Metabolome and Transcriptome Combinatory Profiling Reveals Fluconazole Resistance Mechanisms of Trichosporon asahii and the Role of Farnesol in Fluconazole Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Antifungal Susceptibility Testing
2.3. Transcriptomic Analysis
2.4. Metabolomic Analysis
2.5. Integrative Analysis of Metabolomic and Transcriptomic Data
3. Results
3.1. Effect of Farnesol on Fluconazole Resistance in T. asahii
3.2. Analysis of RNA-Seq Data
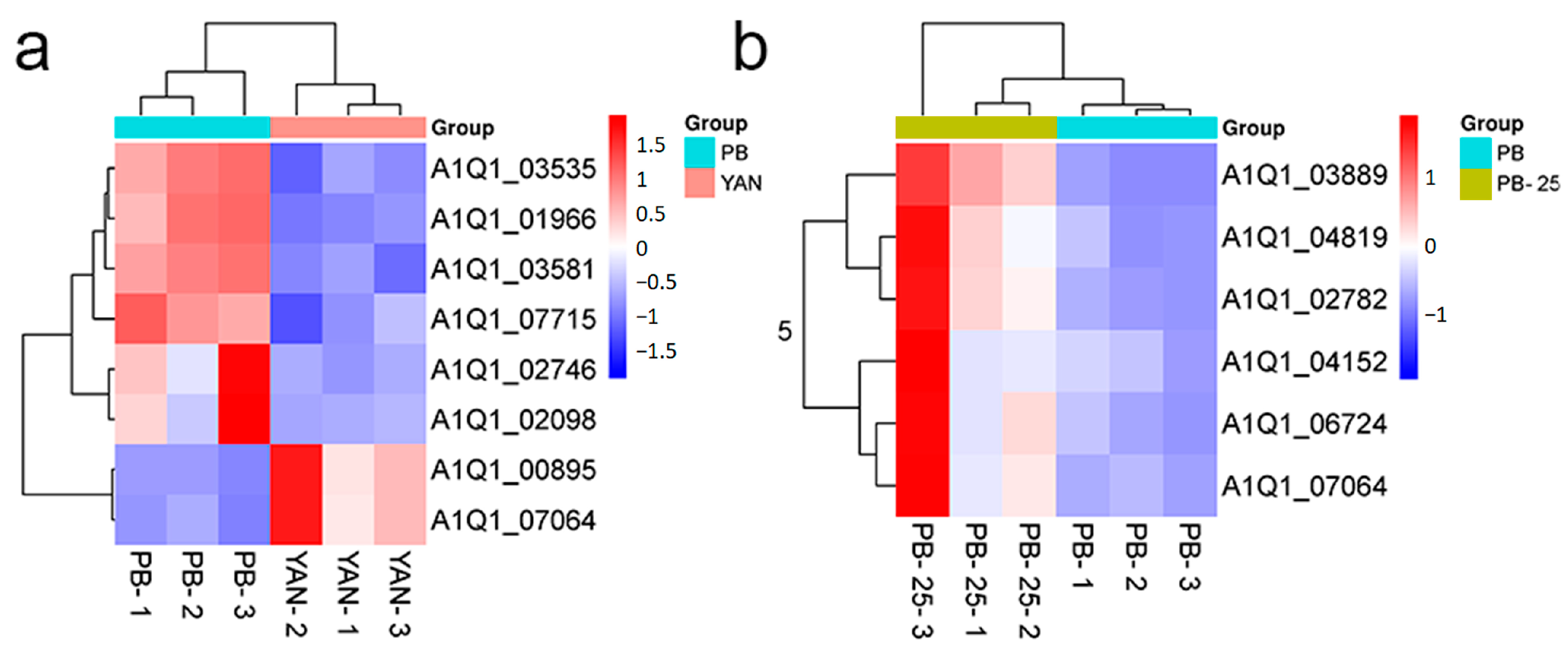
3.3. Differential Expression of Genes between Four Pairwise Strain Combinations
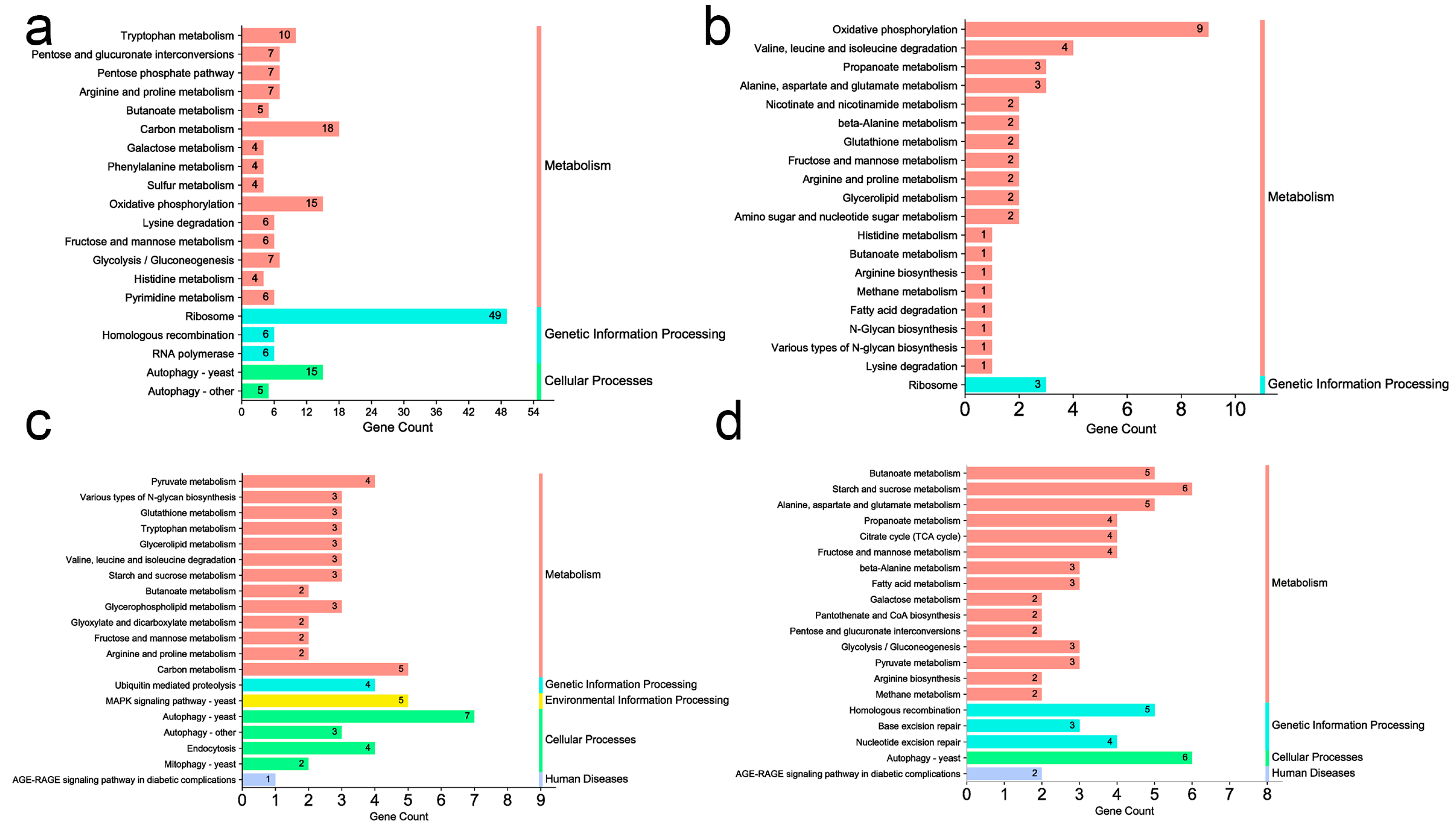
3.4. Gene Ontology (GO) Enrichment and Pathway Analysis of Transcriptomic Data
3.5. Identification of Genes Associated with Efflux Pumps
3.6. Identification of the Genes Associated with Membrane Lipids
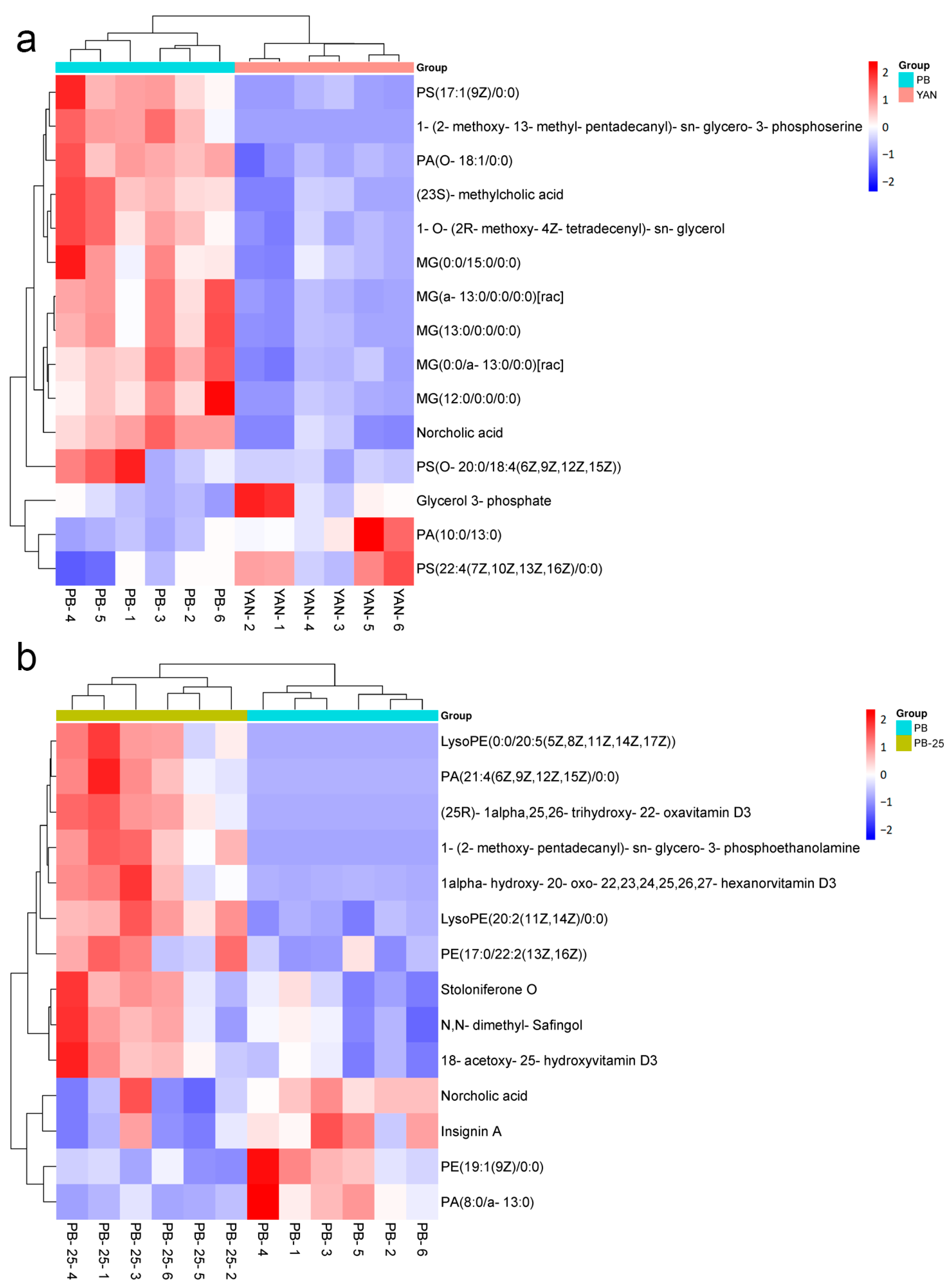
3.7. Differentially Accumulated Metabolites among the Four Comparisons
3.8. Pathway Enrichment of DEMs
3.9. DEMs Related to Membrane Lipids
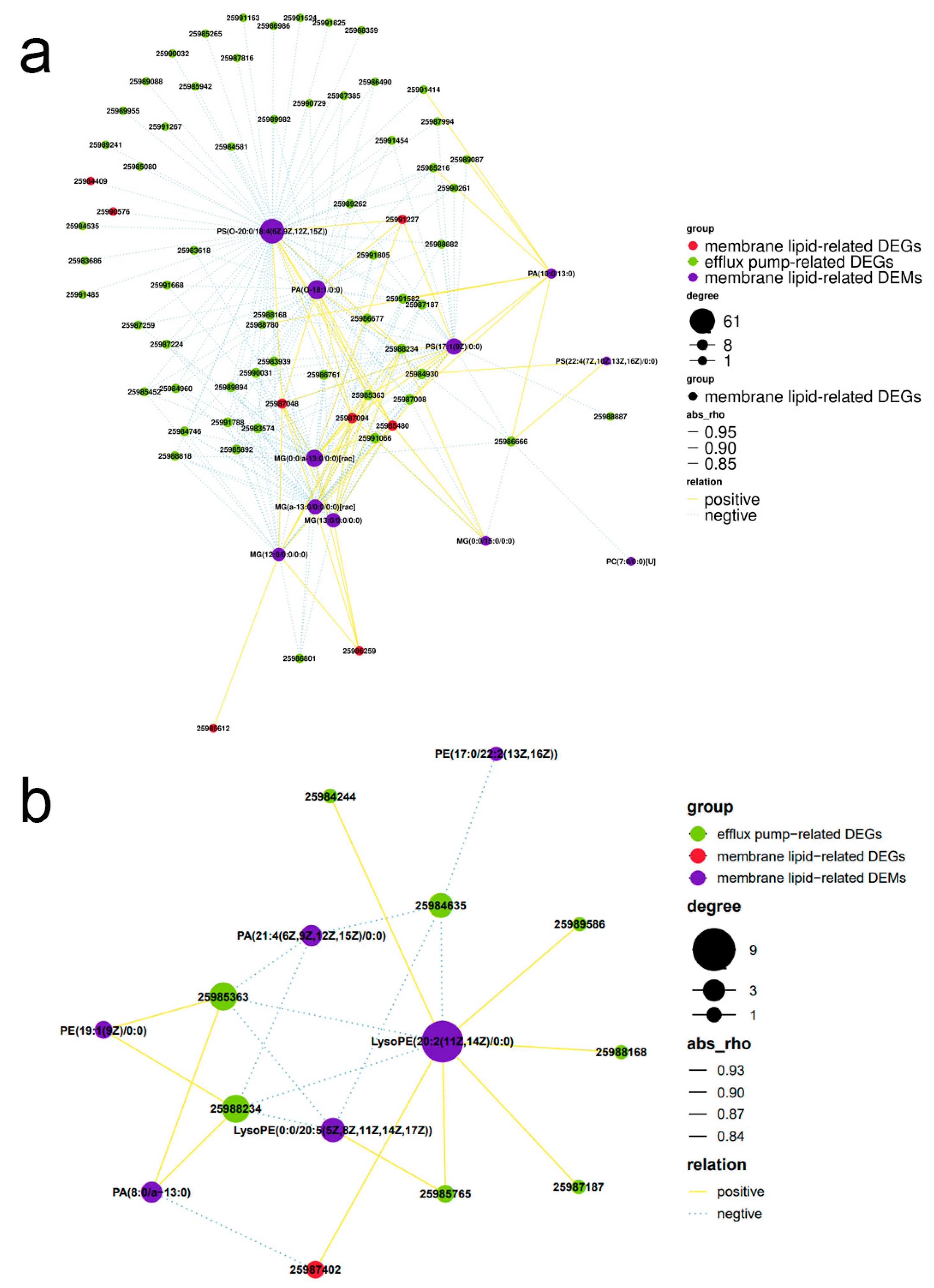
3.10. Association Analysis of DEMs and DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matsumoto, Y.; Yoshikawa, A.; Nagamachi, T.; Sugiyama, Y.; Yamada, T.; Sugita, T. A critical role of calcineurin in stress responses, hyphal formation, and virulence of the pathogenic fungus Trichosporon asahii. Sci. Rep. 2022, 12, 16126. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Ichikawa, T.; Matsukura, M.; Sueda, M.; Takashima, M.; Ikeda, R.; Nishikawa, A.; Shinoda, T. Genetic diversity and biochemical characteristics of Trichosporon asahii isolated from clinical specimens, houses of patients with summer-type-hypersensitivity pneumonitis, and environmental materials. J. Clin. Microbiol. 2001, 39, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Ebright, J.R.; Fairfax, M.R.; Vazquez, J.A. Trichosporon asahii, a non-candida yeast that caused fatal septic shock in a patient without cancer or neutropenia. Clin. Infect. Dis. 2001, 33, E28–E30. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.G.; Falk, R.; Hacham, M.; Theelen, B.; Boekhout, T.; Scorzetti, G.; Shapiro, M.; Block, C.; Salkin, I.F.; Polacheck, I. Multidrug-resistant Trichosporon asahii infection of nongranulocytopenic patients in three intensive care units. J. Clin. Microbiol. 2001, 39, 4420–4425. [Google Scholar] [CrossRef]
- Nakajima, M.; Sugita, T.; Mikami, Y. Granuloma associated with Trichosporon asahii infection in the lung: Unusual pathological findings and PCR detection of Trichosporon DNA. Med. Mycol. 2007, 45, 641–644. [Google Scholar] [CrossRef]
- Lo, C.; Kang, C.L.; Sun, P.L.; Yu, P.H.; Li, W.T. Disseminated Fungal Infection and fungemia Caused by Trichosporon asahii in a Captive Plumed Basilisk (Basiliscus plumifrons). J. Fungi 2021, 7, 1003. [Google Scholar] [CrossRef]
- Martin, S.W.; Konopka, J.B. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot. Cell 2004, 3, 675–684. [Google Scholar] [CrossRef]
- Kelly, S.L.; Arnoldi, A.; Kelly, D.E. Molecular genetic analysis of azole antifungal mode of action. Biochem. Soc. Trans. 1993, 21, 1034–1038. [Google Scholar] [CrossRef]
- Vanden Bossche, H. Biochemical targets for antifungal azole derivatives: Hypothesis on the mode of action. Curr. Top. Med. Mycol. 1985, 1, 313–351. [Google Scholar] [CrossRef]
- Paphitou, N.I.; Ostrosky-Zeichner, L.; Paetznick, V.L.; Rodriguez, J.R.; Chen, E.; Rex, J.H. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob. Agents Chemother. 2002, 46, 1144–1146. [Google Scholar] [CrossRef]
- Kalkanci, A.; Sugita, T.; Arikan, S.; Yucesoy, M.; Ener, B.; Otag, F.; Kiraz, N.; Kustimur, S.; Sancak, B.; Evci, C.; et al. Molecular identification, genotyping, and drug susceptibility of the basidiomycetous yeast pathogen Trichosporon isolated from Turkish patients. Med. Mycol. 2010, 48, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.N.; Yu, S.Y.; Hsueh, P.R.; Al-Hatmi, A.M.S.; Meis, J.F.; Hagen, F.; Xiao, M.; Wang, H.; Barresi, C.; Zhou, M.L.; et al. Invasive infections due to trichosporon: Species distribution, genotyping, and antifungal susceptibilities from a multicenter study in China. J. Clin. Microbiol. 2019, 57, e01505-18. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte resistance to antifungal drugs: Mechanisms and prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Rhome, R.; Del Poeta, M. Lipid signaling in pathogenic fungi. Annu. Rev. Microbiol. 2009, 63, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Décanis, N.; Tazi, N.; Correia, A.; Vilanova, M.; Rouabhia, M. Farnesol, a fungal quorum-sensing molecule triggers Candida albicans morphological changes by downregulating the expression of different secreted aspartyl proteinase genes. Open Microbiol. J. 2011, 5, 119–126. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus aureus response to antimicrobials by the Candida albicans quorum sensing molecule farnesol. Antimicrob. Agents Chemother. 2017, 61, e01573-17. [Google Scholar] [CrossRef]
- Sebaa, S.; Boucherit-Otmani, Z.; Courtois, P. Effects of tyrosol and farnesol on Candida albicans biofilm. Mol. Med. Rep. 2019, 19, 3201–3209. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, H.; Wang, C.; Ding, X.; Zhang, D.; Tan, X.; Chen, J.; Hu, S.; Yang, R. Genomic and transcriptome identification of fluconazole-resistant genes for Trichosporon asahii. Med. Mycol. 2020, 58, 393–400. [Google Scholar] [CrossRef]
- Jakab, Á.; Balla, N.; Ragyák, Á.; Nagy, F.; Kovács, F.; Sajtos, Z.; Tóth, Z.; Borman, A.M.; Pócsi, I.; Baranyai, E.; et al. Transcriptional profiling of the Candida auris response to exogenous farnesol exposure. mSphere 2021, 6, e0071021. [Google Scholar] [CrossRef]
- Han, T.L.; Cannon, R.D.; Villas-Bôas, S.G. The metabolic response of Candida albicans to farnesol under hyphae-inducing conditions. FEMS Yeast Res. 2012, 12, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Desjardins, P.; Conklin, D. NanoDrop microvolume quantitation of nucleic acids. J. Vis. Exp. 2010, 22, 2565. [Google Scholar] [CrossRef]
- Comparison of Agilent 2100 bioanalyzer and Caliper Life Sciences GX II in functionality, total RNA scoring algorithms and reproducibility to evaluate total RNA integrity. J. Biomol. Tech. 2012, 23, S49–S50.
- Patnaik, B.B.; Hwang, H.J.; Kang, S.W.; Park, S.Y.; Wang, T.H.; Park, E.B.; Chung, J.M.; Song, D.K.; Kim, C.; Kim, S.; et al. Transcriptome Characterization for Non-Model Endangered Lycaenids, Protantigius superans and Spindasis takanosis, Using Illumina HiSeq 2500 Sequencing. Int. J. Mol. Sci. 2015, 16, 29948–29970. [Google Scholar] [CrossRef] [PubMed]
- Hestand, M.S.; Ameur, A. The versatility of SMRT sequencing. Genes 2019, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Favre, L.; Ortalo-Magné, A.; Greff, S.; Pérez, T.; Thomas, O.P.; Martin, J.C.; Culioli, G. Discrimination of four marine biofilm-forming bacteria by LC-MS metabolomics and influence of culture parameters. J. Proteome Res. 2017, 16, 1962–1975. [Google Scholar] [CrossRef]
- Mo, C.; Valachovic, M.; Randall, S.K.; Nickels, J.T.; Bard, M. Protein-protein interactions among C-4 demethylation enzymes involved in yeast sterol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 9739–9744. [Google Scholar] [CrossRef]
- Narayanan, V.; Motlekar, S.; Kadhe, G.; Bhagat, S. Efficacy and safety of nadifloxacin for bacterial skin infections: Results from clinical and post-marketing studies. Dermatol. Ther. 2014, 4, 233–248. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef]
- Trof, R.J.; Beishuizen, A.; Debets-Ossenkopp, Y.J.; Girbes, A.R.; Groeneveld, A.B. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007, 33, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Wei, X.; Ma, M.; Chen, X.J.; Xu, S.B. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob. Agents Chemother. 2012, 56, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Quatresooz, P.; Vroome, V.; Borgers, M.; Cauwenbergh, G.; Piérard, G.E. Novelties in the multifaceted miconazole effects on skin disorders. Expert. Opin. Pharmacother. 2008, 9, 1927–1934. [Google Scholar] [CrossRef]
- Wei, J.; Bi, Y.; Xue, H.; Wang, Y.; Zong, Y.; Prusky, D. Antifungal activity of cinnamaldehyde against Fusarium sambucinum involves inhibition of ergosterol biosynthesis. J. Appl. Microbiol. 2020, 129, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Zárate, S.G.; Morales, P.; Świderek, K.; Bolanos-Garcia, V.M.; Bastida, A. A molecular modeling approach to identify novel inhibitors of the major facilitator superfamily of efflux pump transporters. Antibiotics 2019, 8, 25. [Google Scholar] [CrossRef]
- Wu, C.P.; Ambudkar, S.V. The pharmacological impact of ATP-binding cassette drug transporters on vemurafenib-based therapy. Acta Pharm. Sin. B 2014, 4, 105–111. [Google Scholar] [CrossRef]
- Quistgaard, E.M.; Löw, C.; Guettou, F.; Nordlund, P. Understanding transport by the major facilitator superfamily (MFS): Structures pave the way. Nat. Rev. Mol. Cell Biol. 2016, 17, 123–132. [Google Scholar] [CrossRef]
- Gustot, A.; Smriti, J.M.; Ruysschaert, J.M.; McHaourab, H.; Govaerts, C. Lipid composition regulates the orientation of transmembrane helices in HorA, an ABC multidrug transporter. J. Biol. Chem. 2010, 285, 14144–14151. [Google Scholar] [CrossRef]
- Marcoux, J.; Wang, S.C.; Politis, A.; Reading, E.; Ma, J.; Biggin, P.C.; Zhou, M.; Tao, H.; Zhang, Q.; Chang, G.; et al. Mass spectrometry reveals synergistic effects of nucleotides, lipids, and drugs binding to a multidrug resistance efflux pump. Proc. Natl. Acad. Sci. USA 2013, 110, 9704–9709. [Google Scholar] [CrossRef]


| Strain | MIC (μg/mL) |
|---|---|
| YAN | 2 |
| YAN-25 | 1 |
| PB | 32 |
| PB-25 | 128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Yang, W.; Yang, A.; Chen, D.; Wang, C.; Ling, S.; Cao, S.; Zuo, Z.; Wang, Y.; Zhong, Z.; et al. Metabolome and Transcriptome Combinatory Profiling Reveals Fluconazole Resistance Mechanisms of Trichosporon asahii and the Role of Farnesol in Fluconazole Tolerance. Microorganisms 2023, 11, 2798. https://doi.org/10.3390/microorganisms11112798
Ma X, Yang W, Yang A, Chen D, Wang C, Ling S, Cao S, Zuo Z, Wang Y, Zhong Z, et al. Metabolome and Transcriptome Combinatory Profiling Reveals Fluconazole Resistance Mechanisms of Trichosporon asahii and the Role of Farnesol in Fluconazole Tolerance. Microorganisms. 2023; 11(11):2798. https://doi.org/10.3390/microorganisms11112798
Chicago/Turabian StyleMa, Xiaoping, Wanling Yang, Aining Yang, Dong Chen, Chengdong Wang, Shanshan Ling, Sanjie Cao, Zhicai Zuo, Ya Wang, Zhijun Zhong, and et al. 2023. "Metabolome and Transcriptome Combinatory Profiling Reveals Fluconazole Resistance Mechanisms of Trichosporon asahii and the Role of Farnesol in Fluconazole Tolerance" Microorganisms 11, no. 11: 2798. https://doi.org/10.3390/microorganisms11112798
APA StyleMa, X., Yang, W., Yang, A., Chen, D., Wang, C., Ling, S., Cao, S., Zuo, Z., Wang, Y., Zhong, Z., Peng, G., He, M., & Gu, Y. (2023). Metabolome and Transcriptome Combinatory Profiling Reveals Fluconazole Resistance Mechanisms of Trichosporon asahii and the Role of Farnesol in Fluconazole Tolerance. Microorganisms, 11(11), 2798. https://doi.org/10.3390/microorganisms11112798







