Resistance and Biofilm Production Profile of Potential Isolated from Kpètè-Kpètè Used to Produce Traditional Fermented Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Analyses
2.2.1. Identification of Bacterial Strains
2.2.2. Biofilm Formation
2.2.3. Susceptibility of Bacterial Isolates to Tested Antibiotic
2.3. Molecular Characterization of Isolates
2.3.1. DNA Extraction
2.3.2. Search for Alleles of Genes Encoding Virulence
2.4. Data Analysis and Processing
3. Results
3.1. Identification of Pathogenic Bacteria Isolated
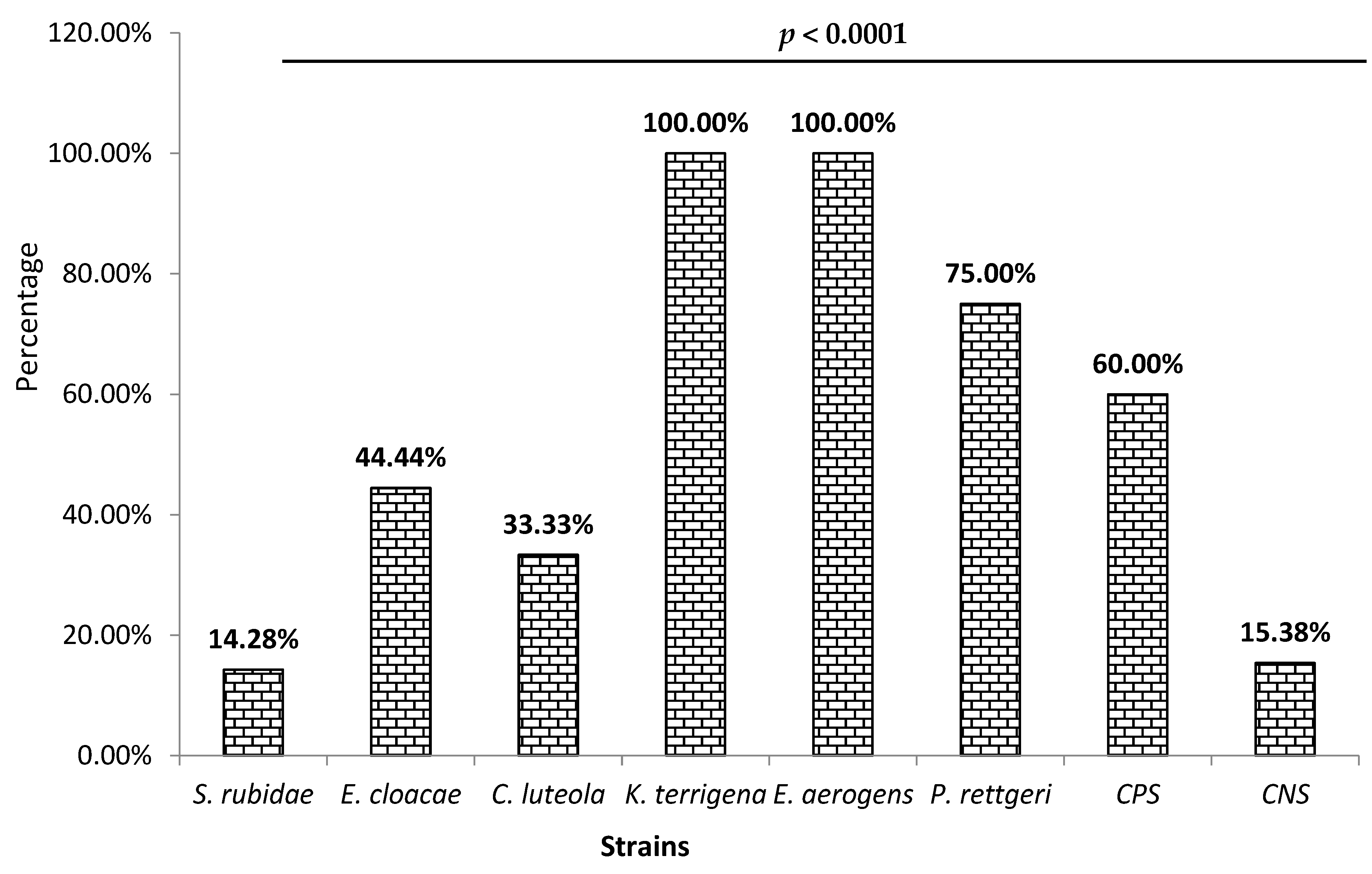
3.2. Biofilm Production
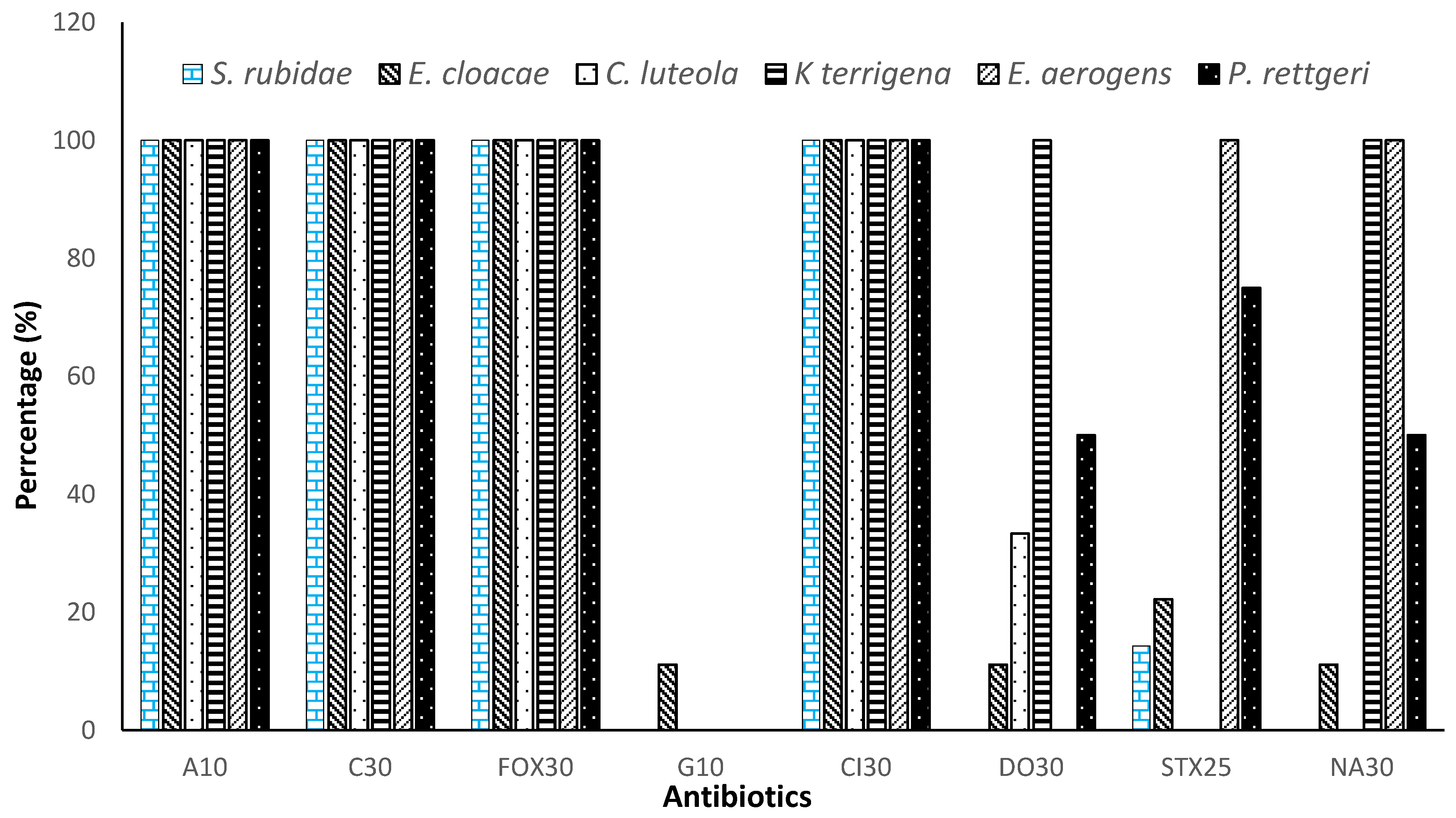
3.3. Resistance Profile of Enterobacteriaceae to Antibiotics by Isolated Species
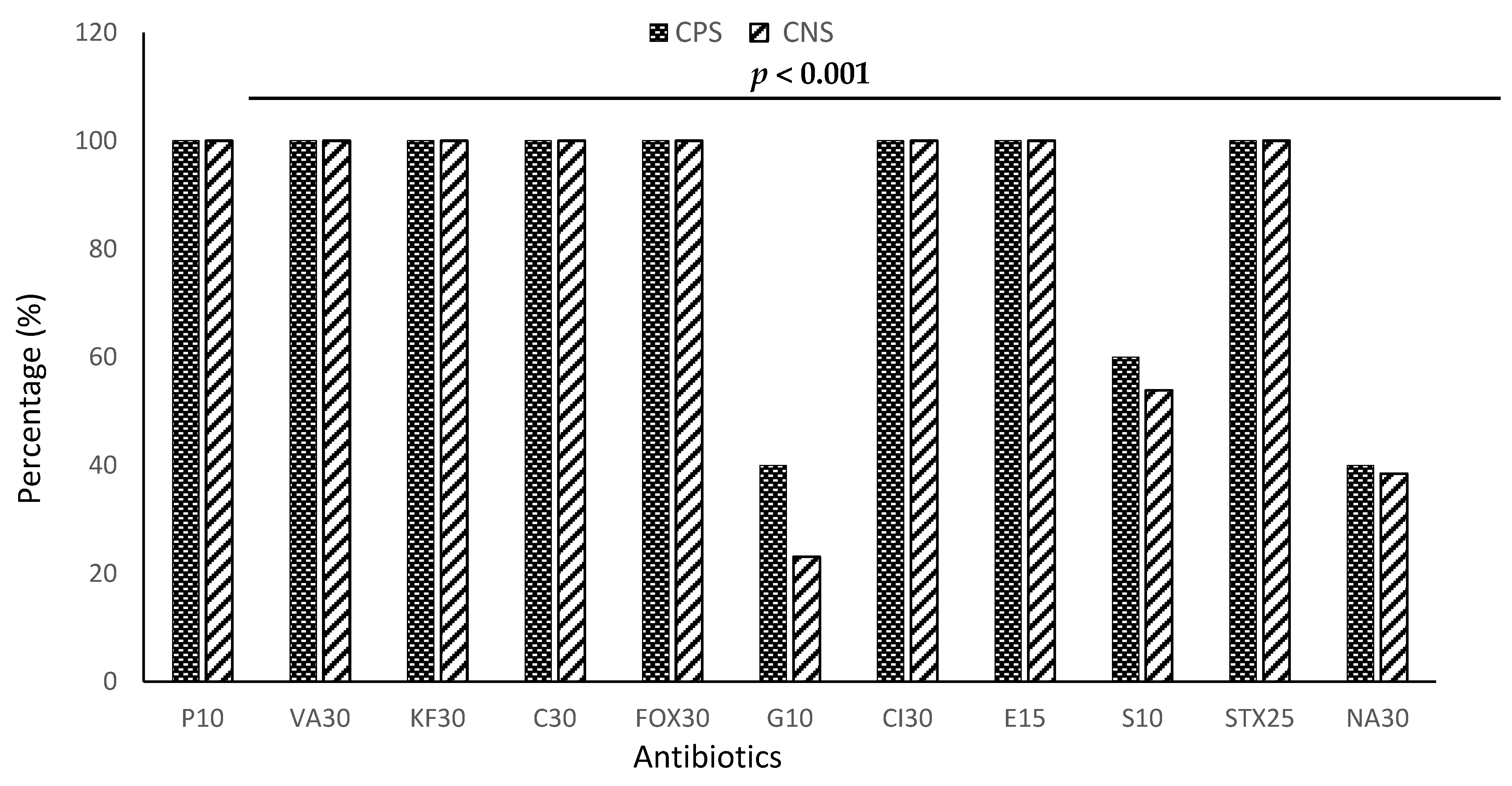
3.4. Resistance Profile of Staphylococci to Antibiotics by Species
3.5. Virulence Genes and Resistance Gene Macrolides
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Rapport OMS: 420 000 Personnes Tuées par l’intoxication Alimentaire; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Osseyi, E.G.; Tagba, P.; Karou, S.D. Stabilization of the Traditional Sorghum Beer “Tchoukoutou” using Rustic Wine- Making Method. Adv. J. Food Sci. Technol. 2011, 3, 254–258. [Google Scholar]
- Kayodé, A.P.P.; Linmemann, A.R.; Nout, M.J.R.; Hounhouigan, D.J.; Stomph, T.J.; Smulders, M.J.M. Diversity and food quality properties of farmers’ varieties of sorghum from Benin. J. Sci. Food Agric. 2006, 86, 1032–1039. [Google Scholar] [CrossRef]
- Missihoun, A.A.; Agbangla, C.; Sagbadja, H.A.; Ahanhanzo, C.; Vodouhe, R. Gestion traditionnelle et statut des ressources génétiques du sorgho (Sorghum bicolor L. Moench) au Nord-Ouest du Bénin. Int. J. Biol. Chem. Sci. 2012, 6, 1003–1018. [Google Scholar] [CrossRef]
- Aholou, C.C. Construction identitaire et urbaine autour du cabaret à Lomé (Togo). Hommes Migr. 2010, 1283, 32–41. [Google Scholar] [CrossRef][Green Version]
- Baba-Moussa, L.; Bokossa, Y.I.; Baba-Moussa, F.; Ahisou, H.; Adeoti, Z.; Yehouenou, B.; Mamadou, A.; Toukourou, F.; Sanni, A. Etude des possibilités de contamination des aliments de rues au Bénin: Cas de la ville de Cotonou. J. Rech. Sci. L’université Lomé (Togo) Série A 2006, 8, 149–156. [Google Scholar]
- Adesetan, T.O.; Efuntoye, M.O.; Babalola, O.O. Profiling of Bacillus Cereus Enterotoxigenic Genes from Retailed Foods and Detection of the Nhe and Hbl Toxins with Immunological Assay. J. Appl. Nat. Sci. 2022, 14, 254–267. [Google Scholar] [CrossRef]
- Imade, E.E.; Omonigho, S.E.; Babalola, O.O.; Enagbonma, B.J. Lactic acid bacterial bacteriocins and their bioactive properties against food-associated antibiotic-resistant bacteria. Ann. Microbiol. 2021, 71, 44. [Google Scholar] [CrossRef]
- Chuah, L.O.; Shamila-Syuhada, A.K.; Liong, M.T.; Rosma, A.; Thong, K.L.; Rusul, G. Physio-chemical, microbiological properties of tempoyak and molecular characterisation of lactic acid bacteria isolated from tempoyak. Food Microbiol. 2016, 58, 95–104. [Google Scholar] [CrossRef]
- Egbuta, M.A.; Mwanza, M.; Babalola, O.O. Health Risks Associated with Exposure to Filamentous Fungi. Int. J. Environ. Res. Public Health 2017, 14, 719. [Google Scholar] [CrossRef]
- Achi, O.K.; Ukwuru, M. Cereal-based fermented foods of Africa as functional foods. Int. J. Microbiol. Appl. 2015, 2, 71–83. [Google Scholar]
- Adesetan, T.O.; Efuntoye, M.O.; Babalola, O.O. Biochemical Characterization and Antimicrobial Susceptibility of Bacillus Cereus Isolates from Some Retailed Foods in Ogun State, Nigeria. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 616–621. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X.; Yu, H. Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef]
- Akaki, K.D.; Aw, S.; Loiseau, G.; Guyot, J.P. Etude du comportement des souches de Bacillus cereus ATCC 9139 et d’Escherichia coli ATCC 25922 par la méthode des challenge-tests lors de la confection de bouillies à base de pâte de mil fermentée en provenance de Ouagadougou (Burkina-Faso). Rev. Ivoirienne Des Sci. Et Technol. 2008, 11, 103–117. [Google Scholar]
- N’tcha, C.; Adéyèmi, A.D.; Kayodé, A.P.P.; Vieira-Dalodé, G.; Agbobatinkpo, B.P.; Codjia, J.C.; Baba-Moussa, L. Indigenous knowledge associated with the production of starters culture used to produce Beninese opaque sorghum beers. J. Appl. Biosci. 2015, 88, 8223–8234. [Google Scholar] [CrossRef][Green Version]
- Ekonomou, S.I.; Parlapani, F.F.; Kyritsi, M.; Hadjichristodoulou, C.; Boziaris, I.S. Preservation status and microbial communities of vacuum-packed hot smoked rainbow trout fillets. Food Microbiol. 2022, 103, 103959. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.; Yogesh, P.; Dhandapani, R. Production of prodigiosin from Serratia marcescens isolated from soil. Indian J. Sci. Technol. 2009, 2, 32–34. [Google Scholar] [CrossRef]
- Rodríguez-Saavedra, M.; González de Llano, D.; Moreno-Arribas, M.V. Beer spoilage lactic acid bacteria from craft brewery microbiota: Microbiological quality and food safety. Food Res. Int. 2020, 138 Pt A, 109762. [Google Scholar] [CrossRef] [PubMed]
- Hounsa, A.; Kouadio, L.; De Mol, P. Self-medication with antibiotics obtained from private pharmacies in Abidjan, Ivory Coast. Médecine Mal. Infect. 2009, 40, 333–340. [Google Scholar] [CrossRef]
- Alsan, M.; Morden, N.; Gottlieb, J.D.; Weiping, Z.; Skinner, J. Antibiotic use in cold and flu season and prescribing quality: A retrospective cohort study. Med. Care 2015, 53, 1066. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Akindolire, M.A.; Babalola, O.O.; Ateba, C.N. Detection of Antibiotic Resistant Staphylococcus aureus from Milk: A Public Health Implication. Int. J. Environ. Res. Public Health 2015, 12, 10254–10275. [Google Scholar] [CrossRef]
- Muylaert, A.; Mainil, J.G. Résistances aux fluoroquinolones: La situation actuelle. Ann. Med. Vet. 2013, 157, 15–26. [Google Scholar]
- Christensen, G.D.; Simpson, W.A.; Bisno, A.L.; Beachy, E.H. Adherence of biofilm producing strains of Staphylococci epidermidis to smooth surfaces. Infect. Immun. 1985, 37, 318–326. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- CASFM. Comité de l’antibiogramme de la Société Française de Microbiologie: Recommandations 2020 V.1.1. 181 p 2020. Available online: https://www.sfm-microbiologie.org/wp-content/uploads/2020/04/CASFM2020_Avril2020_V1.1.pdf (accessed on 1 September 2022).
- Rasmussen, R.S.; Morrissey, M.T. DNA-based methods for the identification of commercial fish and seafood species. Compr. Rev. Food Sci. Food Saf. 2008, 7, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Lamy, M.C.; Dramsi, S.; Billoët, A.; Réglier-Poupet, H.; Tazi, A.; Raymond, J.; Guérin, F.; Couvé, E.; Kunst, F.; Glaser, P. Rapid detection of the highly virulent group B Streptococcus ST-17 clone. Microb. Infect. 2006, 8, 1714–1722. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Budzyńska, A.; Gospodarek, E. Prevalence of genes encoding virulence factors among Escherichia coli wiyh K1 antigen and-K1 E. coli strains. J. Med. Microbiol. 2012, 61, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Moulin-Schouleur, M.; Schouleur, C.; Tailliez, P.; Kao, M.R.; Brée, A.; Germon, P.; Oswald, E.; Mainil, J.; Blanco, M.; Blanco, J. Common Virulence factors and genetic relationships between O18:K1:H7 Escherichia coli isolates of human and avian origin. J. Clin. Microbiol. 2006, 44, 3484–3492. [Google Scholar] [CrossRef]
- Germon, P.; Chen, Y.H.; He, L.; Blanco, J.E.; Bree, A.; Schouler, C.; Huang, S.H.; Moulin-Schouleur, M. IbeA, a virulence factors of avian pathogenic Escherichia coli. Microbiology 2005, 151, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.; Delgaty, K.L.; Ramotar, K.; Toye, B. Prevalence and mechanisms of erythromycin resistance in group A and group B Streptococcus: Implications for reporting susceptibility results. J. Clin. Microbiol. 2004, 42, 5620–5623. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Klaysom, C.; Jaafar, J.; Khan, A.L. An integrated rotating biological contactor and membrane separation process for domestic wastewater treatment. Alex. Eng. J. 2020, 59, 4257–4265. [Google Scholar] [CrossRef]
- Tankoano, A.; Diop, M.B.; Sawadogo-Lingani, H.; Kaoré, D.; Savadogo, A. Les aspects technologiques, microbiologiques et nutritionnels des aliments fermentés à base de lait de mil en Afrique de l’ouest. Int. J. Adv. Res. 2017, 5, 1509–1526. [Google Scholar] [CrossRef]
- Cason, D.E.; Mahlomaholo, J.B.; Taole, M.M.; Abong, O.G.; Vermeulen, J.G.; de Smidt, O.; Vermeulen, M.; Steyn, L.; Valverde, A.; Vijoen, B. Bacterial and Fungal Dynamics during the Fermentation Process of Sesotho, a Traditional Beer of Southern Africa. Front. Microbiol. 2020, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Maria, E.R.; Martinez, A.; Munguia, A.L. Characterization of bacterial diversity in Pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 2004, 235, 273. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Bamforth, C.W.; Mills, D.A. Brewhouse-resident microbiota is responsible for multi-stage fermentation of American coolshipale. PLoS ONE 2012, 7, e35507. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Carreon, F.; De Anda-Mora, K.; Rojas-Barrera, I.C.; Andrade, A. Serratia marcescens antibiotic resistance mechanisms of an opportunistic pathogen: A literature review. Peer J. 2023, 5, e14399. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Chen, L.; Yassin, A.K.; Kelly, P.; Butaye, P.; Li, J.; Gong, J.; Cattley, R.; Qi, K.; et al. Housefly (Musca domestica) and Blow Fly (Protophormia terraenovae) as Vectors of Bacteria Carrying Colistin Resistance Genes. Appl. Environ. Microbiol. 2017, 84, e01736-17. [Google Scholar] [CrossRef] [PubMed]
- Bensakhria, A. Biologie: Staphylococcus aureus (staphylocoques). 2017. Available online: www.magazinescience.com (accessed on 5 May 2022).
- Kostaki, M.; Chorianopoulos, N.; Braxou, E.; Nychas, G.J.; Efstathios, G. Differential biofilm formation and chemical disinfection resistance of sessile cells of Listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica) conditions. Appl. Environ. Microbiol. 2012, 8, 2586–2595. [Google Scholar] [CrossRef]
- Verstraeten, N.; Braeken, K.; Debkumari, B.; Fauvart, M.; Fransaer, J.; Vermant, J.; Michiels, J. Living on a surface: Swarming and biofilm formation. Trends Microbiol. 2008, 16, 496–506. [Google Scholar] [CrossRef]
- Balestrino, D.; Ghigo, J.M.; Charbonnel, N.; Haagensen, J.A.; Forestier, C. The characterization of functions involved in the establishment and maturation of Klebsiella pneumoniae in vitro biofilm reveals dual roles for surface exopolysaccharides. Environ. Microbiol. 2008, 10, 685–701. [Google Scholar] [CrossRef]
- Ahouandjinou, H. Qualité Microbiologique, Antibiorésistance des Souches de Staphylococcus spp et d’Escherichia coli Isolées des Carcasses Bovines au Bénin. Ph.D. Thesis, University of Abomey, Calavi, Benin, 2016. [Google Scholar]
- Lerebour, G.; Cupferman, S.; Bellon-Fontaine, M.N. Adhesion of Staphylococcus aureus and Staphylococcus epidermidis to the Episkin reconstructed epidermis model and to an inert 304 stainless steel substrate. J. Appl. Microbiol. 2004, 97, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.C.; Silva-Rezende, J.G.; de Freitas-Alves, L.A. Formation of biofilms by Staphylococcus aureus on stainless steel and glass surfaces and its resistance to some selected chemical sanitiziers. Braz. J. Microbiol. 2007, 38, 538–543. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Hama, H. Recherche de Bactéries Associées aux Mammites Subcliniques Dans le Lait de Chèvre en Mauritanie et au Togo et Détermination de leur Antibiosensibilité. Ph.D. Thesis, Veterinary Medicine, Dakar, Senegal, 2004. [Google Scholar]
- Anago, E.; Ayi-Fanou, L.; Akpovi, C.D.; Hounkpe, W.B.; Tchibozo, M.; Bankole, H.S. Antibiotic resistance and genotype of beta-lactamase producing Escherichia coli in nosocomial infections in Cotonou, Benin. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 5. [Google Scholar] [CrossRef]
- Touati, A.; Brasme, L.; Benallaoua, S.; Gharout, A.; Madoux, J.; De Champs, C. First report of qnrB-producing Enterobacter cloacae and qnrA-producing Acinetobacter baumannii recovered from Algerian hospitals. Diagn. Microbiol. Infect. Dis. 2008, 60, 287–290. [Google Scholar] [CrossRef]
- Soussy, C.J. Quinolones et bactéries à Gram négatif. In Patrice Courvalin, Roland Leclercq, 2nd ed.; Bingen, E., Ed.; ESKA: Paris, France, 2006; 277p. [Google Scholar]
- Kadja, M.C.; Kane, Y.; Viban, B.V.; Kaboret, Y.; Alambedji, B. Sensibilité aux antibiotiques des Bactéries associées aux mammites Clinique des petits ruminants dans la région de Dakar. Ann. Sci. Agron. 2013, 17, 205–216. [Google Scholar]
- Davin-Regli, A.; Bolla, J.M.; James, C.E.; Lavigne, J.P.; Chevalier, J.; Garnotel, E. Membrane permeability and regulation of drug “influx and efflux” in enterobacterial pathogens. Curr. Drug Targets 2008, 9, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Shafiee, M.; Hasso, M.; Sharif, U. Providencia rettgeri: An unexpected case of Gram-negative cellulitis. Case Rep. Wounds Int. 2015, 6, 30–32. [Google Scholar]
- Sina, H.; Attien, P.; Wélé, M.; Socohou, A.; Boukary-Chabi, A.; Dougnon, V.T.; Baba-Moussa, F.; Adjanohoun, A.; Baba-Moussa, L. Sanitary Risk Factors and Microbial Contamination of Grilled Meats Sold in Cotonou, Benin. J. Food Secur. 2019, 7, 175–182. [Google Scholar]
- Schwarz, S.; Chaslus-Dancla, E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 2001, 32, 201–225. [Google Scholar] [CrossRef]
- Chiquet, C.; Max, M.; Altayrac, J.; Aptel, F.; Boisset, S.; Vandenesch, F.; Carricajo, A. Correlation between clinical data and antibiotic resistance in coagulase negative Staphylococcus species isolated from 68 patients with acute post-cataract endophthalmitis. Clin. Microbiol. Infect. 2015, 21, 592.e1–592.e8. [Google Scholar] [CrossRef] [PubMed]
- Bonnel, A.S.; Quinque, K.; Le Roux, P.; Le Luyer, B. Aspergillose nécrosante semi invasive chez un enfant de 14 ans atteint de mucoviscidose. Rev. Mal. Respir. 2006, 23, 343–347. [Google Scholar] [CrossRef]
- Rodrigues, M.X.; Silva, C.C.N.; Trevillin, H.J.; Cruzado, B.M.; Mui, S.T.; Sanches Duarte, R.F.; Castillo, C.C.; Canniatti-Brazaca, G.S.; Porto, E. Antibiotic resistance and molecular characterization of Staphylococcus species from mastitic milk. Afr. J. Microbiol. Res. 2017, 11, 84–91. [Google Scholar]
- Lesch, C.; Itokazu, G.; Danziger, L.; Weinstein, R. Multi-hospital analysis of antimicrobial usage and resistance trends. Diagn. Microbiol. Infect. Dis. 2001, 41, 149–154. [Google Scholar] [CrossRef]
- Trivedi, K.; Cupakova, S.; Karpiskova, R. Virulence factors and antibiotic resistance in enterococci isolated foodstuffs. Vet. Med. 2011, 56, 352–357. [Google Scholar] [CrossRef]
- Clegg, S.; Hughes, K.T. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J. Bacteriol. 2002, 184, 1209–1213. [Google Scholar] [CrossRef]
- Le Trong, I. Donor strand exchange and conformational changes during E. coli fimbrial formation. J. Struct. Biol. 2008, 172, 380–388. [Google Scholar] [CrossRef]
- Soria-Bustos, J.; Ares, M.A.; Gómez-Aldapa, C.A.; González-y-Merchand, J.A.; Girón, J.A.; De la Cruz, M.A. Two Type VI Secretion Systems of Enterobacter cloacae Are Required for Bacterial Competition, Cell Adherence, and Intestinal Colonization. Front. Microbiol. 2020, 11, 560488. [Google Scholar] [CrossRef]
- Lockman, H.A. Yersinia pseudotuberculosis produces a cytotoxic necrotizing factor. Infect. Immun. 2002, 70, 2708–2714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, X.; Wang, M.; Zhou, Z. The potential impact of naturally produced antibiotics, environmental factors, and anthropogenic pressure on the occurrence of erm genes in urban soils. Environ. Pollut. 2019, 245, 282–289. [Google Scholar] [CrossRef]
- Luthje, P.; Schwarz, S. Antimicrobial resistance of coagulase-negative staphylococci from bovine subclinical mastitis with particular reference to macrolide lincosamide resistance phenotypes and genotypes. J. Antimicrob. Chemother. 2006, 57, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mah, T.F. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef] [PubMed]

| Antibiotics | Percentage of Resistance | p Value |
|---|---|---|
| Ampicillin (A10) | 100.00% | <0.001 |
| Ceftriaxone (Cl30) | 100.00% | |
| Cefoxitin (FOX30) | 100.00% | |
| Chloramphenicol (C30) | 100.00% | |
| Gentamicin (G10) | 1.85% | |
| Doxycycline (DO30) | 32.40% | |
| Trimethoprim-sulfamethoxazole (STX25) | 32.85% | |
| Nalidixic acid (NA30) | 43.51% |
| Antibiotics | Percentage of Resistance | p Value |
|---|---|---|
| Penicillin G (P10) | 100.00% | <0.0001 |
| Ceftriaxone (Cl30) | 100.00% | |
| Cefoxitin (FOX30) | 100.00% | |
| Chloramphenicol (C30) | 100.00% | |
| Cephalothin (KF30) | 100.00% | |
| Vancomycin (VA30) | 100.00% | |
| Erythromycin (E15) | 100.00% | |
| Trimethoprim-sulfamethoxazole (STX25) | 100.00% | |
| Gentamicin (G10) | 31.54% | |
| Nalidixic Acid (NA30) | 39.23% | |
| Streptomycin (S10) | 59.92% |
| fimA | cnf1 | ermB | mefA | |
|---|---|---|---|---|
| Klebsiella terrigena | (0%) | (0%) | NA | NA |
| Enterobacter aerogens | (0%) | (0%) | NA | NA |
| Providencia rettgeri | (0%) | (0%) | NA | NA |
| Chryseomonas luteola | (0%) | (0%) | NA | NA |
| Serratia rubidae | (0%) | (0%) | NA | NA |
| Enterobacter cloacae | (4%) | (0%) | NA | NA |
| S. aureus | NA | NA | (0%) | (0%) |
| SCN | NA | NA | (5.55%) | (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
N’Tcha, C.; Sina, H.; Bourobou, D.N.; Hoteyi, S.M.I.; Boya, B.; Agnimonhan, R.; Mavoungou, J.F.; Adjanohoun, A.; Babalola, O.O.; Baba-Moussa, L. Resistance and Biofilm Production Profile of Potential Isolated from Kpètè-Kpètè Used to Produce Traditional Fermented Beer. Microorganisms 2023, 11, 1939. https://doi.org/10.3390/microorganisms11081939
N’Tcha C, Sina H, Bourobou DN, Hoteyi SMI, Boya B, Agnimonhan R, Mavoungou JF, Adjanohoun A, Babalola OO, Baba-Moussa L. Resistance and Biofilm Production Profile of Potential Isolated from Kpètè-Kpètè Used to Produce Traditional Fermented Beer. Microorganisms. 2023; 11(8):1939. https://doi.org/10.3390/microorganisms11081939
Chicago/Turabian StyleN’Tcha, Christine, Haziz Sina, Dyana Ndiade Bourobou, S. M. Ismaël Hoteyi, Bawa Boya, Raoul Agnimonhan, Jacques François Mavoungou, Adolphe Adjanohoun, Olubukola Oluranti Babalola, and Lamine Baba-Moussa. 2023. "Resistance and Biofilm Production Profile of Potential Isolated from Kpètè-Kpètè Used to Produce Traditional Fermented Beer" Microorganisms 11, no. 8: 1939. https://doi.org/10.3390/microorganisms11081939
APA StyleN’Tcha, C., Sina, H., Bourobou, D. N., Hoteyi, S. M. I., Boya, B., Agnimonhan, R., Mavoungou, J. F., Adjanohoun, A., Babalola, O. O., & Baba-Moussa, L. (2023). Resistance and Biofilm Production Profile of Potential Isolated from Kpètè-Kpètè Used to Produce Traditional Fermented Beer. Microorganisms, 11(8), 1939. https://doi.org/10.3390/microorganisms11081939









