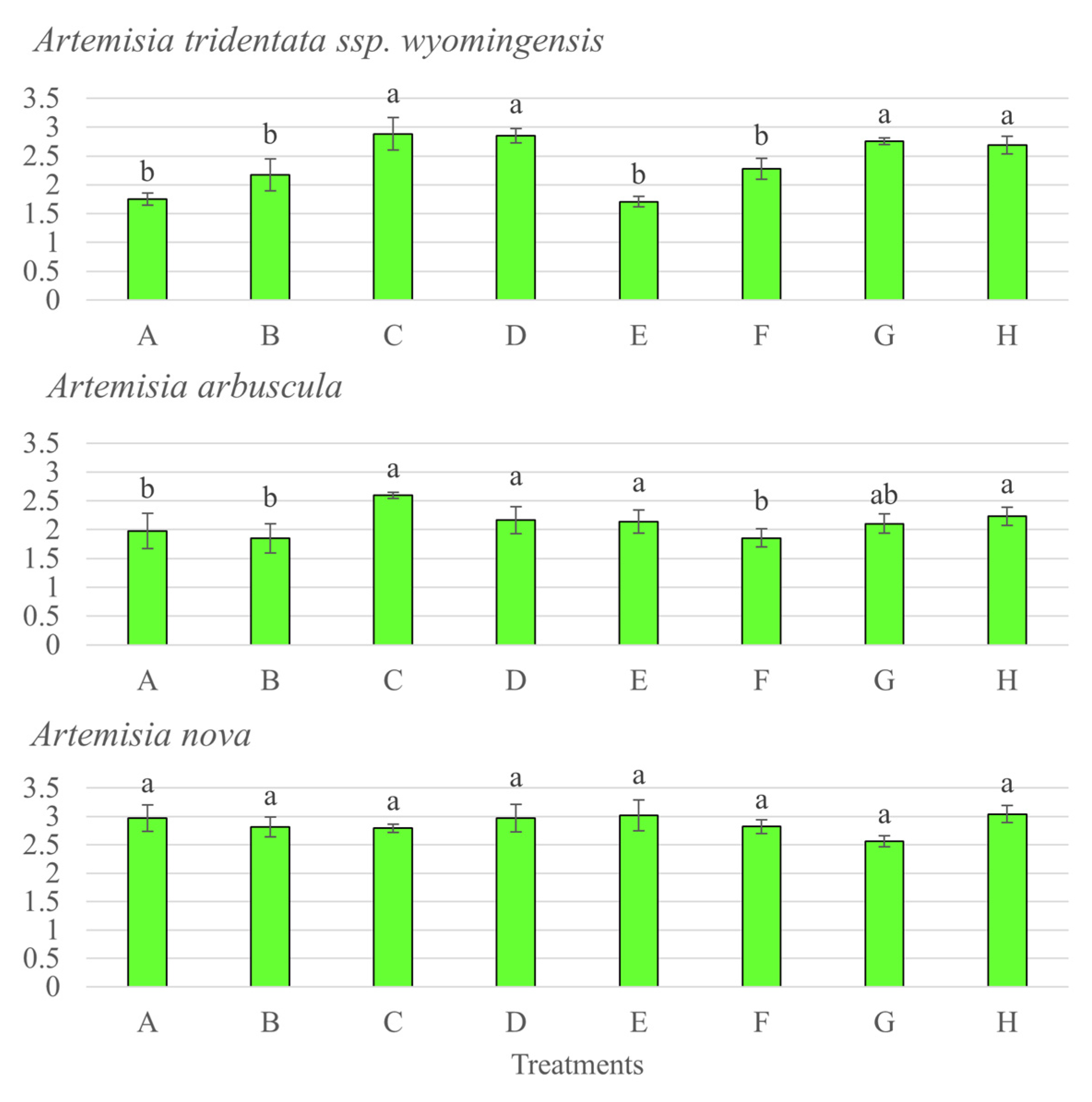
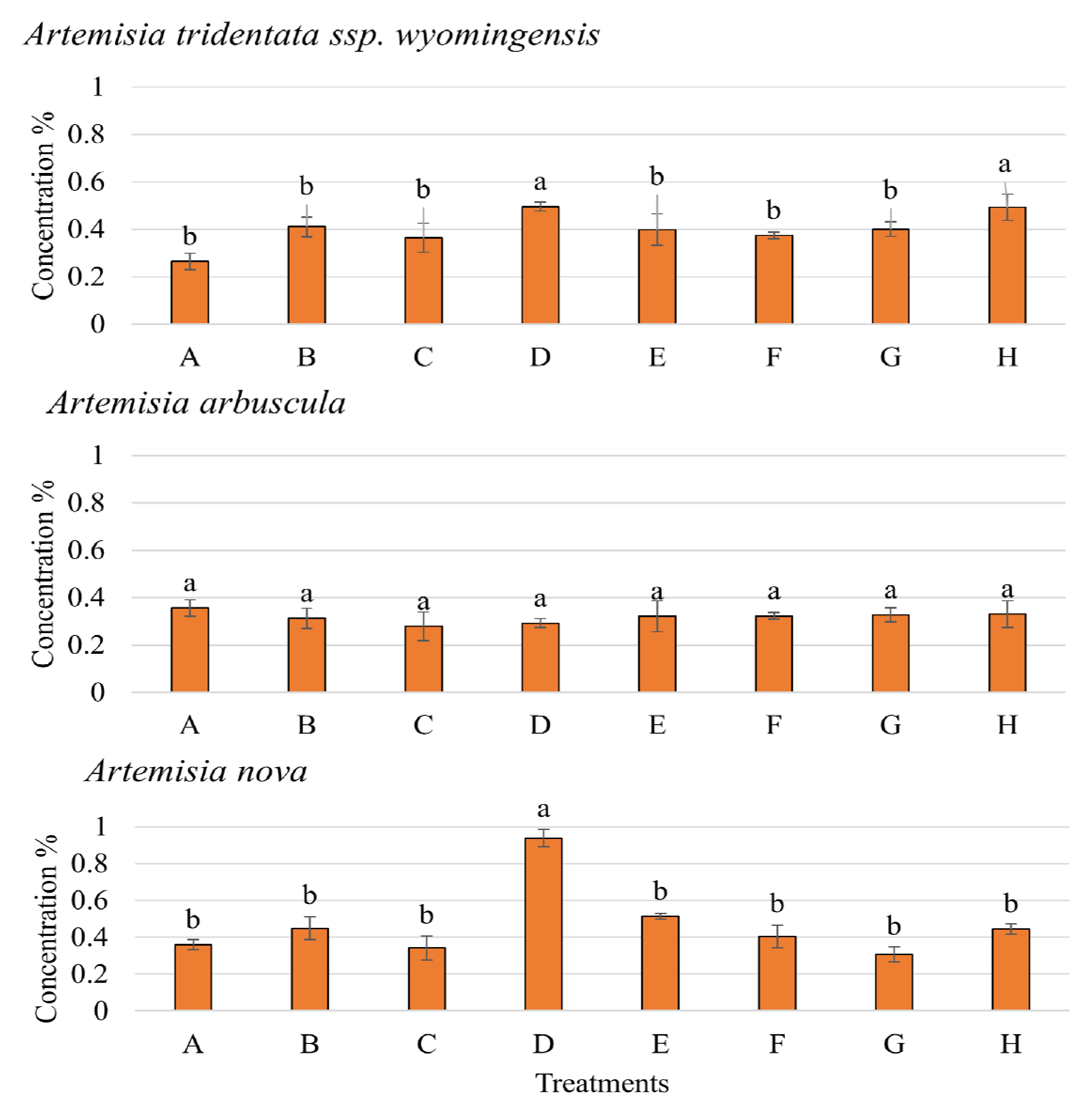
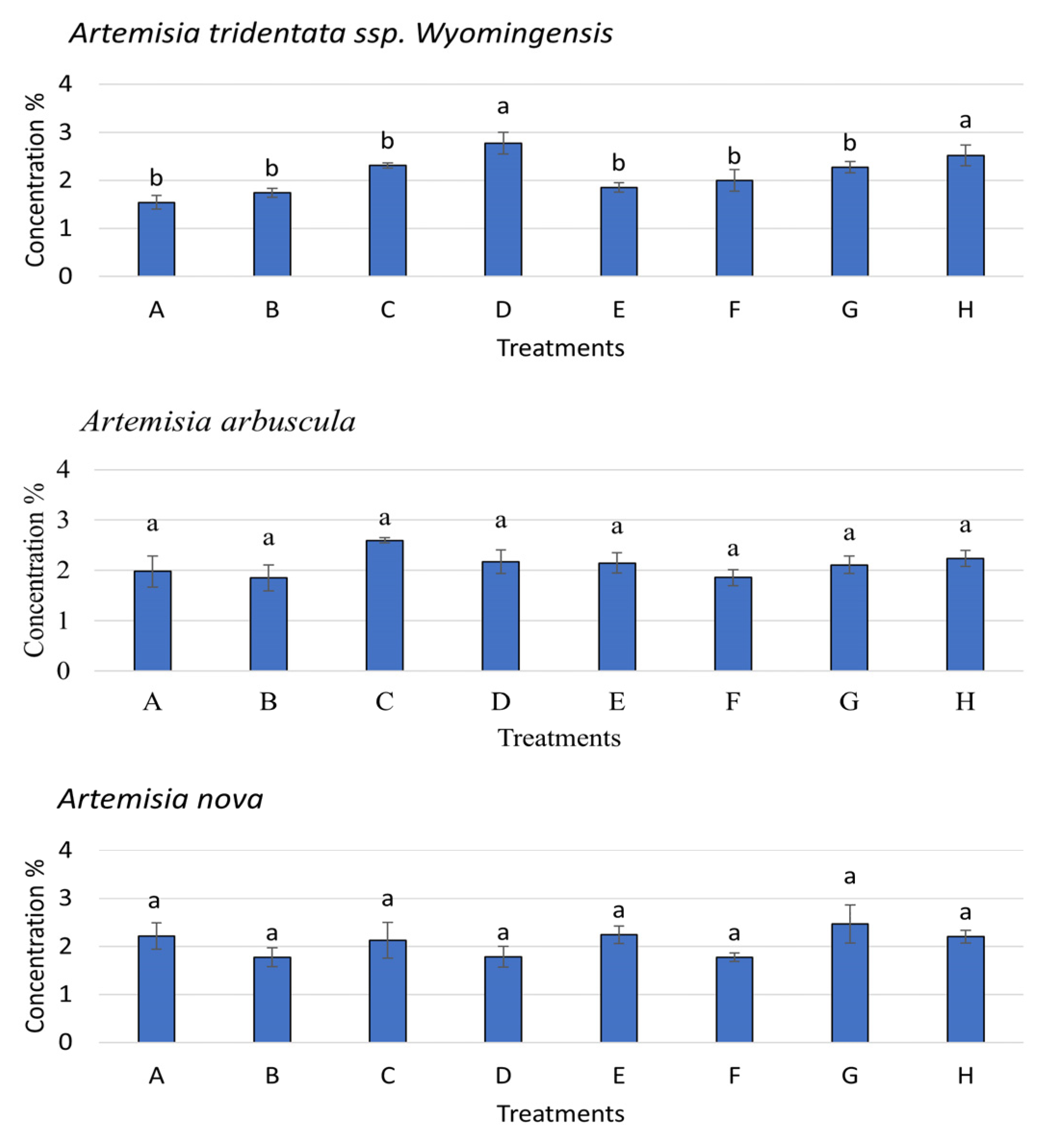
3.5. Nutrient Acquisition of the 3 Artemisia Species
3.6. Nutrient Acquisition of T. caput-medusae
4. Discussion
4.1. Can Environmental Stress Be Ameliorated with a Commercial AMF Inoculum?
4.2. Mycorrhizal Responsiveness of T. caput-medusae
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carbajal-Morón, N.A.; Manzano, M.G.; Mata-González, R. Soil hydrology and vegetation as impacted by goat grazing in Vertisols and Regosols in semi-arid shrublands of northern Mexico. Rangel. J. 2017, 39, 363–373. [Google Scholar] [CrossRef]
- Pyke, D.A.; Chambers, J.C.; Pellant, M.; Knick, S.T.; Miller, R.F.; Beck, J.L.; McIver, J.D. Restoration Handbook for Sagebrush Steppe Ecosystems with Emphasis on Greater Sage-Grouse Habitat-Part 1. Circular 1416; US Department of the Interior: Washington, DC, USA; US Geological Survey: Reston, VA, USA, 2015; p. 43.
- Emam, T. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restor. Ecol. 2016, 24, 35–44. [Google Scholar] [CrossRef]
- Davies, K.W.; Boyd, C.S.; Beck, J.L.; Bates, J.D.; Svejcar, T.J.; Gregg, M.A. Saving the sagebrush sea: An ecosystem conservation plan for big sagebrush plant communities. Biol. Conserv. 2011, 144, 2573–2584. [Google Scholar] [CrossRef] [Green Version]
- Reed-Dustin, C.M.; Mata-González, R.; Rodhouse, T.J. Long-term fire effects on native and invasive grasses in protected area sagebrush steppe. Rangel. Ecol. Manag. 2016, 69, 257–264. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of alien invasive plants on fire regimes. Bioscience 2004, 54, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Mata-González, R.; Reed-Dustin, C.M.; Rodhouse, T.J. Contrasting effects of long-term fire on sagebrush steppe shrubs mediated by topography and plant community. Rangel. Ecol. Manag. 2018, 71, 336–344. [Google Scholar] [CrossRef]
- Abdelaal, K.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of drought damages by exogenous chitosan and yeast extract with modulating the photosynthetic pigments, antioxidant defense system and improving the productivity of garlic plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Khaffagy, A.E.; Mazrou, Y.S.A.; Morsy, A.R.; El-Mansoury, M.A.M.; El-Tokhy, A.I.; Hafez, Y.; Abdelaal, K.; Khedr, R.A. Impact of Irrigation Levels and Weed Control Treatments on Annual Weeds, Physiological Traits and Productivity of Soybean under Clay Soil Conditions. Agronomy 2022, 12, 1037. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal impacts on competitive interactions between Acacia etbaica and Boswellia papyrifera seedlings under drought stress. J. Plant Ecol. 2014, 7, 298–308. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; James, T.Y.A. phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augé, R.M. Water relations, drought, and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [Green Version]
- Merrild, M.P.; Ambus, P.; Rosendahl, S.; Jakobsen, I. Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytol. 2013, 200, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; McCormack, M.L.; Guo, D. Arbuscular mycorrhizal fungal effects on plant competition and community structure. J. Ecol. 2015, 103, 1224–1232. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Chen, Z.L. Mycorrhizal inoculation modulates root morphology and root phytohormone responses in trifoliate orange under drought stress. Emir. J. Food Agric. 2016, 28, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Xavier, L.J.; Germida, J.J. Selective interactions between arbuscular mycorrhizal fungi and Rhizobium leguminosarum bv. viceae enhance pea yield and nutrition. Biol. Fertil. Soils 2003, 37, 261–267. [Google Scholar] [CrossRef]
- Chen, W.; Meng, P.; Feng, H.; Wang, C. Effects of arbuscular mycorrhizal fungi on growth and physiological performance of Catalpa bungei CA Mey. under drought Stress. Forests 2020, 11, 1117. [Google Scholar]
- Prado-Tarango, D.E.; Mata-González, R.; Hovland, M.; Schreiner, R.P. Assessing commercial and early-seral arbuscular mycorrhizal fungi inoculation to aid in restoring sagebrush steppe shrubs. Rangel. Ecol. Manag. 2021, 79, 87–90. [Google Scholar] [CrossRef]
- Hovland, M.; Mata-González, R.; Schreiner, R.P.; Rodhouse, T.J. Fungal facilitation in rangelands: Do arbuscular mycorrhizal fungi mediate resilience and resistance in sagebrush steppe? Rangel. Ecol. Manag. 2019, 72, 678–691. [Google Scholar] [CrossRef]
- Asmelash, F.; Bekele, T.; Birhane, E. The potential role of arbuscular mycorrhizal fungi in the restoration of degraded lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef] [PubMed]
- Scherpenisse, D.S. Mycorrhizae in Sagebrush-Steppe Community Restoration: Mycorrhizal Dependency of Invasive and Native Grasses with Intraspecific and Interspecific Competition. Master’s Thesis, Utah State University, Logan, UT, USA, 2009. [Google Scholar]
- Hovland, M. Mycological Maintenance of Rangelands and the Impacts of a Thatch Forming Invasive Annual Grass (Taeniatherum caput-medusae). Master’s Thesis, Oregon State University, Corvalllis, OR, USA, 2017. [Google Scholar]
- Gornish, E.S.; Fierer, N.; Barberán, A. Associations between an invasive plant (Taeniatherum caput-medusae, Medusahead) and soil microbial communities. PLoS ONE 2016, 11, e0163930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, L.B.; Nowak, R.S. Native, and non-native grasses generate common types of plant–soil feedbacks by altering soil nutrients and microbial communities. Oikos 2013, 122, 199–208. [Google Scholar] [CrossRef]
- Stahl, P.D.; Schuman, G.E.; Frost, S.M.; Williams, S.E. Arbuscular mycorrhizae and water stress tolerance of Wyoming big sagebrush seedlings. Soil Sci. Soc. Am. J. 1998, 62, 1309–1313. [Google Scholar] [CrossRef]
- Rowe, H.I.; Brown, C.S.; Claassen, V.P. Comparisons of mycorrhizal responsiveness with field soil and commercial inoculum for six native montane species and Bromus Tectorum. Restor. Ecol. 2007, 15, 44–52. [Google Scholar] [CrossRef]
- Dettweiler-Robinson, E.; Bakker, J.D.; Evans, J.R.; Newsome, H.; Davies, G.M.; Wirth, T.A.; Pyke, D.A.; Easterly, R.T.; Salstrom, D.; Dunwiddie, P.W. Outplanting Wyoming big sagebrush following wildfire: Stock performance and economics. Rangel. Ecol. Manag. 2013, 66, 657–666. [Google Scholar] [CrossRef]
- Davidson, B.E.; Novak, S.J.; Serpe, M.D. Consequences of inoculation with native arbuscular mycorrhizal fungi for root colonization and survival of Artemisia tridentata ssp. wyomingensis seedlings after transplanting. Mycorrhiza 2016, 26, 595–608. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Lekberg, Y.; Klironomos, J.; Maherali, H. Does responsiveness to arbuscular mycorrhizal fungi depend on plant invasive status? Ecol. Evol. 2017, 7, 6482–6492. [Google Scholar] [CrossRef] [Green Version]
- Mata-González, R.; Sosebee, R.E.; Wan, C. Physiological impacts of biosolids application in desert grasses. Environ. Exp. Bot. 2002, 48, 139–148. [Google Scholar] [CrossRef]
- Sheley, R.L.; Svejcar, T.J. Response of bluebunch wheatgrass and medusahead to defoliation. Rangel. Ecol. Manag. 2009, 62, 278–283. [Google Scholar] [CrossRef]
- Hahl, T.; van Moorsel, S.J.; Schmid, M.W.; Zuppinger-Dingley, D.; Schmid, B.; Wagg, C. Plant responses to diversity-driven selection and associated rhizosphere microbial communities. Funct. Ecol. 2020, 34, 707–722. [Google Scholar] [CrossRef]
- Schreiner, R.P. Effects of native and nonnative arbuscular mycorrhizal fungi on growth and nutrient uptake of ‘Pinot noir’(Vitis vinifera L.) in two soils with contrasting levels of phosphorus. Appl. Soil Ecol. 2007, 36, 205–215. [Google Scholar] [CrossRef]
- Schreiner, R.P. Mycorrhizal colonization of grapevine rootstocks under field conditions. Am. J. Enol. Vitic. 2003, 54, 143–149. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2009, 50, 593–604. [Google Scholar] [CrossRef]
- Prado-Tarango, D.; Mata-González, R.; Melgoza-Castillo, A.; Elias, S.G.; Santellano-Estrada, E. Simulated rainfall sequences affect germination and biomass allocation of Chihuahuan desert native plants. Arid. Land Res. Manag. 2019, 33, 22–36. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. Mutualism, and parasitism: Diversity in function and structure in the arbuscular (VA) mycorrhizal symbiosis. Adv. Bot. Res. 1996, 22, 1–43. [Google Scholar]
- Jones, M.D.; Smith, S.E. Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Canad. J. Bot. 2004, 82, 1089–1109. [Google Scholar] [CrossRef] [Green Version]
- Allen, E.B.; Allen, M.F.; Egerton-Warburton, L.; Corkidi, L.; Gómez-Pompa, A. Impacts of early-and late-seral mycorrhizae during restoration in seasonal tropical forest, Mexico. Ecol. Appl. 2003, 13, 1701–1717. [Google Scholar] [CrossRef]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Smith, F.A.; Grace, E.J.; Smith, S.E. More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 2009, 182, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Blank, R.R.; Sforza, R.; Morgan, T. Medusahead: Available soil N and microbial communities in native and invasive soils. In Proceedings of the Shrublands under Fire: Disturbance and Recovery in a Changing World (RMRS-P-52), Cedar City, UT, USA, 6–8 June 2006; Kitchen, S.G., Pendleton, R.L., Monaco, T.A., Vernon, J., Eds.; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2008; pp. 73–76. [Google Scholar]
- Wilson, G.W.T.; Hartnett, D.C. Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am. J. Bot. 1998, 85, 1732–1738. [Google Scholar] [CrossRef]


| Letter | Treatment Combinations |
|---|---|
| A | High moisture, autoclaved soil, sham inoculum, no competition |
| B | High moisture, field soil, sham inoculum, no competition |
| C | Low moisture, autoclaved soil, sham inoculum, no competition |
| D | Low moisture, field soil, sham inoculum, no competition |
| E | High moisture, autoclaved soil, commercial AMF inoculum, no competition |
| F | High moisture, field soil, commercial AMF inoculum, no competition |
| G | Low moisture, autoclaved soil, commercial AMF inoculum, no competition |
| H | Low moisture, field soil, commercial AMF inoculum, no competition |
| I | High moisture, autoclaved soil, sham inoculum, competition |
| J | High moisture, field soil, sham inoculum, competition |
| K | Low moisture, autoclaved soil, sham inoculum, competition |
| L | Low moisture, field soil, sham inoculum, competition |
| M | High moisture, autoclaved soil, commercial AMF inoculum, competition |
| N | High moisture, field soil, commercial AMF inoculum, competition |
| O | Low moisture, autoclaved soil, commercial AMF inoculum, competition |
| P | Low moisture, field soil, commercial AMF inoculum, competition |
| Treatments | Colonization ± SE | |||||
|---|---|---|---|---|---|---|
| Moisture | Soil | Inoculum | Competition | A. tridentata | A. arbuscula | A. nova |
| High | Autoclaved | Sham | No | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| High | Field | Sham | No | 37.85 ± 4.71 | 8.19 ± 2.72 | 33.8 ± 4.91 |
| Low | Autoclaved | Sham | No | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Low | Field | Sham | No | 28.59 ± 4.06 | 17.61 ± 2.14 | 24.7 ± 1.53 |
| High | Autoclaved | AMF | No | 34.42 ± 7.02 | 4.19 ± 3.03 | 5.14 ± 5.14 |
| High | Field | AMF | No | 33.50 ± 2.52 | 221.61 ± 1.3 | 33 ± 2.42 |
| Low | Autoclaved | AMF | No | 7.32 ± 2.71 | 2.03 ± 2.03 | 13.2 ± 0.63 |
| Low | Field | AMF | No | 29.10 ± 2.27 | 18.73 ± 6.00 | 35.5 ± 2.64 |
| High | Autoclaved | Sham | Yes | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| High | Field | Sham | Yes | 2.04 ± 0.97 | 9.59 ± 2.60 | 2.75 ± 1.66 |
| Low | Autoclaved | Sham | Yes | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Low | Field | Sham | Yes | 1.59 ± 0.77 | 1.01 ± 0.71 | 2.11 ± 1.46 |
| High | Autoclaved | AMF | Yes | 0.54 ± 0.37 | 2.10 ± 2.10 | 0.00 ± 0.00 |
| High | Field | AMF | Yes | 3.30 ± 1.19 | 8.89 ± 1.87 | 2.65 ± 1.33 |
| Low | Autoclaved | AMF | Yes | 0.00 ± 0.00 | 6.63 ± 1.20 | 0.00 ± 0.00 |
| Low | Field | AMF | Yes | 7.52 ± 2.31 | 3.10 ± 0.51 | 4.23 ± 1.31 |
| T. caput-medusae grown with A. tridentata ssp. wyomingensis | ||
| Treatment | Colonization % | SE |
| High moisture, autoclaved soil, sham inoculum, competition | 0.00 | 0.00 |
| High moisture, field soil, sham inoculum, competition | 28.66 | 2.11 |
| Low moisture, autoclaved soil, sham inoculum, competition | 0.00 | 0.00 |
| Low moisture, field soil, sham inoculum, competition | 27.18 | 0.57 |
| High moisture, autoclaved soil, AMF inoculum, competition | 14.60 | 2.39 |
| High moisture, field soil, AMF inoculum, competition | 34.88 | 1.89 |
| Low moisture, autoclaved soil, AMF inoculum, competition | 7.74 | 2.10 |
| Low moisture, field soil, AMF inoculum, competition | 15.04 | 2.28 |
| T. caput-medusae grown with A. arbuscula | ||
| Treatment | Colonization % | SE |
| High moisture, autoclaved soil, sham inoculum, competition | 0.00 | 0.00 |
| High moisture, field soil, sham inoculum, competition | 61.40 | 4.65 |
| Low moisture, autoclaved soil, sham inoculum, competition | 0.00 | 0.00 |
| Low moisture, field soil, sham inoculum, competition | 25.59 | 1.74 |
| High moisture, autoclaved soil, AMF inoculum, competition | 18.58 | 2.16 |
| High moisture, field soil, AMF inoculum, competition | 43.35 | 2.40 |
| Low moisture, autoclaved soil, AMF inoculum, competition | 10.24 | 3.55 |
| Low moisture, field soil, AMF inoculum, competition | 18.92 | 2.10 |
| T. caput-medusae grown with A. nova | ||
| Treatment | Colonization % | SE |
| High moisture, autoclaved soil, sham inoculum, competition | 0.00 | 0.00 |
| High moisture, field soil, sham inoculum, competition | 43.82 | 1.53 |
| Low moisture, autoclaved soil, sham inoculum, competition | 0.00 | 0.00 |
| Low moisture, field soil, sham inoculum, competition | 23.13 | 2.07 |
| High moisture, autoclaved soil, AMF inoculum, competition | 24.68 | 1.38 |
| High moisture, field soil, AMF inoculum, competition | 14.06 | 2.55 |
| Low moisture, autoclaved soil, AMF inoculum, competition | 8.38 | 2.24 |
| Low moisture, field soil, AMF inoculum, competition | 38.14 | 1.50 |
| A. tridentata | Treatment | N (%) | SE | P (%) | SE | K (%) | SE |
| I | 0.54 b | 0.01 | 0.74 ab | 0.01 | 0.39 b | 0.06 | |
| J | 0.68 b | 0.02 | 0.12 a | 0.006 | 0.39 b | 0.03 | |
| K | 0.68 b | 0.04 | 0.09 ab | 0.01 | 0.43 b | 0.05 | |
| L | 0.55 b | 0.01 | 0.07 ab | 0.005 | 0.41 b | 0.05 | |
| M | 0.56 b | 0.04 | 0.07 ab | 0.006 | 0.59 a | 0.06 | |
| N | 0.63 b | 0.03 | 0.10 a | 0.003 | 0.32 b | 0.02 | |
| O | 0.70 a | 0.05 | 0.09 ab | 0.01 | 0.79 a | 0.07 | |
| P | 0.56 b | 0.02 | 0.05 c | 0.006 | 0.41 b | 0.03 | |
| A. arbuscula | Treatment | N (%) | SE | P (%) | SE | K (%) | SE |
| I | 0.60 b | 0.02 | 0.06 b | 0.003 | 0.55 b | 0.03 | |
| J | 0.77 a | 0.02 | 0.06 b | 0.006 | 0.43 b | 0.03 | |
| K | 0.65 b | 0.00 | 0.06 b | 0.003 | 0.33 b | 0.01 | |
| L | 0.62 b | 0.02 | 0.06 b | 0.004 | 0.41 b | 0.03 | |
| M | 0.55 b | 0.03 | 0.06 b | 0.003 | 0.34 b | 0.01 | |
| N | 0.77 a | 0.01 | 0.10 a | 0.003 | 0.32 b | 0.01 | |
| O | 0.71 b | 0.07 | 0.08 a | 0.01 | 0.69 a | 0.12 | |
| P | 0.54 b | 0.01 | 0.05 b | 0.004 | 0.41 b | 0.06 | |
| A. nova | Treatment | N (%) | SE | P (%) | SE | K (%) | SE |
| I | 0.59 b | 0.04 | 0.07 a | 0.01 | 0.40 b | 0.06 | |
| J | 0.59 b | 0.03 | 0.09 a | 0.008 | 0.35 b | 0.02 | |
| K | 0.57 b | 0.04 | 0.09 a | 0.007 | 0.43 b | 0.08 | |
| L | 0.58 b | 0.05 | 0.06 a | 0.01 | 0.40 b | 0.01 | |
| M | 0.82 a | 0.01 | 0.07 a | 0.003 | 0.73 a | 0.07 | |
| N | 0.70 a | 0.04 | 0.08 a | 0.003 | 0.26 b | 0.02 | |
| O | 0.69 b | 0.04 | 0.08 a | 0.01 | 0.51 ab | 0.07 | |
| P | 0.5 b | 0.04 | 0.06 a | 0.01 | 0.43 b | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado-Tarango, D.E.; Mata-Gonzalez, R.; Hovland, M. Drought and Competition Mediate Mycorrhizal Colonization, Growth Rate, and Nutrient Uptake in Three Artemisia Species. Microorganisms 2023, 11, 50. https://doi.org/10.3390/microorganisms11010050
Prado-Tarango DE, Mata-Gonzalez R, Hovland M. Drought and Competition Mediate Mycorrhizal Colonization, Growth Rate, and Nutrient Uptake in Three Artemisia Species. Microorganisms. 2023; 11(1):50. https://doi.org/10.3390/microorganisms11010050
Chicago/Turabian StylePrado-Tarango, David Eduardo, Ricardo Mata-Gonzalez, and Matthew Hovland. 2023. "Drought and Competition Mediate Mycorrhizal Colonization, Growth Rate, and Nutrient Uptake in Three Artemisia Species" Microorganisms 11, no. 1: 50. https://doi.org/10.3390/microorganisms11010050
APA StylePrado-Tarango, D. E., Mata-Gonzalez, R., & Hovland, M. (2023). Drought and Competition Mediate Mycorrhizal Colonization, Growth Rate, and Nutrient Uptake in Three Artemisia Species. Microorganisms, 11(1), 50. https://doi.org/10.3390/microorganisms11010050







