Cross-Reactivity of Antibodies in Intravenous Immunoglobulin Preparation for Protection against SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. IVIG Preparation
2.2. Evaluating the SARS-CoV-2-Derived Antigen-Binding Ability of the IVIG Preparation
2.3. Evaluating the Infection-Preventive Effect of IVIG Preparations Using a Pseudovirus Entry Assay
3. Results
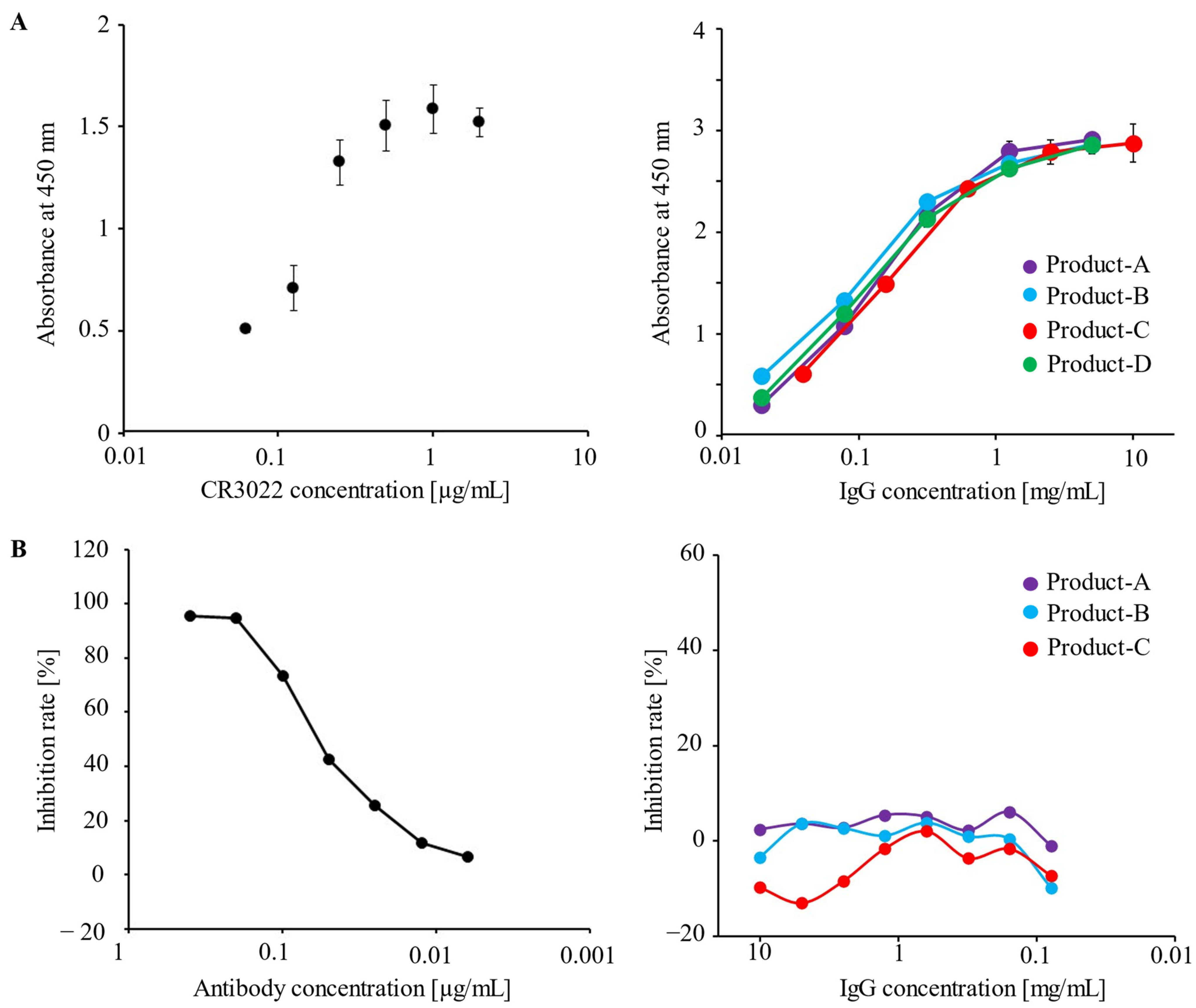
3.1. Human IVIG Preparations Contain IgG Antibodies against the Spike Protein of SARS-CoV-2
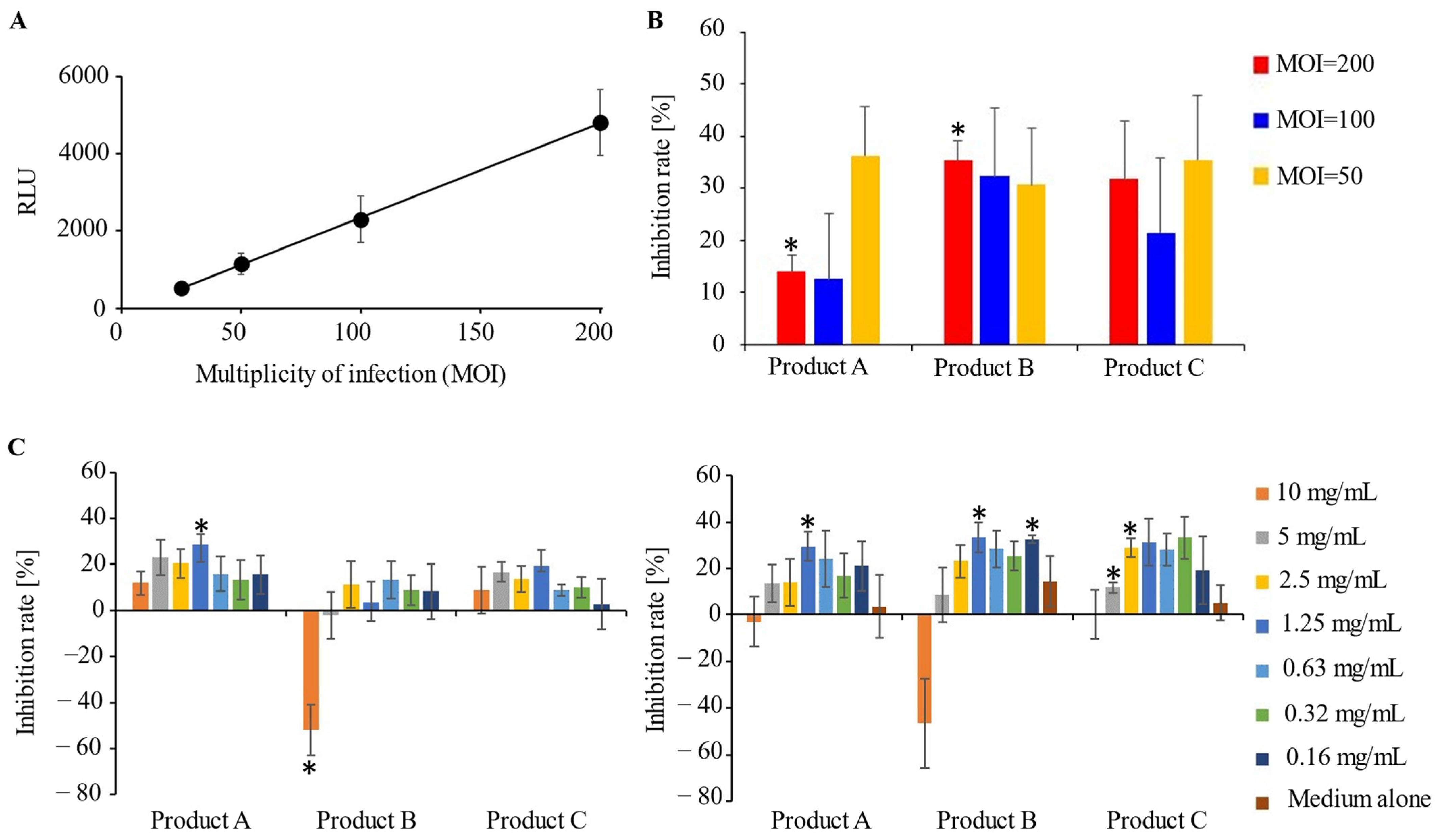
3.2. A Potential Preventive Effect of IVIG Preparations for SARS-CoV-2 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.P.; Prévost, J.; Nayrac, M.; Beaudoin-Bussières, G.; Benlarbi, M.; Gasser, R.; Brassard, N.; Laumaea, A.; Gong, S.Y.; Bourassa, C.; et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep. Med. 2021, 2, 100290. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.; Cloutier, M.; Prévost, J.; Fink, C.; Ducas, É.; Ding, S.; Dussault, N.; Landry, P.; Tremblay, T.; Laforce-Lavoie, A.; et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep. 2021, 34, 108790. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R.; Pawlowski, C.; O’Horo, J.C.; Arndt, L.L.; Arndt, R.; Bierle, D.M.; Borgen, M.D.; Hanson, S.N.; Hedin, M.C.; Lenehan, P.; et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine 2021, 40, 101102. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Murano, K.; Guo, Y.; Siomi, H. The emergence of SARS-CoV-2 variants threatens to decrease the efficacy of neutralizing antibodies and vaccines. Biochem. Soc. Trans 2021, 49, 2879–2890. [Google Scholar] [CrossRef]
- Gelfand, E.W. Intravenous immune globulin in autoimmune and inflammatory diseases. N. Engl. J. Med. 2012, 367, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Takai, T. Fc receptors and their role in immune regulation and autoimmunity. J. Clin. Immunol. 2005, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mazeraud, A.; Jamme, M.; Mancusi, R.L.; Latroche, C.; Megarbane, B.; Siami, S.; Zarka, J.; Moneger, G.; Santoli, F.; Argaud, L.; et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): Multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 158–166. [Google Scholar] [CrossRef]
- Pei, L.; Zhang, S.; Huang, L.; Geng, X.; Ma, L.; Jiang, W.; Li, W.; Chen, D. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: A systematic review and meta-analysis. Pol. Arch. Intern. Med. 2020, 130, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Geriak, M.; Kullar, R.; Greenwood, K.L.; Habib, M.; Vyas, A.; Ghafourian, M.; Dintyala, V.N.K.; Haddad, F. Intravenous Immunoglobulin (IVIG) Significantly Reduces Respiratory Morbidity in COVID-19 Pneumonia: A Prospective Randomized Trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Gharebaghi, N.; Nejadrahim, R.; Mousavi, S.J.; Sadat-Ebrahimi, S.R.; Hajizadeh, R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: A randomized placebo-controlled double-blind clinical trial. BMC Infect. Dis. 2020, 20, 786. [Google Scholar] [CrossRef]
- Shao, Z.; Feng, Y.; Zhong, L.; Xie, Q.; Lei, M.; Liu, Z.; Wang, C.; Ji, J.; Liu, H.; Gu, Z.; et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: A multicenter retrospective cohort study. Clin. Transl. Immunol. 2020, 9, e1192. [Google Scholar] [CrossRef] [PubMed]
- Tabarsi, P.; Barati, S.; Jamaati, H.; Haseli, S.; Marjani, M.; Moniri, A.; Abtahian, Z.; Dastan, A.; Yousefian, S.; Eskandari, R.; et al. Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID-19 cases: A randomized controlled trial. Int. Immunopharmacol. 2021, 90, 107205. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Pharmaceuticals and Medical Devices Safety Information. Available online: https://www.pmda.go.jp/files/000153605 (accessed on 12 February 2023).
- ter Meulen, J.; van den Brink, E.N.; Poon, L.L.; Marissen, W.E.; Leung, C.S.; Cox, F.; Cheung, C.Y.; Bakker, A.Q.; Bogaards, J.A.; van Deventer, E.; et al. Human monoclonal antibody combination against SARS coronavirus: Synergy and coverage of escape mutants. PLoS Med. 2006, 3, e237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Napoli, R.D. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/ (accessed on 12 February 2023).
- Leussink, V.I.; Hartung, H.P.; Kieseier, B.C.; Stettner, M. Subcutaneous immunoglobulins in the treatment of chronic immune-mediated neuropathies. Ther. Adv. Neurol. Disord 2016, 9, 336–343. [Google Scholar] [CrossRef]
- Takahashi, G.; Shibata, S. Study of usefulness of low-dose IgG for patients with septic disseminated intravascular coagulation. Biomark. Med. 2020, 14, 1189–1196. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost 2020, 18, 2103–2109. [Google Scholar] [CrossRef]
- Beaudoin-Bussières, G.; Chen, Y.; Ullah, I.; Prévost, J.; Tolbert, W.D.; Symmes, K.; Ding, S.; Benlarbi, M.; Gong, S.Y.; Tauzin, A.; et al. A Fc-enhanced NTD-binding non-neutralizing antibody delays virus spread and synergizes with a nAb to protect mice from lethal SARS-CoV-2 infection. Cell Rep. 2022, 38, 110368. [Google Scholar] [CrossRef]
- Ilinykh, P.A.; Huang, K.; Santos, R.I.; Gilchuk, P.; Gunn, B.M.; Karim, M.M.; Liang, J.; Fouch, M.E.; Davidson, E.; Parekh, D.V.; et al. Non-neutralizing Antibodies from a Marburg Infection Survivor Mediate Protection by Fc-Effector Functions and by Enhancing Efficacy of Other Antibodies. Cell Host Microbe 2020, 27, 976–991. [Google Scholar] [CrossRef]
- Ko, Y.A.; Yu, Y.H.; Wu, Y.F.; Tseng, Y.C.; Chen, C.L.; Goh, K.S.; Liao, H.Y.; Chen, T.H.; Cheng, T.R.; Yang, A.S.; et al. A non-neutralizing antibody broadly protects against influenza virus infection by engaging effector cells. PLoS Pathog. 2021, 17, e1009724. [Google Scholar] [CrossRef]
- Hioe, C.E.; Li, G.; Liu, X.; Tsahouridis, O.; He, X.; Funaki, M.; Klingler, J.; Tang, A.F.; Feyznezhad, R.; Heindel, D.W.; et al. Non-neutralizing antibodies targeting the immunogenic regions of HIV-1 envelope reduce mucosal infection and virus burden in humanized mice. PLoS Pathog. 2022, 18, e1010183. [Google Scholar] [CrossRef]
- Brown, E.P.; Weiner, J.A.; Lin, S.; Natarajan, H.; Normandin, E.; Barouch, D.H.; Alter, G.; Sarzotti-Kelsoe, M.; Ackerman, M.E. Optimization and qualification of an Fc Array assay for assessments of antibodies against HIV-1/SIV. J. Immunol. Methods 2018, 455, 24–33. [Google Scholar] [CrossRef]
- Adeniji, O.S.; Giron, L.B.; Purwar, M.; Zilberstein, N.F.; Kulkarni, A.J.; Shaikh, M.W.; Balk, R.A.; Moy, J.N.; Forsyth, C.B.; Liu, Q.; et al. COVID-19 Severity Is Associated with Differential Antibody Fc-Mediated Innate Immune Functions. mBio 2021, 12, e00281-21. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Chaigne, B.; Mouthon, L. Mechanisms of action of intravenous immunoglobulin. Transfus. Apher. Sci. 2017, 56, 45–49. [Google Scholar] [CrossRef]
- Liu, Y.; Soh, W.T.; Kishikawa, J.I.; Hirose, M.; Nakayama, E.E.; Li, S.; Sasai, M.; Suzuki, T.; Tada, A.; Arakawa, A.; et al. An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Cell 2021, 184, 3452–3466.e18. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z.; et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020, 94, e02015-19. [Google Scholar] [CrossRef]
- Sharma, N.K.; Sarode, S.C. Low pH and temperature of airway surface liquid are key determinants that potentiate SARS-CoV-2 infectivity. Curr. Mol. Med. 2021, 22, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Grubaugh, N.D. Why does Japan have so few cases of COVID-19? EMBO Mol. Med. 2020, 12, e12481. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.; Nayrac, M.; Benlarbi, M.; Gong, S.Y.; Gasser, R.; Beaudoin-Bussières, G.; Brassard, N.; Laumaea, A.; Vézina, D.; Prévost, J.; et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe 2021, 29, 1137–1150. [Google Scholar] [CrossRef]
- Sablerolles, R.S.G.; Rietdijk, W.J.R.; Goorhuis, A.; Postma, D.F.; Visser, L.G.; Geers, D.; Schmitz, K.S.; Garcia Garrido, H.M.; Koopmans, M.P.G.; Dalm, V.A.S.H.; et al. Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming. N. Engl. J. Med. 2022, 386, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.R.; Cheng, X.; Li, Y.; Luo, W.W.; Zhang, Q.Z.; Peng, W.X. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): A meta-analysis. Int. Immunopharmacol. 2021, 96, 107732. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaka, T.; Yamamoto, Y.; Soma, T.; Yanagisawa, N.; Nagata, S. Cross-Reactivity of Antibodies in Intravenous Immunoglobulin Preparation for Protection against SARS-CoV-2. Microorganisms 2023, 11, 471. https://doi.org/10.3390/microorganisms11020471
Osaka T, Yamamoto Y, Soma T, Yanagisawa N, Nagata S. Cross-Reactivity of Antibodies in Intravenous Immunoglobulin Preparation for Protection against SARS-CoV-2. Microorganisms. 2023; 11(2):471. https://doi.org/10.3390/microorganisms11020471
Chicago/Turabian StyleOsaka, Toshifumi, Yoko Yamamoto, Takehisa Soma, Naoko Yanagisawa, and Satoru Nagata. 2023. "Cross-Reactivity of Antibodies in Intravenous Immunoglobulin Preparation for Protection against SARS-CoV-2" Microorganisms 11, no. 2: 471. https://doi.org/10.3390/microorganisms11020471





