Drug Discovery for Cutaneous Leishmaniasis: A Review of Developments in the Past 15 Years
Abstract
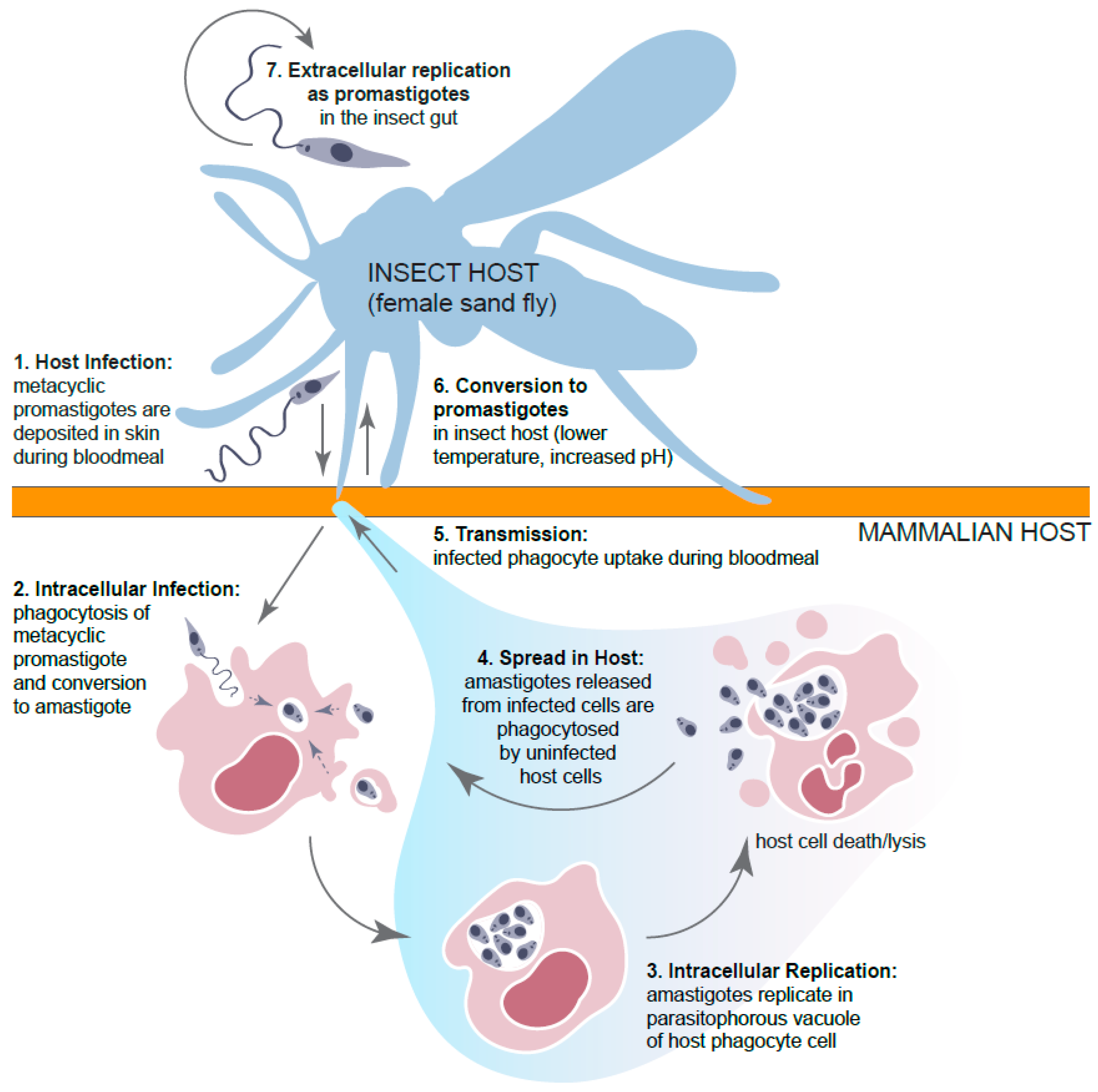
:1. Introduction
2. Drug Discovery Targeting a Single Leishmania Species
3. Drug Discovery Targeting Multiple Species of Leishmania
4. Drug Discovery Targeting Multiple Eukaryotic and Prokaryotic Pathogens
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bari, A.U.; Rahman, S.B. Cutaneous leishmaniasis: An overview of parasitology and host-parasite-vector inter relationship. J. Pak. Assoc. Dermatol. 2008, 18, 42–48. [Google Scholar]
- Pedrique, B.; Strub-Wourgaft, N.; Some, C.; Olliaro, P.; Trouiller, P.; Ford, N.; Pecoul, B.; Bradol, J.H. The drug and vaccine landscape for neglected diseases (2000-11): A systematic assessment. Lancet Glob. Health 2013, 1, e371–e379. [Google Scholar] [CrossRef]
- Harhay, M.O.; Olliaro, P.L.; Vaillant, M.; Chappuis, F.; Lima, M.A.; Ritmeijer, K.; Costa, C.H.; Costa, D.L.; Rijal, S.; Sundar, S.; et al. Who is a typical patient with visceral leishmaniasis? Characterizing the demographic and nutritional profile of patients in Brazil, East Africa, and South Asia. Am. J. Trop. Med. Hyg. 2011, 84, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Malafaia, G. Protein-energy malnutrition as a risk factor for visceral leishmaniasis: A review. Parasite Immunol. 2009, 31, 587–596. [Google Scholar] [CrossRef]
- Ready, P.D. Epidemiology of visceral leishmaniasis. Clin. Epidemiol. 2014, 6, 147–154. [Google Scholar] [CrossRef]
- Zacarias, D.A.; Rolao, N.; de Pinho, F.A.; Sene, I.; Silva, J.C.; Pereira, T.C.; Costa, D.L.; Costa, C.H.N. Causes and consequences of higher Leishmania infantum burden in patients with kala-azar: A study of 625 patients. Trop. Med. Int. Health 2017, 22, 679–687. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Bennis, I.; Thys, S.; Filali, H.; De Brouwere, V.; Sahibi, H.; Boelaert, M. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect. Dis. Poverty 2017, 6, 46. [Google Scholar] [CrossRef]
- Fikre, H.; Mohammed, R.; Atinafu, S.; van Griensven, J.; Diro, E. Clinical features and treatment response of cutaneous leishmaniasis in North-West Ethiopia. Trop. Med. Int. Health 2017, 22, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Marsden, P.D. Mucosal leishmaniasis (“espundia” Escomel, 1911). Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 859–876. [Google Scholar] [CrossRef]
- De Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Cipriano, N.; Cox, B.; Satoskar, A.R. Mechanisms of Immunopathogenesis in Cutaneous Leishmaniasis and Post Kala-azar Dermal Leishmaniasis (PKDL). Front. Cell Infect. Microbiol. 2021, 11, 685296. [Google Scholar] [CrossRef]
- Bahrami, F.; Harandi, A.M.; Rafati, S. Biomarkers of Cutaneous Leishmaniasis. Front. Cell Infect. Microbiol. 2018, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Savioli, L.; Fenwick, A. Neglected tropical diseases of the Middle East and North Africa: Review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 2012, 6, e1475. [Google Scholar] [CrossRef]
- Hotez, P.J.; Woc-Colburn, L.; Bottazzi, M.E. Neglected tropical diseases in Central America and Panama: Review of their prevalence, populations at risk and impact on regional development. Int. J. Parasitol. 2014, 44, 597–603. [Google Scholar] [CrossRef]
- Karimkhani, C.; Wanga, V.; Coffeng, L.E.; Naghavi, P.; Dellavalle, R.P.; Naghavi, M. Global burden of cutaneous leishmaniasis: A cross-sectional analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 584–591. [Google Scholar] [CrossRef]
- Alexander, B.; Maroli, M. Control of phlebotomine sandflies. Med. Vet. Entomol. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Roberts, M.T. Current understandings on the immunology of leishmaniasis and recent developments in prevention and treatment. Br. Med. Bull. 2005, 75–76, 115–130. [Google Scholar] [CrossRef]
- Choudhury, R.; Das, P.; De, T.; Chakraborti, T. 115 kDa serine protease confers sustained protection to visceral leishmaniasis caused by Leishmania donovani via IFN-gamma induced down-regulation of TNF-alpha mediated MMP-9 activity. Immunobiology 2013, 218, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: Drug or vaccine therapy? Drug Des. Devel. Ther. 2018, 12, 25–40. [Google Scholar] [CrossRef]
- Giunchetti, R.C.; Reis, A.B.; da Silveira-Lemos, D.; Martins-Filho, O.A.; Correa-Oliveira, R.; Bethony, J.; Vale, A.M.; da Silva Quetz, J.; Bueno, L.L.; Franca-Silva, J.C.; et al. Antigenicity of a whole parasite vaccine as promising candidate against canine leishmaniasis. Res. Vet. Sci. 2008, 85, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Khalil, E.A.; El Hassan, A.M.; Zijlstra, E.E.; Mukhtar, M.M.; Ghalib, H.W.; Musa, B.; Ibrahim, M.E.; Kamil, A.A.; Elsheikh, M.; Babiker, A.; et al. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: A randomised, double-blind, BCG-controlled trial in Sudan. Lancet 2000, 356, 1565–1569. [Google Scholar] [CrossRef]
- Dinesh, D.S.; Das, M.L.; Picado, A.; Roy, L.; Rijal, S.; Singh, S.P.; Das, P.; Boelaert, M.; Coosemans, M. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl. Trop. Dis. 2010, 4, e859. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.R.; Kaye, P.; Croft, S.L.; Sundar, S. Leishmaniasis: New approaches to disease control. BMJ 2003, 326, 377–382. [Google Scholar] [CrossRef]
- Gonzalez, U.; Pinart, M.; Sinclair, D.; Firooz, A.; Enk, C.; Velez, I.D.; Esterhuizen, T.M.; Tristan, M.; Alvar, J. Vector and reservoir control for preventing leishmaniasis. Cochrane Database Syst. Rev. 2015, 2015, CD008736. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; Kager, P.A.; Bart, A.; de Vries, H.J.; van Thiel, P.P.; Leenstra, T.; de Vries, P.J.; van Vugt, M.; Grobusch, M.P.; van Gool, T. Species-directed therapy for leishmaniasis in returning travellers: A comprehensive guide. PLoS Negl. Trop. Dis. 2014, 8, e2832. [Google Scholar] [CrossRef]
- Weina, P.J.; Neafie, R.C.; Wortmann, G.; Polhemus, M.; Aronson, N.E. Old world leishmaniasis: An emerging infection among deployed US military and civilian workers. Clin. Infect. Dis. 2004, 39, 1674–1680. [Google Scholar] [CrossRef]
- Target Product Profile for Cutaneous Leishmaniasis. Available online: https://dndi.org/diseases/cutaneous-leishmaniasis/target-product-profile/ (accessed on 30 August 2023).
- Lamotte, S.; Aulner, N.; Spath, G.F.; Prina, E. Discovery of novel hit compounds with broad activity against visceral and cutaneous Leishmania species by comparative phenotypic screening. Sci. Rep. 2019, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, B.; Jones, A.J.; Sykes, M.L.; Shelper, T.B.; Davis, R.A.; Avery, V.M. Screening a Natural Product-Based Library against Kinetoplastid Parasites. Molecules 2017, 22, 1715. [Google Scholar] [CrossRef]
- Harris, M.T.; Mitchell, W.G.; Morris, J.C. Targeting protozoan parasite metabolism: Glycolytic enzymes in the therapeutic crosshairs. Curr. Med. Chem. 2014, 21, 1668–1678. [Google Scholar] [CrossRef]
- Sharma, N.; Shukla, A.K.; Das, M.; Dubey, V.K. Evaluation of plumbagin and its derivative as potential modulators of redox thiol metabolism of Leishmania parasite. Parasitol. Res. 2012, 110, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lira, R.; Hansell, E.; McKerrow, J.H.; Roush, W.R. Synthesis of macrocyclic trypanosomal cysteine protease inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 5860–5863. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.K.; Datta, R.; Sen, B. Antiparasitic chemotherapy: Tinkering with the purine salvage pathway. Adv. Exp. Med. Biol. 2008, 625, 116–132. [Google Scholar] [CrossRef]
- Balana-Fouce, R.; Alvarez-Velilla, R.; Fernandez-Prada, C.; Garcia-Estrada, C.; Reguera, R.M. Trypanosomatids topoisomerase re-visited. New structural findings and role in drug discovery. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 326–337. [Google Scholar] [CrossRef]
- Gilbert, I.H. Inhibitors of dihydrofolate reductase in Leishmania and trypanosomes. Biochim. Biophys. Acta 2002, 1587, 249–257. [Google Scholar] [CrossRef]
- Ben Khalaf, N.; De Muylder, G.; Louzir, H.; McKerrow, J.; Chenik, M. Leishmania major protein disulfide isomerase as a drug target: Enzymatic and functional characterization. Parasitol. Res. 2012, 110, 1911–1917. [Google Scholar] [CrossRef]
- Crunkhorn, S. Antiparasitic drugs: Proteasome inhibition combats kinetoplastid infections. Nat. Rev. Drug Discov. 2016, 15, 676–677. [Google Scholar] [CrossRef]
- Gille, C.; Goede, A.; Schloetelburg, C.; Preissner, R.; Kloetzel, P.M.; Gobel, U.B.; Frommel, C. A comprehensive view on proteasomal sequences: Implications for the evolution of the proteasome. J. Mol. Biol. 2003, 326, 1437–1448. [Google Scholar] [CrossRef]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.; Myburgh, E.; Gao, M.Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef]
- Mbang-Benet, D.E.; Sterkers, Y.; Morelle, C.; Kebe, N.M.; Crobu, L.; Portales, P.; Coux, O.; Hernandez, J.F.; Meghamla, S.; Pages, M.; et al. The bacterial-like HslVU protease complex subunits are involved in the control of different cell cycle events in trypanosomatids. Acta Trop. 2014, 131, 22–31. [Google Scholar] [CrossRef]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B.; et al. Discovery and Characterization of Clinical Candidate LXE408 as a Kinetoplastid-Selective Proteasome Inhibitor for the Treatment of Leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef]
- Gazanion, E.; Fernandez-Prada, C.; Papadopoulou, B.; Leprohon, P.; Ouellette, M. Cos-Seq for high-throughput identification of drug target and resistance mechanisms in the protozoan parasite Leishmania. Proc. Natl. Acad. Sci. USA 2016, 113, E3012–E3021. [Google Scholar] [CrossRef] [PubMed]
- Al Khoury, C.; Nemer, G.; Guillot, J.; Tokajian, S. Absolute quantification of gene expression in drug discovery using RT-qPCR: Case of a drug used in the treatment of leishmaniasis. Res. Vet. Sci. 2022, 153, 17–22. [Google Scholar] [CrossRef]
- Gomes, L.I.; Gonzaga, F.M.; de Morais-Teixeira, E.; de Souza-Lima, B.S.; Freire, V.V.; Rabello, A. Validation of quantitative real-time PCR for the in vitro assessment of antileishmanial drug activity. Exp. Parasitol. 2012, 131, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Dashatan, N.; Rezaei-Tavirani, M.; Ahmadi, N. A quantitative proteomic and bioinformatics analysis of proteins in metacyclogenesis of Leishmania tropica. Acta Trop. 2020, 202, 105227. [Google Scholar] [CrossRef]
- Chavali, A.K.; Blazier, A.S.; Tlaxca, J.L.; Jensen, P.A.; Pearson, R.D.; Papin, J.A. Metabolic network analysis predicts efficacy of FDA-approved drugs targeting the causative agent of a neglected tropical disease. BMC Syst. Biol. 2012, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Majid Shah, S.; Ullah, F.; Ayaz, M.; Sadiq, A.; Hussain, S.; Ali Shah, A.U.; Adnan Ali Shah, S.; Wadood, A.; Nadhman, A. beta-Sitosterol from Ifloga spicata (Forssk.) Sch. Bip. as potential anti-leishmanial agent against Leishmania tropica: Docking and molecular insights. Steroids 2019, 148, 56–62. [Google Scholar] [CrossRef]
- Mendez-Cuesta, C.A.; Mendez-Lucio, O.; Castillo, R. Homology modeling, docking and molecular dynamics of the Leishmania mexicana arginase: A description of the catalytic site useful for drug design. J. Mol. Graph. Model. 2012, 38, 50–59. [Google Scholar] [CrossRef]
- Caridha, D.; Parriot, S.; Hudson, T.H.; Lang, T.; Ngundam, F.; Leed, S.; Sena, J.; Harris, M.; O’Neil, M.; Sciotti, R.; et al. Use of Optical Imaging Technology in the Validation of a New, Rapid, Cost-Effective Drug Screen as Part of a Tiered In Vivo Screening Paradigm for Development of Drugs To Treat Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2017, 61, e02048-16. [Google Scholar] [CrossRef] [PubMed]
- Lackovic, K.; Parisot, J.P.; Sleebs, N.; Baell, J.B.; Debien, L.; Watson, K.G.; Curtis, J.M.; Handman, E.; Street, I.P.; Kedzierski, L. Inhibitors of Leishmania GDP-mannose pyrophosphorylase identified by high-throughput screening of small-molecule chemical library. Antimicrob. Agents Chemother. 2010, 54, 1712–1719. [Google Scholar] [CrossRef]
- Fey, P.; Chartomatsidou, R.; Kiefer, W.; Mottram, J.C.; Kersten, C.; Schirmeister, T. New aziridine-based inhibitors of cathepsin L-like cysteine proteases with selectivity for the Leishmania cysteine protease LmCPB2.8. Eur. J. Med. Chem. 2018, 156, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Bandeira, C.C.; de Faria, B.G.; Alves, M.L.R.; Vieira, F.O.; Giunchetti, R.C.; Uzonna, J.E.; Teixeira-Carvalho, A.; Peruhype-Magalhaes, V.; Souza-Fagundes, E.M. An ex vivo multiparametric flow cytometry assay using human whole blood to simultaneously measure cytotoxicity and leishmanicidal activities. Exp. Parasitol. 2020, 216, 107940. [Google Scholar] [CrossRef]
- Schroder, J.; Noack, S.; Marhofer, R.J.; Mottram, J.C.; Coombs, G.H.; Selzer, P.M. Identification of semicarbazones, thiosemicarbazones and triazine nitriles as inhibitors of Leishmania mexicana cysteine protease CPB. PLoS ONE 2013, 8, e77460. [Google Scholar] [CrossRef]
- Alves, K.M.A.; Cardoso, F.J.B.; Honorio, K.M.; de Molfetta, F.A. Design of Inhibitors for Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH) Enzyme of Leishmania mexicana. Med. Chem. 2020, 16, 784–795. [Google Scholar] [CrossRef]
- Costa, C.; Bichara, T.W.; Gomes, G.C.; Dos Santos, A.M.; da Costa, K.S.; Lima, A.; Alves, C.N.; Lameira, J. Unraveling the conformational dynamics of glycerol 3-phosphate dehydrogenase, a nicotinamide adenine dinucleotide-dependent enzyme of Leishmania mexicana. J. Biomol. Struct. Dyn. 2021, 39, 2044–2055. [Google Scholar] [CrossRef]
- Angelo de Souza, L.; Silva, E.B.M.; de Melo Agripino, J.; Souza Onofre, T.; Apaza Calla, L.F.; Heimburg, T.; Ghazy, E.; Bayer, T.; Ferraz da Silva, V.H.; Dutra Ribeiro, P.; et al. Histone deacetylases inhibitors as new potential drugs against Leishmania braziliensis, the main causative agent of new world tegumentary leishmaniasis. Biochem. Pharmacol. 2020, 180, 114191. [Google Scholar] [CrossRef]
- Awada, B.; Hamie, M.; El Hajj, R.; Derbaj, G.; Najm, R.; Makhoul, P.; Ali, D.H.; Abou Fayad, A.G.; El Hajj, H. HAS 1: A natural product from soil-isolated Streptomyces species with potent activity against cutaneous leishmaniasis caused by Leishmania tropica. Front. Pharmacol. 2022, 13, 1023114. [Google Scholar] [CrossRef]
- Peretz, A.; Zabari, L.; Pastukh, N.; Avital, N.; Masaphy, S. In Vitro Antileishmanial Activity of a Black Morel, Morchella importuna (Ascomycetes). Int. J. Med. Mushrooms 2018, 20, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Chadbourne, F.L.; Raleigh, C.; Ali, H.Z.; Denny, P.W.; Cobb, S.L. Studies on the antileishmanial properties of the antimicrobial peptides temporin A, B and 1Sa. J. Pept. Sci. 2011, 17, 751–755. [Google Scholar] [CrossRef]
- Mbekeani, A.J.; Jones, R.S.; Bassas Llorens, M.; Elliot, J.; Regnault, C.; Barrett, M.P.; Steele, J.; Kebede, B.; Wrigley, S.K.; Evans, L.; et al. Mining for natural product antileishmanials in a fungal extract library. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 118–128. [Google Scholar] [CrossRef]
- Braga, M.A.; de Oliveira Rodrigues, R.; Yaochite, J.N.U.; Sasahara, G.L.; Santos, F.A.; Fonseca, F.R.M.; de Castro Rodrigues, N.L.; Teixeira, M.J.; Junior, J.T.C.; Rodrigues, A.L.M.; et al. Astronium fraxinifolium Schott Exerts Leishmanicidal Activity by Providing a Classically Polarized Profile in Infected Macrophages. Acta Parasitol. 2020, 65, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.P.; Tomaz, D.C.; Angelo de Souza, L.; Onofre, T.S.; Aquiles de Menezes, W.; Almeida-Silva, J.; Suarez-Fontes, A.M.; Rogeria de Almeida, M.; Manoel da Silva, A.; Bressan, G.C.; et al. Synthesis of cinnamic acid derivatives and leishmanicidal activity against Leishmania braziliensis. Eur. J. Med. Chem. 2019, 183, 111688. [Google Scholar] [CrossRef]
- McMahon-Pratt, D.; Alexander, J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 2004, 201, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Aulner, N.; Danckaert, A.; Rouault-Hardoin, E.; Desrivot, J.; Helynck, O.; Commere, P.H.; Munier-Lehmann, H.; Spath, G.F.; Shorte, S.L.; Milon, G.; et al. High content analysis of primary macrophages hosting proliferating Leishmania amastigotes: Application to anti-leishmanial drug discovery. PLoS Negl. Trop. Dis. 2013, 7, e2154. [Google Scholar] [CrossRef]
- Agostino, V.S.; Trinconi, C.M.; Galuppo, M.K.; Price, H.; Uliana, S.R.B. Evaluation of NanoLuc, RedLuc and Luc2 as bioluminescent reporters in a cutaneous leishmaniasis model. Acta Trop. 2020, 206, 105444. [Google Scholar] [CrossRef] [PubMed]
- Reimao, J.Q.; Trinconi, C.T.; Yokoyama-Yasunaka, J.K.; Miguel, D.C.; Kalil, S.P.; Uliana, S.R. Parasite burden in Leishmania (Leishmania) amazonensis-infected mice: Validation of luciferase as a quantitative tool. J. Microbiol. Methods 2013, 93, 95–101. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Boechat, N.; Pinheiro, L.C.; Bastos, M.M.; Costa, C.C.; Bartholomeu, J.C.; da Costa, T.H. Novel selective inhibitor of Leishmania (Leishmania) amazonensis arginase. Chem. Biol. Drug Des. 2015, 86, 969–978. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Brogi, S.; Grillo, A.; Campiani, G.; Gemma, S.; Vieira, P.C.; Maquiaveli, C.D.C. Cinnamic acids derived compounds with antileishmanial activity target Leishmania amazonensis arginase. Chem. Biol. Drug Des. 2019, 93, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.R.; Come, J.; Brogi, S.; Calderone, V.; Chemi, G.; Campiani, G.; Oliveira, T.; Pham, T.N.; Pudlo, M.; Girard, C.; et al. Cinnamides Target Leishmania amazonensis Arginase Selectively. Molecules 2020, 25, 5271. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Neto, V.V.; Cunha-Junior, E.F.; Canto-Cavalheiro, M.M.; Atella, G.C.; Fernandes, T.A.; Costa, P.R.; Torres-Santos, E.C. Antileishmanial Activity of Ezetimibe: Inhibition of Sterol Biosynthesis, In Vitro Synergy with Azoles, and Efficacy in Experimental Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2016, 60, 6844–6852. [Google Scholar] [CrossRef]
- Fonseca-Silva, F.; Inacio, J.D.; Canto-Cavalheiro, M.M.; Menna-Barreto, R.F.; Almeida-Amaral, E.E. Oral Efficacy of Apigenin against Cutaneous Leishmaniasis: Involvement of Reactive Oxygen Species and Autophagy as a Mechanism of Action. PLoS Negl. Trop. Dis. 2016, 10, e0004442. [Google Scholar] [CrossRef] [PubMed]
- Marinho, F.A.; Sangenito, L.S.; Oliveira, S.S.C.; De Arruda, L.B.; D’Avila-Levy, C.M.; Santos, A.L.S.; Branquinha, M.H. The potent cell permeable calpain inhibitor MDL28170 affects the interaction of Leishmania amazonensis with macrophages and shows anti-amastigote activity. Parasitol. Int. 2017, 66, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Antonello, A.M.; Sartori, T.; Folmer Correa, A.P.; Brandelli, A.; Heermann, R.; Rodrigues Junior, L.C.; Peres, A.; Romao, P.R.T.; Da Silva, O.S. Entomopathogenic bacteria Photorhabdus luminescens as drug source against Leishmania amazonensis. Parasitology 2018, 145, 1065–1074. [Google Scholar] [CrossRef]
- Do Nascimento, A.M.; Soares, M.G.; da Silva Torchelsen, F.K.; de Araujo, J.A.; Lage, P.S.; Duarte, M.C.; Andrade, P.H.; Ribeiro, T.G.; Coelho, E.A.; do Nascimento, A.M. Antileishmanial activity of compounds produced by endophytic fungi derived from medicinal plant Vernonia polyanthes and their potential as source of bioactive substances. World J. Microbiol. Biotechnol. 2015, 31, 1793–1800. [Google Scholar] [CrossRef]
- Rabito, M.F.; Britta, E.A.; Pelegrini, B.L.; Scariot, D.B.; Almeida, M.B.; Nixdorf, S.L.; Nakamura, C.V.; Ferreira, I.C. In vitro and in vivo antileishmania activity of sesquiterpene lactone-rich dichloromethane fraction obtained from Tanacetum parthenium (L.) Schultz-Bip. Exp. Parasitol. 2014, 143, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, F.; de Oliveira, A.E.R.; Abreu-Silva, A.L.; da Silva Calabrese, K. In vitro activity of Morinda citrifolia Linn. fruit juice against the axenic amastigote form of Leishmania amazonensis and its hydrogen peroxide induction capacity in BALB/c peritoneal macrophages. BMC Res. Notes 2018, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Pinon, A.; Sculli, R.; Setzer, W.N. Chemistry and leishmanicidal activity of the essential oil from Artemisia absinthium from Cuba. Nat. Prod. Commun. 2014, 9, 1799–1804. [Google Scholar]
- Felix, M.B.; de Araujo, R.S.A.; Barros, R.P.C.; de Simone, C.A.; Rodrigues, R.R.L.; de Lima Nunes, T.A.; da Franca Rodrigues, K.A.; Junior, F.; Muratov, E.; Scotti, L.; et al. Computer-Assisted Design of Thiophene-Indole Hybrids as Leishmanial Agents. Curr. Top. Med. Chem. 2020, 20, 1704–1719. [Google Scholar] [CrossRef]
- Felix, M.B.; de Souza, E.R.; de Lima, M.; Frade, D.K.G.; Serafim, V.L.; Rodrigues, K.; Neris, P.; Ribeiro, F.F.; Scotti, L.; Scotti, M.T.; et al. Antileishmanial activity of new thiophene-indole hybrids: Design, synthesis, biological and cytotoxic evaluation, and chemometric studies. Bioorg. Med. Chem. 2016, 24, 3972–3977. [Google Scholar] [CrossRef] [PubMed]
- Luna, I.S.; Souza, T.A.; da Silva, M.S.; Franca Rodrigues, K.A.D.; Scotti, L.; Scotti, M.T.; Mendonca-Junior, F.J.B. Computer-Aided drug design of new 2-amino-thiophene derivatives as anti-leishmanial agents. Eur. J. Med. Chem. 2023, 250, 115223. [Google Scholar] [CrossRef] [PubMed]
- Jacomini, A.P.; Silva, M.J.V.; Silva, R.G.M.; Goncalves, D.S.; Volpato, H.; Basso, E.A.; Paula, F.R.; Nakamura, C.V.; Sarragiotto, M.H.; Rosa, F.A. Synthesis and evaluation against Leishmania amazonensis of novel pyrazolo[3,4-d]pyridazinone-N-acylhydrazone-(bi)thiophene hybrids. Eur. J. Med. Chem. 2016, 124, 340–349. [Google Scholar] [CrossRef]
- Da Silva, E.T.; de Andrade, G.F.; Araujo, A.D.S.; Almeida, A.D.C.; Coimbra, E.S.; de Souza, M.V.N. In vitro Assessment of Camphor Hydrazone Derivatives as an Agent against Leishmania amazonensis. Acta Parasitol. 2020, 65, 203–207. [Google Scholar] [CrossRef]
- Antolinez, I.V.; Barbosa, L.C.A.; Borgati, T.F.; Baldaia, A.; Ferreira, S.R.; Almeida, R.M.; Fujiwara, R.T. Tetroxanes as New Agents against Leishmania amazonensis. Chem. Biodivers. 2020, 17, e2000142. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, A.R.; Trefzger, O.S.; Barbosa, N.V.; Honorato, A.M.; Carvalho, D.B.; Moslaves, I.S.; Kadri, M.C.T.; Yoshida, N.C.; Kato, M.J.; Arruda, C.C.P.; et al. Effect of isoxazole derivatives of tetrahydrofuran neolignans on intracellular amastigotes of Leishmania (Leishmania) amazonensis: A structure-activity relationship comparative study with triazole-neolignan-based compounds. Chem. Biol. Drug Des. 2019, 94, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Soares, D.C.; Barreto-Junior, C.B.; Nascimento, M.T.; Freire-de-Lima, L.; Delorenzi, J.C.; Lima, M.E.; Atella, G.C.; Folly, E.; Carvalho, T.M.; et al. Leishmanicidal effects of piperine, its derivatives, and analogues on Leishmania amazonensis. Phytochemistry 2011, 72, 2155–2164. [Google Scholar] [CrossRef]
- Oliveira, V.G.; Dos Santos Faioes, V.; Goncalves, G.B.R.; Lima, M.F.O.; Boechat, F.C.S.; Cunha, A.C.; de Andrade-Neto, V.V.; de C da Silva, F.; Torres-Santos, E.C.; de Souza, M. Design, Synthesis and Antileishmanial Activity of Naphthotriazolyl-4- Oxoquinolines. Curr. Top. Med. Chem. 2018, 18, 1454–1464. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Cipriano, D.F.; de Freitas, J.C.C.; Junior, M.A.S.; Ocaris, E.R.Y.; Teles, C.B.G.; de Jesus Gouveia, A.; Rodrigues, R.P.; Zanini, M.S.; Villanova, J.C.O. Physicochemical characterization and in vitro biological evaluation of solid compounds from furazolidone-based cyclodextrins for use as leishmanicidal agents. Drug Deliv. Transl. Res. 2020, 10, 1788–1809. [Google Scholar] [CrossRef]
- Coimbra, E.S.; Nora de Souza, M.V.; Terror, M.S.; Pinheiro, A.C.; da Trindade Granato, J. Synthesis, biological activity, and mechanism of action of new 2-pyrimidinyl hydrazone and N-acylhydrazone derivatives, a potent and new classes of antileishmanial agents. Eur. J. Med. Chem. 2019, 184, 111742. [Google Scholar] [CrossRef]
- Fernandes, I.A.; de Almeida, L.; Ferreira, P.E.; Marques, M.J.; Rocha, R.P.; Coelho, L.F.; Carvalho, D.T.; Viegas, C., Jr. Synthesis and biological evaluation of novel piperidine-benzodioxole derivatives designed as potential leishmanicidal drug candidates. Bioorg. Med. Chem. Lett. 2015, 25, 3346–3349. [Google Scholar] [CrossRef]
- Noleto Dias, C.; Nunes, T.A.L.; Sousa, J.M.S.; Costa, L.H.; Rodrigues, R.R.L.; Araujo, A.J.; Marinho Filho, J.D.B.; da Silva, M.V.; Oliveira, M.R.; Carvalho, F.A.A.; et al. Methyl gallate: Selective antileishmanial activity correlates with host-cell directed effects. Chem. Biol. Interact. 2020, 320, 109026. [Google Scholar] [CrossRef]
- Santos, C.C.; Zhang, H.; Batista, M.M.; de Oliveira, G.M.; Demarque, K.C.; da Silva, N.L.; Moreira, O.C.; Ogungbe, I.V.; Soeiro, M.N.C. Phenotypic investigation of 4-nitrophenylacetyl- and 4-nitro-1H-imidazoyl-based compounds as antileishmanial agents. Parasitology 2022, 149, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Do Espirito Santo, R.D.; Velasquez, A.M.A.; Passianoto, L.V.G.; Sepulveda, A.A.L.; da Costa Clementino, L.; Assis, R.P.; Baviera, A.M.; Kalaba, P.; Dos Santos, F.N.; Eberlin, M.N.; et al. N, N′, N″-trisubstituted guanidines: Synthesis, characterization and evaluation of their leishmanicidal activity. Eur. J. Med. Chem. 2019, 171, 116–128. [Google Scholar] [CrossRef]
- Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Uliana, S.R. Tamoxifen is effective in the treatment of Leishmania amazonensis infections in mice. PLoS Negl. Trop. Dis. 2008, 2, e249. [Google Scholar] [CrossRef]
- Trinconi, C.T.; Reimao, J.Q.; Yokoyama-Yasunaka, J.K.; Miguel, D.C.; Uliana, S.R. Combination therapy with tamoxifen and amphotericin B in experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2014, 58, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Fivelman, Q.L.; Adagu, I.S.; Warhurst, D.C. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef] [PubMed]
- Seifert, K.; Croft, S.L. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 2006, 50, 73–79. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Alcantara, L.M.; Ferreira, T.C.S.; Fontana, V.; Chatelain, E.; Moraes, C.B.; Freitas-Junior, L.H. A Multi-Species Phenotypic Screening Assay for Leishmaniasis Drug Discovery Shows That Active Compounds Display a High Degree of Species-Specificity. Molecules 2020, 25, 2551. [Google Scholar] [CrossRef]
- Palacios, G.; Parodi, A.; Upegui, Y.A.; Montoya, A.; Pulido, S.; Velez, I.D.; Robledo, S.M. Studies in vitro on infectivity and sensitivity to antileishmanial drugs in New World Leishmania species transfected with the green fluorescent protein [pIR3(-)-eGFP]. Parasitology 2017, 144, 1718–1725. [Google Scholar] [CrossRef]
- Patel, A.P.; Deacon, A.; Getti, G. Development and validation of four Leishmania species constitutively expressing GFP protein. A model for drug discovery and disease pathogenesis studies. Parasitology 2014, 141, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Alves, A.G.; Maia, A.F.; Cruz, T.; Castro, H.; Tomas, A.M. Development of an automated image analysis protocol for quantification of intracellular forms of Leishmania spp. PLoS ONE 2018, 13, e0201747. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Vasconcelos, C.R.; Rezende, A.M. Systematic in silico Evaluation of Leishmania spp. Proteomes for Drug Discovery. Front. Chem. 2021, 9, 607139. [Google Scholar] [CrossRef] [PubMed]
- Borba, J.V.B.; Silva, A.C.; Ramos, P.I.P.; Grazzia, N.; Miguel, D.C.; Muratov, E.N.; Furnham, N.; Andrade, C.H. Unveiling the Kinomes of Leishmania infantum and L. braziliensis Empowers the Discovery of New Kinase Targets and Antileishmanial Compounds. Comput. Struct. Biotechnol. J. 2019, 17, 352–361. [Google Scholar] [CrossRef]
- Mina, J.G.; Mosely, J.A.; Ali, H.Z.; Shams-Eldin, H.; Schwarz, R.T.; Steel, P.G.; Denny, P.W. A plate-based assay system for analyses and screening of the Leishmania major inositol phosphorylceramide synthase. Int. J. Biochem. Cell Biol. 2010, 42, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Porta, E.O.J.; Isern, J.A.; Kalesh, K.; Steel, P.G. Discovery of Leishmania Druggable Serine Proteases by Activity-Based Protein Profiling. Front. Pharmacol. 2022, 13, 929493. [Google Scholar] [CrossRef]
- Pena-Guerrero, J.; Fernandez-Rubio, C.; Burguete-Mikeo, A.; El-Dirany, R.; Garcia-Sosa, A.T.; Nguewa, P. Discovery and Validation of Lmj_04_BRCT Domain, a Novel Therapeutic Target: Identification of Candidate Drugs for Leishmaniasis. Int. J. Mol. Sci. 2021, 22, 1049. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Rescifina, A.; Micale, N.; Piperno, A.; Schirmeister, T.; Maes, L.; Grassi, G. Ensemble-based ADME-Tox profiling and virtual screening for the discovery of new inhibitors of the Leishmania mexicana cysteine protease CPB2.8DeltaCTE. Chem. Biol. Drug Des. 2018, 91, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.A.H.; Ramos, S.L., Jr.; Tassone, G.; Leitao, A.; Montanari, C.A.; Botta, M.; Mori, M.; Borges, J.C. Discovery of small molecule inhibitors of Leishmania braziliensis Hsp90 chaperone. J. Enzyme Inhib. Med. Chem. 2020, 35, 639–649. [Google Scholar] [CrossRef]
- Parise-Filho, R.; Pasqualoto, K.F.; Magri, F.M.; Ferreira, A.K.; da Silva, B.A.; Damiao, M.C.; Tavares, M.T.; Azevedo, R.A.; Auada, A.V.; Polli, M.C.; et al. Dillapiole as antileishmanial agent: Discovery, cytotoxic activity and preliminary SAR studies of dillapiole analogues. Arch. Pharm. 2012, 345, 934–944. [Google Scholar] [CrossRef]
- Oliveira, S.S.C.; Marques, C.S.F.; de Sousa, D.P.; Andrade, L.N.; Fricks, A.T.; Jain, S.; Branquinha, M.H.; Souto, E.B.; Santos, A.L.S.; Severino, P. Analysis of the mechanisms of action of isopentenyl caffeate against Leishmania. Biochimie 2021, 189, 158–167. [Google Scholar] [CrossRef]
- Van Bocxlaer, K.; Caridha, D.; Black, C.; Vesely, B.; Leed, S.; Sciotti, R.J.; Wijnant, G.J.; Yardley, V.; Braillard, S.; Mowbray, C.E.; et al. Novel benzoxaborole, nitroimidazole and aminopyrazoles with activity against experimental cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 129–138. [Google Scholar] [CrossRef]
- Istanbullu, H.; Bayraktar, G.; Akbaba, H.; Cavus, I.; Coban, G.; Debelec Butuner, B.; Kilimcioglu, A.A.; Ozbilgin, A.; Alptuzun, V.; Erciyas, E. Design, synthesis, and in vitro biological evaluation of novel thiazolopyrimidine derivatives as antileishmanial compounds. Arch. Pharm. 2020, 353, e1900325. [Google Scholar] [CrossRef]
- Barbosa, T.P.; Sousa, S.C.; Amorim, F.M.; Rodrigues, Y.K.; de Assis, P.A.; Caldas, J.P.; Oliveira, M.R.; Vasconcellos, M.L. Design, synthesis and antileishmanial in vitro activity of new series of chalcones-like compounds: A molecular hybridization approach. Bioorg. Med. Chem. 2011, 19, 4250–4256. [Google Scholar] [CrossRef]
- Calvo Alvarez, E.; D’Alessandro, S.; Proverbio, D.; Spada, E.; Perego, R.; Taramelli, D.; Basilico, N.; Parapini, S. In Vitro Antiparasitic Activities of Monovalent Ionophore Compounds for Human and Canine Leishmaniases. Animals 2022, 12, 2337. [Google Scholar] [CrossRef]
- Sanchez-Salgado, J.C.; Bilbao-Ramos, P.; Dea-Ayuela, M.A.; Hernandez-Luis, F.; Bolas-Fernandez, F.; Medina-Franco, J.L.; Rojas-Aguirre, Y. Systematic search for benzimidazole compounds and derivatives with antileishmanial effects. Mol. Divers. 2018, 22, 779–790. [Google Scholar] [CrossRef]
- Mendes, B.; Proano-Bolanos, C.; Gadelha, F.R.; Almeida, J.R.; Miguel, D.C. Cruzioseptins, antibacterial peptides from Cruziohyla calcarifer skin, as promising leishmanicidal agents. Pathog. Dis. 2020, 78, ftaa053. [Google Scholar] [CrossRef]
- Corman, H.N.; Shoue, D.A.; Norris-Mullins, B.; Melancon, B.J.; Morales, M.A.; McDowell, M.A. Development of a target-free high-throughput screening platform for the discovery of antileishmanial compounds. Int. J. Antimicrob. Agents 2019, 54, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Pandharkar, T.; Werbovetz, K. Identification of new antileishmanial leads from hits obtained by high-throughput screening. Antimicrob. Agents Chemother. 2012, 56, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Ben Khalaf, N.; De Muylder, G.; Ratnam, J.; Kean-Hooi Ang, K.; Arkin, M.; McKerrow, J.; Chenik, M. A high-throughput turbidometric assay for screening inhibitors of Leishmania major protein disulfide isomerase. J. Biomol. Screen. 2011, 16, 545–551. [Google Scholar] [CrossRef]
- Ortalli, M.; Varani, S.; Rosso, C.; Quintavalla, A.; Lombardo, M.; Trombini, C. Evaluation of synthetic substituted 1,2-dioxanes as novel agents against human leishmaniasis. Eur. J. Med. Chem. 2019, 170, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Farahat, A.A.; Mattamana, M.; Joice, A.; Pandharkar, T.; Holt, E.; Banerjee, M.; Gragg, J.L.; Hu, L.; Kumar, A.; et al. Synthesis and pharmacological evaluation of mono-arylimidamides as antileishmanial agents. Bioorg. Med. Chem. Lett. 2016, 26, 2551–2556. [Google Scholar] [CrossRef]
- Faioes, V.D.S.; da Frota, L.; Cunha-Junior, E.F.; Barcellos, J.C.F.; Da Silva, T.; Netto, C.D.; Da-Silva, S.A.G.; da Silva, A.J.M.; Costa, P.R.R.; Torres-Santos, E.C. Second-generation pterocarpanquinones: Synthesis and antileishmanial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Barbolla, I.; Hernandez-Suarez, L.; Quevedo-Tumailli, V.; Nocedo-Mena, D.; Arrasate, S.; Dea-Ayuela, M.A.; Gonzalez-Diaz, H.; Sotomayor, N.; Lete, E. Palladium-mediated synthesis and biological evaluation of C-10b substituted Dihydropyrrolo[1,2-b]isoquinolines as antileishmanial agents. Eur. J. Med. Chem. 2021, 220, 113458. [Google Scholar] [CrossRef] [PubMed]
- Freijo, M.B.; Lopez-Arencibia, A.; Pinero, J.E.; McNaughton-Smith, G.; Abad-Grillo, T. Design, synthesis and evaluation of amino-substituted 1H-phenalen-1-ones as anti-leishmanial agents. Eur. J. Med. Chem. 2018, 143, 1312–1324. [Google Scholar] [CrossRef]
- Ortalli, M.; Ilari, A.; Colotti, G.; De Ionna, I.; Battista, T.; Bisi, A.; Gobbi, S.; Rampa, A.; Di Martino, R.M.C.; Gentilomi, G.A.; et al. Identification of chalcone-based antileishmanial agents targeting trypanothione reductase. Eur. J. Med. Chem. 2018, 152, 527–541. [Google Scholar] [CrossRef]
- Collar, C.J.; Zhu, X.; Werbovetz, K.; Boykin, D.W.; Wilson, W.D. Molecular factors governing inhibition of arylimidamides against Leishmania: Conservative computational modeling to improve chemotherapies. Bioorg. Med. Chem. 2011, 19, 4552–4561. [Google Scholar] [CrossRef]
- Ortiz, D.; Forquer, I.; Boitz, J.; Soysa, R.; Elya, C.; Fulwiler, A.; Nilsen, A.; Polley, T.; Riscoe, M.K.; Ullman, B.; et al. Targeting the Cytochrome bc1 Complex of Leishmania Parasites for Discovery of Novel Drugs. Antimicrob. Agents Chemother. 2016, 60, 4972–4982. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Ferro, S.; Buemi, M.R.; Monforte, A.M.; Gitto, R.; Schirmeister, T.; Maes, L.; Rescifina, A.; Micale, N. Discovery of benzimidazole-based Leishmania mexicana cysteine protease CPB2.8DeltaCTE inhibitors as potential therapeutics for leishmaniasis. Chem. Biol. Drug Des. 2018, 92, 1585–1596. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, N.; Agnihotri, P.; Mishra, S.; Singh, S.P.; Kolli, B.K.; Chang, K.P.; Sahasrabuddhe, A.A.; Siddiqi, M.I.; Pratap, J.V. Discovery of novel inhibitors for Leishmania nucleoside diphosphatase kinase (NDK) based on its structural and functional characterization. J. Comput. Aided Mol. Des. 2017, 31, 547–562. [Google Scholar] [CrossRef]
- Mao, W.; Daligaux, P.; Lazar, N.; Ha-Duong, T.; Cave, C.; van Tilbeurgh, H.; Loiseau, P.M.; Pomel, S. Biochemical analysis of leishmanial and human GDP-Mannose Pyrophosphorylases and selection of inhibitors as new leads. Sci. Rep. 2017, 7, 751. [Google Scholar] [CrossRef]
- Brannigan, J.A.; Roberts, S.M.; Bell, A.S.; Hutton, J.A.; Hodgkinson, M.R.; Tate, E.W.; Leatherbarrow, R.J.; Smith, D.F.; Wilkinson, A.J. Diverse modes of binding in structures of Leishmania major N-myristoyltransferase with selective inhibitors. IUCrJ 2014, 1, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.D. The Leishmania mexicana proteasome. Mol. Biochem. Parasitol. 1999, 103, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D.; Allice, T.; Tovo, P.A. Antileishmanial activity of HIV protease inhibitors. Int. J. Antimicrob. Agents 2005, 26, 92–94. [Google Scholar] [CrossRef]
- Silva-Jardim, I.; Horta, M.F.; Ramalho-Pinto, F.J. The Leishmania chagasi proteasome: Role in promastigotes growth and amastigotes survival within murine macrophages. Acta Trop. 2004, 91, 121–130. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 31 August 2023).
- Phan, T.N.; Park, K.P.; Benitez, D.; Comini, M.A.; Shum, D.; No, J.H. Discovery of novel Leishmania major trypanothione synthetase inhibitors by high-throughput screening. Biochem. Biophys. Res. Commun. 2022, 637, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.G.R.; Mercaldi, G.F.; Pereira, J.G.C.; Oliveira, P.S.L.; Rodriguez, A.; Cordeiro, A.T. First Nonphosphorylated Inhibitors of Phosphoglucose Isomerase Identified by Chemical Library Screening. SLAS Discov. 2018, 23, 1051–1059. [Google Scholar] [CrossRef]
- Da Rosa, R.; de Moraes, M.H.; Zimmermann, L.A.; Schenkel, E.P.; Steindel, M.; Bernardes, L.S.C. Design and synthesis of a new series of 3,5-disubstituted isoxazoles active against Trypanosoma cruzi and Leishmania amazonensis. Eur. J. Med. Chem. 2017, 128, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Trefzger, O.S.; das Neves, A.R.; Barbosa, N.V.; Carvalho, D.B.; Pereira, I.C.; Perdomo, R.T.; Matos, M.F.C.; Yoshida, N.C.; Kato, M.J.; de Albuquerque, S.; et al. Design, synthesis and antitrypanosomatid activities of 3,5-diaryl-isoxazole analogues based on neolignans veraguensin, grandisin and machilin G. Chem. Biol. Drug Des. 2019, 93, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, T.G.; Dutra, L.A.; de Almeida, L.; Velasquez, A.M.; Torres, F.A.; Yamasaki, P.R.; dos Santos, M.B.; Regasini, L.O.; Michels, P.A.; Bolzani Vda, S.; et al. Synthesis and evaluation of novel prenylated chalcone derivatives as anti-leishmanial and anti-trypanosomal compounds. Bioorg. Med. Chem. Lett. 2015, 25, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Hicks, J.C.; Chacon-Vargas, K.F.; Hernandez-Rivera, J.L.; Nogueda-Torres, B.; Tamariz, J.; Sanchez-Torres, L.E.; Camacho-Davila, A. Novel prenyloxy chalcones as potential leishmanicidal and trypanocidal agents: Design, synthesis and evaluation. Eur. J. Med. Chem. 2019, 167, 402–413. [Google Scholar] [CrossRef]
- Velasquez, A.M.A.; Ribeiro, W.C.; Venn, V.; Castelli, S.; Camargo, M.S.; de Assis, R.P.; de Souza, R.A.; Ribeiro, A.R.; Passalacqua, T.G.; da Rosa, J.A.; et al. Efficacy of a Binuclear Cyclopalladated Compound Therapy for Cutaneous Leishmaniasis in the Murine Model of Infection with Leishmania amazonensis and Its Inhibitory Effect on Topoisomerase 1B. Antimicrob. Agents Chemother. 2017, 61, 10–128. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.J.V.; Jacomini, A.P.; Goncalves, D.S.; Pianoski, K.E.; Poletto, J.; Lazarin-Bidoia, D.; Volpato, H.; Nakamura, C.V.; Rosa, F.A. Discovery of 1,3,4,5-tetrasubstituted pyrazoles as anti-trypanosomatid agents: Identification of alterations in flagellar structure of L. amazonensis. Bioorg. Chem. 2021, 114, 105082. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.M.; Amado, D.F.; Kouznetsov, V.V.; Escobar, P. In vitro antileishmanial, trypanocidal, and Mammalian cell activities of diverse n,n′-dihetaryl substituted diamines and related compounds. Sci. Pharm. 2013, 81, 43–55. [Google Scholar] [CrossRef]
- Galiana-Rosello, C.; Bilbao-Ramos, P.; Dea-Ayuela, M.A.; Rolon, M.; Vega, C.; Bolas-Fernandez, F.; Garcia-Espana, E.; Alfonso, J.; Coronel, C.; Gonzalez-Rosende, M.E. In vitro and in vivo antileishmanial and trypanocidal studies of new N-benzene- and N-naphthalenesulfonamide derivatives. J. Med. Chem. 2013, 56, 8984–8998. [Google Scholar] [CrossRef] [PubMed]
- Barros Freitas, L.A.; Caroline da Silva Santos, A.; de Cassia Silva, G.; Nayara do Nascimento Albuquerque, F.; Silva, E.D.; Alberto de Simone, C.; Alves Pereira, V.R.; Alves, L.C.; Brayner, F.A.; Lima Leite, A.C.; et al. Structural improvement of new thiazolyl-isatin derivatives produces potent and selective trypanocidal and leishmanicidal compounds. Chem. Biol. Interact. 2021, 345, 109561. [Google Scholar] [CrossRef]
- Fandzloch, M.; Arriaga, J.M.M.; Sanchez-Moreno, M.; Wojtczak, A.; Jezierska, J.; Sitkowski, J.; Wisniewska, J.; Salas, J.M.; Lakomska, I. Strategies for overcoming tropical disease by ruthenium complexes with purine analog: Application against Leishmania spp. and Trypanosoma cruzi. J. Inorg. Biochem. 2017, 176, 144–155. [Google Scholar] [CrossRef]
- Laurella, L.C.; Cerny, N.; Bivona, A.E.; Sanchez Alberti, A.; Giberti, G.; Malchiodi, E.L.; Martino, V.S.; Catalan, C.A.; Alonso, M.R.; Cazorla, S.I.; et al. Assessment of sesquiterpene lactones isolated from Mikania plants species for their potential efficacy against Trypanosoma cruzi and Leishmania sp. PLoS Negl. Trop. Dis. 2017, 11, e0005929. [Google Scholar] [CrossRef] [PubMed]
- Sulsen, V.P.; Lizarraga, E.F.; Elso, O.G.; Cerny, N.; Sanchez Alberti, A.; Bivona, A.E.; Malchiodi, E.L.; Cazorla, S.I.; Catalan, C.A.N. Activity of Estafietin and Analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules 2019, 24, 1209. [Google Scholar] [CrossRef]
- Sulsen, V.P.; Cazorla, S.I.; Frank, F.M.; Laurella, L.C.; Muschietti, L.V.; Catalan, C.A.; Martino, V.S.; Malchiodi, E.L. Natural terpenoids from Ambrosia species are active in vitro and in vivo against human pathogenic trypanosomatids. PLoS Negl. Trop. Dis. 2013, 7, e2494. [Google Scholar] [CrossRef]
- Gonzalez, L.A.; Upegui, Y.A.; Rivas, L.; Echeverri, F.; Escobar, G.; Robledo, S.M.; Quinones, W. Effect of substituents in the A and B rings of chalcones on antiparasite activity. Arch. Pharm. 2020, 353, e2000157. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.J.; Arnold, M.S.; Xu, W.; Lancelot, J.; Lamotte, S.; Spath, G.F.; Prina, E.; Pierce, R.J.; Fairlie, D.P.; Skinner-Adams, T.S.; et al. Effect of clinically approved HDAC inhibitors on Plasmodium, Leishmania and Schistosoma parasite growth. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Gahalawat, S.; Booshehri, L.M.; Niederstrasser, H.; Majumdar, S.; Leija, C.; Bradford, J.M.; Hu, B.; Ready, J.M.; Wetzel, D.M. An Antiparasitic Compound from the Medicines for Malaria Venture Pathogen Box Promotes Leishmania Tubulin Polymerization. ACS Infect. Dis. 2020, 6, 2057–2072. [Google Scholar] [CrossRef]
- Hameed, H.; King, E.F.B.; Doleckova, K.; Bartholomew, B.; Hollinshead, J.; Mbye, H.; Ullah, I.; Walker, K.; Van Veelen, M.; Abou-Akkada, S.S.; et al. Temperate Zone Plant Natural Products-A Novel Resource for Activity against Tropical Parasitic Diseases. Pharmaceuticals 2021, 14, 227. [Google Scholar] [CrossRef]
- Mai, L.H.; Chabot, G.G.; Grellier, P.; Quentin, L.; Dumontet, V.; Poulain, C.; Espindola, L.S.; Michel, S.; Vo, H.T.; Deguin, B.; et al. Antivascular and anti-parasite activities of natural and hemisynthetic flavonoids from New Caledonian Gardenia species (Rubiaceae). Eur. J. Med. Chem. 2015, 93, 93–100. [Google Scholar] [CrossRef]
- Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le Pape, P.; Savrimoutou, S.; Pinaud, N.; Mullie, C.; Daulouede, S.; Vincendeau, P.; et al. Design, synthesis and biological evaluation of novel 4-alkapolyenylpyrrolo[1,2-a]quinoxalines as antileishmanial agents—part III. Eur. J. Med. Chem. 2014, 81, 378–393. [Google Scholar] [CrossRef]
- Mendoza-Martinez, C.; Correa-Basurto, J.; Nieto-Meneses, R.; Marquez-Navarro, A.; Aguilar-Suarez, R.; Montero-Cortes, M.D.; Nogueda-Torres, B.; Suarez-Contreras, E.; Galindo-Sevilla, N.; Rojas-Rojas, A.; et al. Design, synthesis and biological evaluation of quinazoline derivatives as anti-trypanosomatid and anti-plasmodial agents. Eur. J. Med. Chem. 2015, 96, 296–307. [Google Scholar] [CrossRef]
- Colin-Lozano, B.; Leon-Rivera, I.; Chan-Bacab, M.J.; Ortega-Morales, B.O.; Moo-Puc, R.; Lopez-Guerrero, V.; Hernandez-Nunez, E.; Arguello-Garcia, R.; Scior, T.; Barbosa-Cabrera, E.; et al. Synthesis, in vitro and in vivo giardicidal activity of nitrothiazole-NSAID chimeras displaying broad antiprotozoal spectrum. Bioorg. Med. Chem. Lett. 2017, 27, 3490–3494. [Google Scholar] [CrossRef] [PubMed]
- Nava-Zuazo, C.; Chavez-Silva, F.; Moo-Puc, R.; Chan-Bacab, M.J.; Ortega-Morales, B.O.; Moreno-Diaz, H.; Diaz-Coutino, D.; Hernandez-Nunez, E.; Navarrete-Vazquez, G. 2-acylamino-5-nitro-1,3-thiazoles: Preparation and in vitro bioevaluation against four neglected protozoan parasites. Bioorg. Med. Chem. 2014, 22, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.C.; Santana, D.B.; Araujo, R.M.; de Paula, J.E.; do Nascimento, P.C.; Lopes, N.P.; Braz-Filho, R.; Espindola, L.S. Discovery of the rapanone and suberonone mixture as a motif for leishmanicidal and antifungal applications. Bioorg. Med. Chem. 2014, 22, 135–140. [Google Scholar] [CrossRef]
- Fernandes, P.A.S.; Silva, J.; Lima Sales, D.; Ribeiro, P.R.V.; Sousa de Brito, E.; Kerntopf, M.R.; Delmondes, G.A.; Andrade Pinheiro, J.C.; Salazar, G.J.T.; Batista, F.L.A.; et al. Chemical Constituents and Biological Activities of Croton heliotropiifolius Kunth. Antibiotics 2021, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Houel, E.; Gonzalez, G.; Bessiere, J.M.; Odonne, G.; Eparvier, V.; Deharo, E.; Stien, D. Therapeutic switching: From antidermatophytic essential oils to new leishmanicidal products. Mem. Inst. Oswaldo Cruz 2015, 110, 106–113. [Google Scholar] [CrossRef]
- Zahra, S.S.; Ahmed, M.; Qasim, M.; Gul, B.; Zia, M.; Mirza, B.; Haq, I.U. Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complement. Altern. Med. 2017, 17, 443. [Google Scholar] [CrossRef]
- Trefzger, O.S.; Barbosa, N.V.; Scapolatempo, R.L.; das Neves, A.R.; Ortale, M.; Carvalho, D.B.; Honorato, A.M.; Fragoso, M.R.; Shuiguemoto, C.Y.K.; Perdomo, R.T.; et al. Design, synthesis, antileishmanial, and antifungal biological evaluation of novel 3,5-disubstituted isoxazole compounds based on 5-nitrofuran scaffolds. Arch. Pharm. 2020, 353, e1900241. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.M.; Patel, N.B.; Chan-Bacab, M.J.; Rivera, G. Synthesis, biological evaluation and molecular dynamics studies of 1,2,4-triazole clubbed Mannich bases. Comput. Biol. Chem. 2018, 76, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Pena-Carrillo, M.S.; Pinos-Tamayo, E.A.; Mendes, B.; Dominguez-Borbor, C.; Proano-Bolanos, C.; Miguel, D.C.; Almeida, J.R. Dissection of phospholipases A(2) reveals multifaceted peptides targeting cancer cells, Leishmania and bacteria. Bioorg. Chem. 2021, 114, 105041. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Furtado, F.B.; Gomes, A.A.S.; Belut, B.R.; Nascimento, E.A.; Morais, S.A.L.; Martins, C.H.G.; Santos, V.C.O.; da Silva, C.V.; Teixeira, T.L.; et al. Chemical Constituents and Antileishmanial and Antibacterial Activities of Essential Oils from Scheelea phalerata. ACS Omega 2020, 5, 1363–1370. [Google Scholar] [CrossRef]
- Erasmus, C.; Aucamp, J.; Smit, F.J.; Seldon, R.; Jordaan, A.; Warner, D.F.; David, D.D. Synthesis and comparison of in vitro dual anti-infective activities of novel naphthoquinone hybrids and atovaquone. Bioorg. Chem. 2021, 114, 105118. [Google Scholar] [CrossRef]
- Dos Reis, D.B.; Souza, T.C.A.; Lourenco, M.C.S.; de Almeida, M.V.; Barbosa, A.; Eger, I.; Saraiva, M.F. Synthesis and biological evaluation against Mycobacterium tuberculosis and Leishmania amazonensis of a series of diaminated terpenoids. Biomed. Pharmacother. 2016, 84, 1739–1747. [Google Scholar] [CrossRef]
- Hernandez, P.; Rojas, R.; Gilman, R.H.; Sauvain, M.; Lima, L.M.; Barreiro, E.J.; Gonzalez, M.; Cerecetto, H. Hybrid furoxanyl N-acylhydrazone derivatives as hits for the development of neglected diseases drug candidates. Eur. J. Med. Chem. 2013, 59, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Nava-Zuazo, C.; Estrada-Soto, S.; Guerrero-Alvarez, J.; Leon-Rivera, I.; Molina-Salinas, G.M.; Said-Fernandez, S.; Chan-Bacab, M.J.; Cedillo-Rivera, R.; Moo-Puc, R.; Miron-Lopez, G.; et al. Design, synthesis, and in vitro antiprotozoal, antimycobacterial activities of N-2-[(7-chloroquinolin-4-yl)amino]ethylureas. Bioorg. Med. Chem. 2010, 18, 6398–6403. [Google Scholar] [CrossRef]
- Reguera, R.M.; Calvo-Alvarez, E.; Alvarez-Velilla, R.; Balana-Fouce, R. Target-based vs. phenotypic screenings in Leishmania drug discovery: A marriage of convenience or a dialogue of the deaf? Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Hilley, J.D.; Dickens, N.J.; Wilkes, J.; Bates, P.A.; Depledge, D.P.; Harris, D.; Her, Y.; Herzyk, P.; Imamura, H.; et al. Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res. 2011, 21, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Negreira, G.H.; Monsieurs, P.; Imamura, H.; Maes, I.; Kuk, N.; Yagoubat, A.; Van den Broeck, F.; Sterkers, Y.; Dujardin, J.C.; Domagalska, M.A. High throughput single-cell genome sequencing gives insights into the generation and evolution of mosaic aneuploidy in Leishmania donovani. Nucleic Acids Res. 2022, 50, 293–305. [Google Scholar] [CrossRef]
| Ideal | Acceptable | |
|---|---|---|
| Target species | One treatment for all species of Leishmania | L. tropica or L. braziliensis |
| Safety and tolerability | Well tolerated All adverse reactions (AR)s ≤ grade 1 | Safety monitoring at primary health care (PHC) level No major safety concerns Well tolerated in >95% of patients treated Systemic AR ≤ grade 3 in <5% Local AR ≤ grade 2 in <30% No treatment-induced mortality |
| Contraindications | None | Can be assessed at PHC level |
| Efficacy | >95% patients with complete clinical cure (100% epithelialization/flattening of lesions at 3 months from treatment onset) Minimal scarring No relapse or development of Leishmaniasis recidivans or mucocutaneous leishmaniasis (MCL) Parasitological endpoint not required | 60% epithelialization/flattening of lesion(s) for L. tropica and 70% for L. braziliensis patients with complete cure Scarring no worse than natural healing <5% rate of relapse or development of Leishmaniasis recidivans or MCL at 1 year |
| Formulation | Topical or oral | Non-parenteral; few doses if parenteral |
| Treatment regimen | Topical ≤ 14 days Oral < 7 days | Topical: 28 days Oral: twice daily for 28 days Parenteral ≤ 3 injections |
| Target population | No restrictions | >9 months of age No efficacy in immunocompromised patients Not for use in pregnancy |
| Stability | No cold chain At least 3 years at 37 °C | 2 years at 4–8 °C |
| Cost | To be defined | To be defined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corman, H.N.; McNamara, C.W.; Bakowski, M.A. Drug Discovery for Cutaneous Leishmaniasis: A Review of Developments in the Past 15 Years. Microorganisms 2023, 11, 2845. https://doi.org/10.3390/microorganisms11122845
Corman HN, McNamara CW, Bakowski MA. Drug Discovery for Cutaneous Leishmaniasis: A Review of Developments in the Past 15 Years. Microorganisms. 2023; 11(12):2845. https://doi.org/10.3390/microorganisms11122845
Chicago/Turabian StyleCorman, Hannah N., Case W. McNamara, and Malina A. Bakowski. 2023. "Drug Discovery for Cutaneous Leishmaniasis: A Review of Developments in the Past 15 Years" Microorganisms 11, no. 12: 2845. https://doi.org/10.3390/microorganisms11122845
APA StyleCorman, H. N., McNamara, C. W., & Bakowski, M. A. (2023). Drug Discovery for Cutaneous Leishmaniasis: A Review of Developments in the Past 15 Years. Microorganisms, 11(12), 2845. https://doi.org/10.3390/microorganisms11122845







