GC-MS Analysis, Antibacterial, and Anticancer Activities of Hibiscus sabdariffa L. Methanolic Extract: In Vitro and In Silico Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction
2.2. Bacterial Strains
2.3. Antibacterial Assay
2.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.5. Antibiotic Sensitivity Assay
2.6. Synergistic Assay
2.7. Scanning Electron Microscopy
2.8. Anticancer and Cytotoxicity Assay
2.9. Flow Cytometric Analysis
2.10. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.11. Molecular Docking
2.12. Statistical Analysis
3. Results
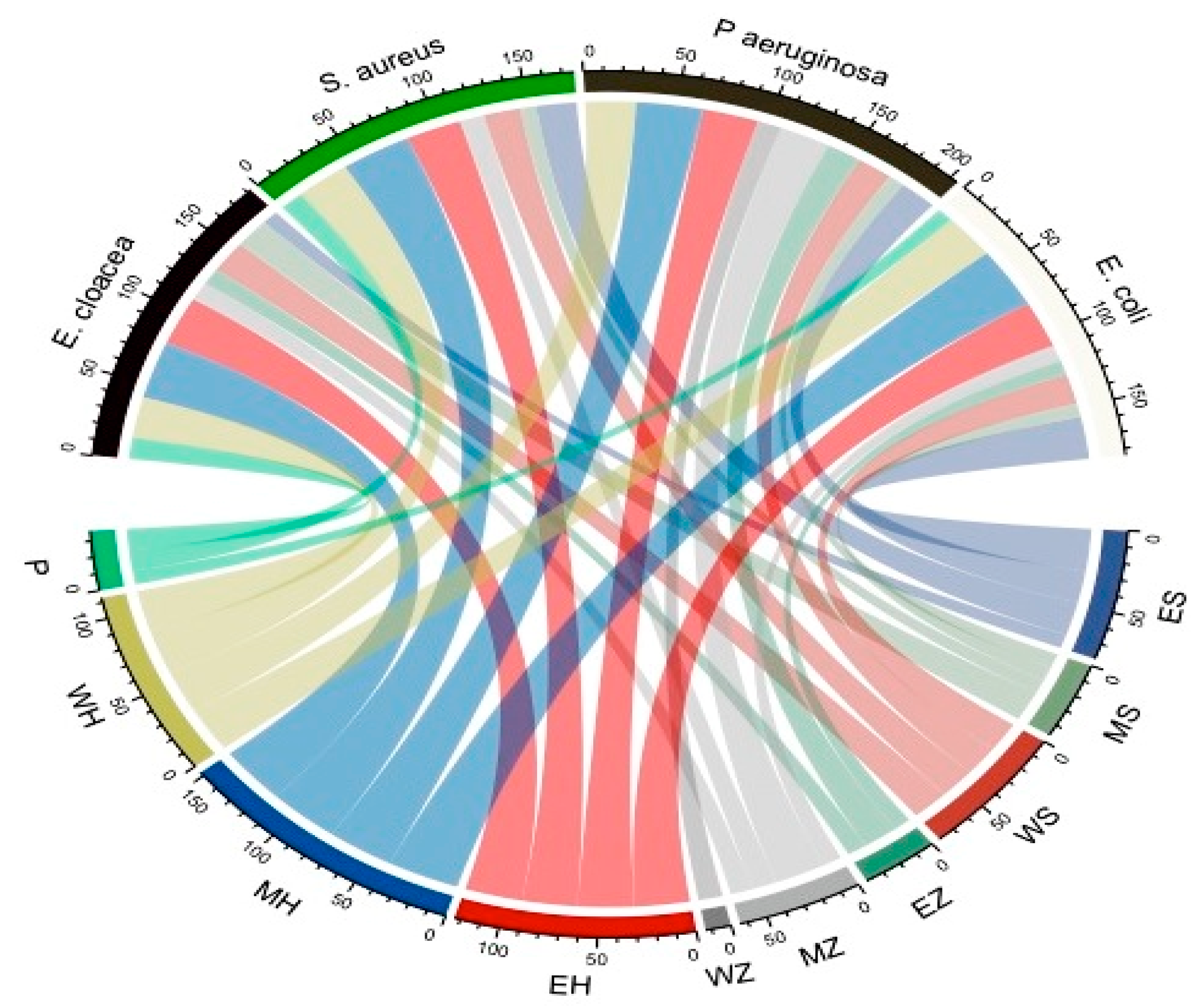
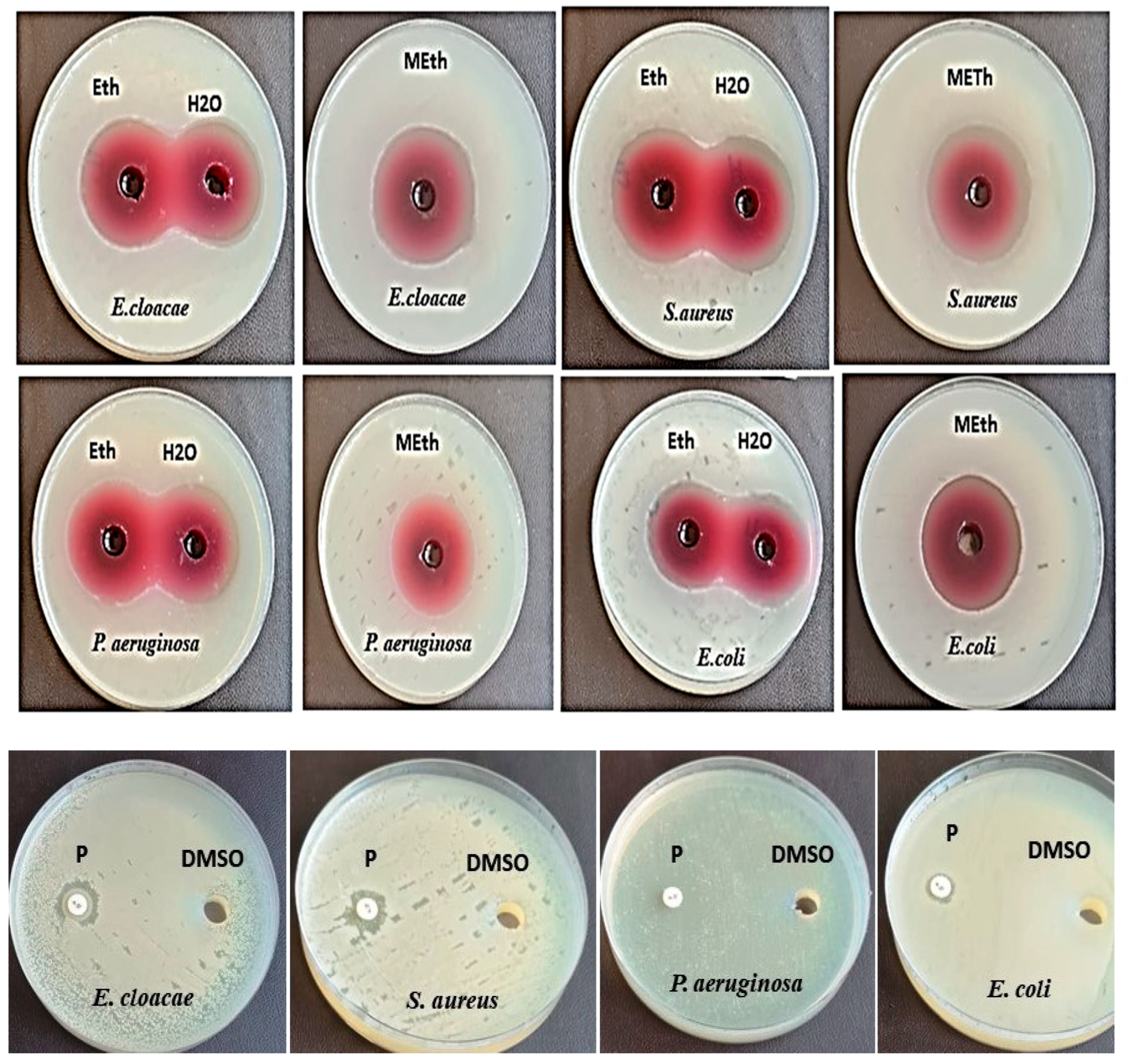
3.1. Antibacterial Impact of Different Plant Extracts Using Different Solvents
3.2. Measurement of MIC and MBC
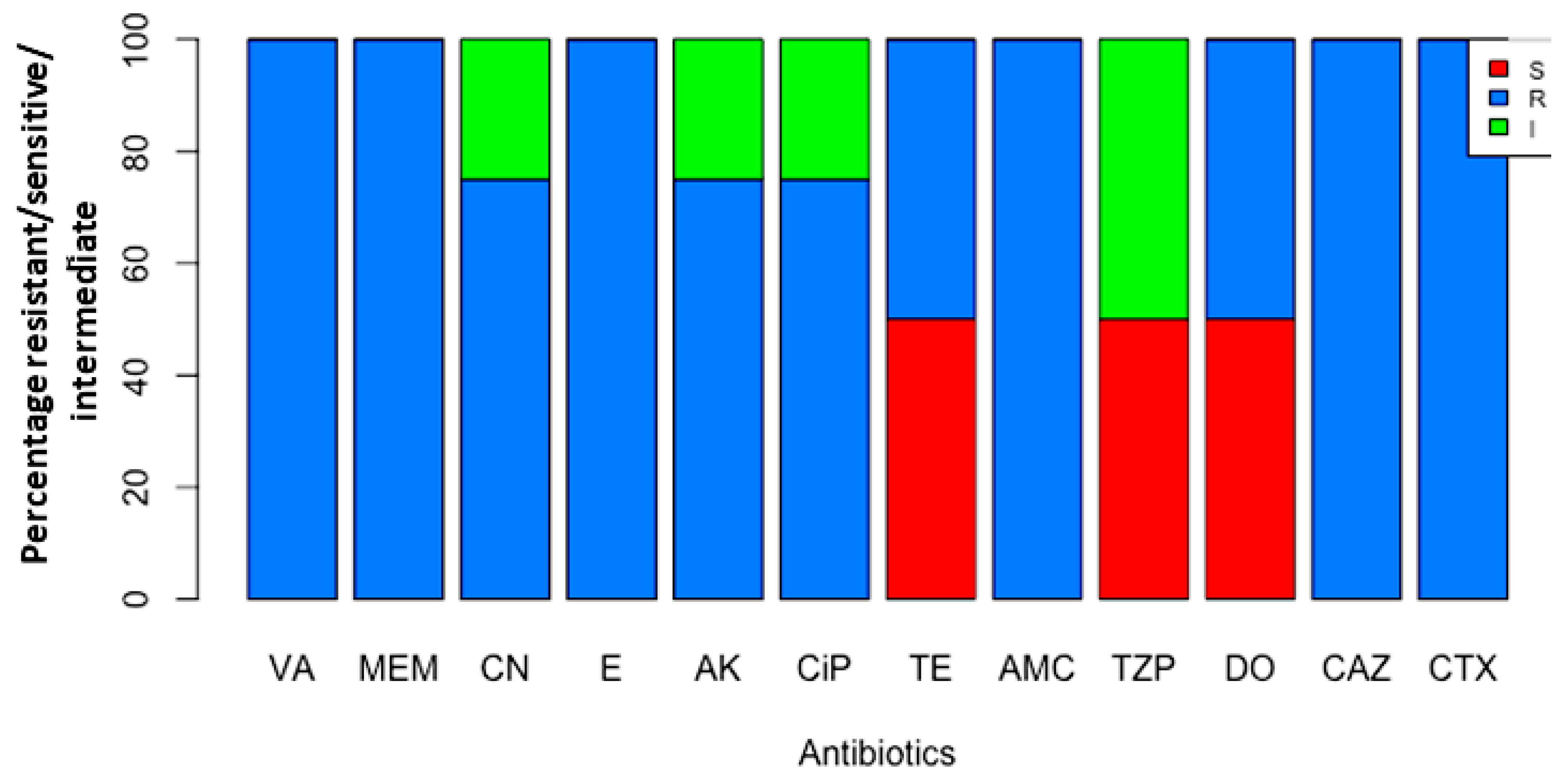
3.3. Testing Impact of Antibiotics
3.4. Testing Synergism among (TZP) and H. sabdariffa L. Extract
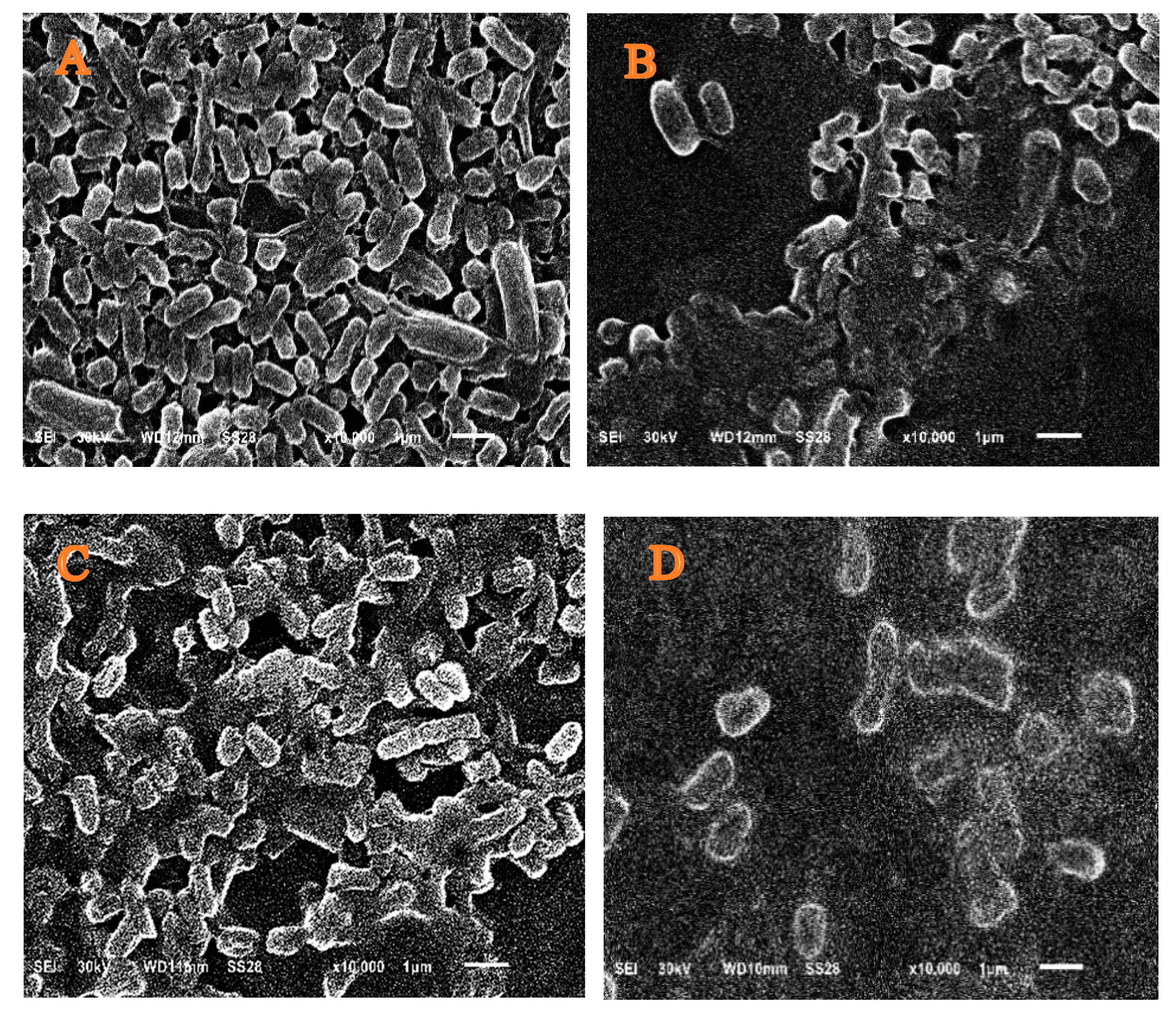
3.5. Scanning Electron Microscopy
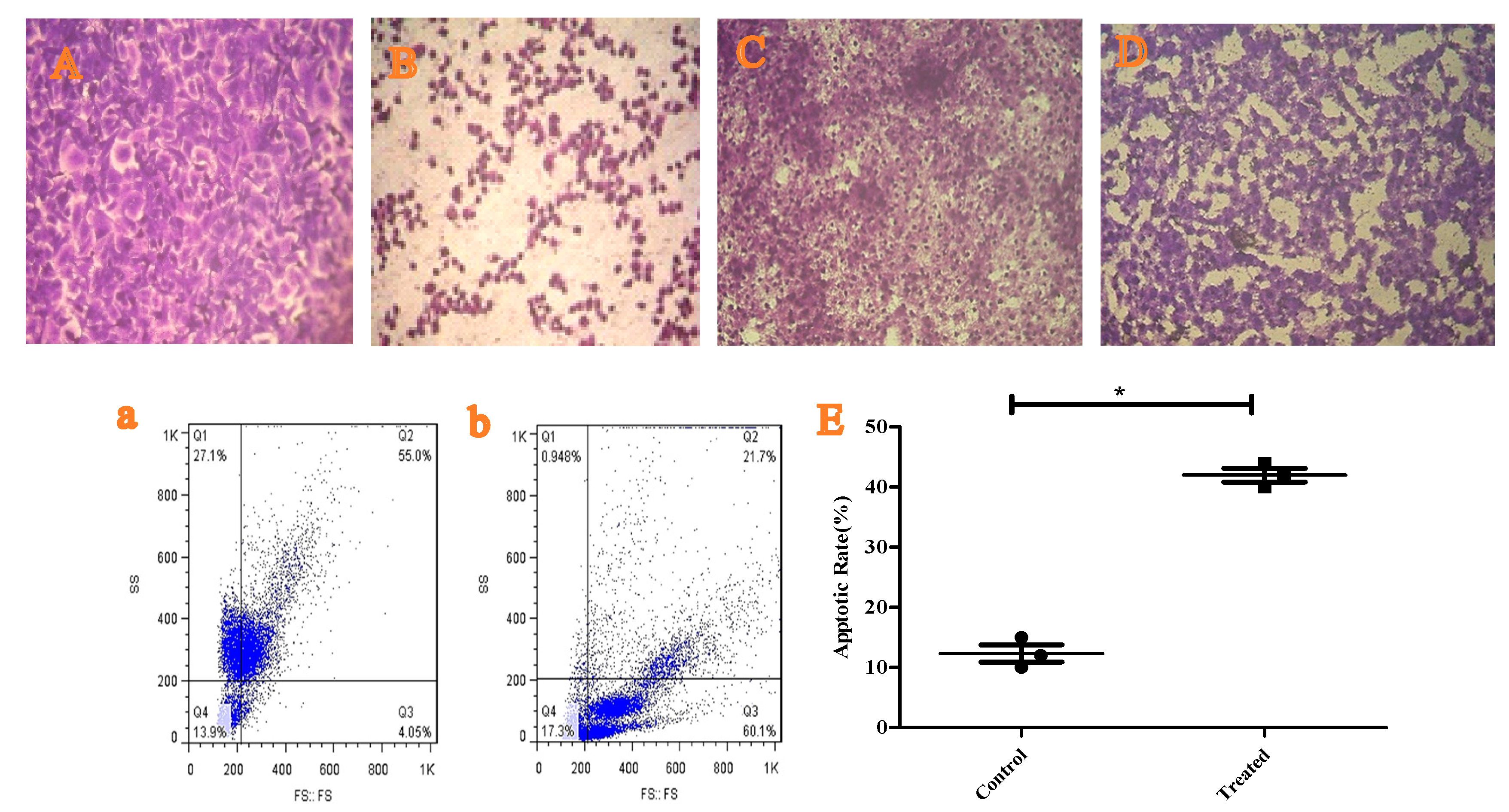
3.6. Anticancer Role of H. sabdariffa L. Extract and Cytotoxicity
3.7. Testing the Apoptotic Role of H. sabdariffa L. Extract
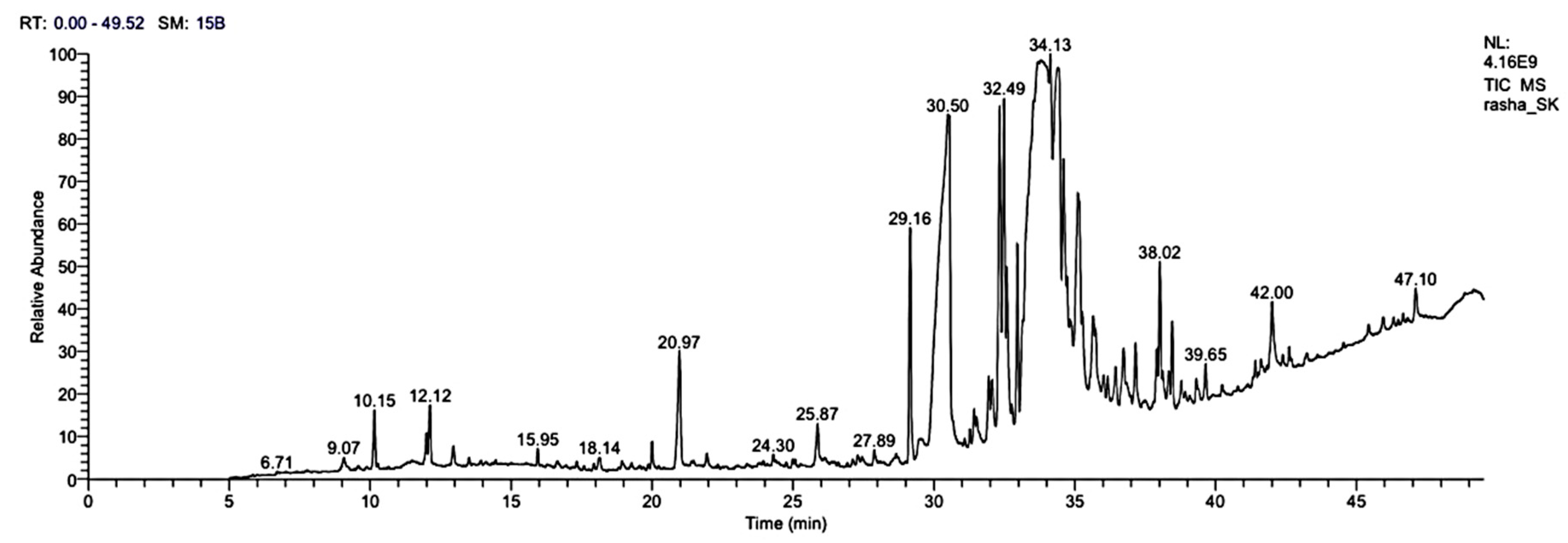
3.8. GC-MS Analysis of H. sabdariffa L. Methanolic Extract
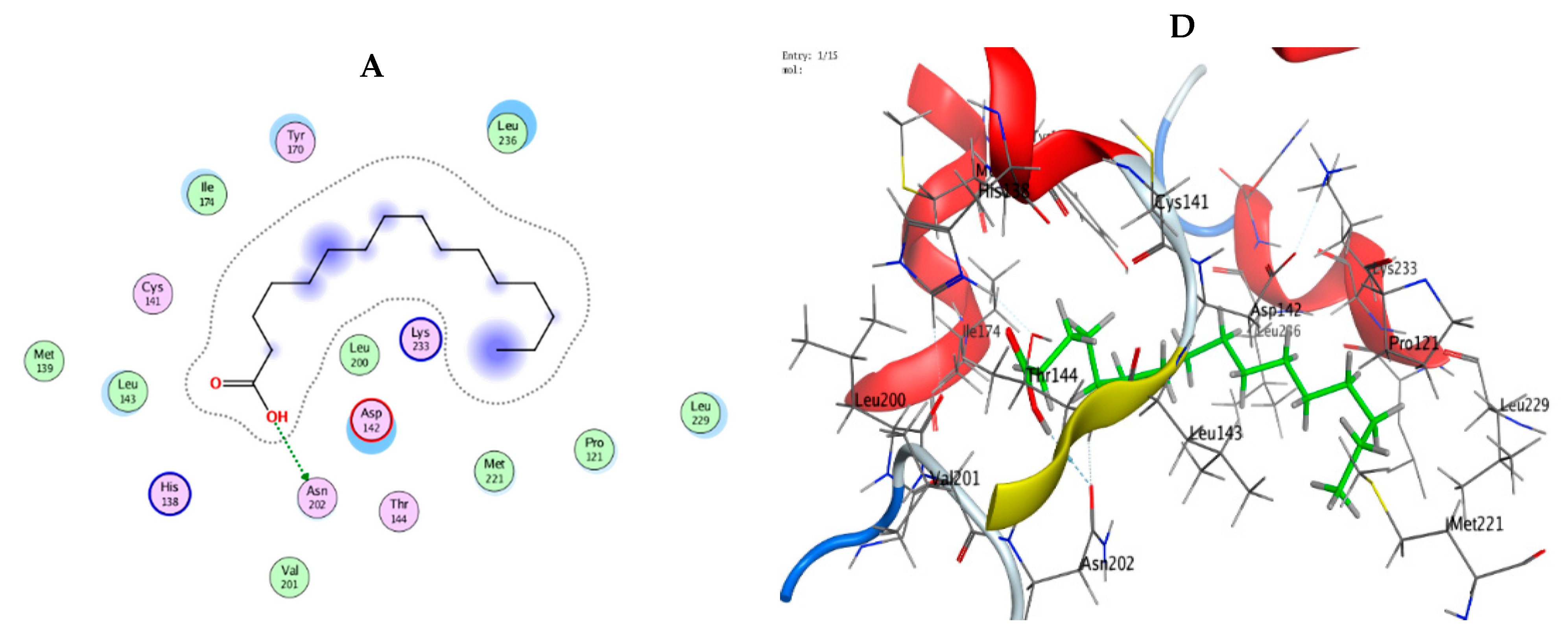
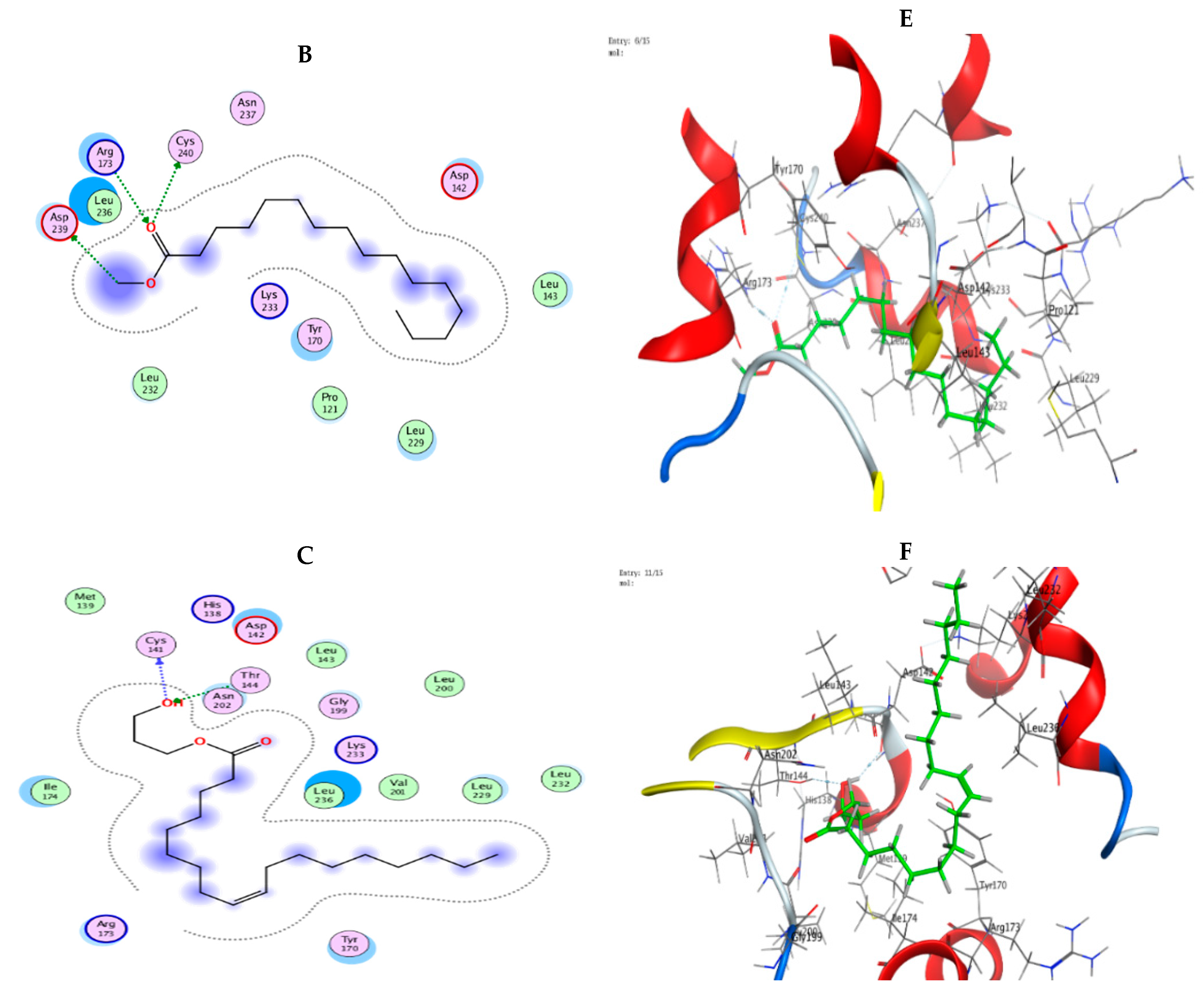
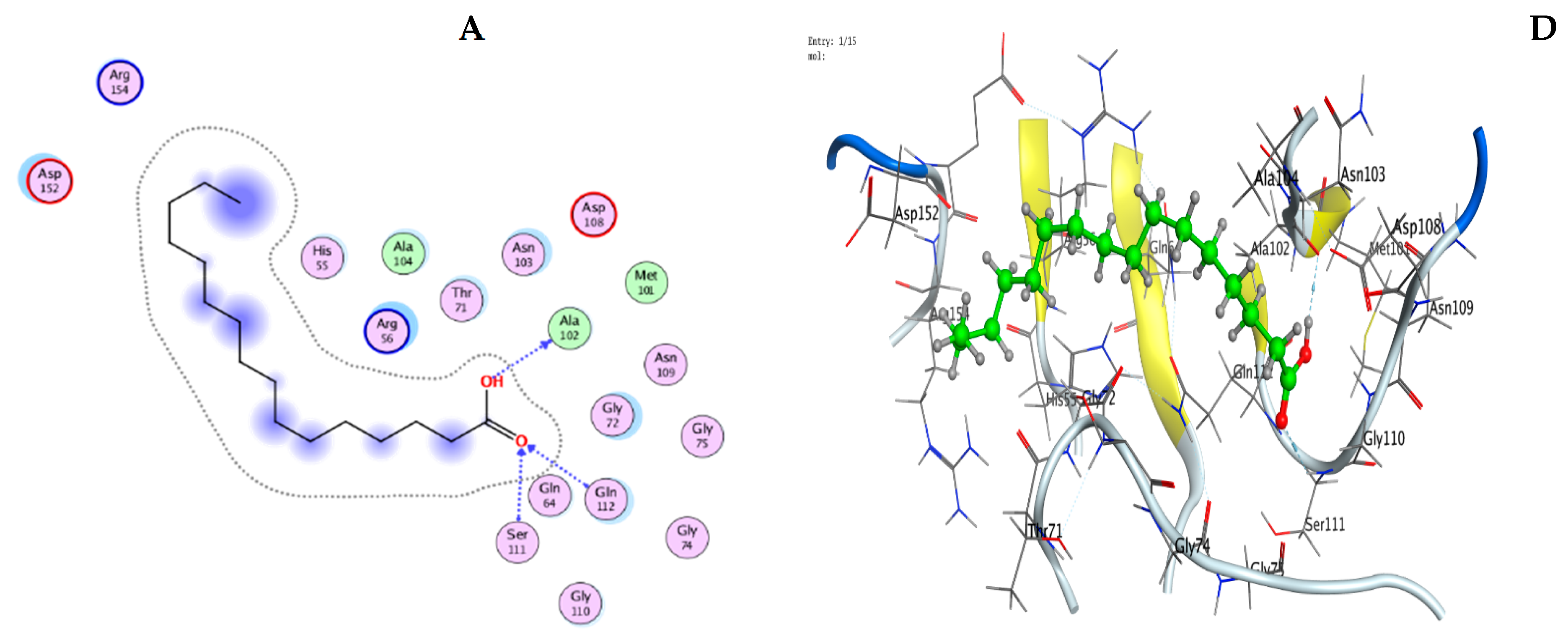
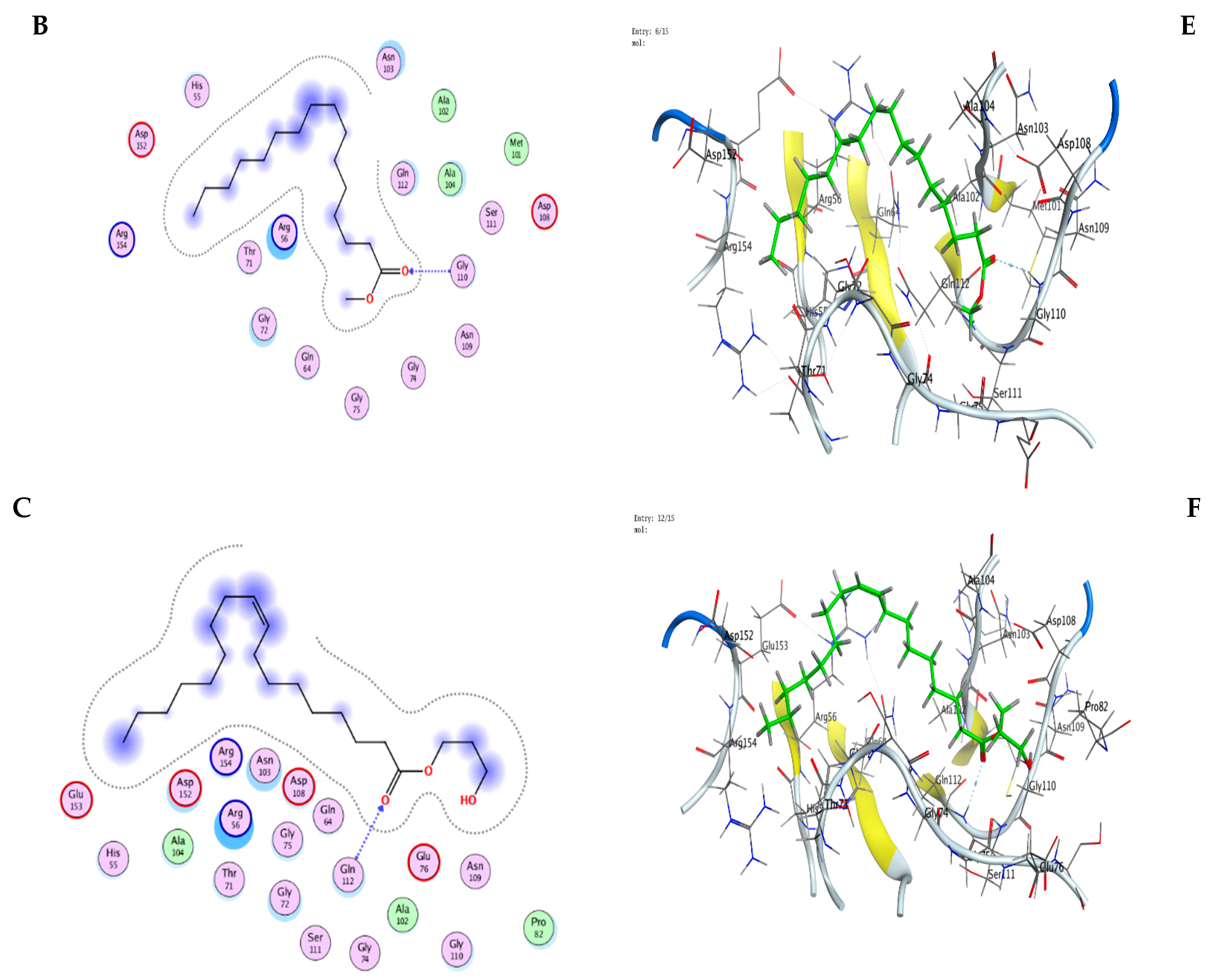
3.9. Molecular Modeling: Docking Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matlock, A.; Garcia, J.A.; Moussavi, K.; Long, B.; Liang, S.Y.T. Advances in novel antibiotics to treat multidrug-resistant gram-negative bacterial infections. Intern. Emerg. Med. 2021, 16, 2231–2241. [Google Scholar] [CrossRef]
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cilloniz, C.; Martin-Loeches, I.; Garcia-Vidal, C.; San Jose, A.; Torres, A. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Patterns. Int. J. Mol. Sci. 2016, 17, 2120. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Monnet, D.L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, S.M. Multidrug-resistant, extensively drug-resistant and Pandrug-resistant bacteria and antimicrobial therapy in combination. Bangladesh J. Med. Microbiol. 2015, 9, 1–2. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Akram, M.; Riaz, M.; Munir, N.; Rasul, A.; Daniyal, M.; Ali Shah, S.M.; Said Khan, F. Progress and prospects in the management of bacterial infections and developments in Phytotherapeutic modalities. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1107–1119. [Google Scholar] [CrossRef]
- Al-Kaabi, H.K.J.; Hmood, B.A.; Jebur, L.S.; Abdullah, S.M.; AL-Qraghi, S.A.A. Determination of Ziziphus spina-christi leaves extracts antibacterial activity against some pathogenic bacteria. Al-Kufa Univ. J. Biol. 2021, 13, 14–20. [Google Scholar]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef]
- Begashaw, B.; Mishra, B.; Tsegaw, A.; Shewamene, Z. Methanol leaves extract Hibiscus micranthus Linn. exhibited antibacterial and wound healing activities. BMC Complement. Altern. Med. 2017, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Anwar, M.N. Antimicrobial activity of crude extract obtained from the root of Plumbago zeylanica. Bangladesh J. Microbiol. 2007, 24, 73–75. [Google Scholar] [CrossRef]
- Valli, M.; Pivatto, M.; Danuello, A.; Castro-Gamboa, I.; Silva, D.H.S.; Cavalheiro, A.J.; Araújo, Â.R.; Furlan, M.; Lopes, M.N.; Bolzani, V.D.S. Tropical biodiversity: Has it been a potential source of secondary metabolites useful for medicinal chemistry? Química Nova 2012, 35, 2278–2287. [Google Scholar] [CrossRef]
- Parvin, S.; Kader, M.A.; Chouduri, A.U.; Rafshanjani, M.A.S.; Haque, M.E. Antibacterial, antifungal, and insecticidal activities of the n-hexane and ethyl-acetate fractions of methanolic extract of the leaves of Calotropis gigantea Linn. J. Pharmacogn. Phytochem. 2014, 2, 47–51. [Google Scholar]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [PubMed]
- Garcia, C.S.C.; Menti, C.; Lambert, A.P.F. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): Antioxidant, and antitumor in mammalian cells. An. Da Acad. Bras. De Ciências 2016, 88, 281–292. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Saied, A.S.; Gebauer, J.; Hammer, K.; Buerkert, A. Ziziphus spina-christi (L.) Willd.: A multipurpose fruit tree. Genet. Resour. Crop Evol. 2008, 55, 929–937. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Salim, S.Y.; Assaf, M.H.; Abdel-Hady, R.H. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J. Ethnopharmacol. 2005, 101, 129–138. [Google Scholar] [CrossRef]
- Albalawi, A.E. Antileishmanial activity of Ziziphus spina-christi leaves extract and its possible cellular mechanisms. Microorganisms 2021, 9, 2113. [Google Scholar] [CrossRef]
- Baghazadeh-Daryaii, L.; Sharifi-Sirchi, G.R.; Samsampoor, D. Morphological, phytochemical and genetic diversity of Ziziphus spina–Christi (L) Des. in South and Southeastern of Iran. J. Appl. Res. Med. Aromat. Plants 2017, 7, 99–107. [Google Scholar] [CrossRef]
- Voon, H.C.; Bhat, R.; Rusul, G. Flower extracts and their essential oils as potential antimicrobial agents for food uses and pharmaceutical applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 34–55. [Google Scholar] [CrossRef]
- Patel, S. Hibiscus sabdariffa: An ideal yet under-exploited candidate for nutraceutical applications. Biomed. Prev. Nutr. 2014, 4, 23–27. [Google Scholar] [CrossRef]
- Abdallah, E.M. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug resistant Acinetobacter baumannii. J. Acute Dis. 2016, 5, 512–516. [Google Scholar] [CrossRef]
- Xu, G.H.; Chen, J.C.; Liu, D.H.; Zhang, Y.H.; Jiang, P.; Ye, X.Q. Minerals, phenolic compounds, and antioxidant capacity of citrus peel extract by hot water. J. Food Sci. 2008, 73, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Stewart, J.; Wu, F.S.; El Shikh, H.; Hassan, F.; Elaasser, M. Effectiveness of different solvents extracts from edible mushrooms in inhibiting the growth of tumor cells. Cancer Biol. 2014, 4, 1–15. [Google Scholar]
- Younis, A.M.; Yosri, M.; Stewart, J.K. In vitro evaluation of pleiotropic properties of wild mushroom Laetiporus sulphureus. Ann. Agric. Sci. 2019, 64, 79–87. [Google Scholar] [CrossRef]
- Durairaj, S.; Srinivasan, S.; Lakshmanaperumalsamy, P. In vitro Antibacterial Activity and Stability of Garlic Extract at Different pH and Temperature. Electr. J. Biol. 2009, 5, 5–10. [Google Scholar]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Rezaei, R.; Mousavi, S.R.; Salari, M.; Ghanavati Behbahan, F. Antimicrobial activities of gold nanoparticles against Salmonella typhimurium. Adv. Herb. Med. 2017, 3, 26–30. [Google Scholar]
- Abass, A.A.; Wasna’a, M.A.; Alsehlawi, Z.S. Characterization of Antimicrobial Activity of gold nanoparticles prepared Sabdariffa L.) calyx decoction as an Indonesian folk medicine beverage. Drug Invent. Today 2020, 13, 719–724. [Google Scholar]
- Zahin, M.; Hasan, S.; Aqil, F.; Khan, M.; Ahmad, S.; Husain, F.M.; Ahmad, I. Screening of certain medicinal plants from India for their anti-quorum sensing activity. Indian J. Exp. Biol. 2010, 48, 1219–1224. [Google Scholar]
- CLSI. Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement M100-S23; CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- Lo Cantore, P.; Iacobellis, N.S.; De Marco, A.; Capasso, F.; Senatore, F. Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller var. vulgare (Miller) essential oils. J. Agric. Food Chem. 2004, 52, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, M.; Pirani, F.; Tagliabracci, A.; Valsecchi, M.; Procopio, A.D.; Busardò, F.P.; Graciotti, L. SARS-CoV-2 identification in lungs, heart and kidney specimens by transmission and scanning electron microscopy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5186–5188. [Google Scholar] [PubMed]
- Sayed, R.; Safwat, N.A.; Amin, B.H.; Yosri, M. Study of the dual biological impacts of aqueous extracts of normal and gamma-irradiated Galleria mellonella larvae. J. Taibah. Univ. Med. Sci. 2022, 17, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Chang, W.L.; Lee, S.C.; Liu, Y.G.; Lin, H.C.; Chen, C.J.; Yen, C.Y.; Yu, D.S.; Lin, S.Z.; Harn, H.J. Acetone Extract of Bupleurum scorzonerifolium Inhibits Proliferation of A549 Human Lung Cancer Cells via Inducing Apoptosis and Suppressing Telomerase Activity. Life Sci. 2003, 73, 2383–2394. [Google Scholar] [CrossRef]
- Ahmed, H.Y.; Kareem, S.M.; Atef, A.; Safwat, N.A.; Shehata, R.M.; Yosri, M.; Youssef, M.; Baakdah, M.M.; Sami, R.; Baty, R.S.; et al. Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities. Antioxidants 2022, 11, 1960. [Google Scholar] [CrossRef]
- Yuan, K.; Mei, J.; Shao, D.; Zhou, F.; Qiao, H.; Liang, Y.; Li, K.; Tang, T. Cerium oxide nanoparticles regulate osteoclast differentiation bidirectionally by modulating the cellular production of reactive oxygen species. Int. J. Nanomed. 2020, 15, 6355–6372. [Google Scholar] [CrossRef]
- Yosri, M.; Elaasser, M.M.; Abdel-Aziz, M.M.; Hassan, M.M.; Alqhtani, A.H.; Al-Gabri, N.; Ali, A.B.A.; Pokoo-Aikins, A.; Amin, B.H. Determination of Therapeutic and Safety Effects of Zygophyllum coccineum Extract in Induced Inflammation in Rats. Biomed. Res. Int. 2022, 2022, 7513155. [Google Scholar] [CrossRef]
- Deyab, M.A.; El-Sheekh, M.M.; Hasan, R.S.; Elsadany, A.Y.; Abu Ahmed, S.E.S. Phytochemical Components of Two Cyanobacterial Local Strains. Sci. J. Damietta Fac. Sci. 2021, 11, 67–75. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, S.S.; Filho, R.M. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Márquez-Rodríguez, A.S.; Nevárez-Baca, S.; Lerma-Hernández, J.C.; Hernández-Ochoa, L.R.; Nevárez-Moorillon, G.V.; Gutiérrez-Méndez, N.; Muñoz-Castellanos, L.N.; Salas, E. In Vitro Antibacterial Activity of Hibiscus sabdariffa L. Phenolic Extract and Its in Situ Application on Shelf-Life of Beef Meat. Foods 2020, 9, 1080. [Google Scholar] [CrossRef]
- Djeussi, D.E.; Noumedem, J.A.; Seukep, J.A.; Fankam, A.G.; Voukeng, I.K.; Tankeo, S.B.; Nkuete, A.H.; Kuete, V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med. 2013, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.S.; Tsao, S.M.; Yin, M.C. In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phytother. Res. 2005, 19, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.M.; Hardy, W.D.; et al. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e61594. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The antibacterial activity of coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella Enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef]
- Malacrida, A.; Erriquez, J.; Hashemi, M.; Rodriguez-Menendez, V.; Cassetti, A.; Cavaletti, G.; Miloso, M. Evaluation of antitumoral effect of Hibiscus sabdariffa extract on human breast cancer cells. Biochem. Biophys. Rep. 2022, 32, 101353. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Choi, H.S.; Park, S.Y.; Kim, J.K.; Seo, K.H.; Kim, H.; Kim, Y.J. Hibiscus syriacus L. cultivated in callus culture exerts cytotoxicity in colorectal cancer via Notch signaling-mediated cholesterol biosynthesis suppression. Phytomedicine 2022, 95, 153870. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, L.; Wie, M.R. The mechanisms of fatty acid-induced proton permeability of the inner mitochondrial membrane. J. Bioenerg. Biomembr. 1999, 31, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Greenway, D.; Dyke, K. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. Microbiology 1979, 115, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Rassem, H.; Nour, A.H.; Yunus, R.M. GC-MS analysis of bioactive constituents of Hibiscus flower. Aust. J. Basic Appl. Sci. 2017, 11, 91–97. [Google Scholar]
- Vijayakumar, S.; Yabesh, J.M.; Arulmozhi, P.; Praseetha, P.K. Identification and isolation of antimicrobial compounds from the flower extract of Hibiscus rosa-sinensis L: In silico and in vitro approaches. Microb. Pathog. 2018, 123, 527–535. [Google Scholar] [CrossRef]
- Mohammed, G.J. Study of the synergistic effect of some antibiotics and aqueous extract of Hibiscus sabdariffa plant against Salmonella typhi. Int. J. Drug Deliv. Technol. 2020, 10, 217–221. [Google Scholar] [CrossRef]
- Gaba, M.; Gaba, P.; Singh, S.; Gupta, G.D. An overview on molecular docking. Int. J. Drug Dev. Res. 2010, 2, 219–231. [Google Scholar]
- Jiang, M.; Cao, Y.; Guo, Z.F.; Chen, M.; Chen, X.; Guo, Z. Menaquinone biosynthesis in Escherichia coli: Identification of 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate as a novel intermediate and re-evaluation of MenD activity. Biochemistry 2007, 46, 10979–10989. [Google Scholar] [CrossRef]
- Li, X.; Liu, N.; Zhang, H.; Knudson, S.E.; Slayden, R.A.; Tonge, P.J. Synthesis and SAR studies of 1, 4-benzoxazine MenB inhibitors: Novel antibacterial agents against Mycobacterium tuberculosis. Bioorganic Med. Chem. Lett. 2010, 20, 6306–6309. [Google Scholar] [CrossRef]
- Matarlo, J.S.; Lu, Y.; Daryaee, F.; Daryaee, T.; Ruzsicska, B.; Walker, S.G.; Tonge, P.J. A methyl 4-oxo-4-phenylbut-2-enoate with in vivo activity against MRSA that inhibits MenB in the bacterial menaquinone biosynthesis pathway. ACS Infect. Dis. 2016, 2, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gething, M.J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.; Halestrap, A. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, T.; Weimann, A.; Borsetti, A.; Walsh, C.T.; Göttlinger, H.G. Active-site residues of cyclophilin A are crucial for its incorporation into human immunodeficiency virus type 1 virions. J. Virol. 1997, 71, 7110–7113. [Google Scholar] [CrossRef]
- Uchida, T.; Takamiya, M.; Takahashi, M.; Miyashita, H.; Ikeda, H.; Terada, T.; Matsuo, Y.; Shirouzu, M.; Yokoyama, S.; Fujimori, F.; et al. Pin1 and Par14 peptidyl prolyl isomerase inhibitors block cell proliferation. Chem. Biol. 2003, 10, 15–24. [Google Scholar] [PubMed]
- Campaner, E.; Rustighi, A.; Zannini, A.; Cristiani, A.; Piazza, S.; Ciani, Y.; Kalid, O.; Golan, G.; Baloglu, E.; Shacham, S.; et al. A covalent PIN1 inhibitor selectively targets cancer cells by a dual mechanism of action. Nat. Commun. 2017, 8, 15772. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, H.; Liao, X.H.; Chen, C.P.; Zhang, A.L.; Lu, W.; Wang, L.; Yang, D.; Wang, J.; Liu, H.; et al. Inhibition of the prolyl isomerase Pin1 enhances the ability of sorafenib to induce cell death and inhibit tumor growth in hepatocellular carcinoma. Oncotarget 2017, 8, 29771. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Yazdanparast, R. Sensitizing effect of juglone is mediated by down regulation of Notch1 signaling pathway in trastuzumab-resistant SKBR3 cells. Apoptosis 2017, 22, 135–144. [Google Scholar] [CrossRef]
- Stanya, K.J.; Liu, Y.; Means, A.R.; Kao, H.Y. Cdk2 and Pin1 negatively regulate the transcriptional corepressor SMRT. J. Cell Biol. 2008, 183, 49–61. [Google Scholar] [CrossRef]
- Min, S.H.; Lau, A.W.; Lee, T.H.; Inuzuka, H.; Wei, S.; Huang, P.; Shaik, S.; Lee, D.Y.; Finn, G.; Balastik, M.; et al. Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol. Cell 2012, 46, 771–783. [Google Scholar] [CrossRef]
| Test Bacterial Strains | Diameter Inhibition Zone (DIZ) (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Salvia officinalis L. | Ziziphus spina-christi L. | Hibiscus sabdariffa L. | Penicillin G | |||||||
| Eth | Meth | Water | Eth | Meth | Water | Eth | Meth | Water | ||
| E. cloacae (ATCC13047) | 9.0 ± 0.0 | 19.6 ± 0.05 | 20.6 ± 0.05 | 11.0 ± 0.1 | 12.0 ± 0.1 | 0.0 | 31.6 ± 0.2 | 37.6 ± 0.15 | 26.6 ± 0.15 | 1.4 ± 0.0 |
| S. aureus (ATCC 25923) | 22.0 ± 0.1 | 9.0 ± 0.0 | 20.0 ± 0.0 | 0.0 | 13.6 ± 0.15 | 0.0 | 30.3 ± 0.05 | 38.6 ± 0.15 | 29.6 ± 0.05 | 1.33 ± 0.11 |
| P. aeruginosa (RCMB008001) | 20.6 ± 0.05 | 9.3 ± 0.05 | 19.3 ± 0.05 | 19.3 ± 0.05 | 28.0 ± 0.26 | 13.3 ± 0.15 | 30.6 ± 0.05 | 36.6 ± 0.05 | 27.6 ± 0.23 | * NA |
| E. coli (RCMB004001) | 27.6 ± 0.3 | 9.0 ± 0.0 | 19.3 ± 005 | 9.6 ± 0.05 | 10.6 ± 0.05 | 0.0 | 30.6 ± 0.11 | 39.6 ± 0.20 | 29.3 ± 03 | 0.9 ± 0.0 |
| Test Bacterial Strains | Hibiscus sabdariffa L. Methanolic Extract (µg/mL) | ||
|---|---|---|---|
| MIC | MBC | MBC/MIC | |
| E. cloacae | 1.9 | 7.8 | 4.1 |
| S. aureus | 31.25 | 62.5 | 2 |
| P. aeruginosa | 15.6 | 31.2 | 2 |
| E. coli | 3.9 | 7.8 | 2 |
| Antibiotics | Conc. (µg/disc) | Resistant (R) No. * (%) | Intermediate (I) No. * (%) | Susceptible (S) No. * (%) |
|---|---|---|---|---|
| Vancomycin (VA) | 30 | 4 (100) | 0 (0) | 0 (0) |
| Meropenem (MEM) | 10 | 4 (100) | 0 (0) | 0 (0) |
| Gentamicin (CN) | 10 | 3 (75) | 1(25) | 0 (0) |
| Erythromycin (E) | 15 | 4 (100) | 0 (0) | 0 (0) |
| Amikacin (AK) | 30 | 3 (75) | 1 (25) | 0 (0) |
| Ciprofloxacin (CIP) | 5 | 3 (75) | 1 (25) | 0 (0) |
| Tetracycline (TE) | 30 | 2 (50) | 0 (0) | 2 (50) |
| Amoxicillin/clavulanic Acid (AMC) | 30 | 4 (100) | 0 (0) | 0 (0) |
| Piperacillin/Tazobactam (TZP) | 110 | 0 (0) | 2 (50) | 2 (50) |
| Doxycycline (DO) | 30 | 2 (50) | 0 (0) | 2 (50) |
| Ceftazidime (CAZ) | 30 | 4 (100) | 0 (0) | 0 (0) |
| Cefotaxime (CTX) | 30 | 4 (100) | 0 (0) | 0 (0) |
| Bacterial Strains | DIZ Antibiotic (TZP) + Methanol Extract of Hibiscus sabdariffa L. (mm) | Outcome |
|---|---|---|
| E. cloacae | 57 | Synergism |
| S. aureus | 30 | antagonism |
| P. aeruginosa | 36 | antagonism |
| E. coli | 63 | Synergism |
| RT | Area% | Compound Name | Molecular Formula | Molecular Weight |
|---|---|---|---|---|
| 9.07 | 0.20 | 1,4-Diphenylbut-3-ene-2-OL | C16H16O | 224 |
| 10.15 | 0.93 | 3-Heptenoic acid, methyl ester | C8H14O2 | 142 |
| 12.12 | 0.96 | 2-Pentenoic acid, 3-ethyl-, methyl ester | C8H14O2 | 142 |
| 15.95 | 0.24 | 1,2,3- Propanetriol, triactate | C9H14O6 | 218 |
| 18.14 | 0.24 | Nonanoic acid, 9-oxo-, methyl ester | C10H18O3 | 186 |
| 20.97 | 2.16 | Butanedioic acid,1-hydroxy-2,2-dimethyl-, dimethyl, (R)- | C8H14O5 | 190 |
| 24.30 | 0.18 | Oleic Acid | C18H34O2 | 282 |
| 25.78 | 0.67 | Tetradecanoic acid | C14H28O2 | 228 |
| 27.89 | 0.27 | Hexadecanoic acid, 2,3-dihydroxypropyl ester | C19H38O4 | 330 |
| 29.16 | 3.93 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 |
| 30.50 | 6.32 | n-Hexadecanoic acid | C16H32O2 | 256 |
| 32.49 | 2.03 | 11-Octadecenoic acid, methyl ester | C19H36O2 | 296 |
| 34.13 | 2.18 | cis-13-Octadecenoic acid | C18H34O2 | 282 |
| 38.02 | 2.27 | Oleic acid, 3-hydroxypropyl ester | C21H40O3 | 340 |
| 39.65 | 0.20 | Linoleic acid ethyl ester | C20H36O2 | 308 |
| 42.00 | 1.29 | 9-Octadecenoic acid (Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C21H40O4 | 356 |
| 47.10 | 0.55 | Stigmast-5-en-3-ol, (3a)- | C29H50O | 414 |
| Compounds | rseq | mseq | S | rmsd_refine | E_conf | E_place | E_score1 | E_refine | E_score2 |
|---|---|---|---|---|---|---|---|---|---|
| n-Hexadecanoic acid | 1 | 1 | −6.86665 | 0.994746 | −12.659 | −53.7632 | −10.7978 | −32.0979 | −6.86665 |
| n-Hexadecanoic acid | 1 | 1 | −6.55267 | 1.277218 | −10.8394 | −59.7529 | −9.55267 | −33.733 | −6.55267 |
| n-Hexadecanoic acid | 1 | 1 | −6.52165 | 1.769185 | −18.0445 | −64.3249 | −10.2547 | −35.0176 | −6.52165 |
| n-Hexadecanoic acid | 1 | 1 | −6.51166 | 1.839089 | −15.5851 | −53.5681 | −9.78798 | −33.7598 | −6.51166 |
| n-Hexadecanoic acid | 1 | 1 | −6.30383 | 1.516807 | −16.9191 | −65.1021 | −10.7465 | −32.7108 | −6.30383 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −6.10658 | 1.385643 | 30.86198 | −47.8591 | −8.56195 | −27.4175 | −6.10658 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −6.07297 | 1.644855 | 25.24213 | −73.9025 | −9.3113 | −30.1375 | −6.07297 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −5.9455 | 2.384998 | 21.9286 | −49.9538 | −8.39467 | −25.3867 | −5.9455 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −5.92716 | 1.321452 | 21.94232 | −57.8695 | −8.42712 | −27.0658 | −5.92716 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −5.8308 | 1.646691 | 20.72942 | −39.7795 | −8.48416 | −26.0097 | −5.8308 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −7.58806 | 1.82872 | 10.58784 | −63.0984 | −9.11248 | −44.3234 | −7.58806 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −6.90692 | 1.904773 | 24.04655 | −69.2759 | −8.38785 | −33.8326 | −6.90692 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −6.90146 | 2.127806 | 17.13374 | −60.4561 | −8.59733 | −32.6049 | −6.90146 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −6.86368 | 2.844606 | 13.11685 | −59.2249 | −9.04413 | −36.2489 | −6.86368 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −6.77453 | 1.80254 | 12.37747 | −38.8555 | −9.28452 | −34.6887 | −6.77453 |
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| n-Hexadecanoic acid | O 49 | OD1 ASN 202 (A) | H-donor | 2.89 | −0.7 |
| Hexadecanoic acid, methyl ester | O 48 | SG CYS 240 (A) | H-donor | 3.38 | −1.3 |
| C 50 | OD2 ASP 239 (A) | H-donor | 3.53 | −0.6 | |
| O 48 | NH1 ARG 173 (A) | H-acceptor | 3.15 | −3.4 | |
| Oleic acid,3-hydroxypropyl ester | O 19 | O CY S 141 (A) | H-donor | 2.95 | −1.2 |
| O 19 | OG1 THR 144 (A) | H-acceptor | 2.85 | −1.4 |
| Compounds | rseq | mseq | S | rmsd_refine | E_conf | E_place | E_score1 | E_refine | E_score2 |
|---|---|---|---|---|---|---|---|---|---|
| n-Hexadecanoic acid | 1 | 1 | −7.06866 | 1.400918 | −13.0742 | −58.1423 | −10.1681 | −39.9907 | −7.06866 |
| n-Hexadecanoic acid | 1 | 1 | −6.83773 | 1.639413 | −19.6424 | −67.4092 | −9.91065 | −38.2725 | −6.83773 |
| n-Hexadecanoic acid | 1 | 1 | −6.79092 | 1.952255 | −19.0811 | −71.4487 | −10.3565 | −35.9714 | −6.79092 |
| n-Hexadecanoic acid | 1 | 1 | −6.75079 | 1.205076 | −18.8722 | −56.279 | −10.7956 | −36.788 | −6.75079 |
| n-Hexadecanoic acid | 1 | 1 | −6.7165 | 1.589987 | −17.4918 | −57.3984 | −10.3913 | −36.4317 | −6.7165 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −6.90753 | 0.883272 | 25.82254 | −70.1823 | −9.63924 | −35.4005 | −6.90753 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −6.59635 | 1.414765 | 20.0315 | −60.5045 | −9.50993 | −36.1628 | −6.59635 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −6.56374 | 1.684296 | 23.14412 | −63.223 | −9.01701 | −36.5304 | −6.56374 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −6.5409 | 1.357839 | 23.56528 | −68.1719 | −9.3606 | −35.875 | −6.5409 |
| Hexadecanoic acid, methyl ester | 1 | 2 | −6.49536 | 2.879772 | 20.43498 | −52.616 | −9.35453 | −35.5943 | −6.49536 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −7.36223 | 1.532105 | 9.535423 | −81.9436 | −10.2876 | −41.8523 | −7.36223 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −7.28274 | 1.355768 | 11.6478 | −69.1213 | −9.73811 | −39.9229 | −7.28274 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −7.27262 | 1.127731 | 13.77589 | −73.9368 | −10.5268 | −41.5078 | −7.27262 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −6.89469 | 1.566679 | 7.721873 | −61.6901 | −9.17655 | −37.0592 | −6.89469 |
| Oleic acid,3-hydroxypropyl ester | 1 | 3 | −6.88245 | 2.300354 | 10.61165 | −81.0547 | −9.84603 | −38.2886 | −6.88245 |
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| n-Hexadecanoic acid | O 49 | O ALA 102 (A) | H-donor | 2.98 | −1.3 |
| O 48 | N SER 111 (A) | H-acceptor | 3.06 | −1.7 | |
| O 48 | N GLN 112 (A) | H-acceptor | 3.17 | −2.3 | |
| Hexadecanoic acid, methyl ester | O 48 | N GLY 110 (A) | H-acceptor | 3.08 | −0.8 |
| Oleic acid, 3-hydroxypropyl ester | O 9 | N GLN 112 (A) | H-acceptor | 3.26 | −0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehim, A.E.; Amin, B.H.; Yosri, M.; Salama, H.M.; Alkhalifah, D.H.; Alwaili, M.A.; Abd Elghaffar, R.Y. GC-MS Analysis, Antibacterial, and Anticancer Activities of Hibiscus sabdariffa L. Methanolic Extract: In Vitro and In Silico Studies. Microorganisms 2023, 11, 1601. https://doi.org/10.3390/microorganisms11061601
Sehim AE, Amin BH, Yosri M, Salama HM, Alkhalifah DH, Alwaili MA, Abd Elghaffar RY. GC-MS Analysis, Antibacterial, and Anticancer Activities of Hibiscus sabdariffa L. Methanolic Extract: In Vitro and In Silico Studies. Microorganisms. 2023; 11(6):1601. https://doi.org/10.3390/microorganisms11061601
Chicago/Turabian StyleSehim, Amira E., Basma H. Amin, Mohammed Yosri, Hanaa M. Salama, Dalal Hussien Alkhalifah, Maha Abdullah Alwaili, and Rasha Y. Abd Elghaffar. 2023. "GC-MS Analysis, Antibacterial, and Anticancer Activities of Hibiscus sabdariffa L. Methanolic Extract: In Vitro and In Silico Studies" Microorganisms 11, no. 6: 1601. https://doi.org/10.3390/microorganisms11061601
APA StyleSehim, A. E., Amin, B. H., Yosri, M., Salama, H. M., Alkhalifah, D. H., Alwaili, M. A., & Abd Elghaffar, R. Y. (2023). GC-MS Analysis, Antibacterial, and Anticancer Activities of Hibiscus sabdariffa L. Methanolic Extract: In Vitro and In Silico Studies. Microorganisms, 11(6), 1601. https://doi.org/10.3390/microorganisms11061601






