Abstract
Bacillus amyloliquefaciens, a Gram-positive bacterium, has emerged as a versatile microorganism with significant applications in various fields, including industry, medicine, and agriculture. This comprehensive review aims to provide an in-depth understanding of the characteristics, genetic tools, and metabolic capabilities of B. amyloliquefaciens, while highlighting its potential as a chassis cell for synthetic biology, metabolic engineering, and protein expression. We discuss the bacterium’s role in the production of chemicals, enzymes, and other industrial bioproducts, as well as its applications in medicine, such as combating infectious diseases and promoting gut health. In agriculture, B. amyloliquefaciens has demonstrated potential as a biofertilizer, biocontrol agent, and stress tolerance enhancer for various crops. Despite its numerous promising applications, B. amyloliquefaciens remains less studied than its Gram-negative counterpart, Escherichia coli. This review emphasizes the need for further research and development of advanced engineering techniques and genetic editing technologies tailored for B. amyloliquefaciens, ultimately unlocking its full potential in scientific and industrial contexts.
1. Introduction
Bacillus amyloliquefaciens, a ubiquitous Gram-positive, aerobic bacterium, is commonly found in soil environments. This versatile organism has been utilized to produce a diverse array of heterologous proteins, including β-glucanases, acid-stable alpha-amylase, mesophilic alpha-amylase, cellulase, acid-soluble proteins, keratinase, and alkaline protease [1,2,3]. These properties make B. amyloliquefaciens a valuable host for synthesising therapeutic proteins and industrially relevant enzymes. Furthermore, B. amyloliquefaciens offers several advantages for applications in agricultural biotechnology. It produces secondary metabolites that exhibit antimicrobial activities against many phytopathogenic microorganisms, promoting plant growth and enhancing overall plant health [4]. These attributes position B. amyloliquefaciens as a promising candidate for developing sustainable and eco-friendly agricultural practices.
Bacillus amyloliquefaciens possesses remarkable physiological characteristics and a highly adaptable metabolism, enabling its cultivation of cost-effective media [5]. This bacterium exhibits rapid growth, with a fermentation cycle of approximately 72 h, compared to the 180-h cycle of Saccharomyces cerevisiae [6]. B. amyloliquefaciens also benefits from robust expression systems with excellent genetic stability and lacks strong codon preferences [7].
B. amyloliquefaciens has been established as an industrially significant bacterium in the production of biological indicators for sterilization and in biodefense research [8]. The unique properties and capabilities of this microorganism make it an attractive candidate for various biotechnological applications.
Over the years, extensive research has led to the development of various genetic modification tools for Bacillus amyloliquefaciens. These tools range from classical counter-selection marker strategies to the recently developed clustered regularly interspaced short palindromic repeats (CRISPR)—based genetic toolkits. The diverse protein secretion systems, along with the novel artificial promoter and ribosome binding site (RBS) libraries, further facilitate the production of extracellular enzymes [9,10,11,12,13].
B. amyloliquefaciens is an ideal multifunctional probiotic, exhibiting significant potential in inhibiting pathogenic bacterial growth and enhancing nutrient assimilation. This microorganism is known to produce a wide array of enzymes, including α-amylase, protease, lipase, cellulase, xylanase, pectinase, aminotransferase, barnase, peroxidase, glucanase, and chitinase [12,14]. BamHI is a type II restriction endonuclease, capable of recognizing short sequences (6 bp) of DNA and specifically cleaving them at a target site [15]. The β-1,3-1,4-glucanase derived from B. amyloliquefaciens showed an important role in protecting against phytopathogenic fungi [16].
The inherent genetic background of B. amyloliquefaciens, combined with well-developed gene manipulation tools, enables the reconstruction of its cellular metabolism. The availability of public knockout collections further enhances its attractiveness as a host for metabolic engineering applications.
In the agricultural sector, research has demonstrated that adding an appropriate quantity of Bacillus amyloliquefaciens can significantly enhance the carbon content in compost, thereby improving soil quality and promoting crop growth [17]. Moreover, B. amyloliquefaciens is among the most prevalent bacteria known to colonize plants endophytically, playing a crucial role in the biocontrol of vascular plant pathogens [18].
B. amyloliquefaciens is also capable of forming complex biofilms, which can serve as living biological materials to produce various functional biomaterials. These include surface growth factors, antibiotics, lysozyme, and antimicrobial peptides for medical applications. The development of biofilms by Bacillus amyloliquefaciens enhances barley’s resistance to salt stress [19]. However, certain bottlenecks currently limit the yield of target heterologous proteins in B. amyloliquefaciens, such as the absence of efficient genetic editing systems, unclear transcriptional regulation of heterologous proteins, and restricted secretion of heterologous proteins [20].
Most research has focused on optimizing factors such as signal peptides, transport channel levels, chaperone protein levels, and promoters in the expression and transport systems to enhance heterologous protein production in B. amyloliquefaciens [20]. Nevertheless, there is a pressing need to direct research efforts toward designing and developing host chassis to construct sustainable, robust, and efficient microbial cell factories for heterologous protein production.
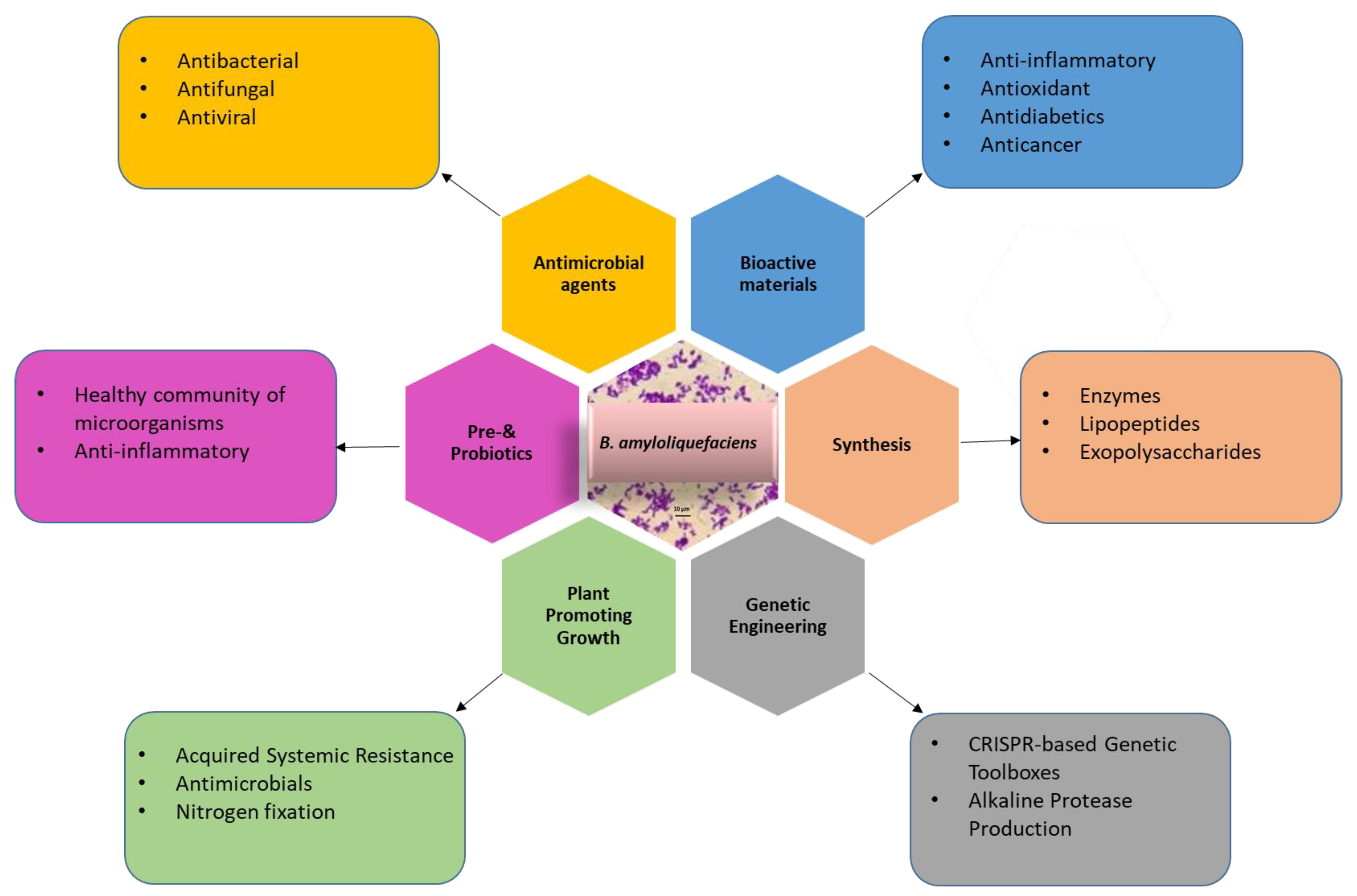
This review summarises the latest advancements in metabolic engineering, protein expression systems, and the diverse industrial, medical, and agricultural applications of Bacillus amyloliquefaciens. Additionally, we have examined the factors that impede the broader utilization of this microorganism and discussed the underlying reasons. This comprehensive overview serves as a valuable resource for researchers seeking to gain a thorough understanding of B. amyloliquefaciens and its myriad applications (Figure 1).
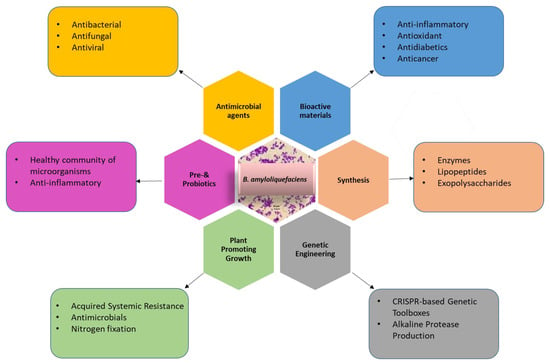
Figure 1.
Application of B. amyloliquefaciens for genetic engineering, production of industrial chemicals or enzymes, agriculture, medicine, and biomaterials.
2. Genetic Manipulation of Bacillus amyloliquefaciens
Bacillus amyloliquefaciens is a bacterium of considerable industrial importance, and various genetic manipulation techniques have been employed to enhance its strains for diverse applications [1,8,10,21]. A critical target for genetic modification in B. amyloliquefaciens is the alpha-amylase gene (amyE), which has been manipulated using innovative plasmid designs for extrachromosomal and intrachromosomal purposes [22].
The amyE gene is important because it includes homologous sequences necessary for integration into the B. amyloliquefaciens chromosome. This integration can serve as a model for evaluating the success of different genetic engineering strategies: the amyE gene’s ability to integrate into the B. amyloliquefaciens chromosome can be a practical tool for checking and measuring the performance of various engineering strategies. For instance, if a modification to the amyE gene successfully integrates and improves the bacterium’s performance, the applied strategy was effective [22]. Previous research has confirmed the successful application of genetic engineering techniques in B. amyloliquefaciens and shown the potential for further optimization and industrial utilization [9,10,13,22,23]. For instance, studies [13,20,23,24,25,26,27] have exhibited enhancements in the production of enzymes, such as protease and lipase, due to genetic engineering. Other research [28,29] has focused on the bacterium’s potential in plant disease control and its role in promoting plant growth. The wide range of applications and the potential for further improvements underline the importance of ongoing research and development.
2.1. Development and Application of CRISPR-Based Genetic Toolboxes in Bacillus amyloliquefaciens Strains
Xin et al. (2022) recently reported the development of a CRISPR-based genetic toolkit designed for efficient gene editing, knockout, and integration in Bacillus amyloliquefaciens LB1ba02. By employing a single-plasmid CRISPR/Cas9n system, the researchers achieved an impressive 93% knockout efficiency for a single gene and simultaneous editing of three loci with 53.3% efficiency using a base editing CRISPR/Cas9n-AID system [9]. This innovative toolkit was successfully applied to four genes (aprE, nprE, wprA, and bamHIR), showcasing its potential as a rapid gene knockout and integration tool for B. amyloliquefaciens LB1ba02.
In a unique study, Zhao et al. (2020) developed a novel genetic toolbox to augment the endogenous expression of the mesophilic α-amylase gene in Bacillus amyloliquefaciens 205 [13]. A key component of this toolbox was the implementation of an efficient interspecific transformation method, which is a technique that allows genetic material to be transferred across different species of Bacillus [30]. This method enhanced the ability to introduce foreign DNA into B. amyloliquefaciens 205, thereby facilitating the genetic manipulation of this bacterium. The toolbox also incorporated functional CRISPR systems, which provided precise editing capabilities to further manipulate the genetic material. This innovative strategy, particularly the implementation of interspecific transformation, holds potential applications across a broad range of Bacillus species. It allows for the transfer of beneficial traits from one species to another, broadening the possibilities for genetic enhancement in this bacterial genus [30].
The researchers applied several genetic engineering techniques to systematically increase gene expression levels. This included sporulation suppression, a technique that limits the bacteria’s natural process of forming spores, which allows more resources to be dedicated to the expression of the target gene. Additionally, transcript activation techniques were used to increase the production of the specific protein from its corresponding gene, and plasmid-based gene overexpression was utilized to boost the quantity of the target protein being produced. These strategic modifications can be applied across many Bacillus species [13].
Furthermore, Chen et al. (2016) successfully promoted spontaneous genetic competence in isolated B. amyloliquefaciens strains by overexpressing the master regulator ComK from Bacillus subtilis (ComKBsu). Utilizing direct transformation of PCR-generated deletion cassettes, they executed tasks such as replicative plasmid distribution and gene knockout. Artificial induction of genetic competence in B. amyloliquefaciens strains carrying the plasmid pUBXC can be achieved through the overexpression of ComKBsu [23].
2.2. Enhancing Alkaline Protease Production and Antifungal Properties of Bacillus amyloliquefaciens through Genetic Engineering
In a study focused on enhancing the alkaline protease production capacity of Bacillus amyloliquefaciens, researchers employed genetic engineering techniques to improve the production of the alkaline protease BSP-1. They cloned the bsp-1 gene from a Bacillus subtilis strain and introduced it into B. amyloliquefaciens. The results demonstrated that the recombinant strain produced a higher quantity of alkaline protease than the wild-type strain, highlighting a potential approach for genetically engineering B. amyloliquefaciens to increase the production of novel alkaline proteases [10].
More recent research has directed efforts toward genetically modifying B. amyloliquefaciens to enhance its antifungal properties and boost the production of eco-friendly antifungal lipopeptides. The study identified several genetic modifications that improved the engineered strains’ antifungal activity and lipopeptide production. These modifications included the deletion of genes involved in branched-chain amino acid biosynthesis and the overexpression of genes associated with lipopeptide synthesis. These findings suggest that genetic engineering can progressively enhance B. amyloliquefaciens’ antifungal capabilities, thereby facilitating the development of sustainable and environmentally friendly antifungal agents [11].
3. Gene Expression Using Bacillus amyloliquefaciens
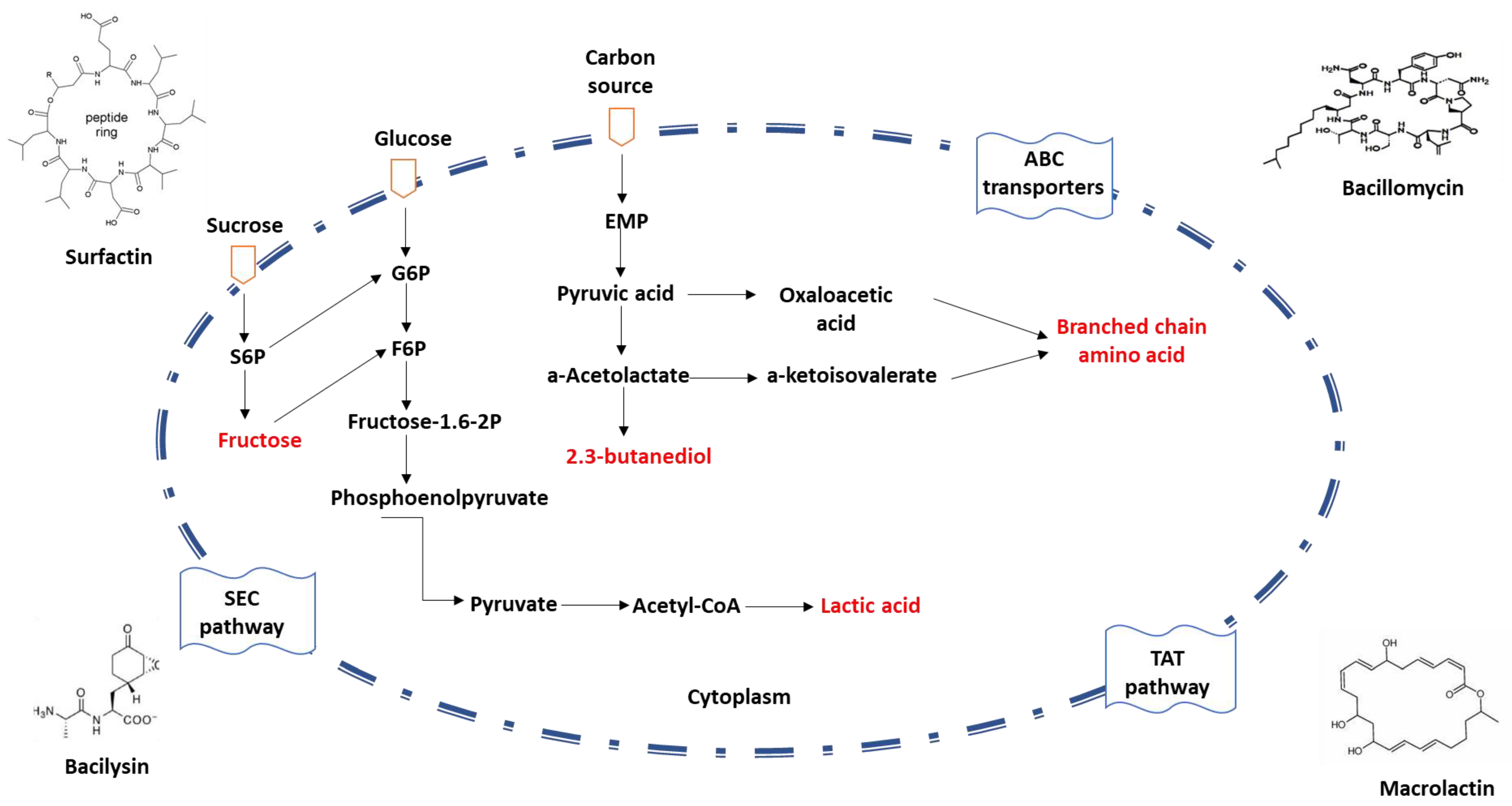
Owing to their remarkable capacity to express and secrete proteins, Bacillus spp. are frequently utilized in the production of commercial enzyme preparations. To accommodate the secretion requirements of diverse proteins, Bacillus spp. possess several promoters and plasmid expression systems. The three conventional protein secretion pathways in Bacillus spp. include the general protein secretion pathway (Sec), the twin-arginine translocation pathway (Tat), and the ATP-binding cassette (ABC) transporters [31,32,33] (Figure 2).
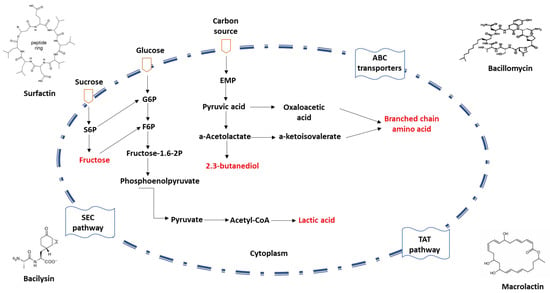
Figure 2.
Schematic diagram of protein secretion pathways in Bacillus spp. The mechanism of the non-classical secretion pathway is not clear.
3.1. Investigating Plant-Bacteria Interaction: Bacillus amyloliquefaciens and Sclerotinia sclerotiorum in Soybean Plants
In a recent investigation, a technique known as digital gene expression profiling was employed to analyze the activity of genes in soybean plants interacting with Bacillus amyloliquefaciens and the plant pathogen Sclerotinia sclerotiorum [34]. Digital gene expression profiling is a next-generation sequencing method that provides an accurate snapshot of gene activity in a given cell or tissue at a particular time. It allows researchers to identify and quantify specific genes being activated or suppressed in response to various conditions or stimuli.
Using this technique, the study found distinct transcriptional responses in soybean plants interacting with these two organisms. B. amyloliquefaciens induced the expression of genes that promote growth and tolerance to stress, while S. sclerotiorum stimulated the expression of genes associated with defense and stress responses. This research provides valuable insights into the genetic dynamics of plant interactions with beneficial bacteria and pathogens. Moreover, the study identified several candidate genes differentially expressed in the soybean plants in response to both microorganisms. These ‘candidate genes’ showed significant changes in their activity levels and may play key roles in the plant’s response to B. amyloliquefaciens and S. sclerotiorum. The study also suggested potential regulatory mechanisms that control the activity of these genes during the plant-bacteria interaction [34].
These regulatory mechanisms can involve various factors and influence how the plant responds to the presence of these microorganisms. This research offers valuable insights into the intricate dynamics between beneficial bacteria, plant pathogens, and their host plants, highlighting how plants can genetically adjust their responses to different microorganisms. Such insights contribute to a deeper understanding of plant-microbe interactions and their implications for crop health and productivity, potentially informing strategies to enhance disease resistance and growth in crops [34].
3.2. Cloning and Expression of Bacillus amyloliquefaciens Transglutaminase Gene in E. coli for Food Industry Applications
Duarte et al. (2020) successfully cloned and expressed the transglutaminase (TGase) gene from Bacillus amyloliquefaciens in Escherichia coli using a bicistronic vector-mediated approach. Transglutaminase enzymes are highly sought after in the food industry due to their ability to enhance food products’ flavor and nutritional value. However, their production has been challenging due to high costs and low yields [25].
To overcome these challenges, the authors constructed a plasmid in which the B. amyloliquefaciens TGase gene was fused to the prodomain of the Streptomyces caniferus protease. This prodomain is crucial in protein folding, ensuring the TGase enzyme is formed correctly. It also prevents the premature activation of TGase within the bacterial cell, which could disrupt normal cellular processes. To activate the enzyme once it is correctly folded and exported from the cell, the 3C protease gene was also incorporated in the plasmid, allowing for in vivo removal of the prodomain and subsequent activation of the enzyme. The bicistronic vector constructed in this way was then used to transform an E. coli strain ready for expression.
The study reported a successful expression of the B. amyloliquefaciens TGase gene in E. coli. The purified enzyme demonstrated activity across various substrates, indicating potential applications in the food industry [25].
3.3. Heterologous Expression and Periplasmic Secretion of an Antifungal Bacillus amyloliquefaciens BLB 369 Endo-β-1,3-1,4-Glucanase in Escherichia coli
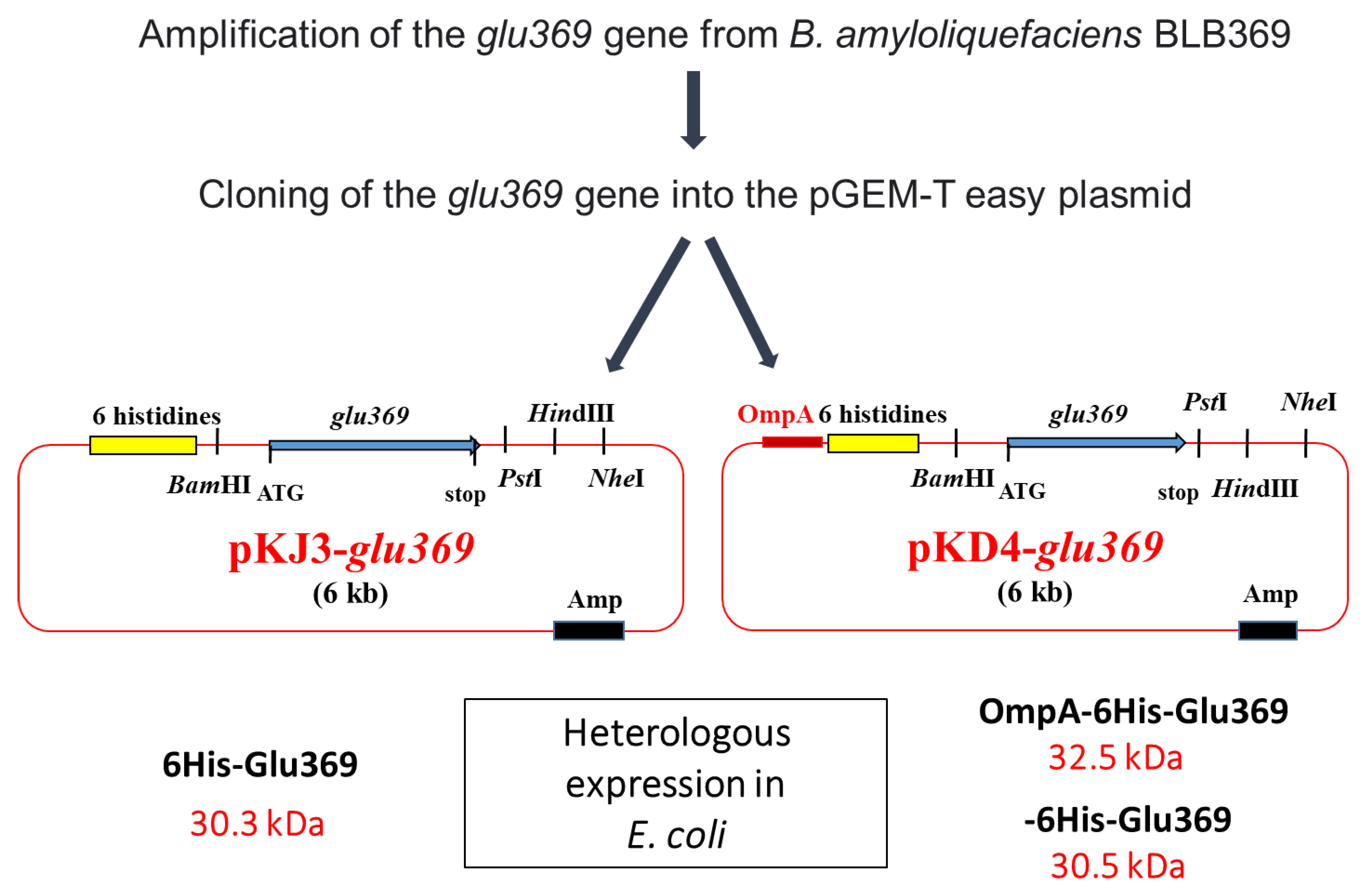
Endo-β-1,3-1,4-glucanases, classified as glycoside hydrolases, play a crucial role in the enzymatic depolymerization of 1,3-1,4 β-glucans and exhibit antifungal properties. B. amyloliquefaciens BLB369 is known to produce this enzyme. Researchers have sequenced, cloned, and effectively expressed the glu369 full-coding sequence of the endo-β-1,3-1,4-glucanase gene in Escherichia coli Top10. To simplify the purification process, the glu369 coding sequence was integrated into the pKJD4 vector. The resultant OmpA-His-Glu369 fusion protein incorporated the OmpA signal sequence for E. coli periplasmic targeting, followed by a 6xHistidine tag for purification purposes. Owing to these beneficial attributes, endo-β-1,3-1,4-glucanase holds significant potential for various biotechnological applications [35] (Figure 3).
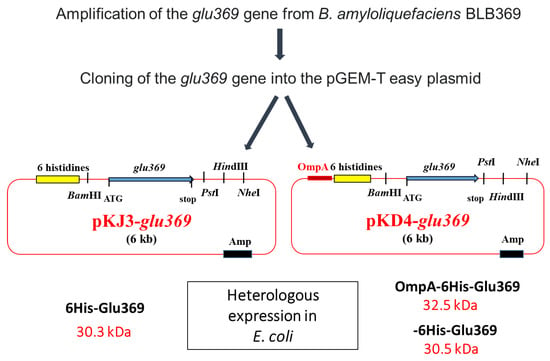
Figure 3.
Strategy for cloning the glu369 gene in pKJ3 and pKJ4 and structure of recombinant plasmids pKJ3-glu369 and pKD4-glu369. Amp, ampicillin resistance; lacZ, the gene encoded by the β-galactosidase; OmpA, signal sequence [35].
To enhance heterologous protein synthesis in B. amyloliquefaciens, most existing research has focused on optimizing factors such as signal peptides, transport channel levels, chaperone protein levels, promoters, and other components of the expression and transport systems [20]. Nonetheless, for the establishment of sustainable, dependable, and efficient heterologous protein-producing microbial cell factories, emphasis should be placed on designing and developing host chassis.
Owing to the unique genetic backgrounds of B. amyloliquefaciens, several model strains have been extensively investigated. Advanced genome editing techniques have been employed in species like B. subtilis and B. licheniformis for heterologous protein generation through meticulous microbial chassis engineering [36]. Therefore, it is essential to augment heterologous protein production utilizing B. amyloliquefaciens, a strain distinct from B. subtilis and B. licheniformis. However, our understanding of the regulatory mechanisms governing specific enzyme secretion remains limited. This knowledge gap leads to challenges in devising accurate secretion route engineering strategies and assembling a toolbox of signal peptide sequences for various heterologous enzymes.
Cell membrane engineering is a specialized field that focuses on manipulating the structure and composition of the cell membrane to enhance its functionality. In the case of Bacillus amyloliquefaciens, this approach can increase the bacterium’s efficiency in producing and secreting proteins—essentially transforming the bacterium into a ‘cell factory’. However, one of the challenges with this approach lies in predicting the most suitable enzymes to target for a given application. Enzymes are proteins that act as catalysts for various biochemical reactions, and different enzymes are involved in different reactions. Therefore, the choice of enzymes to target can greatly influence the efficiency and effectiveness of protein production and secretion. To address this challenge, one potential strategy is to further optimize cell membrane engineering techniques to be more adaptable to different enzymes. This involves creating a modular system where the techniques can be adjusted and applied predictably depending on the specific enzymes targeted.
In other words, this strategy aims to establish a flexible and standardized framework for cell membrane engineering, where different techniques can be plugged in or taken out as needed to optimize the bacterium’s ability to produce and secrete different target enzymes. This could potentially enhance the usefulness of B. amyloliquefaciens in various biotechnological applications.
4. The Importance and Applications of the Bacillus amyloliquefaciens
B. amyloliquefaciens is a versatile and beneficial bacterium with significant importance in various industries due to its ability to produce an array of enzymes and antimicrobial compounds. It is a biocontrol agent in agriculture, promoting plant growth and protecting crops from pathogens. Its ability to produce enzymes such as amylase, protease, and lipase has led to its widespread use in food and beverage, contributing to processes like fermentation, baking, and brewing. Furthermore, B. amyloliquefaciens has applications in biotechnology, where it is employed as a host to produce heterologous proteins and as a source of valuable biomolecules. The diverse applications of B. amyloliquefaciens highlight its importance in advancing sustainable and eco-friendly solutions across multiple sectors [5,8,11,17,21,24,35,36,37,38,39,40,41,42,43].
4.1. Industrial Application of Bacillus amyloliquefaciens
Bacillus amyloliquefaciens has shown the ability to produce a variety of amino acids and peptides, which can be relevant to the industrial sector.
γ-aminobutyric acid (GABA), an inhibitory neurotransmitter, has been utilized in treating conditions such as anxiety and sleep disorders. GABA has also shown potential as an anti-aging factor. Although GABA has been used as a food supplement, its efficacy and precise mechanism warrant further research. Bacillus amyloliquefaciens EH-9 has been employed for the biosynthesis of GABA, presenting a cost-effective and safe approach. In a study by Zayabaatar et al. (2023), B. amyloliquefaciens EH-9 was co-cultivated with germinated rice seeds, and the resulting supernatant was rich in GABA. When this supernatant was applied topically to mice’s dorsal skin, it significantly enhanced type I collagen production. This increase in type I collagen synthesis is attributed to the presence of GABA, as demonstrated by the loss of effect upon knocking down GABA-A receptors in mice [44].
Huang et al., (2023) harnessed B. amyloliquefaciens to produce GABA-rich rice by inoculating grain seeds with the bacterium. Mice fed with B. amyloliquefaciens-inoculated rice exhibited increased serum levels of neuropeptide Y (Low serum Neuropeptide Y is known to be associated with sleep, circadian rhythm, and emotional disturbances) and improved head dips. A head dip is observed when a mouse puts its head in holes when placed in a hole board test to test anxiety levels. The study suggests this effect is mediated by gastrointestinal GABA-B receptors and the vagus nerve [45].
Poly-γ-glutamic acid (γ-PGA) is a biocompatible and biodegradable polypeptide primarily produced by Bacillus species, with diverse applications across various fields [46]. Although B. amyloliquefaciens LL3 has demonstrated the ability to synthesize this polypeptide, the yield remains low. Gao et al. (2019) employed genetic and metabolic engineering techniques to enhance the γ-PGA yield in this glutamic acid-independent γ-PGA-producing bacterial strain. In their study, glutamate metabolism pathways were partially inhibited by deleting the fadR, lysC, aspB, pckA, proAB, rocG, and gudB genes. The promoter Pc 2up was also inserted into the icd gene to regulate NADPH, while the srf and itu operons were removed. These modifications resulted in the development of a new strain, B. amyloliquefaciens NK-A11, which exhibited the highest γ-PGA titer (7.53 g/L), representing a 2.05-fold increase compared to B. amyloliquefaciens LL3Δupp [26]. Furthermore, Fang et al. (2020) demonstrated that B. amyloliquefaciens JX-6 showed high yields of γ-PGA from fermenting corn stoke and soybean meal, where the yield was 112.82 g/L [47].
Pectinases, enzymes employed in the food industry and other sectors, are typically produced by bacteria or fungi; however, bacterial-derived pectinases are often preferred due to the ease of fermentation and the availability of various techniques to increase yield.
A primary challenge in pectinase production is the associated cost. To address this issue, Doan et al. (2021) utilized B. amyloliquefaciens TKU050 to produce pectinases through bioprocessing banana peels, a pectin-containing by-product. By employing banana peels as a carbon source for pectinase synthesis, the approach not only reduced production costs but also promoted environmentally friendly practices. Furthermore, the study suggested that B. amyloliquefaciens TKU050 pectinases could have potential applications in prebiotic production [48]. Devaraj et al. (2019) investigated the synthesis of numerous thermostable enzymes by the B. amyloliquefaciens strain KUB29. By employing a two-stage fermentation strategy, the researchers identified several enzymes exhibiting activity across various temperatures and pH levels, thereby enhancing enzyme synthesis. These findings indicated that the KUB29 strain holds promise for various industrial biotechnology applications, particularly in biofuel production and other bioproducts, as its enzymes provide a cleaner source than traditional methods [24].
In a recent study, a B. amyloliquefaciens D1 strain was isolated from Morchella crassipes. This strain can produce high levels of neutral proteases purified using chromatography and ammonium sulfate precipitation. In this study, the purified protease was used in soybean milk fermentation as an example of an industrial application where they increased the release of essential amino acids, which could improve the nutritional value of the fermented soybean milk [49].
In the food industry, multi-drug-resistant foodborne pathogens are a great concern. A study has demonstrated that fengycin produced by B. amyloliquefaciens JFL21 showed great antifungal activity and antibacterial activity against foodborne Gram-positive and Gram-negative pathogens. Furthermore, antimicrobial compounds produced by the JFL21 were purified, named Anti-JFL21 and were found to be composed of different lipopeptides, including surfactin, fengycin and iturin. This study has found that Anti-JFL21 showed great antimicrobial activity even to the multi-drug resistant pathogens with low impact on the probiotics [50].
Soussi et al. (2019) conducted a study illustrating that B. amyloliquefaciens C5 pro-duced potent antimicrobial surfactant lipopeptides, namely surfactin and bacillomycin D. The growth and antimicrobial activity of this strain were optimized using grape seed flour substrate, which demonstrated optimal performance at 0.2% (w/v) [51]. Iturin A is another surfactant lipopeptide produced by B. amyloliquefaciens, offering diverse applications in the petrochemical, agricultural, and medical sectors. Gao et al. (2022) successfully im-proved the yield of iturin A by enhancing the expression of genes associated with fatty acid biosynthesis, resulting in the development of a new strain, B. amyloliquefaciens HZ-ADFTL2. This strain exhibited an increased iturin A yield of 2.96 g/L, representing a 6.59-fold enhancement compared to the original strain, B. amyloliquefaciens HZ-12 [52]. In a study by Lu et al. (2016), the synthesis of fengycin, an effective antifungal lipopeptide, by B. amyloliquefaciens fmb-60 was enhanced with the addition of fructose to the culture me-dia. A subsequent study revealed that the increased fengycin synthesis in B. amyloliquefa-ciens fmb-60 was attributable to the upregulation of transcriptional factor sigmaH and two-component system degU and degQ, while downregulating the inhibitory regulator AbrB. Additionally, numerous genes involved in amino acid metabolism, fatty acid me-tabolism, and energy metabolism were upregulated or downregulated [53]. Wang et al. (2022) employed a different approach to improve the yield of fengycin and iturin using metabolically engineered B. amyloliquefaciens. They utilized B. amyloliquefaciens WH1 as a wild-type strain and knocked out several genes, including bdh to block the carbon over-flow metabolic pathway, kinA to disrupt bacterial sporulation and extend the production period, dhbF to enhance the availability of amino acids and fatty acids, and rapA to in-crease Spo0A~P levels. The quadruple knockout strain ΔkinAΔbdhΔdhbFΔrapA exhibited enhanced antifungal activity, and after fermentation, iturin and fengycin titers in the bio-reactor increased to 123.5 mg/L (a 22-fold increase) and 1200.8 mg/L (a 15.9-fold increase), respectively [11]. Table 1 presents a summary of representative chemicals produced by B. amyloliquefaciens.

Table 1.
Representative chemicals produced by B. amyloliquefaciens.
4.2. Medical Application of Bacillus amyloliquefaciens
B. amyloliquefaciens SC06 has demonstrated a protective effect against high-fat diet (HFD)-induced obesity in animal models. Wang et al. (2019) found that mice fed an HFD combined with SC06 exhibited reduced fat accumulation, lower obesity levels, and a slight decrease in insulin resistance. Additionally, SC06 treatment provided liver protection and decreased the secretion of serum inflammatory factors, specifically IL-6, TNF-α, and leptin. The study also revealed that SC06 improved the intestinal microbiota composition, contributing to the observed protective effects [55].
Jeong et al. (2020) investigated the impact of B. amyloliquefaciens on neuronal activity, microbiome composition, and glucose metabolism in an ischemic stroke model. In their study, Mongolian gerbils were fed chungkookjang, a soybean product fermented with B. amyloliquefaciens SRCM100730 (CKJ730) and B. amyloliquefaciens SRCM100731 (CKJ731), before inducing ischemic stroke via artery occlusion. The results indicated that the treatment reduced neuronal cell death, ameliorated stroke-related neurological symptoms, enhanced glucose metabolism by preserving β-cells in the pancreas, and improved insulin sensitivity. Furthermore, the treatment exhibited an anti-inflammatory effect, as evidenced by the reduced serum levels of proinflammatory cytokines TNF-α and IL-1β, while preserving the gut microbiome constituents, particularly Bacteroidia and Clostridia [56].
A novel Bacillus amyloliquefaciens strain, X030, has been identified to produce a lipopeptide called bacillomycin Lb, which exhibits anticancer potential against various cancer cell lines [54]. Additionally, bacillomycin Lb has demonstrated antimicrobial activity [57]. A study by Zhou et al. (2022) revealed that using B. amyloliquefaciens X030 improved the microbiome composition in grass carp by promoting the presence of probiotics and reducing opportunistic bacteria in the intestine. The strain was also found to produce macrolactin A, an antibacterial agent that collectively enhanced grass carp resistance to certain infections. Furthermore, grass carp fed with B. amyloliquefaciens X030 exhibited increased expression of IL-8 (a chemokine), C3 (involved in liver stress tolerance), and IgM, thereby improving the immunity of grass carp against Aeromonas hydrophila X040 and A. veronii X005 infections [58].
In hospital settings, Acinetobacter spp. is a nosocomial pathogen known for forming robust biofilms and exhibiting increasing antibiotic resistance, making the treatment of infections caused by this pathogen challenging. Al-Dulaimi et al., (2021) conducted a preliminary study evaluating the potential use of a probiotic strain, B. amyloliquefaciens B-1895 cell-free supernatant (CFS), against Acinetobacter spp. isolates from selected clinical cases. CFS’s antimicrobial and anti-biofilm activities were tested alone or in combination with polymyxin E. The study demonstrated that at high concentrations, CFS of B. amyloliquefaciens B-1895 inhibited both the growth of Acinetobacter spp. and biofilm formation. The combination of polymyxin E and CFS exhibited a synergistic effect, with lower minimum inhibitory concentration (MIC) and minimum biofilm inhibitory concentration (MBIC) values compared to each agent used individually [59].
B. amyloliquefaciens also plays a significant role in animal health. In a 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis model in mice, camel milk enriched with B. amyloliquefaciens PBT-3 (BEY) demonstrated beneficial effects [60]. BEY treatment reduced colitis progression and prevented the deterioration of intestinal epithelia in mice by decreasing proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, modulating IL-8 activity, and upregulating the anti-inflammatory cytokine IL-4 [60].
In a comprehensive investigation of subclinical necrotic enteritis in broiler chickens, researchers observed that pretreatment with Bacillus amyloliquefaciens BLCC1-0238, either alone or in conjunction with mannan-oligosaccharides (MOS), led to a decrease in mortality rates, reduced intestinal mucosal lesions, and improved growth performance. This outcome appears to be associated with the elevated expression of claudin-3 and peroxisome proliferator-activated receptor-gamma coactivator-1α in both pretreatment groups. Furthermore, the addition of MOS to the bacterial treatment resulted in upregulation of mucin-2 [61]. In a separate study conducted by Morozova et al. (2022), it was demonstrated that feeding Muc2−/− mice, which possess impaired intestinal barriers, an autoclaved diet devoid of Bacillus spp. led to an increased incidence of intestinal inflammation due to alterations in the gut microbiome. Additionally, a decline in fertility was observed [62]. Table 2 provides a comprehensive overview of the medical and agricultural applications of B. amyloliquefaciens.

Table 2.
List of medical and agricultural applications using B. amyloliquefaciens.
4.3. Application of Bacillus amyloliquefaciens in Agriculture
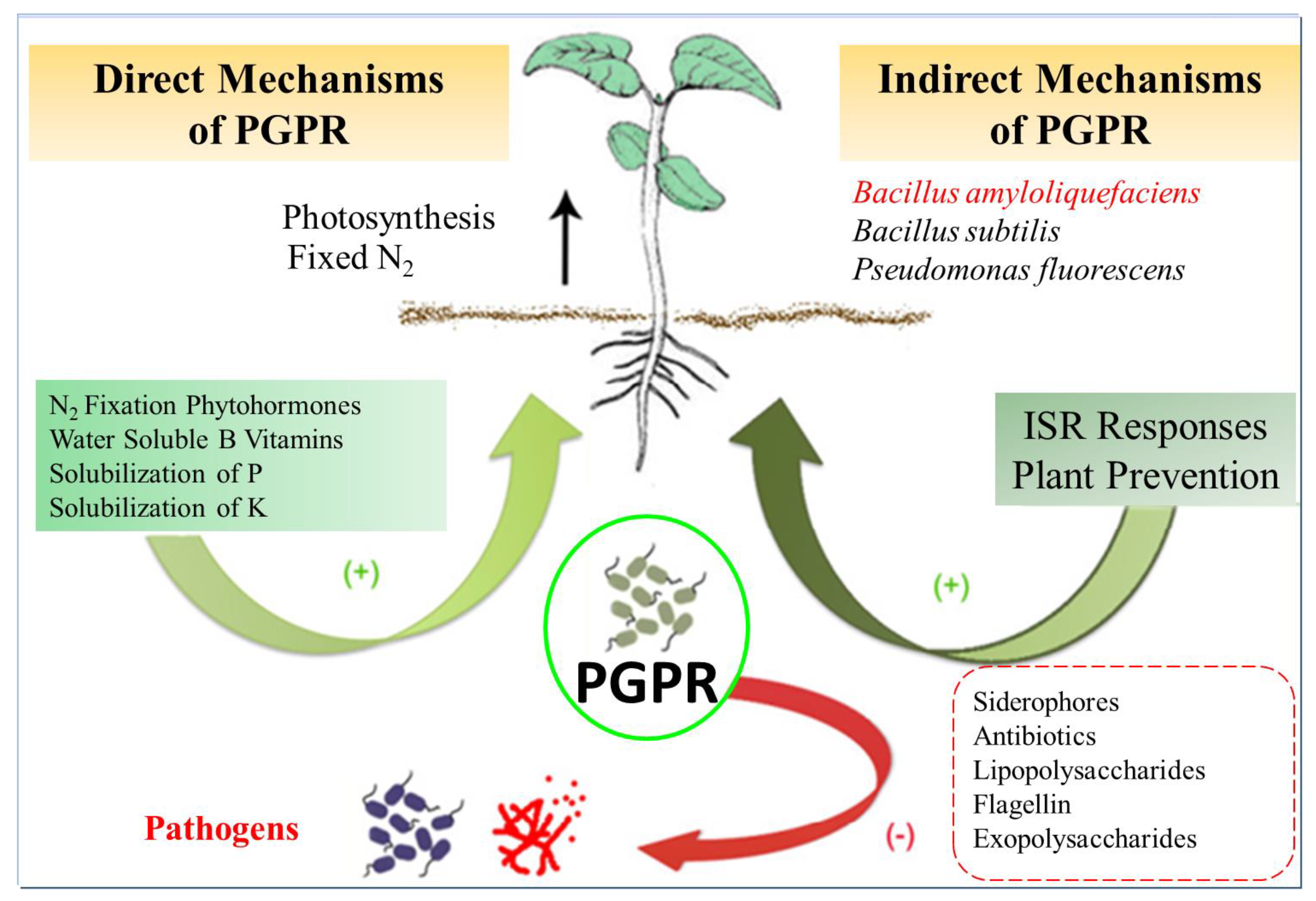
Bacillus amyloliquefaciens, when isolated from the rhizosphere, has exhibited notable antifungal and antibacterial properties and the ability to promote plant growth, making it a valuable resource in agriculture. Plant growth-promoting rhizobacteria (PGPR) are recognized for their capacity to safeguard plants from biotic and abiotic stress, enhance plant growth, and increase crop yields (Figure 4). The composition of PGPR communities can be influenced by factors such as soil temperature, pH, humidity, and nutrient availability [38]. Rhizobacteria have demonstrated various roles in nutrient acquisition and assimilation, improving soil structure, and producing an array of molecules, including hormones, secondary metabolites, antibiotics, and signaling molecules, all contributing to enhanced plant growth and yield [71]. B. amyloliquefaciens has exhibited potential as a biofertilizer, offering a sustainable alternative to conventional synthetic fertilizers [42]. Additionally, the antimicrobial activity of B. amyloliquefaciens supports its use in biological control or biocontrol strategies, wherein non-hazardous materials are employed to manage plant diseases, ultimately improving crop yields [43].
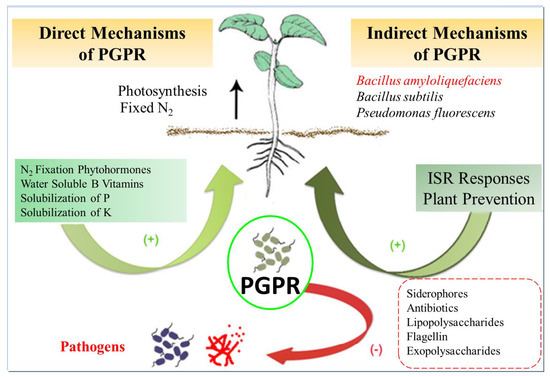
Figure 4.
PGPR (Plant growth-promoting rhizobacteria) mechanisms of action. Plant growth-promoting rhizobacteria are microbes associated with plant roots that promote plant growth, supplying improved mineral nutrition, creating hormones or other molecules that stimulate plant growth and strengthen the plant defenses against biotic and abiotic stresses, or defending plants from pathogens by reducing the survival of pathogenic microorganisms. ISR: Induced Systemic Resistance.
Bacillus amyloliquefaciens, when utilized as a biofertilizer in conjunction with chemical fertilizers in alkaline farmland soil, has been associated with reduced ammonia volatilization loss, improved crop yields, and enhanced nitrogen recovery. These effects appear to be linked to the inhibition of urease activity and the promotion of potential ammonia oxidation. Moreover, B. amyloliquefaciens has been shown to alter the microbial community structure and composition, leading to an increased abundance of ammonia-oxidizing bacteria [42]. In a study involving degraded soil, Bacillus amyloliquefaciens B14 was applied to seed inoculation, resulting in improved nutrient availability, enhanced soil enzyme activity, increased microbial respiration, elevated biomass carbon, and altered soil microbial community composition. Collectively, these factors contributed to superior crop growth and yield [17].
Bacillus amyloliquefaciens is recognized as a beneficial rhizobacterium for watermelon plants, displaying potential in combating Fusarium wilt, a devastating fungal disease caused by Fusarium oxysporum f.sp. niveum (Fon). Wu et al. (2019) demonstrated that both diffusible and volatile organic compounds (VOCs) produced by B. amyloliquefaciens L3 exhibited antifungal activity, particularly against Fon while promoting watermelon growth. The study identified 2-nonanone and 2-heptanone as the most potent antifungal VOCs, with acetoin and 2,3-butanediol acting as growth promoters in a dose-dependent manner [41]. Additionally, Al-Mutar et al. (2023) reported that B. amyloliquefaciens DHA55 displayed antifungal activity against Fon and other phytopathogenic fungi, mediated by the production of lipopeptides such as iturin, surfactin, and fungycin [63].
In a separate study, the antifungal effect of B. amyloliquefaciens LZN01 against Fon was attributed to the production of myriocin, which disrupts fungal growth at the cellular level. The antifungal mechanism appears to involve myriocin’s impact on the expression of numerous membrane-related genes, primarily affecting sphingolipid metabolism, steroid biosynthesis, and glycerophospholipid metabolism. Additionally, myriocin disturbs protein processing in the endoplasmic reticulum and influences ABC transporters. These effects lead to decreased membrane fluidity and compromised cell membrane integrity, resulting in shrinkage and eventual death of Fon cells [64]. Similarly, B. amyloliquefaciens subsp. plantarum FZB42 (isolate DMK-7-2) demonstrated antifungal activity against Fon and promoted watermelon growth [29].
Similarly, Bacillus amyloliquefaciens B1408 has demonstrated enhanced cucumber growth and biocontrol effects against Fusarium oxysporum f.sp. cucumerinum (Foc), which causes Fusarium wilt in cucumber plants. The beneficial effects of B. amyloliquefaciens B1408 appear to be mediated by inducing abnormalities, deformation, and cytoplasmic extravasation in Foc hyphal morphology. Additionally, B1408 alters the rhizosphere microbial community, providing protection against Fusarium wilt [65]. In another study, B. amyloliquefaciens B9601-Y2 was found to promote maize seedling growth, improve soil quality by enhancing enzyme activity, increase chlorophyll content, and reduce the infection index by antagonizing Bipolaris maydis, which causes southern corn leaf blight [37].
Gautam et al. (2019) reported that B. amyloliquefaciens S1 exhibits biocontrol effects against Clavibacter michiganensis, the causative agent of bacterial canker in tomato plants, when tested under net house conditions. The biocontrol effect is attributed to the production of siderophores, lytic enzymes, and antimicrobial metabolites. Moreover, in vitro experiments demonstrated that B. amyloliquefaciens S1 possesses significant phosphorus solubilization and indole acetic acid production capabilities [66]. In a safety assessment,
Anastassiadou et al. (2020) evaluated the use of B. amyloliquefaciens AH2 as a fungicide on grapes. The fungicidal effect of B. amyloliquefaciens AH2 was deemed effective against grey mold, the target pathogen. Furthermore, the toxicity of B. amyloliquefaciens AH2 was assessed in workers handling the bacterium, with no reported side effects or hypersensitivity reactions. However, a few individuals experienced allergic reactions to the bacterium’s metabolites. In mammalian studies, no adverse effects were observed, and the bacterium did not cause infectious disease in rats. However, the toxic effects of the bacterium’s metabolites remain inconclusive, necessitating further assessment. As a result, the study could not exclude the possibility of toxic effects from the bacterium’s products or metabolites [67].
Plant growth-promoting rhizobacteria (PGPR) has been demonstrated to mitigate soil salinization, an abiotic stressor (salt/salinity stress) that negatively impacts crop growth and yield by reducing agricultural land availability [72]. Research has suggested that halotolerant plant growth-promoting bacteria (PGPB), such as Bacillus amyloliquefaciens SQR9, are more effective in treating saline soil and restoring balance to enhance crop yield [68]. The potential mechanism underlying the beneficial effects of B. amyloliquefaciens SQR9 may involve increased total soluble sugar content, decreased reactive oxygen species, and reduced sodium ion levels, ultimately restoring sodium balance in the soil [73]. Furthermore, B. amyloliquefaciens is effective in countering heat stress in plants [69]. In a study examining the effects of Bacillus amyloliquefaciens NBRI-SN13 inoculation on rice cv. Saryu-52 seedlings subjected to various abiotic stresses (salt, drought, heat, cold, freezing, and desiccation) and phytohormone treatments, it was found that B. amyloliquefaciens NBRI-SN13 promoted proline and total soluble sugar (TSS) production and increased the expression of late embryogenesis abundant (LEA) and dehydrin (DHN) genes, all of which are associated with enhanced stress tolerance. Additionally, SN13 upregulated the expression of glutathione S-transferase (GSTs), an important antioxidant enzyme [70].
Li et al. (2023) raised concerns regarding the toxic effects of polylactic acid microplastic (PLA MPs) exposure on B. amyloliquefaciens, which led to oxidative stress, damage to cell wall components, and negative impacts on energy metabolism and cell growth. However, co-exposure to copper ions did not exhibit any synergistic effects. The study demonstrated that the bacterium resisted the toxicity of PLA MPs through sporulation [74].
5. Adaptation of Bacillus amyloliquefaciens to Acidic Environments: Understanding Acid Tolerance Mechanisms
Recent research has been conducted to investigate the adaptation mechanisms of Bacillus amyloliquefaciens in response to acidic environments as defined by the pH scale [75]. The study examined the effects of varying pH levels, notably slight to extremely acidic conditions, on bacterial cell density and their tolerance to low pH stress. The findings revealed that exposure to moderately acidic pH (around 5–6 on the pH scale) enhances the bacterium’s ability to withstand extremely acidic conditions (a pH of 3 or lower), and bacterial cell density plays a crucial role in low pH tolerance. The researchers also identified an acid tolerance response (ATR) mechanism employed by the bacteria to improve their growth under acidic stress. Additionally, the study highlighted the influence of pH levels, as identified on the pH scale, on the bacterium’s gene expression, enabling it to adapt to acid stress. The research provides insights into the molecular pathways through which B. amyloliquefaciens adjust to acidic environments [75].
Understanding these adaptation mechanisms may be valuable in developing strategies to mitigate the negative effects of acid stress on bacterial survival and growth, ultimately benefiting various applications of B. amyloliquefaciens in agriculture, food production, and environmental management.
6. Conclusions and Perspectives
The Gram-positive bacterium Bacillus amyloliquefaciens possesses a comprehensive array of genetic tools, promoters, and plasmid expression systems, making it a valuable resource in synthetic biology, metabolic engineering, and protein expression. Furthermore, it can potentially produce chemicals, enzymes, and other industrial bioproducts. This review discussed the advantages of B. amyloliquefaciens as a chassis cell in terms of genetic engineering, heterologous gene expression, and its applications in industry, medicine, and agriculture. Despite its numerous promising applications, B. amyloliquefaciens remains less studied than its Gram-negative counterpart, Escherichia coli, even in the context of advanced methodologies and rapid tool development.
One primary barrier to the widespread adoption of B. amyloliquefaciens is its less efficient plasmid synthesis than E. coli. To address this issue, future research could focus on developing modified B. amyloliquefaciens strains that enable direct plasmid synthesis. Conversely, its high recombination rate offers certain advantages for the development of genome editing tools. B. amyloliquefaciens has already been established as a significant platform strain for the commercial production of various chemicals and enzymes. The availability of advanced engineering techniques and cutting-edge genetic editing technologies for controlling and regulating metabolic pathways further enhances the potential of Bacillus strains as exceptional industrial production hosts.
This review aimed to provide readers with a comprehensive understanding of the characteristics of Bacillus amyloliquefaciens, enabling them to leverage its properties for various applications and research endeavors. The continuous advancement of fundamental and applied studies further solidifies the prominent position of B. amyloliquefaciens as one of the leading microbes in the fermentation industry. We anticipate that this evaluation will aid metabolic engineers in effectively harnessing established engineering approaches to guide metabolic engineering efforts for optimizing B. amyloliquefaciens cellular factories. Moreover, we hope that increased emphasis will be placed on fostering the development of metabolic engineering strategies and techniques specifically tailored for B. amyloliquefaciens.
As more scientists and engineers contribute to research on B. amyloliquefaciens, a greater variety of technologies, tools, and methodologies will be employed in the future, enhancing its potential for application in both scientific and industrial contexts.
Funding
This research was funded by an internal grant from Liwa College, Abu Dhabi, United Arab Emirates (I.Z.K).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zakataeva, N.P.; Nikitina, O.V.; Gronskiy, S.V.; Romanenkov, D.V.; Livshits, V.A. A simple method to introduce marker-free genetic modifications into the chromosome of naturally nontransformable Bacillus amyloliquefaciens strains. Appl. Microbiol. Biotechnol. 2010, 85, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Lv, Y.; Bai, Y.; Luo, H.; Shi, P.; Huang, H.; Yao, B. Construction of a Rapid Feather-Degrading Bacterium by Overexpression of a Highly Efficient Alkaline Keratinase in Its Parent Strain Bacillus amyloliquefaciens K11. J. Agric. Food Chem. 2016, 64, 78–84. [Google Scholar] [CrossRef]
- Feng, J.; Gu, Y.; Quan, Y.; Cao, M.; Gao, W.; Zhang, W.; Wang, S.; Yang, C.; Song, C. Improved poly-gamma-glutamic acid production in Bacillus amyloliquefaciens by modular pathway engineering. Metab. Eng. 2015, 32, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Shim, H.; Shin, J.H.; Kim, K.S. Antagonistic Activities of Bacillus spp. Strains Isolated from Tidal Flat Sediment towards Anthracnose Pathogens Colletotrichum acutatum and C. gloeosporioides in South Korea. Plant Pathol. J. 2015, 31, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zalila-Kolsi, I.; Kessentini, S.; Tounsi, S.; Jamoussi, K. Optimization of Bacillus amyloliquefaciens BLB369 Culture Medium by Response Surface Methodology for Low Cost Production of Antifungal Activity. Microorganisms 2022, 10, 830. [Google Scholar] [CrossRef]
- Ye, M.; Sun, L.; Yang, R.; Wang, Z.; Qi, K. The optimization of fermentation conditions for producing cellulase of Bacillus amyloliquefaciens and its application to goose feed. R. Soc. Open Sci. 2017, 4, 171012. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, B.; Li, X.; Xu, X.; Li, D.; Zeng, F.; Zhou, C.; Liu, Y.; Li, Y.; Lu, F. Multiple Modular Engineering of Bacillus amyloliquefaciens Cell Factories for Enhanced Production of Alkaline Proteases From B. Clausii. Front. Bioeng. Biotechnol. 2022, 10, 866066. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A Review on the Biotechnological Applications of the Operational Group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614. [Google Scholar] [CrossRef]
- Xin, Q.; Chen, Y.; Chen, Q.; Wang, B.; Pan, L. Development and application of a fast and efficient CRISPR-based genetic toolkit in Bacillus amyloliquefaciens LB1ba02. Microb. Cell Fact. 2022, 21, 99. [Google Scholar] [CrossRef]
- Jiang, C.; Ye, C.; Liu, Y.; Huang, K.; Jiang, X.; Zou, D.; Li, L.; Han, W.; Wei, X. Genetic engineering for enhanced production of a novel alkaline protease BSP-1 in Bacillus amyloliquefaciens. Front. Bioeng. Biotechnol. 2022, 10, 977215. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Zhao, X.; Ma, G.; Liu, N.; Zheng, Y.; Tan, J.; Qi, G. Systemically engineering Bacillus amyloliquefaciens for increasing its antifungal activity and green antifungal lipopeptides production. Front. Bioeng. Biotechnol. 2022, 10, 961535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huo, K.; Song, X.; Quan, Y.; Wang, S.; Zhang, Z.; Gao, W.; Yang, C. Engineering of a genome-reduced strain Bacillus amyloliquefaciens for enhancing surfactin production. Microb. Cell Fact. 2020, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, H.; Zhen, J.; Shu, W.; Yang, S.; Xu, J.; Song, H.; Ma, Y. Multiplex genetic engineering improves endogenous expression of mesophilic alpha-amylase gene in a wild strain Bacillus amyloliquefaciens 205. Int. J. Biol. Macromol. 2020, 165, 609–618. [Google Scholar] [CrossRef]
- Su, Y.T.; Liu, C.; Long, Z.; Ren, H.; Guo, X.H. Improved Production of Spores and Bioactive Metabolites from Bacillus amyloliquefaciens in Solid-State Fermentation by a Rapid Optimization Process. Probiotics Antimicrob. Proteins 2019, 11, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Wilson, G.A.; Young, F.E. Recognition sequence of specific endonuclease Bam HI from Bacillus amyloliquefaciens H. Nature 1977, 265, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Long, Z.; Liang, X.; Guo, S.; Ning, N.; Yang, L.; Wang, X.; Lu, B.; Gao, J. The role of a β-1, 3-1, 4-glucanase derived from Bacillus amyloliquefaciens FS6 in the protection of ginseng against Botrytis cinerea and Alternaria panax. Biol. Control 2021, 164, 104765. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Brandán, C.P. Bacillus amyloliquefaciens strain enhances rhizospheric microbial growth and reduces root and stem rot in a degraded agricultural system. Rhizosphere 2022, 22, 100544. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016, 61, 217–227. [Google Scholar] [CrossRef]
- Cai, D.; Rao, Y.; Zhan, Y.; Wang, Q.; Chen, S. Engineering Bacillus for efficient production of heterologous protein: Current progress, challenge and prospect. J. Appl. Microbiol. 2019, 126, 1632–1642. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef]
- Vehmaanpera, J.; Steinborn, G.; Hofemeister, J. Genetic manipulation of Bacillus amyloliquefaciens. J. Biotechnol. 1991, 19, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Ji, J.B.; Liu, Y.C.; Ye, B.; Zhou, C.Y.; Yan, X. Artificial induction of genetic competence in Bacillus amyloliquefaciens isolates. Biotechnol. Lett. 2016, 38, 2109–2117. [Google Scholar] [CrossRef]
- Devaraj, K.; Aathika, S.; Periyasamy, K.; Manickam Periyaraman, P.; Palaniyandi, S.; Subramanian, S. Production of thermostable multiple enzymes from Bacillus amyloliquefaciens KUB29. Nat. Prod. Res. 2019, 33, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.S.; Barse, L.Q.; Dalberto, P.F.; da Silva, W.T.S.; Rodrigues, R.C.; Machado, P.; Basso, L.A.; Bizarro, C.V.; Ayub, M.A.Z. Cloning and expression of the Bacillus amyloliquefaciens transglutaminase gene in E. coli using a bicistronic vector construction. Enzym. Microb. Technol. 2020, 134, 109468. [Google Scholar] [CrossRef]
- Gao, W.; He, Y.; Zhang, F.; Zhao, F.; Huang, C.; Zhang, Y.; Zhao, Q.; Wang, S.; Yang, C. Metabolic engineering of Bacillus amyloliquefaciens LL3 for enhanced poly-gamma-glutamic acid synthesis. Microb. Biotechnol. 2019, 12, 932–945. [Google Scholar] [CrossRef]
- Gould, A.R.; May, B.K.; Elliott, W.H. Release of extracellular enzymes from Bacillus amyloliquefaciens. J. Bacteriol. 1975, 122, 34–40. [Google Scholar] [CrossRef]
- Xue, M.; Wu, Y.; Hong, Y.; Meng, Y.; Xu, C.; Jiang, N.; Li, Y.; Liu, W.; Fan, Y.; Zhou, Y. Effects of dietary Bacillus amyloliquefaciens on the growth, immune responses, intestinal microbiota composition and disease resistance of yellow catfish, Pelteobagrus fulvidraco. Front. Cell Infect. Microbiol. 2022, 12, 1047351. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Components of rhizospheric bacterial communities of barley and their potential for plant growth promotion and biocontrol of Fusarium wilt of watermelon. Braz. J. Microbiol. 2019, 50, 749–757. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, J.; Tan, M.; Yu, Z.; Yang, S.; Zheng, H.; Song, H. Construction of a plasmid interspecific transfer system in Bacillus species with the counter-selectable marker mazF. J. Ind. Microbiol. Biotechnol. 2018, 45, 417–428. [Google Scholar] [CrossRef]
- Tjalsma, H.; Antelmann, H.; Jongbloed, J.D.; Braun, P.G.; Darmon, E.; Dorenbos, R.; Dubois, J.Y.; Westers, H.; Zanen, G.; Quax, W.J.; et al. Proteomics of protein secretion by Bacillus subtilis: Separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 2004, 68, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, J.D.; Antelmann, H.; Hecker, M.; Nijland, R.; Bron, S.; Airaksinen, U.; Pries, F.; Quax, W.J.; van Dijl, J.M.; Braun, P.G. Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J. Biol. Chem. 2002, 277, 44068–44078. [Google Scholar] [CrossRef] [PubMed]
- Freudl, R. Signal peptides for recombinant protein secretion in bacterial expression systems. Microb. Cell Fact. 2018, 17, 52. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; He, H.; Zhang, X.; Guo, J.; Bai, J.; Cheng, Y. Digital gene expression profiling of the transcriptional response to Sclerotinia sclerotiorum and its antagonistic bacterium Bacillus amyloliquefaciens in soybean. Front. Microbiol. 2022, 13, 1025771. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Sellami, S.; Tounsi, S.; Jamoussi, K. Heterologous expression and periplasmic secretion of an antifungal Bacillus amyloliquefaciens BLB 369 endo-β-1, 3-1, 4-glucanase in Escherichia coli. J. Phytopathol. 2018, 166, 28–33. [Google Scholar] [CrossRef]
- Contesini, F.J.; Melo, R.R.; Sato, H.H. An overview of Bacillus proteases: From production to application. Crit. Rev. Biotechnol. 2018, 38, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; He, P.; Munir, S.; He, P.; Li, X.; Li, Y.; Wu, J.; Wu, Y.; Yang, L.; He, P. Efficacy of plant growth promoting bacteria Bacillus amyloliquefaciens B9601-Y2 for biocontrol of southern corn leaf blight. Biol. Control 2019, 139, 104080. [Google Scholar] [CrossRef]
- do Nascimento Silva, J.; Mendes, L.W.; Antunes, J.E.L.; Melo, V.M.M.; da Silva Oliveira, F.A.; de Almeida Lopes, A.C.; da Silva, V.B.; de Araujo Pereira, A.P.; Valente, S.E.S.; Araujo, A.S.F. Diversity, structure, and composition of plant growth-promoting bacteria in soil from Brazilian Cerrado. Rhizosphere 2021, 20, 100435. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, Y.; Du, S.; Zhu, Y.; Pan, F.; Wang, R.; Xu, Z.; Xu, X.; Li, S.; Xu, H. Recent advances and prospects of Bacillus amyloliquefaciens as microbial cell factories: From rational design to industrial applications. Crit. Rev. Biotechnol. 2022, 1–19. [Google Scholar] [CrossRef]
- Ren, L.; Yuan, Z.; Xie, T.; Wu, D.; Kang, Q.; Li, J.; Li, J. Extraction and characterization of cyclic lipopeptides with antifungal and antioxidant activities from Bacillus amyloliquefaciens. J. Appl. Microbiol. 2022, 133, 3573–3584. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiologyopen 2019, 8, e00813. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Sun, B.; Yang, Y.; Jin, B.; Zhuang, G.; Bai, Z.; Zhuang, X. Efficiency and mechanism of reducing ammonia volatilization in alkaline farmland soil using Bacillus amyloliquefaciens biofertilizer. Environ. Res. 2021, 202, 111672. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shiraishi, S.; Kawagoe, Y.; Mochizuki, M.; Suzuki, S. Impact of Bacillus amyloliquefaciens S13-3 on control of bacterial wilt and powdery mildew in tomato. Pest. Manag. Sci. 2015, 71, 722–727. [Google Scholar] [CrossRef]
- Zayabaatar, E.; Huang, C.M.; Pham, M.T.; Ganzorig, B.; Wang, S.M.; Chen, C.C. Bacillus amyloliquefaciens Increases the GABA in Rice Seed for Upregulation of Type I Collagen in the Skin. Curr. Microbiol. 2023, 80, 128. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Zayabaatar, E.; Wang, S.-M.; Keshari, S.; Peng, W.-H.; Kung, H.-N.; Lee, Y.-H. Bacillus amyloliquefaciens-Inoculated GABA-Rich Rice Upregulate Neuropeptide Y to Relieve Psychological Stress through Mediations of GABAB Receptor and Vagus Nerves. Biol. Bull. 2023, 50, 186–193. [Google Scholar] [CrossRef]
- Zhang, Z.; He, P.; Cai, D.; Chen, S. Genetic and metabolic engineering for poly-gamma-glutamic acid production: Current progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2022, 38, 208. [Google Scholar] [CrossRef]
- Fang, J.; Liu, Y.; Huan, C.; Xu, L.; Ji, G.; Yan, Z. Comparison of poly-γ-glutamic acid production between sterilized and non-sterilized solid-state fermentation using agricultural waste as substrates. J. Clean. Prod. 2020, 255, 120248. [Google Scholar] [CrossRef]
- Doan, C.T.; Chen, C.L.; Nguyen, V.B.; Tran, T.N.; Nguyen, A.D.; Wang, S.L. Conversion of Pectin-Containing By-Products to Pectinases by Bacillus amyloliquefaciens and Its Applications on Hydrolyzing Banana Peels for Prebiotics Production. Polymers 2021, 13, 1483. [Google Scholar] [CrossRef]
- Du, L.; Wang, J.; Chen, W.; Chen, J.; Zheng, Q.; Fang, X.; Liao, Z. Isolation and Purification of Bacillus amyloliquefaciens D1 Protease and Its Application in the Fermentation of Soybean Milk to Produce Large Amounts of Free Amino Acids. Appl. Biochem. Biotechnol. 2023, 195, 451–466. [Google Scholar] [CrossRef]
- Lin, L.Z.; Zheng, Q.W.; Wei, T.; Zhang, Z.Q.; Zhao, C.F.; Zhong, H.; Xu, Q.Y.; Lin, J.F.; Guo, L.Q. Isolation and Characterization of Fengycins Produced by Bacillus amyloliquefaciens JFL21 and Its Broad-Spectrum Antimicrobial Potential against Multidrug-Resistant Foodborne Pathogens. Front. Microbiol. 2020, 11, 579621. [Google Scholar] [CrossRef]
- Soussi, S.; Essid, R.; Hardouin, J.; Gharbi, D.; Elkahoui, S.; Tabbene, O.; Cosette, P.; Jouenne, T.; Limam, F. Utilization of Grape Seed Flour for Antimicrobial Lipopeptide Production by Bacillus amyloliquefaciens C5 Strain. Appl. Biochem. Biotechnol. 2019, 187, 1460–1474. [Google Scholar] [CrossRef]
- Gao, L.; She, M.; Shi, J.; Cai, D.; Wang, D.; Xiong, M.; Shen, G.; Gao, J.; Zhang, M.; Yang, Z.; et al. Enhanced production of iturin A by strengthening fatty acid synthesis modules in Bacillus amyloliquefaciens. Front. Bioeng. Biotechnol. 2022, 10, 974460. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Qian, S.; Muhammad, U.; Jiang, X.; Han, J.; Lu, Z. Effect of fructose on promoting fengycin biosynthesis in Bacillus amyloliquefaciens fmb-60. J. Appl. Microbiol. 2016, 121, 1653–1664. [Google Scholar] [CrossRef]
- Lu, J.Y.; Zhou, K.; Huang, W.T.; Zhou, P.; Yang, S.; Zhao, X.; Xie, J.; Xia, L.; Ding, X. A comprehensive genomic and growth proteomic analysis of antitumor lipopeptide bacillomycin Lb biosynthesis in Bacillus amyloliquefaciens X030. Appl. Microbiol. Biotechnol. 2019, 103, 7647–7662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, B.; Xu, H.; Mei, X.; Xu, X.; Zhang, X.; Ni, J.; Li, W. Bacillus amyloliquefaciens SC06 Protects Mice against High-Fat Diet-Induced Obesity and Liver Injury via Regulating Host Metabolism and Gut Microbiota. Front. Microbiol. 2019, 10, 1161. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Jeong, S.Y.; Zhang, T.; Wu, X.; Qiu, J.Y.; Park, S. Chungkookjang, a soy food, fermented with Bacillus amyloliquefaciens protects gerbils against ishcmeic stroke injury, and post-stroke hyperglycemia. Food Res. Int. 2020, 128, 108769. [Google Scholar] [CrossRef]
- WoldemariamYohannes, K.; Wan, Z.; Yu, Q.; Li, H.; Wei, X.; Liu, Y.; Wang, J.; Sun, B. Prebiotic, Probiotic, Antimicrobial, and Functional Food Applications of Bacillus amyloliquefaciens. J. Agric. Food Chem. 2020, 68, 14709–14727. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, W.; Zhu, Z.; Zhou, K.; Luo, S.; Hu, S.; Xia, L.; Ding, X. Comparative Study of Bacillus amyloliquefaciens X030 on the Intestinal Flora and Antibacterial Activity against Aeromonas of Grass Carp. Front. Cell Infect. Microbiol. 2022, 12, 815436. [Google Scholar] [CrossRef]
- Al-Dulaimi, M.; Algburi, A.; Abdelhameed, A.; Mazanko, M.S.; Rudoy, D.V.; Ermakov, A.M.; Chikindas, M.L. Antimicrobial and Anti-Biofilm Activity of Polymyxin E Alone and in Combination with Probiotic Strains of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 against Clinical Isolates of Selected Acinetobacter spp.: A Preliminary Study. Pathogens 2021, 10, 1574. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Sheikh, A.; Ibrahim, H.I.M. Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model. Nutrients 2022, 14, 1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Xu, H.; Liang, C.; Zhai, Z. Bacillus amyloliquefaciens BLCC1-0238 Alone or in Combination with Mannan-Oligosaccharides Alleviates Subclinical Necrotic Enteritis in Broilers. Probiotics Antimicrob. Proteins 2022, 14, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Morozova, M.V.; Kalmykova, G.V.; Akulova, N.I.; Ites, Y.V.; Korkina, V.I.; Litvinova, E.A. Autoclaved Diet with Inactivated Spores of Bacillus spp. Decreased Reproductive Performance of Muc2(−/−) and Muc2(+/−) Mice. Animals 2022, 12, 2399. [Google Scholar] [CrossRef]
- Al-Mutar, D.M.K.; Alzawar, N.S.A.; Noman, M.; Azizullah; Li, D.; Song, F. Suppression of Fusarium Wilt in Watermelon by Bacillus amyloliquefaciens DHA55 through Extracellular Production of Antifungal Lipopeptides. J. Fungi 2023, 9, 336. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Liu, Z.; Wang, K.; Xu, W. Membrane disruption of Fusarium oxysporum f. sp. niveum induced by myriocin from Bacillus amyloliquefaciens LZN01. Microb. Biotechnol. 2021, 14, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, Z.; Li, N.; Wang, Y.; Feng, J.; Zhang, X. Bacillus amyloliquefaciens B1408 suppresses Fusarium wilt in cucumber by regulating the rhizosphere microbial community. Appl. Soil. Ecol. 2019, 136, 55–66. [Google Scholar] [CrossRef]
- Gautam, S.; Chauhan, A.; Sharma, R.; Sehgal, R.; Shirkot, C.K. Potential of Bacillus amyloliquefaciens for biocontrol of bacterial canker of tomato incited by Clavibacter michiganensis ssp. michiganensis. Microb. Pathog. 2019, 130, 196–203. [Google Scholar] [CrossRef]
- European Food Safety, A.; Anastassiadou, M.; Arena, M.; Auteri, D.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; Crivellente, F.; et al. Peer review of the pesticide risk assessment of the active substance Bacillus amyloliquefaciens strain AH2. EFSA J. 2020, 18, e06156. [Google Scholar] [CrossRef] [PubMed]
- Sunita, K.; Mishra, I.; Mishra, J.; Prakash, J.; Arora, N.K. Secondary Metabolites from Halotolerant Plant Growth Promoting Rhizobacteria for Ameliorating Salinity Stress in Plants. Front. Microbiol. 2020, 11, 567768. [Google Scholar] [CrossRef]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Pande, A.; Imran, M.; Kang, S.M.; Rahim, W.; Khan, S.A.; Bhatta, D.; Kwon, E.H.; et al. A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants. Microorganisms 2022, 10, 1286. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, V.; Chauhan, P.S.; Lata, C. Bacillus amyloliquefaciens Confers Tolerance to Various Abiotic Stresses and Modulates Plant Response to Phytohormones through Osmoprotection and Gene Expression Regulation in Rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Choudhary, M.; Chandra, P.; Dixit, B.; Nehra, V.; Choudhary, U.; Choudhary, S. Plant growth-promoting microbes: Role and prospective in amelioration of salt stress. Commun. Soil Sci. Plant Anal. 2022, 53, 1692–1711. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Veronican Njeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Li, R.; Tao, J.; Huang, D.; Zhou, W.; Gao, L.; Wang, X.; Chen, H.; Huang, H. Investigating the effects of biodegradable microplastics and copper ions on probiotic (Bacillus amyloliquefaciens): Toxicity and application. J. Hazard. Mater. 2023, 443, 130081. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Goswami, G.; Boro, R.C.; Barooah, M. A pH-Dependent Gene Expression Enables Bacillus amyloliquefaciens MBNC to Adapt to Acid Stress. Curr. Microbiol. 2021, 78, 3104–3114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).