Effects of Increasing Levels of Purified Beta-1,3/1,6-Glucans on the Fecal Microbiome, Digestibility, and Immunity Variables of Healthy Adult Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Animals
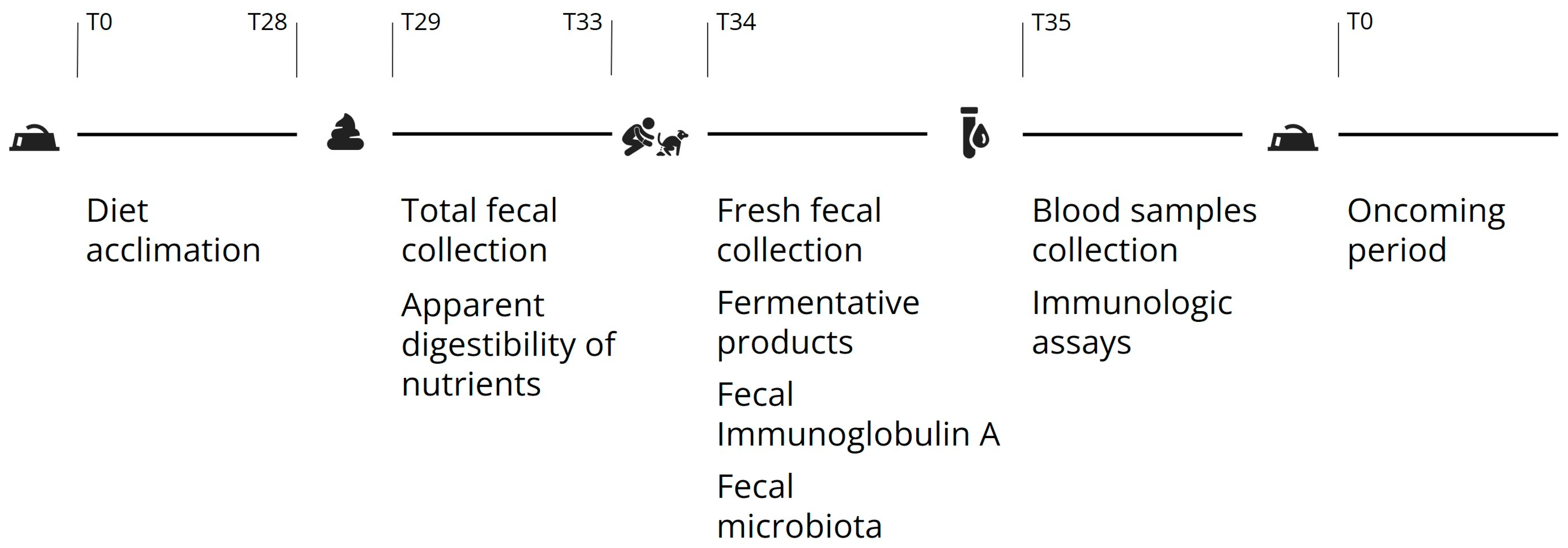
2.2. Diets and Experimental Design
2.3. Fecal Microbiota
2.4. Apparent Digestibility Coefficients of Nutrients
2.5. Fermentative Products
2.5.1. Fecal Ammoniacal Nitrogen, Short-Chain Fatty Acids, and Branched-Chain Fatty Acids
2.5.2. Lactic Acid Analysis
2.5.3. Fecal pH Measurement
2.6. Immunological Variables
2.6.1. Lymphocyte Immunophenotyping
2.6.2. Phagocytosis and Oxidative Burst Test
2.6.3. Fecal Immunoglobulin A Analysis
2.7. Statistical Analyses
3. Results
3.1. Animals and Diet Compositions
3.2. Fecal Microbiota
3.3. Apparent Digestibility Coefficients of Nutrients
3.4. Fermentative Products
3.5. Immunologic Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet. J. 2016, 215, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Intestinal Microbiota of Dogs and Cats: A Bigger World than We Thought. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 261–272. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Tizard, I.R.; Jones, S.W. The Microbiota Regulates Immunity and Immunologic Diseases in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 307–322. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Kerr, K.R.; Dilger, R.N.; Fahey, G.C., Jr.; Guérin-Deremaux, L.; Lynch, G.L.; Wils, D.; Suchodolski, J.S.; Steer, J.M.; Dowd, S.E.; et al. Modulation of the faecal microbiome of healthy adult dogs by inclusion of potato fibre in the diet. Br. J. Nutr. 2015, 113, 125–133. [Google Scholar] [CrossRef]
- Handl, S.; German, A.J.; Holden, S.L.; Dowd, S.E.; Steiner, J.M.; Heilmann, R.M.; Grant, R.W.; Swanson, K.S.; Suchodolski, J.S. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Future aspects and perceptions of companion animal nutrition and sustainability. J. Anim. Sci. 2015, 93, 823–834. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Morales-Medina, J.C.; Palmieri, B.; Pezzuto, F.; Cocco, R.; Flores, G.; Iannitti, T. Functional foods in pet nutrition: Focus on dogs and cats. Res. Vet. Sci. 2017, 112, 161–166. [Google Scholar] [CrossRef]
- Redfern, A.; Suchodolski, J.; Jergens, A. Role of the gastrointestinal microbiota in small animal health and disease. Vet. Record 2017, 181, 370. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Horvat, D.; Lalić, A.; Komlenić, D.K.; Abičić, I.; Zdunić, Z. Distribution of β-Glucan, Phenolic Acids, and Proteins as Functional Phytonutrients of Hull-Less Barley Grain. Foods 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Murphy, E.A.; Davis, J.M.; Carmichael, M.D. Immune modulating effects of betaglucan. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 656–661. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.A.F.; Vetvicka, V.; Zanuzzo, F.S. β-Glucan successfully stimulated the immune system in different jawed vertebrate species. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Vetvicka, V.; de Oliveira, C.A.F. β(1-3)(1-6)-D-glucans Modulate Immune Status and Blood Glucose Levels in Dogs. Br. J. Pharm. Med. Res. 2014, 4, 981–991. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Vendramini, T.H.A.; Amaral, A.R.; Rentas, M.F.; Ernandes, M.C.; da Silva, F.L.; Oba, P.M.; Filho, F.O.R.; Brunetto, M.A. Metabolic variables of obese dogs with insulin resistance supplemented with yeast beta-glucan. BMC Vet. Res. 2022, 18, 14. [Google Scholar] [CrossRef]
- Stuyven, E.; Verdonck, F.; Hoek, I.V.; Daminet, S.; Duchateau, L.; Remon, J.P.; Goddeeris, B.M.; Cox, E. Oral administration of beta-1,3/1,6-glucan to dogs temporally changes total and antigen-specific IgA and IgM. Clin. Vaccine Immunol. 2010, 17, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, A.; Nieradka, R.; Kander, M.; Nowicki, M.; Wdowiak, M.; Sawerska, A.K. The effectiveness of natural and synthetic immunomodulators in the treatment of inflammatory bowel disease in dogs. Acta Vet. Hung. 2013, 61, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D. Development and Validation of a Body Condition Score System for Dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; The European Pet Food Industry Federation: Bruxelas, Belgium, 2021. [Google Scholar]
- AAFCO. Association of American Feed Control Officials; Official Publication: Washington, DC, USA, 2019. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of the Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Vieira, P.F. Efeito do Formaldeído na Proteção de Proteínas e Lipídios em Rações para Ruminantes. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 1980. [Google Scholar]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Pryce, J.D. A modification of the Barker-Summerson method for the determination of latic acid. Analyst 1969, 94, 1121–1151. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Silva, L.P.; Perdomo, D.M.X. Biological response of rats to resistant starch. Rev. Inst. Adolfo Lutz 2005, 64, 252–257. [Google Scholar]
- Peters, I.R.; Calvert, E.L.; Hall, E.J.; Day, M.J. Measurement of immunoglobulin concentrations in the feces of healthy dogs. Clin. Diagn. Lab. Immunol. 2004, 11, 841–848. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dogs and Cats; National Research Council: Washington, DC, USA, 2006. [Google Scholar]
- Beynen, A.; Legerstee, E. Influence of Dietary Beta-1,3/1,6-Glucans on Clinical Signs of Canine Osteoarthritis in a Double-Blind, Placebo-Controlled Trial. Am. J. Anim. Vet. 2010, 5, 97–101. [Google Scholar] [CrossRef]
- Beynen, A.; Saris, D.H.J.; Paap, P.M.; Altena, F.; Visser, E.A.; Middelkoop, J.; Jong, L.; Staats, M. Dietary Beta-1,3/1,6-Glucans Reduce Clinical Signs of Canine Atopy. Am. J. Anim. Vet. 2011, 6, 146–152. [Google Scholar] [CrossRef]
- Theodoro, S.S.; Putarov, T.C.; Tiemi, C.; Volpe, L.M.; de Oliveira, C.A.F.; Glória, M.B.A.; Carciofi, A.C. Effects of the solubility of yeast cell wall preparations on their potential prebiotic properties in dogs. PLoS ONE 2019, 14, e0225659. [Google Scholar] [CrossRef]
- Rentas, M.F.; Pedreira, R.S.; Perini, M.P.; Risolia, L.W.; Zafalon, R.V.A.; Alvarenga, I.C.; Vendramini, T.H.A.; Balieiro, J.C.C.; Pontieri, C.F.F.; Brunetto, M.A. Galactoligosaccharide and a prebiotic blend improve colonic health and immunity of adult dogs. PLoS ONE 2020, 15, e0238006. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.M.; Risolia, L.W.; Rentas, M.F.; Amaral, A.R.; Rodrigues, R.B.A.; Urrego, M.I.G.; Vendramini, T.H.A.; Ventura, R.V.; Balieiro, J.C.C.; Massoco, C.O.; et al. Saccharomyces cerevisiae dehydrated culture modulates fecal microbiota and improves innate immunity of adult dogs. Fermentation 2022, 8, 2. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.D. Rarefaction, alpha diversity, and statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. The role of the phylogenetic diversity measure, PD, in bioinformatics: Getting the definition right. Evol. Bioinform. 2006, 2, 277–283. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8823. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Macedo, H.T.; Rentas, M.F.; Vendramini, T.H.A.; Macegoza, M.V.; Amaral, A.R.; Jeremias, J.T.; Balieiro, J.C.C.; Pfrimer, K.; Ferriolli, E.; Pontieri, C.F.F.; et al. Weight-loss in obese dogs promotes important shifts in fecal microbiota profile to the extent of resembling microbiota of lean dogs. Anim. Microbiome 2022, 4, 6. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 2011, 89, 1520–1530. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Seong, C.N.; Kang, J.W.; Lee, J.H.; Seo, S.Y.; Woo, J.J.; Park, C.; Bae, K.S.; Kim, M.S. Taxonomic hierarchy of the phylum Firmicutes and novel Firmicutes species originated from various environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kelder, T. Correlation network analysis reveals relationships between diet-induced changes in human gut microbiota and metabolic health. Nutr. Diabetes 2014, 4, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ 2017, 5, e3019. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef]
- Vazquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 2016, 1, 16177. [Google Scholar] [CrossRef]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Foster, M.L.; Sohail, M.U.; Leutenegger, C.; Queen, E.V.; Steiner, J.M.; Marks, S.L. The fecal microbiome in cats with diarrhea. PLoS ONE 2015, 10, e0127378. [Google Scholar] [CrossRef]
- Allen-Vercoe, E.; Strauss, J.; Chadee, K. Fusobacterium nucleatum: An emerging gut pathogen? Gut Microbes 2011, 2, 294–298. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Theissig, F.; Rückert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kühn, S.; et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 2011, 60, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Alshawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, 136. [Google Scholar] [CrossRef] [PubMed]
- Middelbos, I.S.; Boler, B.M.V.; Qu, A.; White, B.A.; Swanson, K.S.; Fahey, G.C., Jr. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS ONE 2010, 5, e9768. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Alexander, C.; Steelman, A.J.; Warzecha, C.M.; Godoy, M.R.C.; Swanson, K.S. Effects of a Saccharomyces cerevisiae fermentation product on fecal characteristics, nutrient digestibility, fecal fermentative end-products, fecal microbial populations, immune function, and diet palatability in adult dogs. J. Anim. Sci. 2019, 97, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Barcenas-Walls, J.R.; Suchodolski, J.S.; Steiner, J.M. Molecular assessment of the fecal microbiota in healthy cats and dogs before and during supplementation with fructo-oligosaccharides (FOS) and inulin using high-throughput 454-pyrosequencing. PeerJ 2017, 5, e3184. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Frantz, N.; Khoo, C.; Gibson, G.R.; Rastall, R.A.; McCartney, A.L. Investigation of the faecal microbiota associated with canine chronic diarrhoea. FEMS Microbiol. Ecol. 2010, 71, 304–312. [Google Scholar] [CrossRef]
- Bell, J.A.; Kopper, J.J.; Turnbull, J.Á.; Barbu, N.I.; Murphy, A.J.; Mansfield, L.S. Ecological Characterization of the Colonic Microbiota of Normal and Diarrheic Dogs. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 149694. [Google Scholar] [CrossRef]
- Chaban, B.; Links, M.G.; Hill, J.E. A Molecular Enrichment Strategy Based on cpn60 for Detection of Epsilon-Proteobacteria in the Dog Fecal Microbiome. Microb. Ecol. 2012, 63, 348–357. [Google Scholar] [CrossRef]
- Strickling, J.A.; Harmon, D.L.; Dawson, K.A.; Gross, K.L. Evaluation of oligosaccharide addition to dog diets: Influences on nutrient digestion and microbial populations. Anim. Feed Sci. Technol. 2000, 86, 205–219. [Google Scholar] [CrossRef]
- Middelbos, I.S.; Godoy, M.R.; Fastinger, N.D.; Fahey, G.C., Jr. A dose-response evaluation of spray-dried yeast cell wall supplementation of diets fed to adult dogs: Effects on nutrient digestibility, immune indices, and fecal microbial populations. J. Anim. Sci. 2007, 85, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- Zentek, J.; Marquat, B.; Pietrzak, T. Intestinal effects of mannanoligosaccharides, transgalactooligosaccharides, lactose and lactulose in dogs. J. Nutr. 2002, 132, 1682–1684. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.A.; Hopkins, A.C.; Middelbos, I.S.; Price, N.P.; Fahey, G.C., Jr. Galactoglucomannan oligosaccharide supplementation affects nutrient digestibility, fermentation end-product production, and large bowel microbiota of the dog. J. Anim. Sci. 2011, 89, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, E.A.; Wolf, B.W.; Garleb, K.A.; Chow, J.; Leyer, G.J.; Johns, P.W.; Fahey, G.C., Jr. Glucose-based oligosaccharides exhibit different in vitro fermentation patterns and affect in vivo apparent nutrient digestibility and microbial populations in dogs. J. Nutr. 2000, 130, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106–116. [Google Scholar] [CrossRef]
- Ephraim, E.; Cochrane, C.-Y.; Jewell, D.E. Varying Protein Levels Influence Metabolomics and the Gut Microbiome in Healthy Adult Dogs. Toxins 2020, 12, 517. [Google Scholar] [CrossRef]
- Jackson, A.A. Salvage of urea-nitrogen and protein requirements. Proc. Nutr. Soc. 1995, 54, 535–547. [Google Scholar] [CrossRef]
- Nogueira, J.P.S.; He, F.; Mangian, H.F.; Oba, P.M.; Godoy, M.R.C. Dietary supplementation of a fiber-prebiotic and saccharin-eugenol blend in extruded diets fed to dogs. J. Anim. Sci. 2019, 97, 4519–4531. [Google Scholar] [CrossRef]
- Nery, J.; Goudez, R.; Biourge, V.; Tournier, C.; Leray, V.; Martin, L.; Thorin, C.; Nguyen, P.; Dumon, H. Influence of dietary protein content and source on colonic fermentative activity in dogs differing in body size and digestive tolerance. J. Anim. Sci. 2012, 90, 2570–2580. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef]
- Thornton, J.R. High colonic pH promotes colorectal cancer. Lancet 1981, 1, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Healy, H.P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C., Jr. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 2002, 132, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, E.A.; Schreijen, E.M.W.C.; Patil, A.R.; Hussein, H.S.; Grieshop, C.M.; Merchen, N.R.; Fahey, G.C., Jr. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 2003, 81, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Moens, F.; Abbeele, P.V.; Basit, A.W.; Dodoo, C.; Chatterjee, R.; Smith, B.; Gaisford, S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int. J. Pharm. 2019, 555, 1–10. [Google Scholar] [CrossRef]
- Pawar, M.M.; Pattanaik, A.K.; Sinha, D.K.; Goswami, T.K.; Sharma, K. Effect of dietary mannanoligosaccharide supplementation on nutrient digestibility, hindgut fermentation, immune response and antioxidant indices in dogs. J. Anim. Sci. Technol. 2017, 59, 11. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Novak, M.; Vetvicka, V. Beta-glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. A new receptor for β-glucans. Nature 2001, 413, 36. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, J.T.; Kim, Y.; Han, S.B. Stimulatory Effect of β-glucans on Immune Cells. Immune Netw. 2011, 11, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. An evaluation of the immunological activities of commercially available β-1,3-glucans. J. Am. Nutr. Assoc. 2007, 10, 25–31. [Google Scholar]
- Odiere, M.R.; Scott, M.E.; Leroux, L.P.; Dzierszinski, F.S.; Koski, K.G. Maternal protein deficiency during a gastrointestinal nematode infection alters developmental profile of lymphocyte populations and selected cytokines in neonatal mice. J. Nutr. 2013, 143, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Saracino, A.; Monno, L.; Angarano, G. The Revival of an “Old” Marker: CD4/CD8 Ratio. AIDS Rev. 2017, 19, 81–88. [Google Scholar] [PubMed]
- Blount, D.G.; Pritchard, D.; Heaton, P.R. Age-related alterations to immune parameters in Labrador retriever dogs. Vet. Immunol. Immunopathol. 2005, 108, 399–407. [Google Scholar] [CrossRef]
- Singh, S.K.; Dimri, U.; Sharma, M.C.; Sharma, B.; Saxena, M. Determination of CD4+ and CD8+ T cells in the peripheral blood of dogs with demodicosis. Parasitology 2010, 137, 1921–1924. [Google Scholar] [CrossRef]
- Hellweg, P.; Krammer-Lukas, S.; Strasser, A.; Zentek, J. Effects of bovine lactoferrin on the immune system and the intestinal microflora of adult dogs. Arch. Anim. Nutr. 2008, 62, 152–161. [Google Scholar] [CrossRef]
- Day, M.J. Ageing, Immunosenescence and Inflammageing in the Dog and Cat. J. Comp. Pathol. 2010, 142, S60–S69. [Google Scholar] [CrossRef]







| Item | Diets | |||
|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | |
| Ingredients (%) | ||||
| Corn grain | 33.26 | 33.19 | 33.12 | 32.98 |
| Common viscera meal | 26.38 | 26.38 | 26.38 | 26.38 |
| Broken rice | 15.00 | 15.00 | 15.00 | 15.00 |
| Corn gluten | 7.99 | 7.99 | 7.99 | 7.99 |
| Beet pulp | 4.00 | 4.00 | 4.00 | 4.00 |
| Fish oil | 0.82 | 0.82 | 0.82 | 0.82 |
| Potassium chloride | 0.42 | 0.42 | 0.42 | 0.42 |
| Mineral and vitamin premix 1 | 0.50 | 0.50 | 0.50 | 0.50 |
| Common salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Choline | 0.17 | 0.17 | 0.17 | 0.17 |
| Whole egg powder | 0.15 | 0.15 | 0.15 | 0.15 |
| Antifungal | 0.10 | 0.10 | 0.10 | 0.10 |
| Antioxidant | 0.07 | 0.07 | 0.07 | 0.07 |
| Methionine | 0.03 | 0.03 | 0.03 | 0.03 |
| Poultry viscera fat | 6.81 | 6.81 | 6.81 | 6.81 |
| Swine fat | 4.00 | 4.00 | 4.00 | 4.00 |
| Purified beta-1,3/1,6-glucan 2 | 0.00 | 0.07 | 0.14 | 0.28 |
| Total | 100 | 100 | 100 | 100 |
| Item | Diets | |||
|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | |
| Dry matter (%) | 93.11 | 94.31 | 94.00 | 93.13 |
| Chemical composition in DM (%) | ||||
| Organic matter | 92.13 | 92.07 | 92.04 | 91.93 |
| Crude protein | 25.25 | 25.07 | 27.81 | 28.24 |
| Fat | 17.69 | 17.82 | 17.71 | 17.42 |
| Ash | 7.87 | 7.93 | 7.96 | 8.07 |
| Crude fiber | 10.17 | 9.92 | 10.15 | 8.28 |
| Nitrogen-free extract 1 | 39.02 | 39.26 | 36.37 | 37.99 |
| Calcium | 2.09 | 2.07 | 2.13 | 2.06 |
| Phosphorus | 1.19 | 1.17 | 1.18 | 1.17 |
| Metabolizable energy (kcal/g) 2 | 4.10 | 4.15 | 4.14 | 4.09 |
| Phyla (%) | Diets | p | |||
|---|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | ||
| Actinobacteria | 0.72 ± 0.49 a | 0.51 ± 0.35 b | 0.90 ± 0.61 a | 0.15 ± 0.10 c | <0.0001 |
| Bacteroidetes | 26.38 ± 6.99 a | 19.75 ± 5.70 c | 21.30 ± 6.03 b | 21.73 ± 6.12 b | <0.0001 |
| Firmicutes | 34.18 ± 9.16 c | 46.59 ± 10.13 b | 58.54 ± 9.88 a | 45.54 ± 10.10 b | <0.0001 |
| Fusobacteria | 32.32 ± 5.76 a | 23.33 ± 4.72 b | 14.09 ± 3.19 c | 23.92 ± 4.80 b | <0.0001 |
| Proteobacteria | 2.21 ± 1.08 a | 1.72 ± 0.84 b | 1.42 ± 0.69 c | 1.67 ± 0.82 b | <0.0001 |
| Families (%) | Diets | p | |||
|---|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | ||
| Acidaminococcaceae | 0.46 ± 0.09 a | 0.42 ± 0.09 a | 0.38 ± 0.08 a | 0.16 ± 0.04 b | <0.0001 |
| Bacteroidaceae | 16.17 ± 3.53 a | 13.77 ± 3.09 b | 10.99 ± 2.55 c | 11.76 ± 2.70 c | <0.0001 |
| Clostridiaceae | 0.94 ± 0.54 ab | 0.74 ± 0.43 b | 1.14 ± 0.65 a | 1.02 ± 0.59 ab | 0.0011 |
| Coriobacteriaceae | 0.20 ± 0.10 b | 0.35 ± 0.17 a | 0.37 ± 0.18 a | 0.22 ± 0.11 b | 0.0111 |
| Erysipelatoclostridiaceae | 0.17 ± 0.07 b | 0.10 ± 0.04 b | 0.37 ± 0.16 a | 0.54 ± 0.23 a | <0.0001 |
| Erysipelotrichaceae | 2.99 ± 1.72 c | 4.56 ± 2.58 b | 9.94 ± 5.29 a | 2.02 ± 1.17 d | <0.0001 |
| Fusobacteriaceae | 32.32 ± 5.49 a | 23.33 ± 4.49 b | 14.09 ± 3.04 c | 23.92 ± 4.57 b | <0.0001 |
| Lachnospiraceae | 15.18 ± 4.62 d | 19.77 ± 5.69 b | 16.91 ± 5.04 c | 22.89 ± 6.33 a | <0.0001 |
| Peptococcaceae | 0.31 ± 0.09 | 0.30 ± 0.09 | 0.37 ± 0.11 | 0.32 ± 0.09 | 0.3061 |
| Peptostreptococcaceae | 1.57 ± 0.35 c | 1.95 ± 0.43 a | 1.89 ± 0.42 ab | 1.58 ± 0.35 bc | 0.0003 |
| Prevotellaceae | 7.74 ± 3.04 a | 4.37 ± 1.78 b | 7.85 ± 3.08 a | 7.99 ± 3.13 a | <0.0001 |
| Ruminococcaceae | 5.20 ± 1.11 c | 5.98 ± 1.27 b | 9.66 ± 1.97 a | 6.44 ± 1.36 b | <0.0001 |
| Selenomonadaceae | 0.41 ± 0.20 c | 0.64 ± 0.32 b | 1.49 ± 0.73 a | 0.60 ± 0.30 b | <0.0001 |
| Succinivibrionaceae | 0.45 ± 0.14 b | 0.86 ± 0.26 a | 0.39 ± 0.12 b | 0.59 ± 0.18 ab | <0.0001 |
| Sutterellaceae | 1.47 ± 0.96 a | 0.86 ± 0.57 b | 0.82 ± 0.53 b | 0.86 ± 0.57 b | <0.0001 |
| Tannerellaceae | 0.16 ± 0.03 a | 0.07 ± 0.00 b | 0.06 ± 0.00 c | 0.09 ± 0.00 a | 0.0438 |
| Genera (%) | Diets | p | |||
|---|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | ||
| Allobaculum | 0.32 ± 1.76 c | 0.73 ± 4.03 b | 1.65 ± 9.03 a | 0.01 ± 0.03 d | <0.0001 |
| Alloprevotella | 1.78 ± 0.48 a | 0.84 ± 0.23 c | 1.09 ± 0.30 b | 0.69 ± 0.19 c | <0.0001 |
| Anaerobiospirillum | 0.44 ± 0.14 b | 0.83 ± 0.26 a | 0.39 ± 0.12 b | 0.58 ± 0.18 ab | 0.0002 |
| Bacteroides | 16.17 ± 3.53 a | 13.77 ± 3.09 b | 10.99 ± 2.55 c | 11.76 ± 2.70 c | <0.0001 |
| Blautia | 8.91 ± 2.49 b | 10.78 ± 2.96 a | 8.71 ± 2.44 b | 11.03 ± 3.02 a | <0.0001 |
| Clostridium sensu stricto 1 | 0.88 ± 0.50 ab | 0.71 ± 0.40 b | 1.09 ± 0.61 a | 0.96 ± 0.54 ab | 0.0012 |
| Collinsella | 0.20 ± 0.10 b | 0.35 ± 0.17 a | 0.37 ± 0.18 a | 0.22 ± 0.11 b | 0.0111 |
| Erysipelatoclostridium | 0.06 ± 0.02 b | 0.06 ± 0.03 b | 0.15 ± 0.06 ab | 0.36 ± 0.11 a | 0.0008 |
| Faecalibacterium | 4.76 ± 1.06 c | 5.48 ± 1.21 b | 9.01 ± 1.91 a | 6.03 ± 1.32 b | <0.0001 |
| Fournierella | 0.26 ± 0.06 ab | 0.24 ± 0.06 b | 0.33 ± 0.08 ab | 0.55 ± 0.14 a | 0.0075 |
| Fusobacterium | 32.33 ± 5.46 a | 23.18 ± 4.45 b | 14.09 ± 3.03 c | 23.94 ± 4.55 b | <0.0001 |
| Holdemanella | 0.08 ± 0.03 | 0.17 ± 0.06 | 0.14 ± 0.04 | 0.17 ± 0.06 | 0.2969 |
| Lachnospira | 1.30 ± 0.56 a | 0.41 ± 0.18 b | 0.42 ± 0.18 b | 0.44 ± 0.19 b | 0.0002 |
| Lachnospiraceae NC2004 | 0.32 ± 0.11 b | 0.52 ± 0.19 a | 0.51 ± 0.18 a | 0.22 ± 0.08 b | <0.0001 |
| Lachnospiraceae UCG-004 | 0.44 ± 0.09 a | 0.55 ± 0.12 a | 0.16 ± 0.04 b | 0.43 ± 0.09 a | <0.0001 |
| Megamonas | 0.41 ± 0.20 c | 0.64 ± 0.32 b | 1.48 ± 0.72 a | 0.60 ± 0.30 b | <0.0001 |
| Parabacteroides | 0.16 ± 0.00 a | 0.07 ± 0.00 bc | 0.07 ± 0.00 c | 0.09 ± 0.00 b | 0.0191 |
| Parasuterella | 0.49 ± 0.14 a | 0.12 ± 0.04 b | 0.15 ± 0.05 b | 0.12 ± 0.04 b | 0.0002 |
| Peptoclostridium | 1.15 ± 0.30 ab | 1.41 ± 0.37 a | 1.13 ± 0.30 b | 0.98 ± 0.26 b | <0.0001 |
| Peptococcus | 0.31 ± 0.09 | 0.30 ± 0.09 | 0.37 ± 0.11 | 0.32 ± 0.09 | 0.3061 |
| Phascolarctobacterium | 0.46 ± 0.09 a | 0.42 ± 0.09 a | 0.38 ± 0.08 a | 0.16 ± 0.04 b | <0.0001 |
| Prevotella | 4.94 ± 2.12 b | 3.34 ± 1.46 c | 7.32 ± 3.06 a | 7.81 ± 3.25 a | <0.0001 |
| Prevotellaceae Ga6A1 | 1.11 ± 0.37 a | 0.52 ± 0.18 b | 0.47 ± 0.16 b | 0.58 ± 0.20 b | <0.0001 |
| Romboutsia | 0.06 ± 0.03 | 0.18 ± 0.10 | 0.12 ± 0.05 | 0.10 ± 0.04 | 0.2994 |
| Sutterella | 1.04 ± 0.70 a | 0.75 ± 0.51 b | 0.73 ± 0.50 b | 0.77 ± 0.52 b | 0.0002 |
| Turicibacter | 2.56 ± 0.83 b | 0.83 ± 0.28 c | 2.82 ± 0.91 b | 3.74 ± 1.20 a | <0.0001 |
| Ruminococcus | 1.58 ± 0.46 b | 2.00 ± 0.58 a | 1.67 ± 0.49 ab | 1.10 ± 0.32 c | <0.0001 |
| Uncultured | 1.25 ± 0.46 c | 1.45 ± 0.53 c | 1.97 ± 0.71 b | 2.44 ± 0.87 a | <0.0001 |
| Item (%) | Diets | SEM | p | |||
|---|---|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | |||
| Dry matter | 82.25 | 82.75 | 82.72 | 83.29 | 0.44 | 0.3162 |
| Organic matter | 87.83 | 88.32 | 88.34 | 88.71 | 0.31 | 0.1546 |
| Crude protein | 85.85 b | 85.99 b | 87.50 a | 88.19 a | 0.33 | <0.0001 |
| Ethereal extract | 96.58 | 96.20 | 96.26 | 96.52 | 0.26 | 0.3703 |
| Ash | 69.47 | 81.55 | 77.89 | 79.09 | 5.41 | 0.4065 |
| Crude fiber | 17.01 | 18.11 | 17.75 | 21.52 | 2.15 | 0.3613 |
| Nitrogen-free extract | 87.17 | 87.94 | 88.04 | 87.61 | 0.53 | 0.5585 |
| Item | Diets | SEM | p | |||
|---|---|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | |||
| Fermentative products | ||||||
| Ammoniacal nitrogen (mmol/kg of DM) | 6.97 | 6.92 | 7.28 | 7.13 | 0.62 | 0.9576 |
| Lactic acid (mmol/kg of DM) | 4.80 | 5.41 | 5.65 | 5.21 | 0.72 | 0.5905 |
| Total fatty acids (mmol/kg of DM) | 54.66 | 54.18 | 58.26 | 56.15 | 2.40 | 0.3792 |
| Fecal pH | 6.68 | 6.66 | 6.53 | 6.59 | 0.12 | 0.7406 |
| Short-chain fatty acids (mmol/kg of DM) | ||||||
| Acetic acid | 33.13 | 31.75 | 32.98 | 32.91 | 1.77 | 0.8785 |
| Propionic acid | 13.77 | 14.34 | 15.64 | 15.13 | 1.00 | 0.1219 |
| Butyric acid | 6.26 | 6.58 | 7.72 | 6.57 | 1.00 | 0.6592 |
| Branched-chain fatty acids (mmol/kg of DM) | ||||||
| Valeric acid | 0.10 | 0.29 | 0.18 | 0.11 | 0.09 | 0.4069 |
| Isovaleric acid | 0.76 | 0.69 | 1.00 | 0.81 | 0.10 | 0.0664 |
| Isobutyric acid | 0.66 | 0.61 | 0.76 | 0.65 | 0.06 | 0.1466 |
| Total BCFA | 1.49 | 1.52 | 1.92 | 1.54 | 0.18 | 0.1534 |
| Item | Diets | SEM | p | |||
|---|---|---|---|---|---|---|
| 0.0% | 0.07% | 0.14% | 0.28% | |||
| Phagocytosis (%) | 68.63 | 75.25 | 76.79 | 78.04 | 4.15 | 0.0692 |
| Phagocytosis intensity | 32.85 | 36.56 | 30.33 | 37.80 | 7.47 | 0.2546 |
| Oxidative burst PMA | 894.00 | 809.37 | 785.62 | 1029.12 | 160.39 | 0.4423 |
| T CD4+ lymphocytes (%) | 37.9 | 42.49 | 44.53 | 42.60 | 2.45 | 0.2210 |
| T CD8+ lymphocytes (%) | 29.81 | 27.33 | 23.35 | 26.23 | 2.48 | 0.1237 |
| T CD4+:CD8+ ratio | 1.33 b | 1.71 ab | 1.99 a | 1.74 ab | 0.19 | 0.0368 |
| Fecal IgA fecal (pg/mL) | 225.091 | 271.41 | 242.35 | 229.73 | 53.81 | 0.9254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, P.H.; Vendramini, T.H.A.; Zafalon, R.V.A.; Príncipe, L.d.A.; Cesar, C.G.L.; Perini, M.P.; Putarov, T.C.; Gomes, C.O.M.S.; Balieiro, J.C.d.C.; Brunetto, M.A. Effects of Increasing Levels of Purified Beta-1,3/1,6-Glucans on the Fecal Microbiome, Digestibility, and Immunity Variables of Healthy Adult Dogs. Microorganisms 2024, 12, 113. https://doi.org/10.3390/microorganisms12010113
Marchi PH, Vendramini THA, Zafalon RVA, Príncipe LdA, Cesar CGL, Perini MP, Putarov TC, Gomes COMS, Balieiro JCdC, Brunetto MA. Effects of Increasing Levels of Purified Beta-1,3/1,6-Glucans on the Fecal Microbiome, Digestibility, and Immunity Variables of Healthy Adult Dogs. Microorganisms. 2024; 12(1):113. https://doi.org/10.3390/microorganisms12010113
Chicago/Turabian StyleMarchi, Pedro Henrique, Thiago Henrique Annibale Vendramini, Rafael Vessecchi Amorim Zafalon, Leonardo de Andrade Príncipe, Cinthia Gonçalves Lenz Cesar, Mariana Pamplona Perini, Thaila Cristina Putarov, Cristina Oliveira Massoco Salles Gomes, Júlio Cesar de Carvalho Balieiro, and Marcio Antonio Brunetto. 2024. "Effects of Increasing Levels of Purified Beta-1,3/1,6-Glucans on the Fecal Microbiome, Digestibility, and Immunity Variables of Healthy Adult Dogs" Microorganisms 12, no. 1: 113. https://doi.org/10.3390/microorganisms12010113
APA StyleMarchi, P. H., Vendramini, T. H. A., Zafalon, R. V. A., Príncipe, L. d. A., Cesar, C. G. L., Perini, M. P., Putarov, T. C., Gomes, C. O. M. S., Balieiro, J. C. d. C., & Brunetto, M. A. (2024). Effects of Increasing Levels of Purified Beta-1,3/1,6-Glucans on the Fecal Microbiome, Digestibility, and Immunity Variables of Healthy Adult Dogs. Microorganisms, 12(1), 113. https://doi.org/10.3390/microorganisms12010113





