Eight Up-Coming Biotech Tools to Combat Climate Crisis
Abstract
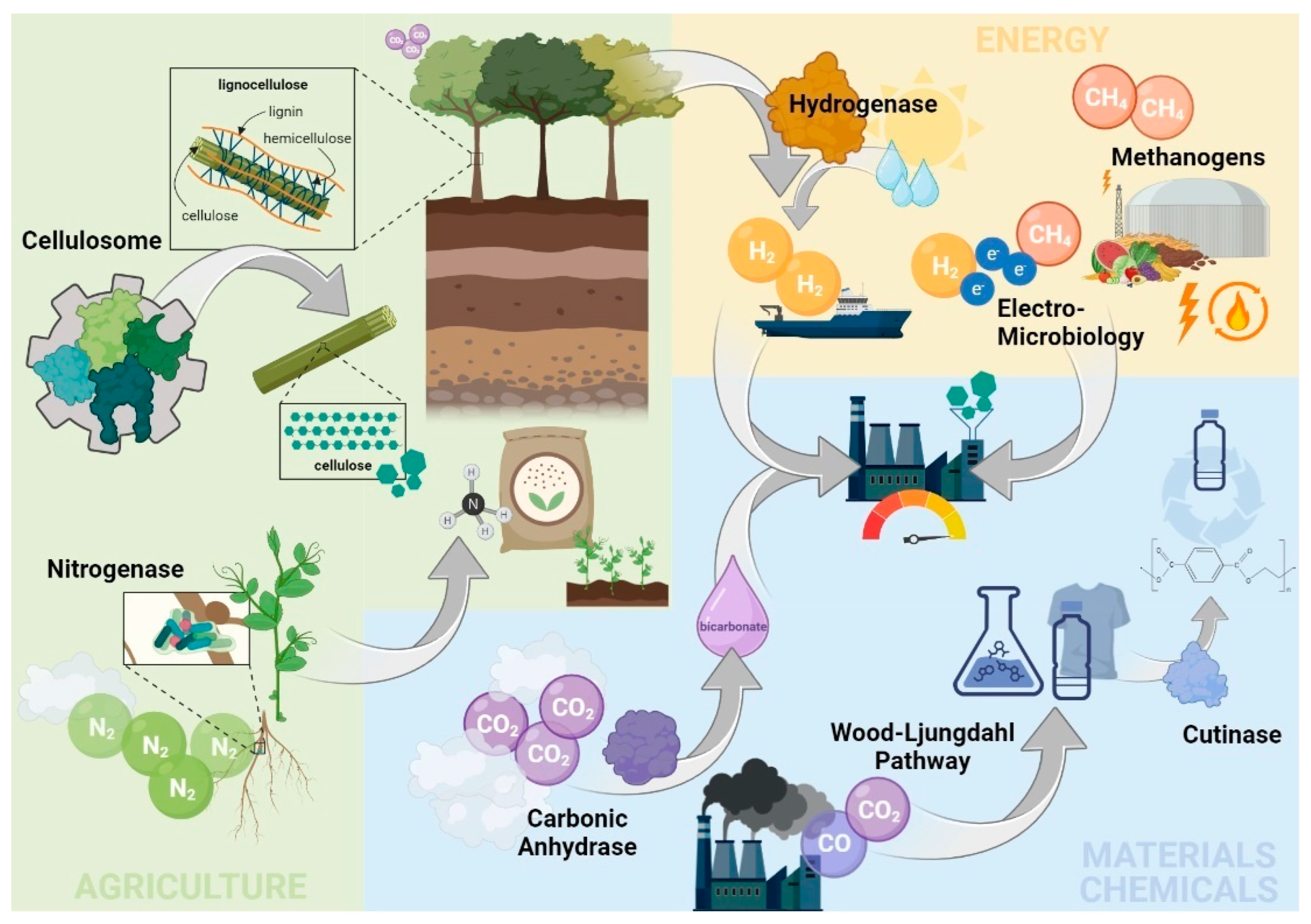
1. Introduction
2. Selected Biotech Tools to Mitigate GHG Emissions with Major Bioeconomy Sectors
2.1. Materials and Chemical Sector
2.1.1. The Wood–Ljungdahl Pathway High-Throughput Microbial CO2 Fixation
2.1.2. Carbonic Anhydrase: An Efficient Enzymatic Capture of CO2
2.1.3. Cutinases: Old Enzymes with a High Relevance for the Future
2.2. Energy Sector
2.2.1. Methanogens: Suppliers of Renewable Natural Gas
2.2.2. Electro Microbiology: Merging Electricity and Biology
2.2.3. Hydrogenases: A Key Enzyme behind the Green Hydrogen Economy
2.3. Agricultural Sector
2.3.1. Cellulosomes: Extracellular Nanomachines for Dismantling Plant Polysaccharides
2.3.2. Nitrogenase: A Bio-Based Approach to Substitute the Energy-Intensive Haber–Bosch Process
3. Potential Impact of Selected Biotechnological Tools
3.1. Wood–Ljungdahl Pathway
3.2. Carbonic Anhydrase
3.3. Cutinase
3.4. Methanogens
3.5. Electro-Microbiology
3.6. Hydrogenases
3.7. Cellulosome
3.8. Nitrogenase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 6223. [Google Scholar] [CrossRef]
- UNEP. Emissions Gap Report 2022: The Closing Window—Climate Crisis Calls for Rapid Transformation of Societies. Available online: https://www.unep.org/emissions-gap-report-2022 (accessed on 21 December 2022).
- EurEau. Europe’s Water in Figures, An Overview of the European Drinking Water and Waste Water Sectors. Available online: https://www.eureau.org/resources/publications/eureau-publications/5824-europe-s-water-in-figures-2021/file (accessed on 21 December 2022).
- US EPA. EPA Facility Registry Service (FRS): Wastewater Treatment Plants. Available online: https://edg.epa.gov/data/PUBLIC/OEI/OIC/FRS_Wastewater.zip (accessed on 21 December 2022).
- Jain, S. Global Potential of Biogas. Available online: https://www.worldbiogasassociation.org/wp-content/uploads/2019/09/WBA-globalreport-56ppa4_digital-Sept-2019.pdf (accessed on 21 December 2022).
- Bajpai, P. Global Production of Bioethanol. In Developments in Bioethanol. Green Energy and Technology; Springer: Singapore, 2021; pp. 177–196. ISBN 978-981-15-8779-5. [Google Scholar]
- IEA. Implementation of Bioenergy in Brazil—2021 update. Available online: https://www.ieabioenergy.com/wp-content/uploads/2021/11/CountryReport2021_Brazil_final.pdf (accessed on 1 June 2023).
- García, J.L.; Galán, B. Integrating greenhouse gas capture and C1 biotechnology: A key challenge for circular economy. Microb. Biotechnol. 2022, 15, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Li, F.-L.; Wang, Y.; Wangikar, P.P.; Guarnieri, M.T.; Luan, G. Editorial: Bioconversion and Biorefinery of C1 Compounds. Front. Microbiol. 2021, 12, 778962. [Google Scholar] [CrossRef]
- Berg, I.A.; Kockelkorn, D.; Ramos-Vera, W.H.; Say, R.F.; Zarzycki, J.; Hügler, M.; Alber, B.E.; Fuchs, G. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol. 2010, 8, 447–460. [Google Scholar] [CrossRef]
- Fuchs, G. Alternative pathways of carbon dioxide fixation: Insights into the early evolution of life. Annu. Rev. Microbiol. 2011, 65, 631–658. [Google Scholar] [CrossRef] [PubMed]
- Eden, G.; Fuchs, G. Total synthesis of acetyl coenzyme a involved in autotrophic CO2 fixation in Acetobacterium woodii. Arch. Microbiol. 1982, 133, 66–74. [Google Scholar] [CrossRef]
- Varma, S.J.; Muchowska, K.B.; Chatelain, P.; Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2018, 2, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Chowdhury, N.; Basen, M. Electron bifurcation: A long-hidden energy-coupling mechanism. Annu. Rev. Microbiol. 2018, 72, 331–353. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Dürre, P. Gas fermentation for commodity chemicals and fuels. Microb. Biotechnol. 2017, 10, 1167–1170. [Google Scholar] [CrossRef]
- Katsyv, A.; Müller, V. Overcoming energetic barriers in acetogenic C1 conversion. Front. Bioeng. Biotechnol. 2020, 8, 621166. [Google Scholar] [CrossRef]
- Mock, J.; Zheng, Y.; Mueller, A.P.; Ly, S.; Tran, L.; Segovia, S.; Nagaraju, S.; Köpke, M.; Dürre, P.; Thauer, R.K. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation. J. Bacteriol. 2015, 197, 2965–2980. [Google Scholar] [CrossRef]
- Heffernan, J.K.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Casini, I.; Plan, M.; Tappel, R.; Simpson, S.D.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-Valorization using Clostridium autoethanogenum for sustainable fuel and chemicals production. Front. Bioeng. Biotechnol. 2020, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Demler, M.; Weuster-Botz, D. Reaction engineering analysis of hydrogenotrophic production of acetic acid by Acetobacterium woodii. Biotechnol. Bioeng. 2011, 108, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Kantzow, C.; Mayer, A.; Weuster-Botz, D. Continuous gas fermentation by Acetobacterium woodii in a submerged membrane reactor with full cell retention. J. Biotechnol. 2015, 212, 11–18. [Google Scholar] [CrossRef]
- Steger, F.; Ergal, İ.; Daubek, A.; Loibl, N.; Rachbauer, L.; Fuchs, W.; Rittmann, S.K.-M.R.; Bochmann, G. Trickle-bed bioreactors for acetogenic H2/CO2 conversion. Front. Energy Res. 2022, 10, 842284. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Production of acids and alcohols from syngas in a two-stage continuous fermentation process. Bioresour. Technol. 2018, 253, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Jing, Y.; Lin, Y.; Zhang, S.; An, D. A novel concept for syngas biomethanation by two-stage process: Focusing on the selective conversion of syngas to acetate. Sci. Total Environ. 2018, 645, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mohan, S.V.; Chang, Y.-C. Medium-chain fatty acids (MCFA) production through anaerobic fermentation using Clostridium kluyveri: Effect of ethanol and acetate. Appl. Biochem. Biotechnol. 2018, 185, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Roghair, M.; Liu, Y.; Strik, D.P.B.T.B.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Development of an effective chain elongation process from acidified food waste and ethanol into n-Caproate. Front. Bioeng. Biotechnol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Stamatopoulou, P.; Malkowski, J.; Conrado, L.; Brown, K.; Scarborough, M. Fermentation of organic residues to beneficial chemicals: A review of medium-chain fatty acid production. Processes 2020, 8, 1571. [Google Scholar] [CrossRef]
- Venkateswar Reddy, M.; Kumar, G.; Mohanakrishna, G.; Shobana, S.; Al-Raoush, R.I. Review on the production of medium and small chain fatty acids through waste valorization and CO2 fixation. Bioresour. Technol. 2020, 309, 123400. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, G.; Farooq, R. Bio-electrochemical synthesis of commodity chemicals by autotrophic acetogens utilizing CO2 for environmental remediation. J. Biosci. 2016, 41, 367–380. [Google Scholar] [CrossRef]
- Candry, P.; Huang, S.; Carvajal-Arroyo, J.M.; Rabaey, K.; Ganigue, R. Enrichment and characterisation of ethanol chain elongating communities from natural and engineered environments. Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Seedorf, H.; Fricke, W.F.; Veith, B.; Brüggemann, H.; Liesegang, H.; Strittmatter, A.; Miethke, M.; Buckel, W.; Hinderberger, J.; Li, F.; et al. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl. Acad. Sci. USA 2008, 105, 2128–2133. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.-Y.; Park, B.; Choi, I.-G.; Chang, I.S. Genetic engineering system for syngas-utilizing acetogen, Eubacterium limosum KIST612. Bioresour. Technol. Rep. 2020, 11, 100452. [Google Scholar] [CrossRef]
- Jin, S.; Bae, J.; Song, Y.; Pearcy, N.; Shin, J.; Kang, S.; Minton, N.P.; Soucaille, P.; Cho, B.-K. Synthetic biology on acetogenic bacteria for highly efficient conversion of C1 gases to biochemicals. Int. J. Mol. Sci. 2020, 21, 7639. [Google Scholar] [CrossRef]
- Jia, D.; He, M.; Tian, Y.; Shen, S.; Zhu, X.; Wang, Y.; Zhuang, Y.; Jiang, W.; Gu, Y. Metabolic engineering of gas-fermenting Clostridium ljungdahlii for efficient co-production of isopropanol, 3-hydroxybutyrate, and ethanol. ACS Synth. Biol. 2021, 10, 2628–2638. [Google Scholar] [CrossRef]
- Liew, F.E.; Nogle, R.; Abdalla, T.; Rasor, B.J.; Canter, C.; Jensen, R.O.; Wang, L.; Strutz, J.; Chirania, P.; de Tissera, S.; et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat. Biotechnol. 2022, 40, 335–344. [Google Scholar] [CrossRef]
- Heijstra, B.D.; Leang, C.; Juminaga, A. Gas fermentation: Cellular engineering possibilities and scale up. Microb. Cell Fact. 2017, 16, 1. [Google Scholar] [CrossRef]
- Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of syngas composition on anaerobic fermentation. Reactions 2021, 2, 391–407. [Google Scholar] [CrossRef]
- US Patent & Trademark Office. Available online: www.uspto.gov/ (accessed on 19 February 2023).
- EEA. Total Greenhous Gas Emission Trends and Projections in Europe. Available online: https://www.eea.europa.eu/ims/total-greenhouse-gas-emission-trends (accessed on 27 September 2022).
- Walsh, B.; Ciais, P.; Janssens, I.A.; Peñuelas, J.; Riahi, K.; Rydzak, F.; van Vuuren, D.P.; Obersteiner, M. Pathways for balancing CO2 emissions and sinks. Nat. Commun. 2017, 8, 14856. [Google Scholar] [CrossRef] [PubMed]
- Skea, J.; Shukla, P.R.; Reisinger, A.; Slade, R.; Pathak, M.; Khourdajie, A.A.; van Diemen, R.; Abdulla, A.; Akimoto, K.; Babiker, M.; et al. Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2022—Mitigation of Climate Change. Available online: https://www.ipcc.ch/report/ar6/wg3/downloads/report/IPCC_AR6_WGIII_FullReport.pdf (accessed on 25 February 2023).
- Brickett, L. Carbon Dioxide Capture Handbook. Available online: https://netl.doe.gov/sites/default/files/netl-file/Carbon-Dioxide-Capture-Handbook-2015.pdf (accessed on 25 February 2023).
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Orlov, A.A.; Valtz, A.; Coquelet, C.; Rozanska, X.; Wimmer, E.; Marcou, G.; Horvath, D.; Poulain, B.; Varnek, A.; de Meyer, F. Computational screening methodology identifies effective solvents for CO2 capture . Commun. Chem. 2022, 5, 1. [Google Scholar] [CrossRef]
- Gervasi, J.; Dubois, L.; Thomas, D. Simulation of the post-combustion CO2 capture with Aspen HysysTM software: Study of different configurations of an absorptionregeneration process for the application to cement flue gases. Energy Procedia 2014, 63, 1018–1028. [Google Scholar] [CrossRef]
- Øi, L.E. Aspen HYSYS simulation of CO2 removal by amine absorption from a gas based power plant. In Proceedings of the 48th Scandinavian Conference on Simulation and Modeling (SIMS 2007), Göteborg (Särö), Sweden, 30–31 October 2007; pp. 73–81. [Google Scholar]
- House, K.Z.; Harvey, C.F.; Aziz, M.J.; Schrag, D.P. The energy penalty of post-combustion CO2 capture & storage and its implications for retrofitting the U.S. installed base. Energy Environ. Sci. 2009, 2, 193–205. [Google Scholar] [CrossRef]
- Grant, T.; Anderson, C.; Hooper, B. Comparative life cycle assessment of potassium carbonate and monoethanolamine solvents for CO2 capture from post combustion flue gases. Int. J. Greenh. Gas Control 2014, 28, 35–44. [Google Scholar] [CrossRef]
- Mazari, S.A.; Si Ali, B.; Jan, B.M.; Saeed, I.M.; Nizamuddin, S. An overview of solvent management and emissions of amine-based CO2 capture technology. Int. J. Greenh. Gas Control 2015, 34, 129–140. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Han, P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef]
- Obert, R.; Dave, B.C. Enzymatic conversion of carbon dioxide to methanol: Enhanced methanol production in silica sol-gel matrices. J. Am. Chem. Soc. 1999, 121, 12192–12193. [Google Scholar] [CrossRef]
- Xu, S.W.; Lu, Y.; Li, J.; Jiang, Z.-Y.; Wu, H. Efficient conversion of CO2 to methanol catalyzed by three dehydrogenases co-encapsulated in an alginate-silica (ALG-SiO2) hybrid gel. Ind. Eng. Chem. Res. 2006, 45, 4567–4573. [Google Scholar] [CrossRef]
- Jain, S.; Dietrich, H.M.; Müller, V.; Basen, M. Formate is required for growth of the thermophilic acetogenic bacterium Thermoanaerobacter kivui lacking hydrogen-dependent carbon dioxide reductase (HDCR). Front. Microbiol. 2020, 11, 59. [Google Scholar] [CrossRef]
- Schwarz, F.M.; Müller, V. Whole-cell biocatalysis for hydrogen storage and syngas conversion to formate using a thermophilic acetogen. Biotechnol. Biofuels 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.M.; Kumar, G.; Yang, Y.H. Carbon dioxide capture and bioenergy production using biological system—A review. Renew. Sustain. Energy Rev. 2019, 110, 143–158. [Google Scholar] [CrossRef]
- Boucif, N.; Roizard, D.; Favre, E. The Carbonic Anhydrase Promoted Carbon Dioxide Capture. In Membranes for Environmental Applications. Environmental Chemistry for a Sustainable World; no. 42; Zhang, Z., Zhang, W., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–44. ISBN 978-3-030-33978-4. [Google Scholar]
- Jensen, E.L.; Maberly, S.C.; Gontero, B. Insights on the functions and ecophysiological relevance of the diverse carbonic anhydrases in microalgae. Int. J. Mol. Sci. 2020, 21, 2922. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. A highlight on the inhibition of fungal carbonic anhydrases as drug targets for the antifungal armamentarium. Int. J. Mol. Sci. 2021, 22, 4324. [Google Scholar] [CrossRef]
- Angeli, A.; Carta, F.; Supuran, C.T. Carbonic anhydrases: Versatile and useful biocatalysts in chemistry and biochemistry. Catalysts 2020, 10, 1008. [Google Scholar] [CrossRef]
- Ozensoy Guler, O.; Capasso, C.; Supuran, C.T. A magnificent enzyme superfamily: Carbonic anhydrases, their purification and characterization. J. Enzym. Inhib. Med. Chem. 2016, 31, 689–694. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Supuran, C.T. A simple yet multifaceted enzyme. Rev. Chim. 2020, 71, 1–16. [Google Scholar] [CrossRef]
- Lin, J.Y.; Sri Wahyu Effendi, S.; Ng, I.-S. Enhanced carbon capture and utilization (CCU) using heterologous carbonic anhydrase in Chlamydomonas reinhardtii for lutein and lipid production. Bioresour. Technol. 2022, 351, 127009. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- de Oliveira Maciel, A.; Christakopoulos, P.; Rova, U.; Antonopoulou, I. Carbonic anhydrase to boost CO2 sequestration: Improving carbon capture utilization and storage (CCUS). Chemosphere 2022, 299, 134419. [Google Scholar] [CrossRef]
- di Fiore, A.; Alterio, V.; Monti, S.M.; de Simone, G.; D’Ambrosio, K. Thermostable carbonic anhydrases in biotechnological applications. Int. J. Mol. Sci. 2015, 16, 15456–15480. [Google Scholar] [CrossRef]
- Feron, P.H.M. Exploring the potential for improvement of the energy performance of coal fired power plants with post-combustion capture of carbon dioxide. Int. J. Greenh. 2010, 4, 152–160. [Google Scholar] [CrossRef]
- US 2013/0171718A1; Highly Stable Beta-Class Carbonic Anhydrases Useful in Carbon Capture Systems. Codexis, Inc.: Redwood City, CA, USA, 2013.
- WO 2012/025577 A1; Heat-Stable Persephonella Carbonic Anhydrases and Their Use. WIPO: Geneva, Switzerland, 2012.
- US7892814B2; Heat-Stable Carbonic Anhydrases and Their Use. Codexis, Inc.: Redwood City, CA, USA, 2011.
- WO 201406699 Al; Techniques for CO2 Capture Using Sulfurihydrogenibium sp. Carbonic Anhydrase. WIPO: Geneva, Switzerland, 2014.
- WO2013064195Al; A New Heat-Stable Carbonic Anhydrase and Uses Thereof. WIPO: Geneva, Switzerland, 2013.
- Fradette, L.; Lefebvre, S.; Carley, J. Demonstration results of enzyme-accelerated CO2 capture. Energy Procedia 2017, 114, 1100–1109. [Google Scholar] [CrossRef]
- Alvizo, O.; Nguyen, L.J.; Savile, C.K.; Bresson, J.A.; Lakhapatri, S.L.; Solis, E.O.P.; Fox, R.J.; Broering, J.M.; Benoit, M.R.; Zimmerman, S.A.; et al. Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas. Proc. Natl. Acad. Sci. USA 2014, 111, 16436–16441. [Google Scholar] [CrossRef]
- Littlechild, J.A. Enzymes from extreme environments and their industrial applications. Front. Bioeng. Biotechnol. 2015, 3, 161. [Google Scholar] [CrossRef]
- Steger, F.; Reich, J.; Fuchs, W.; Rittmann, S.K.-M.R.; Gübitz, G.M.; Ribitsch, D.; Bochmann, G. Comparison of carbonic anhydrases for CO2 sequestration. Int. J. Mol. Sci. 2022, 23, 957. [Google Scholar] [CrossRef]
- Fabbricino, S.; del Prete, S.; Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. In vivo immobilized carbonic anhydrase and its effect on the enhancement of CO2 absorption rate. J. Biotechnol. 2021, 336, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-J.; Cheng, J.-C.; Hu, C.-J.; Yu, C.-Y. Entrapment of the fastest known carbonic anhydrase with biomimetic silica and its application for CO2 sequestration. Polym. J. 2021, 13, 2452. [Google Scholar] [CrossRef]
- Rasouli, H.; Iliuta, I.; Bougie, F.; Garnier, A.; Iliuta, M.C. Enhanced CO2 capture in packed-bed column bioreactors with immobilized carbonic anhydrase. J. Chem. Eng. 2022, 432, 134029. [Google Scholar] [CrossRef]
- Rasouli, H.; Nguyen, K.; Iliuta, M.C. Recent advancements in carbonic anhydrase immobilization and its implementation in CO2 capture technologies: A review. Sep. Purif. Technol. 2022, 296, 121299. [Google Scholar] [CrossRef]
- Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. Immobilization of carbonic anhydrase for CO2 capture and utilization. Appl. Microbiol. Biotechnol. 2022, 106, 3419–3430. [Google Scholar] [CrossRef]
- Saipem. 2020. Available online: https://www.saipem.com/en/media/press-releases/2020-01-22/press-note-saipem-acquired-co2-capture-technology (accessed on 21 December 2022).
- Zaks, A.; Reardon, J. Advanced Low Energy Enzyme Catalyzed Solvent for CO2 Capture. 2013. Available online: https://www.osti.gov/biblio/1121752 (accessed on 21 December 2022).
- Arya, G.C.; Sarkar, S.; Manasherova, E.; Aharoni, A.; Cohen, H. The Plant Cuticle: An Ancient Guardian Barrier Set Against Long-Standing Rivals. Front. Plant. Sci. 2021, 12, 663165. [Google Scholar] [CrossRef]
- Arya, G.C.; Cohen, H. The Multifaceted Roles of Fungal Cutinases during Infection. J. Fungi 2022, 8, 199. [Google Scholar] [CrossRef]
- Liang, X.; Zou, H. Biotechnological application of cutinase: A powerful tool in synthetic biology. SynBio 2022, 1, 54–64. [Google Scholar] [CrossRef]
- Nikolaivits, E.; Kanelli, M.; Dimarogona, M.; Topakas, E. A middle-aged enzyme still in its prime: Recent advances in the field of cutinases. Catalysts 2018, 8, 612. [Google Scholar] [CrossRef]
- Kalantzi, S.; Kekos, D.; Mamma, D. Bioscouring of cotton fabrics by multienzyme combinations: Application of Box–Behnken design and desirability function. Cellulose 2019, 26, 2771–2790. [Google Scholar] [CrossRef]
- Wakelyn, P.J.; Bertoniere, N.R.; French, A.D.; Thibodeaux, D.P.; Triplett, B.A.; Rousselle, M.-A.; Goynes, J.; Edwards, J.V.; Hunter, L.; McAlister, D.D. Cotton Fiber Chemistry and Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-042-914-126-3. [Google Scholar]
- Agrawal, P.B.; Nierstrasz, V.A.; Bouwhuis, G.H.; Warmoeskerken, M.M.C.G. Cutinase and pectinase in cotton bioscouring: An innovative and fast bioscouring process. Biocatal. Biotransform. 2008, 26, 412–421. [Google Scholar] [CrossRef]
- Degani, O. Synergism between cutinase and pectinase in the hydrolysis of cotton fibers’ cuticle. Catalysts 2021, 11, 84. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, W.S.; Kim, H.R. Cutinase treatment of cotton fabrics. Fibers Polym. 2009, 10, 802–806. [Google Scholar] [CrossRef]
- Liu, Z.Z. Application of Cutinase in Textile Industry. In Cutinase: Preparation and Application; BP International, Inc.: Arcadia, CA, USA, 2021; pp. 100–121. [Google Scholar] [CrossRef]
- Wilson, A. Big Benefits with Biotech. Innovation in Textiles. 7 November 2022. Available online: https://www.innovationintextiles.com/big-benefits-with-biotech/ (accessed on 21 December 2022).
- Ito, S.; Kobayashi, T.; Hatada, Y.; Horikoshi, K. Enzymes in Modern Detergents. In Microbial Enzymes and Biotransformations; Barredo, J.L., Ed.; Humana Press: Totova, NJ, USA, 2005; Volume 17, pp. 151–161. ISBN 978-1-59259-846-5. [Google Scholar]
- Novozymes. Enzymes—No Compromise on Sustainability. Available online: https://biosolutions.novozymes.com/en/laundry/insights/sustainability (accessed on 21 December 2022).
- BlueWeave. Polyethylene Terephthalate (PET) Resin Market Size, Share & Growth. 2022. Available online: https://www.blueweaveconsulting.com/report/polyethylene-terephthalate-pet-resin-market (accessed on 21 December 2022).
- Eurostat. EU Recycled 41% of Plastic Packaging Waste in 2019. 27 October 2021. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20211027-2 (accessed on 21 December 2022).
- Ronkvist, A.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Herrero Acero, E.; Ribitsch, D.; Steinkellner, G.; Gruber, K.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Wei, R.; Zimmermann, W.; Zinn, M.; et al. Enzymatic surface hydrolysis of PET: Effect of structural diversity on kinetic properties of cutinases from Thermobifida. Macromolecules 2011, 44, 4632–4640. [Google Scholar] [CrossRef]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Carbios. Carbios Publishes First Sustainability Report and Underscores Commitment to Circularity and Environmental Responsibilities. 15 December 2022. Available online: https://www.carbios.com/en/carbios-publishes-first-sustainability-report-and-underscores-commitment-to-circularity-and-environmental-responsibilities/ (accessed on 21 December 2022).
- Ramanathan, V.; Cicerone, R.J.; Singh, H.B.; Kiehl, J.T. Trace gas trends and their potential role in climate change. J. Geophys. Res. 1985, 90, 5547–5566. [Google Scholar] [CrossRef]
- Monaco, A.; Ross, K.; Waskow, D.; Ge, M. How Methane Emissions Contribute to Climate Change. Available online: https://www.wri.org/insights/methane-gas-emissions-climate-change (accessed on 19 February 2023).
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Wolfe, R.S. An Historical Overview of Methanogenesis. In Methanogenesis: Chapman & Hall Microbiology Series; Ferry, J.G., Ed.; Springer: Boston, MA, USA, 1993; ISBN 978-1-4615-2391-8. [Google Scholar]
- Baker, B.J.; de Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J.G. Archaea and the origin of eukaryotes. Nat. Rev. Microbiol. 2017, 15, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Rittmann, S.K.-M.R. Haloarchaea as emerging big players in future polyhydroxyalkanoate bioproduction: Review of trends and perspectives. Curr. Res. Biotechnol. 2022, 4, 377–391. [Google Scholar] [CrossRef]
- Rittmann, S.K.-M.R.; Pfeifer, K.; Palabikyan, H.; Ergal, İ.; Schuster, B. Archaea in biotechnology | Archaea in der Biotechnologie. BioSpektrum 2021, 27, 96–98. [Google Scholar] [CrossRef]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.-M.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef]
- Lyu, Z.; Rotaru, A.-E.; Pimentel, M.; Zhang, C.-J.; Rittmann, S.K.-M.R. Editorial: The methane moment—Cross-boundary significance of methanogens: Preface. Front. Microbiol. 2022, 13, 1055494. [Google Scholar] [CrossRef] [PubMed]
- Ermler, U.; Grabarse, W.; Shima, S.; Goubeaud, M.; Thauer, R.K. Crystal structure of methyl-coenzyme M reductase: The key enzyme of biological methane formation. Science 1997, 278, 1457–1462. [Google Scholar] [CrossRef]
- Kumari ChitturiS, C.M.; Jeevana Lakshmi, J.P.; Hymavathi, D. Methanotrophs—Role in environmental engineering specific to reversal of global warming. Int. J. Sci. Manag. Eng. 2017, 2, 3. [Google Scholar]
- Leu, A.O.; Cai, C.; McIlroy, S.J.; Southam, G.; Orphan, V.J.; Yuan, Z.; Hu, S.; Tyson, G.W. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae. ISME J. 2020, 14, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, M.; Ya, M.; Xu, C. The denitrifying anaerobic methane oxidation process and microorganisms in the environments: A review. Front. Mar. Sci. 2022, 9, 1038400. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic consortia. Front. Microbiol. 2015, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Whitman, W.B. Transplanting the pathway engineering toolbox to methanogens. Curr. Opin. Biotechnol. 2019, 59, 46–54. [Google Scholar] [CrossRef] [PubMed]
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth. 2020. Available online: https://iea.blob.core.windows.net/assets/03aeb10c-c38c-4d10-bcec-de92e9ab815f/Outlook_for_biogas_and_biomethane.pdf (accessed on 21 December 2022).
- Antukh, T.; Lee, I.; Joo, S.; Kim, H. Hydrogenotrophs-Based Biological Biogas Upgrading Technologies. Front. Bioeng. Biotechnol. 2022, 10, 833482. [Google Scholar] [CrossRef] [PubMed]
- Rittmann, S.K.-M.R. A Critical Assessment of Microbiological Biogas to Biomethane Upgrading Systems. In Biogas Science and Technology, Advances in Biochemical Engineering/Biotechnology; Guebitz, G., Bauer, A., Bochmann, G., Gronauer, A., Weiss, S., Eds.; Springer: Cham, Switzerland, 2015; pp. 117–135. ISBN 978-3-319-21993-6. [Google Scholar]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sust. Energ. Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Mauerhofer, L.M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.S.; Paulik, C.; Rittmann, S.K.-M.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Rittmann, S.K.-M.R.; Seifert, A.H.; Bernacchi, S. Kinetics, multivariate statistical modelling, and physiology of CO2-based biological methane production. Appl. Energy 2018, 216, 751–760. [Google Scholar] [CrossRef]
- Wulf, C.; Zapp, P.; Schreiber, A. Review of Power-to-X demonstration projects in Europe. Front. Energy Res. 2020, 8, 191. [Google Scholar] [CrossRef]
- Thema, M.; Weidlich, T.; Hörl, M.; Bellack, A.; Mörs, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T.; et al. Biological CO2-Methanation: An Approach to Standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef]
- Zauner, A.; Fazeni-Fraisl, K.; Wolf-Zoellner, P.; Veseli, A.; Holzleitner, M.-T.; Lehner, M.; Bauer, S.; Pichler, M. Multidisciplinary Assessment of a Novel Carbon Capture and Utilization Concept including Underground Sun Conversion. Energies 2022, 15, 1021. [Google Scholar] [CrossRef]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground bio-methanation: Concept and potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the edge of research and technological application: A critical review of electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef] [PubMed]
- Sydow, A.; Krieg, T.; Mayer, F.; Schrader, J.; Holtmann, D. Electroactive bacteria—Molecular mechanisms and genetic tools. Appl. Microbiol. Biotechnol. 2014, 98, 8481–8495. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.B.A.; Verstraete, W. 100 years of microbial electricity production: Three concepts for the future. Microb. Biotechnol. 2012, 5, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Dang, Y.; Walker, D.J.F.; Lovley, D.R. The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microb. Genom. 2016, 2, 8. [Google Scholar] [CrossRef]
- Faustino, M.M.; Fonseca, B.M.; Costa, N.L.; Lousa, D.; Louro, R.O.; Paquete, C.M. Crossing the wall: Characterization of the multiheme cytochromes involved in the extracellular electron transfer pathway of Thermincola ferriacetica. Microorganisms 2021, 9, 293. [Google Scholar] [CrossRef]
- Ducluzeau, A.-L.; Nitschke, W. When Did Hemes Enter the Scene of Life? On the Natural History of Heme Cofactors and Heme-Containing Enzymes. In Cytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling. Advances in Photosynthesis and Respiration; Cramer, W., Kallas, T., Eds.; Springer: Dodrecht, The Netherlands, 2016; Volume 41, pp. 13–24. [Google Scholar] [CrossRef]
- Gong, Z.; Yu, H.; Zhang, J.; Li, F.; Song, H. Microbial electro-fermentation for synthesis of chemicals and biofuels driven by bi-directional extracellular electron transfer. Synth. Syst. Biotechnol. 2020, 5, 304–313. [Google Scholar] [CrossRef]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How To Drive Fermentation Using Electrochemical Systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sust. Energ. Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Ghosh, D. Advances in fermentative biohydrogen production: The way forward. Trends Biotechnol. 2009, 27, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Hasibar, B.; Ergal, İ.; Abreu Dias, S.; Bochmann, G.; Rittmann, S.K.-M.R.; Fuchs, W. Competing acetate consumption and production inside a microbial electrolysis cell. J. Environ. Chem. Eng. 2020, 8, 4. [Google Scholar] [CrossRef]
- Litti, Y.V.; Russkova, Y.I.; Zhuravleva, E.A.; Parshina, S.N.; Kovalev, A.A.; Kovalev, D.A.; Nozhevnikova, A.N. Electromethanogenesis: A Promising Biotechnology for the Anaerobic Treatment of Organic Waste. Appl. Biochem. Microbiol. 2022, 58, 19–36. [Google Scholar] [CrossRef]
- Cambrian. Cambrian Innovation. Available online: https://www.cambrianinnovation.com/resources (accessed on 1 June 2023).
- Brahic, C. The electricity eaters. New Sci. 2014, 223, 8–9. [Google Scholar] [CrossRef]
- Walker, G.M.; Ramsey, J.M.; Cavin, R.K.; Herr, D.J.C.; Merzbacher, C.I.; Zhirnov, V. A Framework for Bioelectronics—Discovery and Innovation. 2009. Available online: https://www.nist.gov/system/files/documents/pml/div683/bioelectronics_report.pdf (accessed on 21 December 2022).
- Ukhurebor, K.E.; Adetunji, C.O. Relevance of biosensor in climate smart organic agriculture and their role in environmental sustainability: What has been done and what we need to do? In Biosensors in Agriculture: Recent Trends and Future Perspectives. Concepts and Strategies in Plant Sciences; Pudake, R.N., Jain, U., Kole, C., Eds.; Springer: Cham, Switzerland, 2021; ISBN 978-3-030-66165-6. [Google Scholar]
- Fontecilla-Camps, J.C.; Frey, M.; Garcin, E.; Hatchikian, C.; Montet, Y.; Piras, C.; Vernède, X.; Volbeda, A. Hydrogenase: A hydrogen-metabolizing enzyme. What do the crystal structures tell us about its mode of action. Biochimie 1997, 79, 661–666. [Google Scholar] [CrossRef]
- Vignais, P.M.; Billoud, B. Occurrence, classification, and biological function of hydrogenases: An overview. Chem. Rev. 2007, 107, 4206–4272. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2021. Available online: https://www.iea.org/reports/global-hydrogen-review-2021/executive-summary (accessed on 21 December 2022).
- Cracknell, J.A.; Vincent, K.A.; Armstrong, F.A. Enzymes as working or inspirational electrocatalysts for fuel cells and electrolysis. Chem. Rev. 2008, 108, 2439–2461. [Google Scholar] [CrossRef]
- Wu, C.H.; McTernan, P.M.; Walter, M.E.; Adams, M.W. Production and Application of a Soluble Hydrogenase from Pyrococcus furiosus. Archaea 2015, 2015, 912582. [Google Scholar] [CrossRef]
- Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef]
- Burger, Y.; Schwarz, F.M.; Müller, V. Formate-driven H2 production by whole cells of Thermoanaerobacter kivui. Biotechnol. Biofuels Bioprod. 2022, 15, 48. [Google Scholar] [CrossRef]
- Rittmann, S.K.-M.R.; Lee, H.S.; Lim, J.K.; Kim, T.W.; Lee, J.H.; Kang, S.G. One-carbon substrate-based biohydrogen production: Microbes, mechanism, and productivity. Biotechnol. Adv. 2015, 33, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Ogata, H. Hydrogenases, Structure and Function. In Encyclopedia of Biological Chemistry, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 562–567. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Goenrich, M.; Schick, M.; Hiromoto, T.; Shima, S. Hydrogenases from Methanogenic Archaea, Nickel, a Novel Cofactor, and H2 Storage. Annu. Rev. Biochem. 2010, 79, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Schoelmerich, M.C.; Müller, V. Energy-converting hydrogenases: The link between H2 metabolism and energy conservation. Cell Mol. Life Sci. 2020, 77, 1461–1481. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K. The Wolfe cycle comes full circle. Proc. Natl. Acad. Sci. USA 2012, 109, 15084–15085. [Google Scholar] [CrossRef]
- Buckel, W.; Thauer, R.K. Flavin-Based Electron Bifurcation, A New Mechanism of Biological Energy Coupling. Chem. Rev. 2018, 118, 3862–3886. [Google Scholar] [CrossRef]
- Bothe, H.; Schmitz, O.; Yates, M.G.; Newton, W.E. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 529–551. [Google Scholar] [CrossRef]
- Mertens, R.; Liese, A. Biotechnological applications of hydrogenases. Curr. Opin. Biotechnol. 2004, 15, 343–348. [Google Scholar] [CrossRef]
- Zaborsky, O.R.; Benemann, J.R.; Matsunaga, T.; Miyake, J.; San Pietro, A. (Eds.) BioHydrogen; Springer: New York, NY, USA, 1998; ISBN 978-0-585-35132-2. [Google Scholar]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen production from biomass sources: Metabolic pathways and economic analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Hardt, S.; Stapf, S.; Filmon, D.T.; Birrell, J.A.; Rüdiger, O.; Fourmond, V.; Léger, C.; Plumeré, N. Reversible H2 Oxidation and Evolution by Hydrogenase Embedded in a Redox Polymer Film. Nat. Catal. 2021, 4, 251–258. [Google Scholar] [CrossRef]
- McPherson, I.J.; Vincent, K.A. Electrocatalysis by hydrogenases: Lessons for building bio-inspired devices. J. Braz. Chem. Soc. 2014, 25, 427–441. [Google Scholar] [CrossRef]
- Park, S.-G.; Rajesh, P.P.; Sim, Y.-U.; Jadhav, D.A.; Noori, M.T.; Kim, D.-H.; Al-Qaradawi, S.Y.; Yang, E.; Jang, J.-K.; Chae, K.-J. Addressing scale-up challenges and enhancement in performance of hydrogen-producing microbial electrolysis cell through electrode modifications. Energy Rep. 2022, 8, 2726–2746. [Google Scholar] [CrossRef]
- Dietrich, H.M.; Righetto, R.D.; Kumar, A.; Wietrzynski, W.; Trischler, R.; Schuller, S.K.; Wagner, J.; Schwarz, F.M.; Engel, B.D.; Müller, V.; et al. Membrane-anchored HDCR nanowires drive hydrogen-powered CO2 fixation. Nature 2022, 607, 823–830. [Google Scholar] [CrossRef]
- Prasad, S.; Ingle, A.P. Impacts of sustainable biofuels production from biomass. In Sustainable Bioenergy: Advances and Impacts; Rai, M., Ingle, A.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–346. ISBN 978-012-817-654-2. [Google Scholar]
- Chen, H. Lignocellulose Biorefinery Engineering: Principles and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780081001455. [Google Scholar]
- Fontes, C.M.G.A.; Gilbert, H.J. Cellulosomes: Highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 2010, 79, 655–681. [Google Scholar] [CrossRef]
- Bayer, E.A.; Lamed, R.; White, B.A.; Flints, H.J. From cellulosomes to cellulosomics. Chem. Rec. 2008, 8, 364–377. [Google Scholar] [CrossRef]
- Duarte, M.; Viegas, A.; Alves, V.D.; Prates, J.A.M.; Ferreira, L.M.A.; Najmudin, S.; Cabrita, E.J.; Carvalho, A.L.; Fontes, C.M.G.A.; Bule, P. A dual cohesin-dockerin complex binding mode in Bacteroides cellulosolvens contributes to the size and complexity of its cellulosome. J. Biol. Chem. 2021, 296, 100552. [Google Scholar] [CrossRef]
- Artzi, L.; Bayer, E.A.; Moraïs, S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 2017, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.H.; Kosugi, A.; Murashima, K.; Tamaru, Y.; Han, S.O. Cellulosomes from mesophilic bacteria. J. Bacteriol. 2003, 185, 5907–5914. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A. Cellulosomes and designer cellulosomes: Why toy with Nature? Environ. Microbiol. Rep. 2017, 9, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Stern, J.; Kahn, A.; Galanopoulou, A.P.; Yoav, S.; Shamshoum, M.; Smith, M.A.; Hatzinikolaou, D.G.; Arnold, F.H.; Bayer, E.A. Enhancement of cellulosome-mediated deconstruction of cellulose by improving enzyme thermostability. Biotechnol. Biofuels 2016, 9, 1. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Sharma, N.K.; Kumar, S. Bioprospecting thermostable cellulosomes for efficient biofuel production from lignocellulosic biomass. Bioresour. Bioprocess. 2015, 2, 1. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.J.; Odaneth, A.A. Industrial application of cellulases. In Current Status and Future Scope of Microbial Cellulases; Tuli, D.K., Kuila, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 10; pp. 189–209. ISBN 978-012-821-882-2. [Google Scholar]
- Behera, B.C.; Sethi, B.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Microbial cellulases—Diversity & biotechnology with reference to mangrove environment: A review. J. Genet. Eng. Biotechnol. 2017, 15, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Resch, M.G.; Donohoe, B.S.; Baker, J.O.; Decker, S.R.; Bayer, E.A.; Beckham, G.T.; Himmel, M.E. Fungal cellulases and complexed cellulosomal enzymes exhibit synergistic mechanisms in cellulose deconstruction. Energy Environ. Sci. 2013, 6, 1858–1867. [Google Scholar] [CrossRef]
- Uekotter, F.; Smil, V. Enriching the earth. Fritz Haber, Carl Bosch, and the transformation of world food production. Environ. Hist. Durh. N. C. 2001, 7, 3. [Google Scholar] [CrossRef]
- Guidehouse Insights. Green Ammonia and the Electrification of the Haber-Bosch Process Reduce Carbon Emissions. 2021. Available online: https://guidehouseinsights.com/news-and-views/green-ammonia-and-the-electrification-of-the-haber-bosch-process-reduce-carbon-emissions (accessed on 21 December 2022).
- Jensen, L.S.; Schjoerring, J.K.; van der Hoek, K.W.; Poulsen, H.D.; Zevenbergen, J.F.; Pallière, C.; Lammel, J.; Brentrup, F.; Jongbloed, A.W.; Willems, J.; et al. Benefits of nitrogen for food, fibre and industrial production from Part I—Nitrogen in Europe: The present position. In The European Nitrogen Assessment Sources, Effects and Policy Perspectives; Sutton, M.A., Howard, C.M., Erisman, J.W., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H., Grizzetti, B., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 32–61. [Google Scholar] [CrossRef]
- Hu, Y.; Ribbe, M.W. A journey into the active center of nitrogenase. J. Biol. Inorg. Chem. 2014, 19, 731–736. [Google Scholar] [CrossRef]
- Falkowski, P.G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 1997, 387, 272–275. [Google Scholar] [CrossRef]
- Boyd, E.S.; Peters, J.W. New insights into the evolutionary history of biological nitrogen fixation. Front. Microbiol. 2013, 4, 201. [Google Scholar] [CrossRef]
- Voora, V.; Larrea, C.; Bermúdez, S. Global Market Report: Soybeans. 2020. Available online: https://www.iisd.org/publications/report/global-market-report-soybeans (accessed on 21 December 2022).
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; Dileo, M.V. From the lab to the farm: An industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016, 7, 1110. [Google Scholar] [CrossRef]
- Burén, S.; Rubio, L.M. State of the art in eukaryotic nitrogenase engineering. FEMS Microbiol. Lett. 2018, 365, 2. [Google Scholar] [CrossRef]
- Xiang, N.; Guo, C.; Liu, J.; Xu, H.; Dixon, R.; Yang, J.; Wang, Y.-P. Using synthetic biology to overcome barriers to stable expression of nitrogenase in eukaryotic organelles. Proc. Natl. Acad. Sci. USA 2020, 117, 16537–16545. [Google Scholar] [CrossRef]
- Wen, A.; Havens, K.; Bloch, S.; Shah, N.; Higgins, D.; Davis-Richardson, A.; Sharon, J.; Rezaei, F.; Mohiti-Asli, M.; Johnson, A.; et al. Enabling Biological Nitrogen Fixation for Cereal Crops in Fertilized Fields. ACS Synth. Biol. 2021, 10, 3264–3277. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, R.; Tian, Y.; Zhu, Y.; Gao, J.; Li, Z.; Fu, X.; Xu, J.; Han, H.; Wang, L.; et al. Endowing plants with the capacity for autogenic nitrogen fixation. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; Irving, T.B.; Maia, L.G.S.; Ané, J.-M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 2019, 17, 1. [Google Scholar] [CrossRef]
- Vessey, J.K.; Pawlowski, K.; Bergman, B. Root-based N2-fixing symbioses: Legumes, actinorhizal plants, Parasponia sp. and cycads. Plant Soil 2005, 275, 51–78. [Google Scholar] [CrossRef]
- Levy-Varon, J.H.; Batterman, S.A.; Medvigy, D.; Xu, X.; Hall, J.S.; van Breugel, M.; Hedin, L.O. Tropical carbon sink accelerated by symbiotic dinitrogen fixation. Nat. Commun. 2019, 10, 1. [Google Scholar] [CrossRef]
- Nygren, P.; Fernández, M.P.; Harmand, J.-M.; Leblanc, H.A. Symbiotic dinitrogen fixation by trees: An underestimated resource in agroforestry systems. Nutr. Cycl. Agroecosyst. 2012, 94, 123–160. [Google Scholar] [CrossRef]
- Rachbauer, L.; Bochmann, G.; Fuchs, W. Gas fermentation. In The Autotrophic Biorefinery: Raw Materials from Biotechnology; Kourist, R., Schmidt, S., Eds.; De Gruyter: Berlin, Germany, 2021; Chapter 4; pp. 85–112. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Yang, Z.-Y.; Duval, S.; Dean, D.R. Nitrogenase reduction of carbon-containing compounds. Biochim. Biophys. Acta. Bioenerg. 2013, 1827, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, N.N.; Rebelein, J.G. The conversion of carbon monoxide and carbon dioxide by nitrogenases. Chem. Bio. Chem. 2022, 23, 8. [Google Scholar] [CrossRef]
- Fixena, K.R.; Zhenga, Y.; Harrisb, D.F.; Shawb, S.; Yangb, Z.-Y.; Deanc, D.R.; Seefeldt, L.C.; Harwooda, C.S. Light-driven carbon dioxide reduction to methane by nitrogenase in a photosynthetic bacterium. Proc. Natl. Acad. Sci. USA 2016, 113, 10163–10167. [Google Scholar] [CrossRef]
- Porc, O.; Carus, M.; Kähler, F.; vom Berg, C. Global Carbon Demand for Chemicals and Materials by Sectors—Reference Years: 2015–2020. Renewable Carbon Publications 2021. Nova Institute. Available online: https://renewable-carbon.eu/publications/product/global-carbon-demand-for-chemicals-and-materials-by-sectors-pdf/ (accessed on 17 April 2023).
- Saadatlu, E.A.; Barzinpour, F.; Yaghoubi, S. A sustainable model for municipal solid waste system considering global warming potential impact: A case study. Comput. Ind. Eng. 2022, 169, 108127. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Xue, X.; Chen, D.; Song, X.; Xiaohu, D. Hydrothermal and Pyrolysis Treatment for Sewage Sludge: Choice from Product and from Energy Benefit. Energy Procedia 2015, 66, 301–304. [Google Scholar] [CrossRef]
- Simpson, S.; Kopf, M.H. Alternative Carbon Sources for Chemical Value Chains. BASF Research Press Conference 2022. Available online: https://www.basf.com/global/documents/en/news-and-media/events/2022/research-press-conference/BASF-Research-Press-Conference-2022_BASF-and-LanzaTech.pdf (accessed on 28 April 2023).
- International Energy Agency (IEA). Carbon Capture, Utilisation and Storage. 2021. Available online: https://www.iea.org/fuels-and-technologies/carbon-capture-utilisation-and-storage (accessed on 28 March 2023).
- da Silveira Cachola, C.; Ciotta, M.; dos Santos, A.A.; Peyerl, D. Deploying of the carbon capture technologies for CO2 emission mitigation in the industrial sectors. Carbon Capture Sci. Technol. 2023, 7, 100102. [Google Scholar] [CrossRef]
- Talekar, S.; Byung Hoon Jo, B.H.; Dordick, J.S.; Kim, J. Carbonic anhydrase for CO2 capture, conversion and utilization. Curr. Opin. Biotechnol. 2022, 74, 230–240. [Google Scholar] [CrossRef]
- Soong, Y.-H.V.; Sobkowicz, M.J.; Xie, D. Recent advances in biological recycling of polyethylene terephthalate (PET) plastic wastes. Bioengineering 2022, 9, 98. [Google Scholar] [CrossRef]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of bioplastics: Routes and benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Turner, D.A.; Williams, I.D.; Kemp, S. Greenhouse gas emission factors for recycling of source-segregated waste materials. Resour. Conserv. Recy. 2015, 105, 186–197. [Google Scholar] [CrossRef]
- Guidehouse. Biomethane Production Potentials in the EU—Feasibility of REPowerEU 2030 Targets, Production Potentials in the Member States and Outlook to 2050. July 2022. Available online: https://gasforclimate2050.eu/wp-content/uploads/2022/10/Guidehouse_GfC_report_design_final_v3.pdf (accessed on 3 April 2023).
- Market Observatory for Energy DG Energy. Quarterly Report on European Gas Markets—With Focus on 2021, an Extraordinary Year on the European and Global Gas Markets. 2021. Available online: https://energy.ec.europa.eu/system/files/2022-04/Quarterly%20report%20on%20European%20gas%20markets_Q4%202021.pdf (accessed on 3 April 2023).
- Zhang, M.; Ma, Y. Energy use and challenges in current wastewater treatment plants. In A-B Processes: Towards Energy Self-sufficient Municipal Wastewater Treatment; IWA Publishing: London, UK, 2019; Chapter 1. [Google Scholar] [CrossRef]
- Flörke, M.; Kynast, E.; Bärlund, I.; Eisner, S.; Wimmer, F.; Alcamo, J. Domestic and industrial water uses of the past 60 years as a mirror of socio-economic development: A global simulation study. Glob. Environ. Chang. 2013, 23, 144–156. [Google Scholar] [CrossRef]
- Sato, T.; Qadir, M.; Yamamoto, S.; Endo, T.; Zahoor, A. Global, regional, and country level need for data on wastewater generation, treatment, and use. Agric. Water Manag. 2013, 130, 1–13. [Google Scholar] [CrossRef]
- European Investment Bank (EIB). EIB Project Carbon Footprint Methodologies—Methodologies for the Assessment of Project Greenhouse Gas Emissions and Emission Variations, Version 11.3. 2023. Available online: https://www.eib.org/attachments/lucalli/eib_project_carbon_footprint_methodologies_2023_en.pdf (accessed on 27 April 2023).
- Ning, X.; Lin, R.; O’Shea, R.; Wall, D.; Deng, C.; Wu, B.; Murphy, J.D. Emerging bioelectrochemical technologies for biogas production and upgrading in cascading circular bioenergy systems. iScience 2021, 24, 102998. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). The Future of Hydrogen—Seizing Today’s Opportunities. 2019. Available online: https://iea.blob.core.windows.net/assets/9e3a3493-b9a6-4b7d-b499-7ca48e357561/The_Future_of_Hydrogen.pdf (accessed on 30 March 2023).
- International Renewable Energy Agency (IRENA). Global Energy Transformation: A Roadmap to 2050. 2018. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Apr/IRENA_Report_GET_2018.pdf (accessed on 20 March 2023).
- International Energy Agency (IEA). Hydrogen. 2021. Available online: https://www.iea.org/reports/hydrogen (accessed on 20 March 2023).
- de Kleijne, K.; de Coninck, H.; van Zelm, R.; Huijbregts, M.A.J.; Hanssen, S.V. The many greenhouse gas footprints of green hydrogen. Sustain. Energy Fuels 2022, 6, 4383. [Google Scholar] [CrossRef] [PubMed]
- Zappi, A.; Hernandez, R.; Holmes, W.E. A review of hydrogen production from anaerobic digestion. Int. J. Environ. Sci. Technol. 2021, 18, 4075–4090. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Rajesh Banu, J.; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Moraïs, S.; Himmel, M.E.; Bayer, E.A. New Paradigms for Engineering Plant Cell Wall Degrading Enzymes. In Direct Microbial Conversion of Biomass to Advanced Biofuels; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 8; pp. 129–149. [Google Scholar] [CrossRef]
- Reducing enzyme costs, novel combinations, and advantages of enzymes could lead to improved cost-effective biofuels’ production. In Bioenergy: Biomass to Biofuels and Waste to Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 573–579. [CrossRef]
- Biomass R&D Board. The Billion Ton Bioeconomy Initiative: Challenges & Opportunities. 2016. Available online: https://biomassboard.gov/sites/default/files/pdfs/the_bioeconomy_initiative.pdf (accessed on 13 May 2023).
- Rogers, J.N.; Stokes, B.; Dunn, J.; Cai, H.; Wu, M.; Haq, Z.; Baumes, H. An assessment of the potential products and economic and environmental impacts resulting from a billion ton bioeconomy. Biofuels Bioprod. 2017, 11, 110–128. [Google Scholar] [CrossRef]
- Food and Agriclture Organization of the United Nations. Fertilizers by Nutrient. Available online: https://fenix.fao.org/faostat/internal/en/#data/RFN (accessed on 10 May 2023).
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Hoben, J.P.; Gehl, R.J.; Millar, N.; Grace, P.R.; Robertson, G.P. Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Glob. Chang. Biol. 2011, 17, 1140–1152. [Google Scholar] [CrossRef]
- Minamisawa, K. Mitigation of greenhouse gas emission by nitrogen-fixing bacteria. Biosci. Biotechnol. Biochem. 2023, 87, 7–12. [Google Scholar] [CrossRef]
- Guo, K.; Yang, J.; Yu, N.; Luo, L.; Wang, E. Biological nitrogen fixation in cereal crops: Progress, strategies, and perspectives. Plant Commun. 2023, 4, 100499. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Research and Innovation; Platt, R.; Bauen, A.; Reumerman, P.; Geier, C.; van Ree, R.; Gursel, I.V.; Garcia, L.; Behrens, M.; et al. EU Biorefinery Outlook to 2030: Studies on Support to Research and Innovation Policy in the Area of Bio-Based Products and Services. 2021. Available online: https://data.europa.eu/doi/10.2777/103465 (accessed on 21 December 2022).
- Bioenergy Technologies Office. Sustainable Aviation Fuel Grand Challenge. Available online: https://www.energy.gov/eere/bioenergy/sustainable-aviation-fuel-grand-challenge (accessed on 21 December 2022).
- Chandel, A.K.; Segato, F. (Eds.) Production of Top 12 Biochemicals Selected by USDOE from Renewable Resources, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-012-823-654-3. [Google Scholar]
- Republic of Korea. 2050 Carbon Neutral Strategy of the Republic of Korea—Towards a Sustainable and Green Society. 2020. Available online: https://unfccc.int/sites/default/files/resource/LTS1_RKorea.pdf (accessed on 21 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, W.; Rachbauer, L.; Rittmann, S.K.-M.R.; Bochmann, G.; Ribitsch, D.; Steger, F. Eight Up-Coming Biotech Tools to Combat Climate Crisis. Microorganisms 2023, 11, 1514. https://doi.org/10.3390/microorganisms11061514
Fuchs W, Rachbauer L, Rittmann SK-MR, Bochmann G, Ribitsch D, Steger F. Eight Up-Coming Biotech Tools to Combat Climate Crisis. Microorganisms. 2023; 11(6):1514. https://doi.org/10.3390/microorganisms11061514
Chicago/Turabian StyleFuchs, Werner, Lydia Rachbauer, Simon K.-M. R. Rittmann, Günther Bochmann, Doris Ribitsch, and Franziska Steger. 2023. "Eight Up-Coming Biotech Tools to Combat Climate Crisis" Microorganisms 11, no. 6: 1514. https://doi.org/10.3390/microorganisms11061514
APA StyleFuchs, W., Rachbauer, L., Rittmann, S. K.-M. R., Bochmann, G., Ribitsch, D., & Steger, F. (2023). Eight Up-Coming Biotech Tools to Combat Climate Crisis. Microorganisms, 11(6), 1514. https://doi.org/10.3390/microorganisms11061514





