Abstract
The microorganisms that inhabit the gastrointestinal tract are responsible for multiple chains of reactions that affect their environment and modify the internal metabolism, their study receives the name of microbiome, which has become more relevant in recent years. In the near future, the challenges related to feeding are anticipated to escalate, encompassing the nutritional needs to sustain an overpopulated world. Therefore, it is expected that a better understanding of the interactions between microorganisms within the digestive tract will allow their modulation in order to provide an improvement in the immune system, feed efficiency or the promotion of nutritional characteristics in production animals, among others. In the present study, the main effects of experimental diets in production animals were described, emphasizing the diversity of the bacterial populations found in response to the diets, ordering them between polygastric and monogastric animals, and then describing the experimental diets used and their effect on the microorganisms. It is hoped that this study will help as a first general approach to the study of the role of the microbiome in production animals under different diets.
Keywords:
animal production; bacteria community; gut; monogastric; new feedstuff; livestock; ruminant 1. Introduction
In 2050, the population will exceed 9.7 billion inhabitants and food shortage problems will reach a critical state, aggravated by environmental problems and nutrient shortages on earth [1]. Under this context, the number of research projects related to solving food problems has increased, studies based on the microbiome being a focus of great interest, especially those related to diets in animals. To begin with, it is necessary to differentiate between the terms microbiota and microbiome, the former being defined as the “set of microorganisms present in a defined environment” [2], while microbiome can refer to the microorganisms present in a given habitat that have different physicochemical properties, interact with their environment and can confer microbiological resistance to their host [3]. Furthermore, the microbiome involves and considers interactions between bacteria, archaea, viruses, and fungi, as well as between biotic and abiotic factors involved [4,5]. The alteration of the microorganisms involved in the microbiome can cause dysbiosis, which consists of the imbalance of a healthy microbiome, where the symbiotic relationships of the microbiota are diminished, altering productivity, and causing health problems [6]. Although, studies on the microbiome have increased during the last years, they are mostly based on humans, existing a smaller number related to animals destined to food production [7].
Feed is a key element in the microbiota of animals, since it has a direct effect on microbial populations. For example, in ruminants it is proposed that polyphenols affect carbohydrate fermentation in the rumen, in addition to affecting cellular molecules such as proteins, lipids and nucleic acids [8]. At the same time, studies highlight the action of tannins to reduce fiber degradation, as well as polyphenols to help reduce methane gas production [9]. Other studies have proposed that the concentration of polyphenols affects the intestinal epithelial barrier of pigs in vitro using chestnut extracts, an important source of polyphenols, which at low concentrations can have a protective effect on intestinal epithelial cells [10]. In monogastric animals such as chickens and pigs the consumption of dietary fiber exerts significant effects on the integrity of the intestinal mucosa. Therefore, a dietary fiber deficiency leads to loss of functions of the intestinal mucosa due to the degradation of polysaccharides by bacteria [11]. In addition, the microbiota has been implicated in the immune response as demonstrated in mouse experiments [12].
The gut microbiome possesses effects on microbiological resistance, for example in regulating the physiology of metazoans, multicellular eukaryotic species considered within the group of microorganisms [13]. This implies that a better understanding of the microbiota could avoid the use of synthetic agents such as antibiotics in the fight against pathogens. This coincides with the vision of consumers to produce healthier animals and decrease the promotion of resistance genes [14]. On the other hand, the interactions between dominant microorganisms and variations within the microbiota have been scarcely described in animals, where the multiple variants, including genetic and functional, and diverse life spans between individuals make it even more difficult to assess. Therefore, more studies are required to compile and structure the information generated and thus help to improve animal production and its derivatives in an economic and healthy environment that ensures a benefit to the animal [15]. The present review aims to describe the main effects of experimental diets in production animals, according to changes in the composition of the basal diet and inclusion of feed additives, and their relationship with the proliferation or inhibition of different microorganisms present in the intestinal microbiome. A summary of the diets can be found in the supplementary material (Table S1).
2. Microbiome
The microbiome is the interaction between the different microbial groups and the surrounding internal environment. It is made up of the microbiota, which includes microorganisms such as bacteria, archaea, protozoa, fungi, cyanobacteria, and algae [16]. These interact in a homeostasis within the organism in a complex system where, by any alteration, microbial populations can trigger chains of metabolic reactions that influence at a structural level and affect in a physiological way the development and growth of intestinal mucosa [17]. These components interact within the organism in a multi-complex system, maintaining a state of homeostasis. However, by any alteration, microbial populations can trigger cascades of metabolic reactions that profoundly influence the structural and physiological aspects, ultimately affecting the development and growth of the intestinal mucosa [18]. The composition of microbial populations is diverse; for example, it is estimated that just within the human gastrointestinal tract live about 100 trillion microorganisms, which are taxonomically classified by genus, family, order, and phylum [19]. The onset of microbiome formation is not entirely clear, theorizing that newborn mammals have a sterile microbiome and that it is created at the first interaction outside the womb [20]. It has also been indicated that in mammals colonization begins at birth and starts with the colonization of aerobic and facultative anaerobic bacteria in the first instance through the passage of the vaginal canal, which can vary between species and leaves doubts in cases where cesarean sections are performed [21]. In turn, it is established that initial colonization is fundamental for the development of the neonate; for example, in ruminants the period from birth to weaning is of great importance for the colonization of the microbiome in the rumen and is linked to the concept of coevolution between the microorganisms and the individual [22]. It has also been pointed out in other studies, based on the immune metabolism and microorganisms, that stress caused at weaning can influence immune development and cause a dysbiosis in the microbiota that alters the homeostasis between microorganisms with the promotion of pathogenic colonies [23]. Other studies add concepts such as the “social microbiome”, highlighting that individuals of the same group have a greater similarity between their microbial populations compared to those that do not share the same environment and, therefore, lacking interactions. However, these studies also indicate that diets, genetics, and geographical locations contribute to similarities in microbial composition between species, suggesting that it is not necessarily related to the social transmission of microorganisms between individuals [24]. Additionally, it has been established that factors associated with captivity, such as zoos, farms, and laboratories, can trigger metabolic problems, and modify digestion and reproduction by increasing stress [25]. So, comparing the same species that have been placed in captivity or in the wild, differences in the relative abundance of bacterial taxa can be found [26]. However, it has been determined that the main influencing factor on the microbiota is the diet used in animals, whether in captivity or in a controlled environment [25]. And to a lesser degree it has been reported that contact with soil, water or other exogenous elements contribute to the conformation of the microbial community; for example, in ruminants it has been described that up to 3% of the microbial community is composed of these exogenous microorganisms [27].
Studies that have focused on feed diets that influence the microbiome of production animals are diverse, encompassing those that use modifications of the standard diet. For instance, in the case of ruminants, different proportions of forage and concentrate, as well as the substitution of certain typical constituents, exemplify the diverse dietary adjustment made [28]. On the other hand, there is research that analyzes the change of the microbiome using specific supplements in diets, such as additives, hormones, amino acids, probiotics and vitamins. To help exemplify productive animals, the following is a review of experimental diets that influenced the microbiome ordered according to type and species. The studies are categorized into two groups: firstly, those that made modifications to the basal diet, involving alterations in composition ratios, the addition of foods with distinct qualities, or replacement of specific components. Secondly, we explore those investigations that added supplements, such as probiotics, hormones, amino acids or vitamins, among others relevant factors.
3. Ruminant Animals
Ruminants possess three pre-stomachs: reticulum, rumen, and omasum, and a true stomach called the abomasum [29]. The function of the different compartments is diverse and highly specialized. The feed once ingested by the ruminant enters the esophagus and then the pre-stomach (rumen, reticulum, and omasum) where a process of fermentation and decomposition occurs, and then enters the abomasum where chemical and enzymatic digestion occurs [30]. The rumen is constituted by different microorganisms and is where volatile fatty acids are generated in addition to other proteins for the ruminant in the fermentation process carried out especially by archaea. On the other hand, it is important to highlight that the whole process involves the use and expulsion of nitrogen into the environment in the form of methane gas (CH4), which has no nutritional value for the animal and so it is eliminated as a by-product of rumen fermentation, a product of the archaea using carbon dioxide (CO2) and hydrogen (H2) present in the rumen after fiber degradation [31]. At least 65% of emissions of pollutant gases such as methane gas that contaminate the terrestrial atmosphere are produced by the livestock sector, most of them produced by ruminants, and cattle destined for meat or milk production being mainly responsible as generating most of the CH4 emissions [32]. Studies have highlighted that there are communities of microorganisms responsible for lower methane gas elimination in ruminants; specifically in studies with lambs it was described that Fibrobacter spp., Kandleria vitulina, Olsenella spp., Prevotella bryantii, succinate,and Sharpea azabuensis are associated with lower H2 production [33]. In turn, studies in dairy cows show that CH4 emission is mainly linked to H2-producing bacteria such as Ruminococcaceae, Christensenellaceae, and Lachnospiraceae, and that in the high CH4 emission group, Succinivibrionaceae and Methanosphaera spp. communities are found in lower abundance [34]. Thus, research to reduce CH4 emissions is a latent problem, for which different alternatives have been evaluated [35].
In comparison with monogastric and polygastric animals, ruminants have a great advantage; they are efficient in converting cellulosic matter into energy, as they have a more specialized fermentative digestion. As mentioned above, digestion is developed in regions lacking oxygen, providing an anaerobic environment necessary for the proliferation of microorganisms. In addition, facultative aerobic microorganisms interact to help reduce the oxygen coming from the outside [36]. The temperature inside the rumen ranges between 38 to 42 °C and the pH is normally between 5.5 to 7.0, depending on the diet. The microorganisms present are composed of bacteria, archaea, protozoa and fungi, of which bacteria have a concentration between 1010 to 1011 bacteria per milliliter in the rumen [37].
Archaea are a group of microorganisms similar to bacteria discovered in 1977 by Carl Woese; since then, more varieties have been discovered and have been grouped to date into 20 phyla in total [38]. Among those classified as methanogens, that is, CH4 producers, the genera Methanobrevibacter gottschalkii and Methanobrevibacter ruminantium can be highlighted, which comprise 74% of rumen archaea [39]. The rumen archaea generally use H2 for the production of CH4; it is estimated that a reduction or inhibition of the archaea in the rumen would produce an increase in H2 that would affect the functions of the enzymes, reducing the efficiency in the conversion of food into nutrients [40]. However, it is known that there are varieties that can use formate, acetate, methyl compounds, and ethanol as an alternative to H2 [39].
The protozoa present in the rumen are in a concentration between 104 and 106 per milliliter, reaching half of the microbial mass, and are responsible for 30 to 40% of the total fiber digestion. They are classified in two groups: entodinomorphic and holotrophic, fulfilling mainly a fibrolytic role. Recent research has described that they can produce pectin esterase, cathepsin and glycosyl hydrolase enzymes, also playing a predatory role for bacteria and fungi [41]. The concentration of fungi in the rumen is 103 and 106 zoospores per milliliter, belonging to the class Neocallimastigomycetes, being anaerobic fungi found in the digestive tract composed of six genera: Anaeromyces, Caecomyces, Cyllamyces, Neocallimastix, Orpinomyces, and Piromyces. They possess amylolytic and proteolytic activity, their main function being fiber digestion by degrading structural polymers of plant origin [37,42].
3.1. Cattle
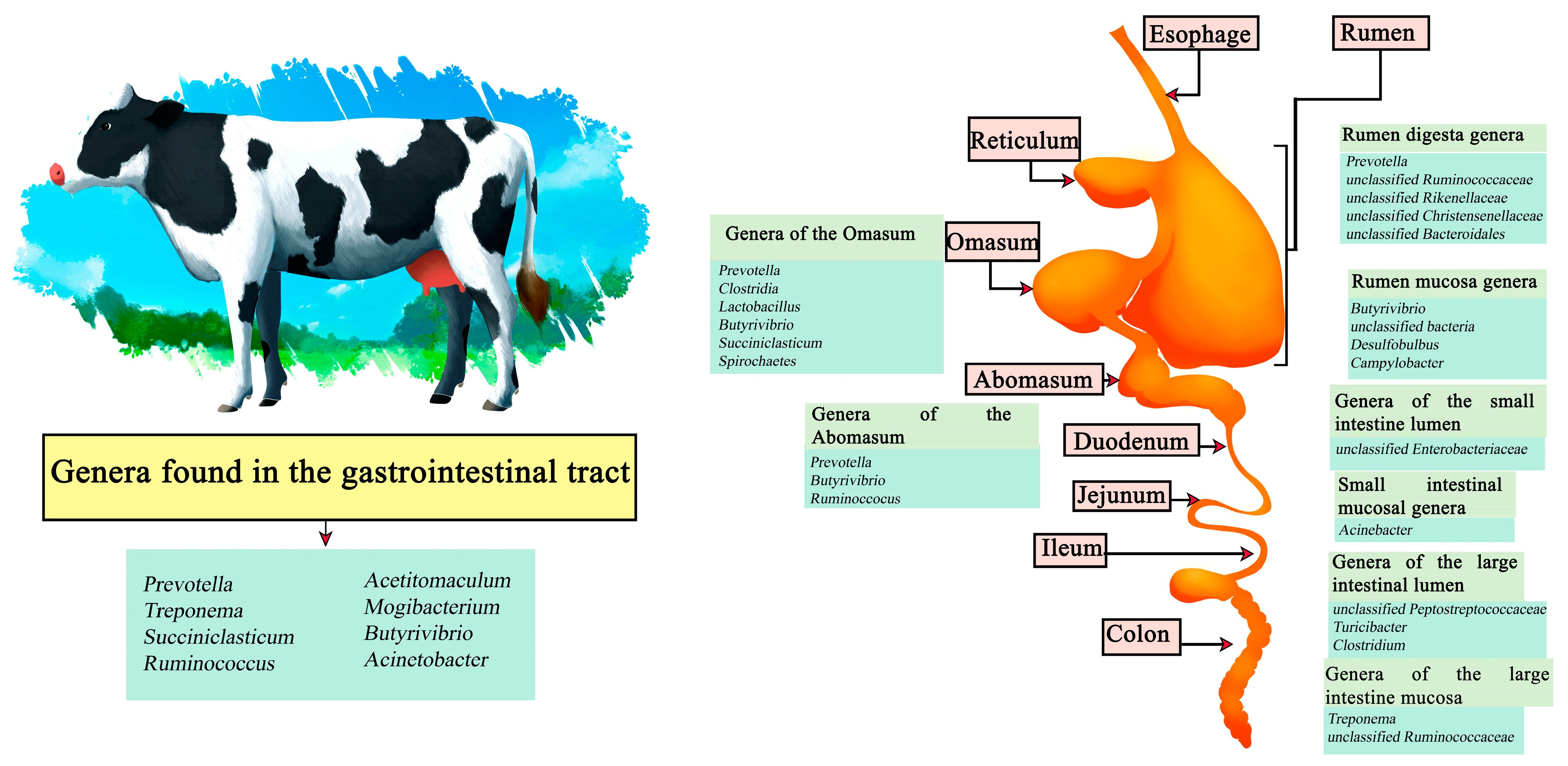
Cattle are one of the main sources of food worldwide, both in the meat and dairy businesses, with meat being a source of protein whose demand is increasing [43]. By 2021, beef production reached a total of 58.152 million tons, where the United States is the largest beef producer reaching 12.730 million tons followed by Brazil and China [44]. By 2030, beef production is expected to reach 44 million tons worldwide [45]. On the other hand, in 2021, dairy production reached a production of 544.072 million tons of cow’s milk, with the European Union being the main producer with 145.700 million tons, followed by the United States and India [46]. Demand for dairy products is estimated to increase over the next 50 years [47]. In cattle it has been described that Prevotella, Treponema, Succiniclasticum, Ruminococcus, Acetitomaculum, Mogibacterium, Butyrivibrio, and Acinetobacter are common genera that can be found throughout the gastrointestinal tract. In turn, genera such as Prevotella, unclassified Ruminococcaceae, unclassified Rikenellaceae, unclassified Christensenellaceae, and unclassified Bacteroidales have been reported in the rumen digesta, and Butyrivibrio, unclassified bacteria, Desulfobulbus, and Campylobacter in the rumen mucosa. In the Omasum the genera Prevotella, Clostridia, Lactobacillus, Butyrivibrio, Succiniclasticum, and Spirochaetes have been found, and in the abomasum, Prevotella, Butyrivibrio, and Ruminoccocus have been described. Other genera such as unclassified Enterobacteriaceae have been described in the small intestine lumen and Acinebacter in samples of the small intestine mucosa. Finally, in the large intestine section, unclassified Peptostreptococcaceae, Turicibacter, and Clostridium genera have been seen in lumen samples and Treponema and unclassified Ruminococcaceae in the mucosa (Figure 1) [48,49].
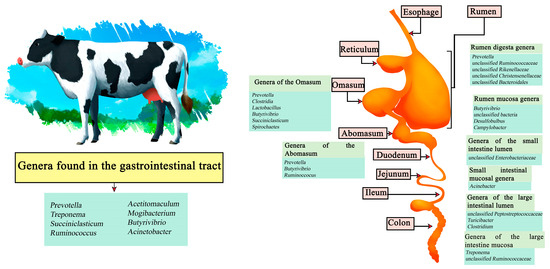
Figure 1.
Digestive system of cows. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
Concentrations of forage and concentrate can alter the microbiota of cattle. An investigation developed by Wang et al. (2020) in Holstein cows used two diets, one containing a high proportion of forage (70%, high forage, HF) and another with 30% concentrate (high concentrate, HC), on a dry matter basis. There it was shown that a diet rich in forage (HF) has an effect on the diversity of microorganisms; these changes were due to the low pH present at the rumen level, which affects some bacteria. The results for both treatments indicated that the phylum with the highest relative content was Bacteroidetes, followed by Firmicutes and Proteobacteria, with no changes between treatments. On the other hand, the Prevotella genus was more abundant in diets rich in fiber (HF), which have the capacity to degrade hemicelluloses, starch, and proteins. Furthermore, in HF diets, the family Ruminococcoccaceae, Veillonellaceae, and the genus Selenomonas were found to be more abundant, suggesting that their abundance is due to a highly fermentable diet [50].
Regarding the use of seaweeds in experimental diets of dairy cattle, Ascophyllum nodosum was incorporated as a dietary iodine fortifier in the diet of Holstein Friesian cows. Iodine is a trace element constituent of triiodothyronine and thyroxine hormones, and its shortage can lead to health problems in populations. The implementation of the diet was carried out in cows using a total mixed concentration (TMR) based on: corn silage (58%), sainfoin hay (13%), second-cut alfalfa hay (6%), and concentrate (23%). One treatment considered this concentrate as a control (CC) and another experimental treatment included Ascophyllum nodosum concentrate (EC). The results showed that the inclusion of Ascophyllum nodosum presented the microbial populations at phylum level in the following proportions: Firmicutes (57.14%), and Proteobacteria, Bacteroidetes and Actinobacteria at 14.28% each. Seven Operational Taxonomic Units (OTU) were identified in the milk. At the genus level, Lactococcus, Pseudomonas, and in smaller quantities Staphylococcus, Enterococcus, Clostridium, Bacteroides, Actinobacteria, and Microbacterium were found, with a smaller quantity of Pseudomonas, Lactococcus raffinolactis, Staphylococcus spp. and Pseudomonas jessenii. However, a higher population of Lactococcus lactis and Lactococcus garvieae was found [51].
Several studies have been conducted with experimental diets in Holstein dairy cows. One of them used the yeast Saccharomyces cerevisiae, a type of fungus commonly used in industrial processes and that provides health advantages in adequate doses [52]. This yeast was used to modify ruminal fermentation and maximize forage yield. The observed results showed an increase in populations of cellulolytic bacteria highlighting Ruminococcus flavefaciens and Fibrobacter succinogenes that possess specific mechanisms for cellulose adhesion and breakdown [53], accompanied by a reduction in lactate-producing bacteria such as Streptococcus bovis. This shift among populations led to an increase in volatile fatty acids (VFA) and an increase in the amount of protein due to a decrease in the protozoan Entodinium, which is responsible for ingesting bacteria and reducing the microbial protein supply to the small intestine. Therefore, the use of Saccharomyces cerevisiae provided higher energy efficiency and better nitrogen utilization [54].
A study by Kasparovska et al. (2016) proposed using isoflavone extract (12.5 g) as a dietary supplement in lactating cows of the Fleckvieh x Holstein cross, a type of phytoestrogen found in legumes and reported to support milk production in ruminants [55]. The results of this experiment indicated that, in the supplemented experimental group, the proportion of Bacteroidetes was higher than that of Firmicutes. At the family level the populations found in the experimental groups were Prevotella, Fibrobacteraceae and Burkholderiales. However, in control animals it was found that in populations of the phylum Firmicutes, the orders Clostridiales, Erysipelotrichales and Lactobacillales were more abundant than in experimental cows [56].
Yoshimura et al. (2018) posited that the use of three components in an experimental diet: flaxseed oil, propolis, and vitamin E, can have a synergistic effect in Holstein dairy cows. Flaxseed oil is rich in α-linolenic acid (18:3), a fatty acid known for its beneficial effects [57]. The experiment was conducted on three groups of supplemented cows; a control diet (25 g flaxseed oil/kg DM (FO); a diet with 25 g flaxseed oil/kg DM and 1.2 g propolis-based product/kg DM (PBP); and a diet with flaxseed oil, PBP and 375 IU vitamin E/kg DM (PBP-E). The results indicated that the diet supplied with flaxseed oil (FO) produced a decrease in protozoa, with a greater number of Entodinium and a lower number of Isotricha, an increase in bacterial species Butyrivibrio fibrisolvens, and a reduction in the populations of Anaerovibrio lipolytica and Methanobrevibacter ruminantium. Therefore, PBP and PBP-E treatments did not have a significant impact on microbial populations. However, they possess positive effects on milk quality by antioxidant activity and do not affect digestive parameters [58].
3.2. Sheep
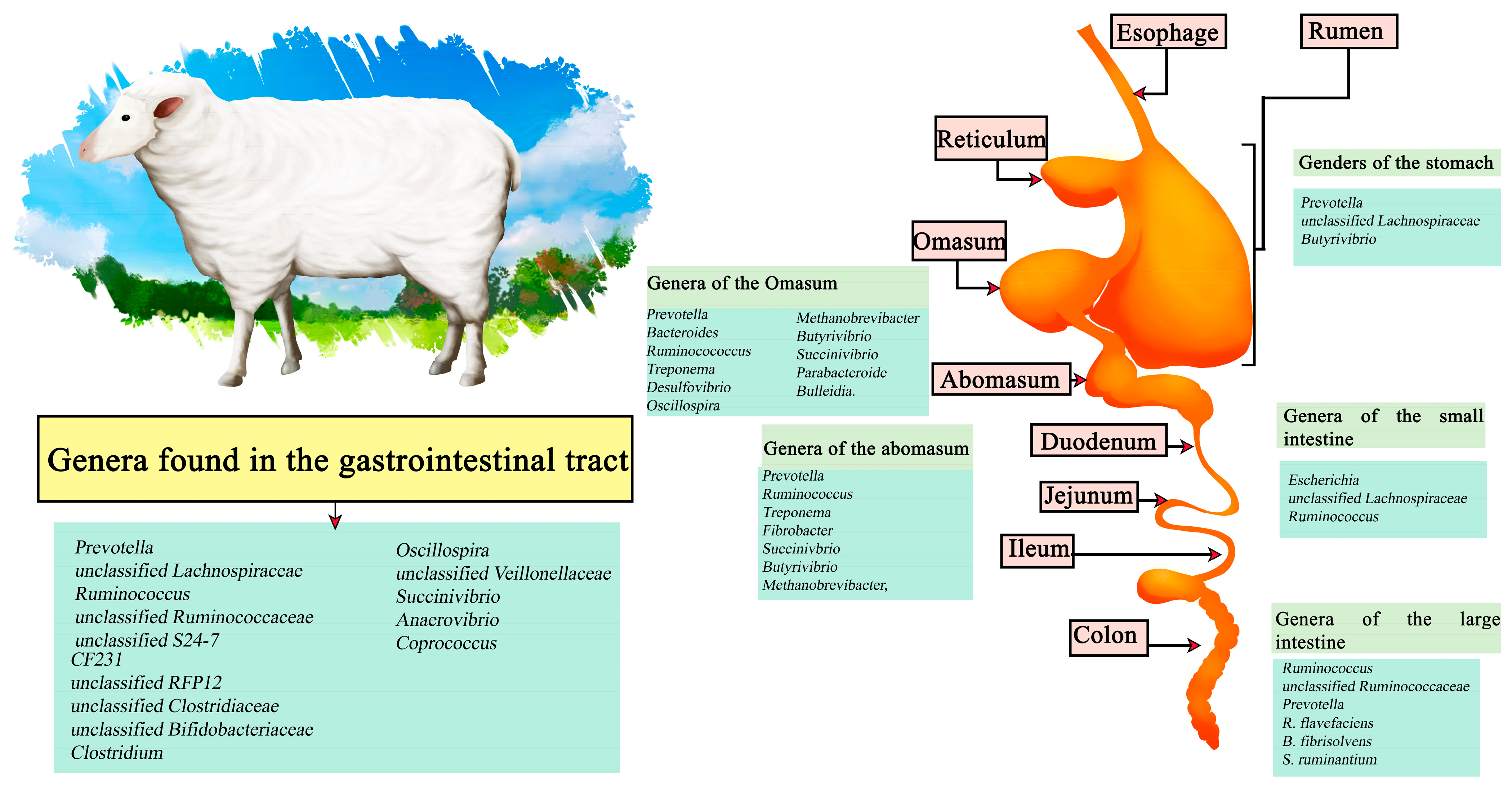
The sheep meat market includes the sale of meat, milk, wool, and skin, depending on the country of origin. Worldwide, there are an estimated 1 billion sheep, distributed mainly in northern Europe, Asia, South America, Australia, and New Zealand, with a consumption of 2.5 kg per person per year. In general, sheep production systems are classified into 3 categories: extensive production, intensive production, and traditional grazing; the latter has been reduced mainly due to the advance of agriculture that has reduced the available space, in addition to problems in yield [59]. In sheep studies the predominant microorganisms in the regions encompassing the sheep stomach have been Prevotella, unclassified Lachnospiraceae, and Butyrivibrio. In the abomasum these are Prevotella, Ruminococcus, Treponema, Fibrobacter, Succinivbrio, Butyrivibrio, and Methanobrevibacter, while in the Omasum Prevotella, Bacteroides, Ruminocococcus, Treponema, Desulfovibrio, Oscillospira, Methanobrevibacter, Butyrivibrio, Succinivibrio, Parabacteroide, and Bulleidia have been described. On the other hand, the three most dominant genera in the small intestine are Escherichia, unclassified Lachnospiraceae, and Ruminocococcus; in the small intestine Ruminocococcus, unclassified Ruminococcaceae and Prevotella. R. flavefaciens, B. fibrisolvens and S. ruminantium are found. Predominant throughout the gastrointestinal tract are the genera of Prevotella, unclassified Lachnospiraceae, Ruminococcus, unclassified Ruminococcaceae, unclassified S24-7, CF231, unclassified RFP12, unclassified Clostridiaceae, unclassified Bifidobacteriaceae, Clostridium, Oscillospira, unclassified Veillonellaceae, Succinivibrio, Anaerovibrio, and Coprococcus (Figure 2) [60,61].
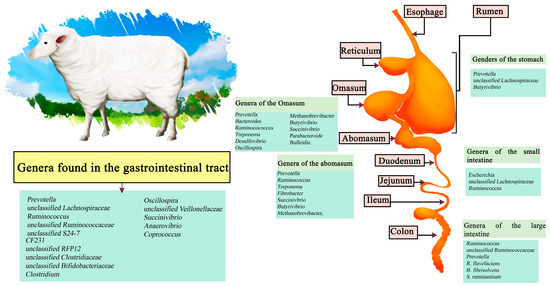
Figure 2.
Digestive tract of sheep. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
Alfalfa (Medicago sativa) is an excellent forage. However, its availability is limited. A study sought to replace alfalfa with native grass in the diet of Ujimqin lambs. For this purpose, three experimental diets were used: a high percentage alfalfa diet (30%, HA) with 10% native grass; a moderate percentage alfalfa diet (20%, MA) with 20% native grass; and a low percentage alfalfa diet (10%, LA) with 30% native grass. It was found that the proportions of microorganisms that constitute the microbiota in the three treatments were different, showing no significant differences. However, the presence of the Prevotella, Muribaculaceae, and Rikenellaceae gut groups, and abundance of the Bacteroidales gut group in MA diets was highlighted [62].
Forage oilseed rape (Brassica napus) is the second most important oilseed crop in the world used for research in breeding studies. It is used in crop rotation in Europe, Australia, Canada, and China, in turn helping to maintain soil fertility achieving sustainable production [63]. An experiment designed by E. Du et al. (2022) used castrated male lambs of the Hu breed and supplemented them with diets based on total mixed ration (TMR) with different inclusion levels of forage canola, from 0, 100, 200, 200, 300 and 400 g/kg−1, in order to know the effects on meat quality and the changes caused in the microbiome. Results at the phylum level indicated that Bacteroidetes and Firmicutes accounted for ~92.5% of the total taxa and dominated all rumen microbial communities. In contrast, Proteobacteria, Patescibacteria, Spirochaetes, Tenericutes, and Euryarchaeota were less abundant, representing 0.1–10.0% of the total bacteria for all lambs. In addition, among the cellulose degrading microorganisms, the following genera were found Fibrobacter, Eubacterium and Ruminiclostridium. The conclusion was that the inclusion of forage canola increases the levels of amino acids and intramuscular α-linolenic acid in terms of meat quality, in addition to promoting the growth of cellulolytic bacteria in the rumen [57].
Antioxidants can help to combat the stress caused by dietary changes in young animals. Grape pomace (Vitis vinifera L. var. Moschato) has been proven as an important source of polyphenols that can act as powerful antioxidants in lambs [64]. Research by Kafantaris et al. (2017) used grape pomace in male Chios lambs to use polyphenols as prebiotic supplements to improve intestinal health. For this purpose, they used diets containing a standard diet for the control group. The experimental group used a diet containing silage with grape pomace polyphenolic additives, based on: corn silage (51% solids), grape pomace (9%), and water (40%). The results indicated a reduction in Enterobacteriaceae in the fecal microflora, accompanied by a reduction in Escherichia coli, as well as other pathogenic bacteria. In turn, the population of Bifidobacterium, beneficial bacteria in polyphenol treatment, increased [65].
Energy and protein levels in diets are capable of altering rumen growth and development by modifying its physiological and anatomical properties, which affects milk quality and production. When high quality forage such as corn silage and alfalfa hay are ingested, the productive performance of ruminants can be increased [66]. One study used Hu Chinese lambs randomized in a 2 × 2 factorial arrangement with a first factor of high metabolizing energy (HE) and low metabolizing energy (LE), with 4 treatments of high protein (HP) and low protein (LP) for the second factor, respectively. The results detected 16 phyla, highlighting Bacteroidetes as the most dominant, followed by Firmicutes, Proteobacteria and Spirochaetes. At the genus level, 126 were identified, highlighting Prevotella, the gut group, Succinivibrionaceae, and Ruminococcaceae, present in all treatments. At the genus level, the dominant members of the high metabolizing energy (HE) group samples contained mainly Veillonellaceae, Succinivibrionaceae, and Veillonellaceae. Low metabolizing energy (LE) groups were dominated by Bacteroidales and Lachnospiraceae. The results obtained give a profile of bacteria present in the microbiome under varying energy and protein, thereby offering a valuable profile for future studies [67].
In order to improve gut health in an economical manner, the effects of rumen-protected methionine (Met) and lysine (Lys) were studied. These were used in experimental low protein (LP) diets, which have been shown to be able to reduce costs associated with protein sources and reduced nitrogen emissions in varied studies, such as chickens and pigs [68,69]. Gebeyew et al. (2021) used male Hulunbuir lambs fed diets with normal protein (NP), low protein (LP) and an LP diet supplemented with Met and Lys (LP + RML). The results indicated that LP + RML diets increased the Paenarthrobacter population in the jejunum and ileum. Ruminococcus and Clostridium were reduced in the jejunum and enriched in the ileum; Saccharimonas decreased in the duodenum, jejunum and ileum; and Gastranaerophilales proliferation increased in the duodenum. This study concluded that the inclusion of methionine and lysine does not result in adverse alterations in the microbiota gut, conversely, contributes to a healthier microbial community [70].
3.3. Goats
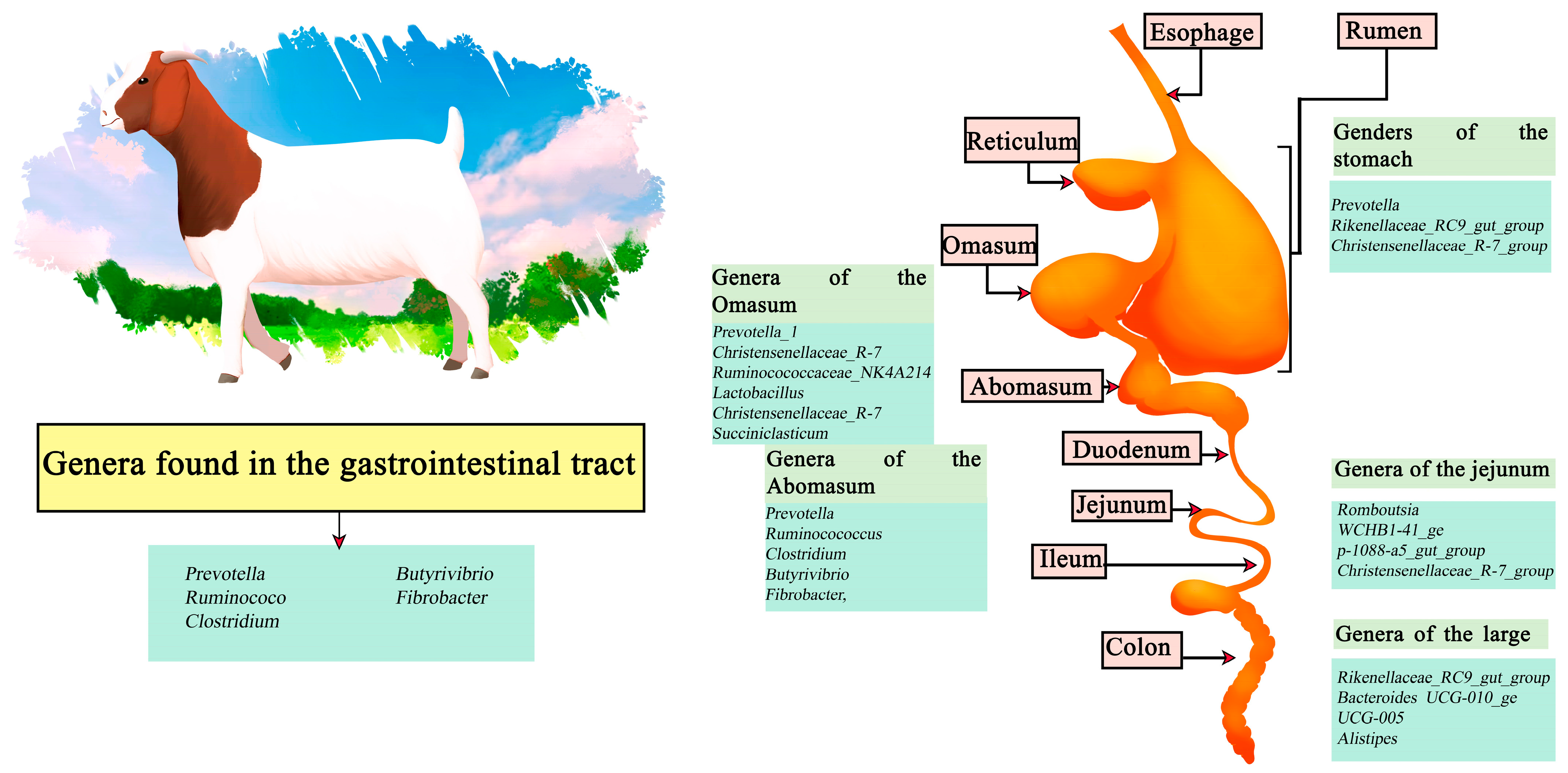
Goats are small ruminants that have been domesticated since ancient times; their uptake is more popular in the East and reduced in the West. However, they have been gaining more relevance recently [71]. The cause of their popularity is due to the healthier nutritional qualities present in goat meat (Chevon), containing a lower level of fat, saturated fat and cholesterol. It also has a higher content of polyunsaturated fatty acids (PUFA), being healthier than other red meats [72]. Among the genera identified in the stomach are Prevotella, Rikenellaceae_RC9_gut_group, and Christensenellaceae_R-7_group. Meanwhile, the abomasum is composed of Prevotella, ruminocococcus, Clostridium, butyrivibrio, and fibrobacter, followed by, in the omasum, Prevotella_1, Christensenellaceae_R-7, Ruminocococcaceae_NK4A214, Lactobacillus, Christensenellaceae_R-7, Succiniclasticum, Clostridiales_vadinBB60, and Clostridiales_vadinBB60. The jejunum section is dominated by Romboutsia, WCHB1-41_ge, p-1088-a5_gut_group, and Christensenellaceae_R-7_group. Finally, the large intestine region presents a higher abundance of the genera Rikenellaceae_RC9_gut_group, Bacteroides UCG-010_ge, UCG-005, and Alistipes [73]. In contrast, along the intestine the main genera involved are Prevotella, ruminococcus, Clostridium, Butyrivibrio, and Fibrobacter (Figure 3) [74,75].
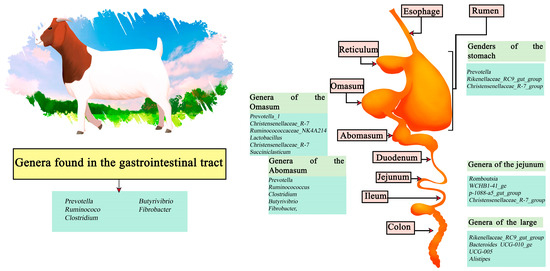
Figure 3.
Digestive tract of goats. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
Milk from ruminants is an important source of nutrients providing protein, vitamins, and minerals. However, goat milk stands out for its nutritional qualities, being a good source of potassium, calcium, and phosphorus. In addition, it possesses many fatty acids, such as conjugated linoleic acid (CLA), these being a mixture of isomers of linoleic acid and alpha linoleic acid that enhance the health properties of milk [76,77]. A study conducted in alpine goats used hemp seeds (linoleic acid) and flaxseeds (alpha linoleic acid) as feed supplements. Three treatments were carried out: control diet; diet supplemented with flaxseed; and diet supplemented with hempseed. The results for the two experimental treatments showed the predominance of Bacteroidetes and Firmicutes phyla with a high abundance of Prevotellaceae and Veillonellaceae and a low abundance of Ruminococcaceae, Prevotellaceae, and Lachnospiraceae. The Prevotellaceae family was dominant in the experimental diets at both sampling times. In all treatments, the genus Methanobrevibacter was the main Archaea found. At the genus level, flaxseed supplementation increased Succinivibrio spp. and Fibrobacter spp. populations, while decreasing the relative abundance of Prevotella spp. Based on this research, it is manifested that fatty acid intake in the diet of alpine goats modifies the intestinal microbiome, which would also affect the fatty acids presented in milk [78].
The ingestion of medicinal plants has the potential to modify ruminal fermentation. In a study developed by Yusuf et al. (2017) it was proposed to used Andrographis paniculata (AP) for this purpose. This medicinal plant is of Asian origin, belongs to the Acanthaceae family and is used in humans to treat inflammatory diseases, having antioxidant properties [79]. The research used Boer goats, providing a control diet (basal diet without additives) plus two experimental diets: a basal diet +1.5% (w/w) of Andrographis paniculata leaf powder (APL); and a basal diet +1.5% (w/w) of Andrographis paniculata with whole plant powder (APW). The results found that the treatment that included Andrographis paniculata powder favored the growth of the genus Ruminococcus (albus, flavefaciens) and Fibrobacter succinogenes due to a higher ruminal pH [80].
In the search for alternatives to the use of antibiotics, Zhou et al. (2020) described as an alternative the use of bioactive compounds or essential oils from plants as a viable option. This approach is considered environmentally safe and cost-effective. The researchers proposes lolot (Piper sarmentosum, PSE) as a medicinal plant, due to its high number of bioactive compounds and antimicrobial activity. The study was conducted on Hainan black goats and employed four treatments with 0, 300, 600 or 1200 mg/kg of PSE extract with zeolite powder. The results showed a decrease in protozoa, fungi, Ruminococcus flavefaciens, and Fibrobacter succinogenes in the 1200 mg/kg PSE treatment. However, the populations of Ruminococcus albus, methanogens, and Butyrivibrio fibrisolvens present in the rumen were not altered by the treatment. Therefore, the experimental diets provided antioxidants properties to the goats, affecting the microbiota populations [81].
To evaluate the effects produced by rice intake in goats, one study used Liuyang Black goats with a control treatment (concentrate/hay 55:45) and a rice-rich diet (concentrate/hay 90:10, HR) were compared. Rice supplementation resulted in a decrease in ruminal pH, followed by an increase in the populations of the genus Ruminococcaceae and a decrease in Christensenellaceae and Bacteroidales, resulting in a loss of gut microbial diversity [82].
Methionine is an important amino acid for the correct nutritional balance in production animals, mainly in ruminants. Upon reaching the rumen, not all amino acids supplied can be absorbed correctly, so dietary protected proteins are used, which have shown improvements in milk production parameters and growth rates [83]. In addition, there are methionine analogues such as HMB (2-hydroxy-4-(methylthio)-butanoic acid) and isopropyl ester HMBi, which are usually supplied at the post-ruminal stage. HMBi can consistently provide methionine by degrading at slower rate in the rumen. In addition, it provides multiple benefits in terms of biological activity, nutrient digestibility, and milk composition. Chen et al. (2020) tested the effects of HMBi on different parameters in Xiang Dong black goats. They used a control supplied by a basal diet and implemented with HMBi at the following proportions: 0%; 0.05%; 0.10%; and 0.20%. At the microbiota level, the most frequently found phylum was Bacteroidetes followed by Firmicutes; at the genus level, 86 taxa were identified, including: Prevotella; Succinivibrio; Selenomonas; Succiniclasticum; YRC22; and Ruminococcus. Correlation analysis determined that Succinivibrio, Bilophila, and Ruminobacter could be related to increased feed intake, although further studies are needed to determine their role. It was concluded that methionine administration had no detrimental effects on goats [84].
4. Monogastric Animals
Monogastric animals have a similar gastrointestinal system in many respects, being classified into carnivores, herbivores, and omnivores. However, there are structural differences between them, for example in the cecum and/or colon. Since they do not have a stomach for fermentative digestion, these structures (cecum and colon) have developed in greater proportion. They are larger in herbivores, as they require bacterial fermentation of fiber [85]. In turn, the microorganisms present in the microbiome are dependent on multiple factors including diet. However, external factors like temperature can cause heat stress in animals, where in animals which, in turn, affects the intestinal composition and microbiota. Animals with absence of sweat glands such as chickens and pigs, experience differences in their bacterial composition as a result [86]. Other sources of stress such as confinement, geographical location, husbandry systems or factors such as breed and age are also determinants [87]. Therefore, the microorganisms found among monogastrics are different from each other.
4.1. Chickens
Within poultry, chicken meat (Gallus gallus domesticus) provided from broilers is one of the most consumed food products in the world, with a high demand due to its nutritional properties with a high content of protein, B-complex vitamins and minerals, and a low level of saturated fats. Its demand is expected to be increasing with the growing increase in population [88].
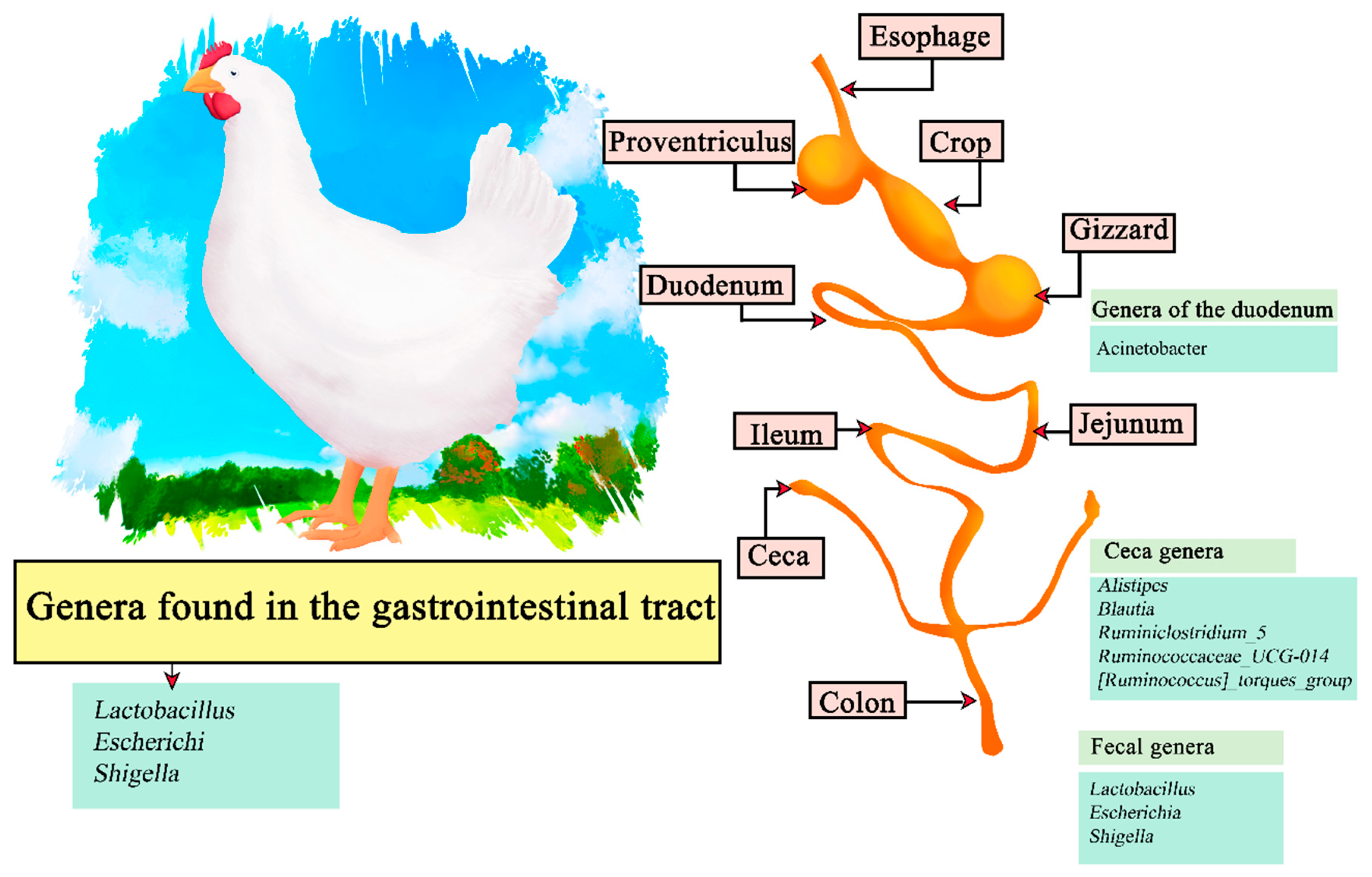
The digestive system of poultry consists of the oral cavity (beak), esophagus, crop, stomach (constituted of two parts; proventriculus and gizzard), small intestine (composed of duodenum, jejunum and ileum), liver, cecum, large intestine, and cloaca (Figure 4). The ingested food is hydrated, ground into small particles, acidified and attacked by endogenous enzymes to obtain the macronutrients. The stomach has oxyntopeptic cells responsible for secreting both hydrochloric acid and pepsinogen, and lipase resulting from reflux from the duodenum can also be found. The functions of the proventriculus and gizzard (ventriculus) are, respectively, to initiate primary digestion using digestive enzymes and to exert a mechanical function by grinding the food to a suitable size to continue through the digestive tract [89,90]. The pH values present in some areas of the digestive system are: beak (6.7); crop (6.4); ileum (6.7); rectum (7.1). As described, the pH in chickens is more acidic than in other mammals and does not change during the life span. On the other hand, the constituent microorganisms of the microflora are obtained by the interaction of newly hatched chicks and the eggshell surface, changing over time [91]. It has been recorded that Clostridium_sensu_stricto_1 is predominant in newly hatched chicks in the cecum, duodenum, and feces. However, Lactobacillus is more abundant in later stages such as after 35 days. Therefore, the genera found in feces are lactobacillus and Escherichia-Shigella, in the cecum Alistipes Blautia, Ruminiclostridium_5, Ruminococcaceae_UCG-014, and the [Ruminococcus]_torques_group, and finally the genus Acinetobacter in the duodenum [92]. Among the most recurrent genera within the gastrointestinal tract are Lactobacillus, Escherichi, and Shigella (Figure 4) [93].
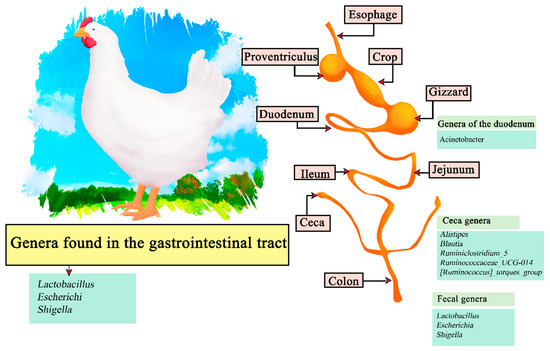
Figure 4.
Digestive tract of chickens. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
The perennial plant Achyranthes japonica, known in oriental countries as a medicinal plant, stands out for its anti-inflammatory, hepatoprotective, antioxidant and anticarcinogenic functions. Its active ingredients such as saponin, phytoecdysteroids, 20-hydroxyecdysone, triterpenoids and inokosterone have been used in animals such as pigs [94]. Recent research proposed the use of the active components present in Achyranthes japonica (AJE) as a feed supplement in Ross 308 broiler chickens [66]. Therefore, four experimental diets were used, a basal diet (CON), followed by treatments containing both the basal diet and different percentages of AJE extract: T1 (0.025% AJE), T2 (0.05% AJE), and T3 (0.1% AJE). In the fecal microflora, it was found that the Lactobacillus increased as AJE increased, while E. coli and Salmonella populations decreased as AJE increased. Thus, Park & Kim (2020) conclude that diets with AJE can improve parameters in broilers, such as growth performance, dry matter digestibility, and nitrogen.
A study by Hafsa et al. (2018) used grape seeds, rich in polyphenols, as a dietary additive in Cobb-500 broiler chickens. Subsequently, their ileum was removed and digestive contents were collected at 42 days of age. Results indicated a reduction in E. coli and Streptococcus populations in the ileum and an increase in ileal populations of beneficial bacteria such as Lactobacillus. It was reported that polyphenols may have bacteriostatic or bactericidal action, or may act to inhibit the adhesion of infection-causing bacteria within the cells of the intestinal tract. Thus, the active components of herbal derivatives, including grape seeds, could help prevent the growth of pathogenic bacteria and promote the population of non-pathogenic bacteria, like Lactobacillus spp. and Bifidobacterium spp. In the particular case of Lactobacillus, possess the ability to metabolize phenolic compounds supplying energy to the cells and positively impacting bacterial metabolism [95].
An alternative to replace food of plant origin consists of using flour from Tenebrio molitor larvae (TM flour). This worm, also known as yellow mealworm, has been one of the most used insect species in feed due to its high nutritional value, with a high protein and lipid content. It is also used in production animals. In a study conducted by Rumbos et al. (2020), Arbor Acres broilers were used and subjected to various diets during the rearing phase. The first of these was corresponding to the initial phase (first 10 days), a second corresponding to the growth phase (11 to 25 days), followed by a finishing diet (26 to 42 days). The study included a control and two experimental diets that replaced 2.5% (MT 2.5) and 5% of the basal diet with TM meal (MT 5). The results showed that the feed conversion rate was higher in the control than in the 2.5% MT treatment. However, no changes were observed in the other treatments in the rearing phases. On the other hand, samples obtained at day 25 from the cecal region indicated a reduction in E. coli content in the 5% TM treatment, demonstrating a linearity of E. coli reduction at higher TM supplementation. It is concluded that TM intake improves immune activity due to a prebiotic effect found in chitin from larvae [96].
Xylanase is a carbohydrase used to improve feed digestibility and has been used in broilers to facilitate digestion of dietary fiber [97]. An investigation tested the effect of xylanase inclusion in ROSS 308 broilers by applying four experimental diets: Control (CON), control plus xylanase (XYL); control plus emulsifier (EMU); and control plus xylanase and emulsifier (XYL + EMU). These diets were administered according to an initial phase (1 to 11 days), emphasizing the use of corn and rapeseed oil as supplementary fat, and in the growth and finishing phases (from 11 to 25 and from 25 to 42 days) beef tallow was integrated. Analyses showed that there were no significant differences in the populations of Bifidobacterium, Lactobacillus, and Escherichia coli in the treatments containing xylanase or emulsifiers. On the other hand, Clostridium (butyric acid-producing bacteria) showed a decreased relative abundance in the emulsifier-containing groups. However, the other butyric acid-producing bacteria were largely unaffected by emulsifier treatment. Consequently, fermentation in the cecum and enzymatic digestion in the duodenum and ileum were favored upon EMU and XYL ingestion [98].
Currently, there has been increased awareness of the use of growth-promoting antibiotics and their effect on the emergence of resistant strains, so a replacement to these in productive use is being sought. Sophorolipids (SPL) are biosurfactants or biological detergents composed of a hydroxylated fatty acid and a glucose disaccharide (Sophorose) from non-pathogenic yeasts. Due to their biodegradability and low toxicity, the food industry has been focused on their use. [99]. It is proposed that the application in Ross 308 chickens can improve intestinal health and improve the growth performance. A study conducted by Kwak et al. (2021) used three experimental diets in this type of chickens: CON (basal diet); BAM a growth-promoting antibiotic (10 mg/kg bambermycin); and SPL (10 mg/kg SPL). Microbial populations in the SPL diet at the phylum level were enhanced in the Firmicutes population over Bacteroidetes. At the genus level Lactobacillus increased in abundance and Streptococcus decreased. At the species level Lactobacillus helveticus, Lactobacillus salivarius and Akkermansia muciniphila showed an increase while Streptococcus gallolyticus was reduced in both the BAM and SPL treatments. It was concluded that the SPL diet contributed to improve intestinal defenses and alleviate inflammation, which promotes growth in animals [100].
4.2. Pigs
Pigs are omnivorous animals and form part of the most consumed type of meat worldwide. Within global meat production, world pork consumption is expected to increase to 129 Mt in ten years, with an estimated 35.6 kg/year of pork consumption by 2030 [101]. Its digestive system is composed of the mouth where the food is introduced, and the stomach which is made up of four regions and includes the esophageal, cardiac, fundic, and pyloric regions (Figure 5). The function of the cardia region is to mix the food by means of a mucus, then the gastric glands of the fundus digest the food with hydrochloric acid and digestive enzymes, and thus the food is directed to the fundus. The pyloric region subsequently secretes mucus into the digestive membranes that serve as protection. Other structures present are the small intestine (duodenum, jejunum, and ileum), where food absorption takes place; the liver secretes bile fluid and the pancreas secretes enzymes, followed by the large intestine constituted by the cecum which digests the fibrous part, and then the colon and rectum. The gastric system found in pigs performs similar functions as in humans. However, it presents some differences in size and shape; the porcine cecum is relatively larger, the porcine colon is spiral oriented and pigs lack an appendix [90,102]. Therefore, the anatomical, physiological, and immunological similarities between humans and pigs mean that the microorganisms present in pigs have similarities to those found in the human digestive system [103,104]. The microorganisms normally found in pigs can be classified into the following phyla: Firmicutes, Proteobacteria, and Bacteroidetes, highlighting the presence of Firmicutes phylum populations in the duodenum and jejunum, and of the Proteobacteria phylum in the gastric and ileal compartments [105].
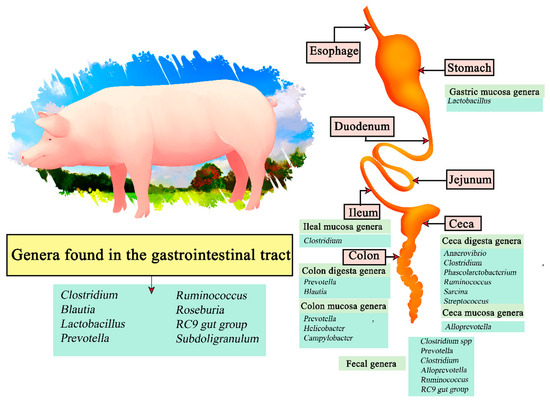
Figure 5.
Digestive tract of pigs. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
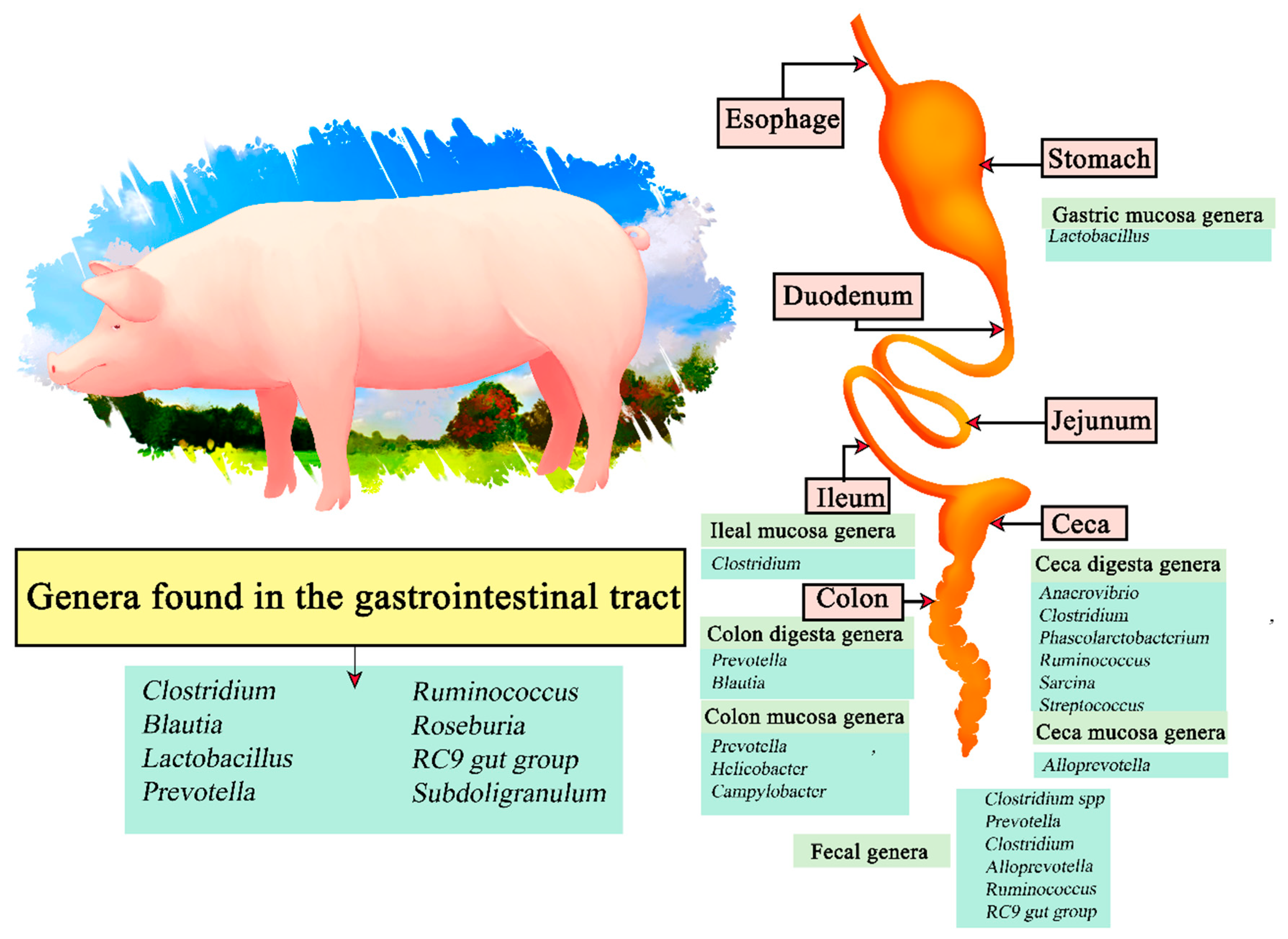
The genus Lactobacillus was found to be more abundant in the gastric mucosa, and Clostridium in samples from the ileal mucosa. Anaerovibrio, Clostridium, Phascolarctobacterium, Ruminocococcus, Sarcina, and Streptococcus were present in the ceca digesta, and Alloprevotella in the cecal mucosa. Prevotella and Blautia were present in colon digesta samples, and Prevotella, Helicobacter, and Campylobacter in the mucosa. Finally, Clostridium spp. Prevotella, Clostridium, Alloprevotella, Ruminococcus, RC9 gut group and Treponema were present in fecal samples [106]. Therefore, the genera Clostridium, Blautia, Lactobacillus, Prevotella, Ruminococcus, Roseburia, RC9 gut group, and Subdoligranulum are present in most of the gastrointestinal tract of swine.
The search for alternatives in the use of carbohydrates in animal diets has become more important due to the limitations of food sources that have been reduced in the agricultural sector due to environmental changes and the problems associated with the decrease in nutrients in the soil, added to the loss of food that is wasted worldwide [107]. Therefore, the search for sustainable carbohydrate alternatives for animal consumption has intensified, and studies have evaluated the administration of processed carbohydrates not suitable for human consumption that fall under the classification of Former foodstuff products (FFPs) [108], in Large White × Landrace pigs using a standard diet for post-weaning piglets for the control group (CTR) and in the experimental group (FFPs) a standard diet including 30% residues of bakery products and wheat-derived varieties as carbohydrate sources. The results indicated that an inclusion of FFPs decreased microbial diversity and decreased the proportion of the genus lactobacillus, and increased the genera of bacteria belonging to the proteobacteria; however, it did not generate major changes in the microbial community and no problems in growth performance were observed [109].
Grape seeds are an important source of flavonoids, which are known for their beneficial effects on health. Polyphenols oxidized in the presence of aflatoxin B1(AF1) can form a complex that helps prevent AF1 from exerting its toxic action in the gut. An experiment conducted by Grosu et al. (2019a) used TOPIGS-40 hybrid pigs and supplemented them with four diets in different treatments: (1) Standard (Control); (2) Diet contaminated with 320 µg/kg AFB1 (AFB1); (3) Diet supplemented with grape seed meal (GC); and (4) Diet with 320 µg/kg AFB1 and 8% grape seed meal (AFB1 + GC). The results indicated, at the phylum level from highest to lowest abundance, the presence of: Firmicutes, Bacteroidetes, Proteobacteria, Spirochaetes, Tenericutes, and Actinobacteria, with traces of Euryarchaeota, Fibrobacteres, Cyanobacteria, and Deferribacteres. Although their proportions were altered, for example in the AFB1 + GC treatment, the proportion of Bacteroidetes increased, while that of Firmicutes decreased compared to the control diet. At the genus level, the treatment including GC increased the populations of Megasphera, Clostridiales, Anaerovibrio, and Prevotella, and decreased the genera of Lactobacillus, Lachnospiraceae, Bacteroidales, and Campylobacter. The authors noted that the inclusion of GC may help to mitigate the negative effects caused by AFB1 contamination in pig diets. The mechanisms that alter the microbiota by the action of aflatoxin B1 and grape seed meal are unknown [110].
The excessive use of antibiotics in the livestock industry has aroused the interest of researchers in the search for replacements with non-harmful feed additives. In this regard, short-chain fatty acids such as butyrate, whose active element is butyric acid, have been shown to help improve intestinal health by regulating proteins present in the mucosa [111]. Butyric acid can be found in salt form as sodium butyrate or as a triglyceride called tributyrin, both of which are used in humans and animals as prodrugs [112]. A work by Sun et al. (2020) sought to apply sodium butyrate in the diet of pigs using pigs from Yorkshire-Landrace × Duroc boar crosses. The study considered three treatments: (1) basal diet; (2) basal diet + 40 ppm zinc bacitracin; and (3) basal diet + 0.2% sodium butyrate. The results indicated that at the phylum level, Firmicutes, Bacteroidetes, Proteobacteria, Verrucomicrobia, Actinobacteria, Tenericutes, Cyanobacteria, and Synergistetes were detected in the cecum, with abundance from highest to lowest, respectively. At the genus level, treatment 3, containing sodium butyrate compared to treatment 1, showed a higher relative abundance of Anaerovibrio, Megasphaera, and Prevotella, and a decrease in Flavobacterium populations compared to group 1. On the other hand, the populations of the genus Dorea, Blautia, Desulfovibrio, Coprococcus, Succinivibrio, and Ruminococcus were decreased in the group with sodium butyrate, in contrast to group 2 containing zinc bacitracin. The authors proposed that the addition of sodium butyrate to the diet improves the small intestinal mucosa, promoting epithelial cell growth, and increasing villus length. In addition, it decreases carbohydrates and proteins entering the cecum and consequently decreases the relative abundance of Firmicutes and Proteobacteria [113]. On the other hand, Wang et al. (2019) proposed the use of tributyrin over sodium salt, pointing out that the latter tends to be absorbed by the upper digestive tract, whereas tributyrin can cross this barrier and be absorbed by the lower digestive tract. In this study, different levels of tributyrin were used in pigs (Duroc × (Landrace × Yorkshire)) and a control diet was used followed by three dietary treatments with 250, 500 and 750 mg/kg of tributyrin. The results showed that the use of tributyrin increased the Lactobacillus/E. coli ratio, which is indicative of good intestinal health, thus reducing the negative health effects of E. coli infection [114].
Apart from the aforementioned methods to replace antibiotics, it is possible to use probiotics such as Bacillus coagulans (BC). The latter produces enzymes and organic acids such as lactic and acetic acid [115], which acidify the pH of the intestinal tract, controlling pathogens and yeast hydrolysates that are sensitive to pH. In addition, Bacillus coagulans bacteria contain active substances such as β-glucan and mannan which are constituents of the cell wall [116]. Therefore, it is expected that the use of Bacillus coagulans can help improve the health of the gastric system in animals and strengthen the immune system by functioning as a prebiotic. In a study by Shin et al. (2019), [Landrace × Yorkshire] × Duroc pigs and three treatments were used: (1) Control diet (CON) and enramycin (20 mg/kg); (2) Diet with Bacillus coagulans (20 mg/kg, BC group); and (3) Diet with yeast hydrolysates (3000 mg/kg, YH group). The diet belonging to the BC group significantly increased the number of Bifidobacterium in the colon, followed by a decrease in E. coli counts. The YH diet increased Lactobacillus and Bacillus counts in the cecum, apart from increasing Bifidobacterium and Bacillus in the colon. Therefore, an increase in microbial populations beneficial to the host could be detected, demonstrating the immunological role of the probiotic Bacillus coagulans [117]. Another probiotic alternative is the use of Lactiplantibacillus plantarum proposed by authors Shin et al. (2019), which can enhance and stabilize the microbiota as well as other lactic acid bacteria [118]. The study used pigs (Landrace × Yorkshire × Duroc) with control diets and diets supplied with JDFM LP11 probiotics (2.5 × 10 7 CFU/mL) in a weaning diet. Aliquots of 50 mL/kg diet, 1.25 × 109 CFU/kg diet were fed to the experimental group. The most abundant phyla were Bacteroidetes and Firmicutes in all diets; at the family level 19 different families of bacteria were found with significant differences in Prevotellaceae, Erysipelotrichaceae, Sphaerochaetaceae, Spirochaetaceae, and Christensenellaceae, with a higher abundance in Prevotellaceae and Ruminococcaceae. Therefore, a higher abundance of Spirochaetaceae, Eryspelotrichaceae, Sphaerochaetaceae, and Christensenellaceae was found in the experimental diet and a lower abundance of Prevotellaceae. It was concluded that the microorganisms involved have effects at the immunological level and on the weight gain of the animals. On the other hand, the experimental diet helped in the development and integrity of the intestinal epithelium [119].
5. Fish
Aquatic resources such as fish possess excellent nutritional value as a source of protein, fatty acids, vitamins, minerals, and essential micronutrients [120,121]. In 2020, global fish production from aquaculture reached an all-time high of 214 million tons, distributed among other marine products such as aquatic animals and algae [122]. Efforts in this industry are focused on increasing yields and decreasing costs associated with production by optimizing fish farming. Understanding the microbiome is also fundamental in fish, as it is involved in the development and efficiency of the gastrointestinal tract, resisting infections and maintaining the homeostasis of the organism [123]. With respect to the colonization and formation of the microbiome in fish, it is estimated that it starts in the eggs, by the influence of the surrounding water and in the content of the first food, initially giving discrepancies in the identification of native microbial communities, concluding that there are species-specific colonizing bacteria with which it starts [124].
It is now recognized that the predominant phyla in fish are Proteobacteria, Firmicutes, Fusobacteria, and Actinobacteria from the highest to lowest abundance, respectively, although of course there are variations due to diet [125].
5.1. Salmon
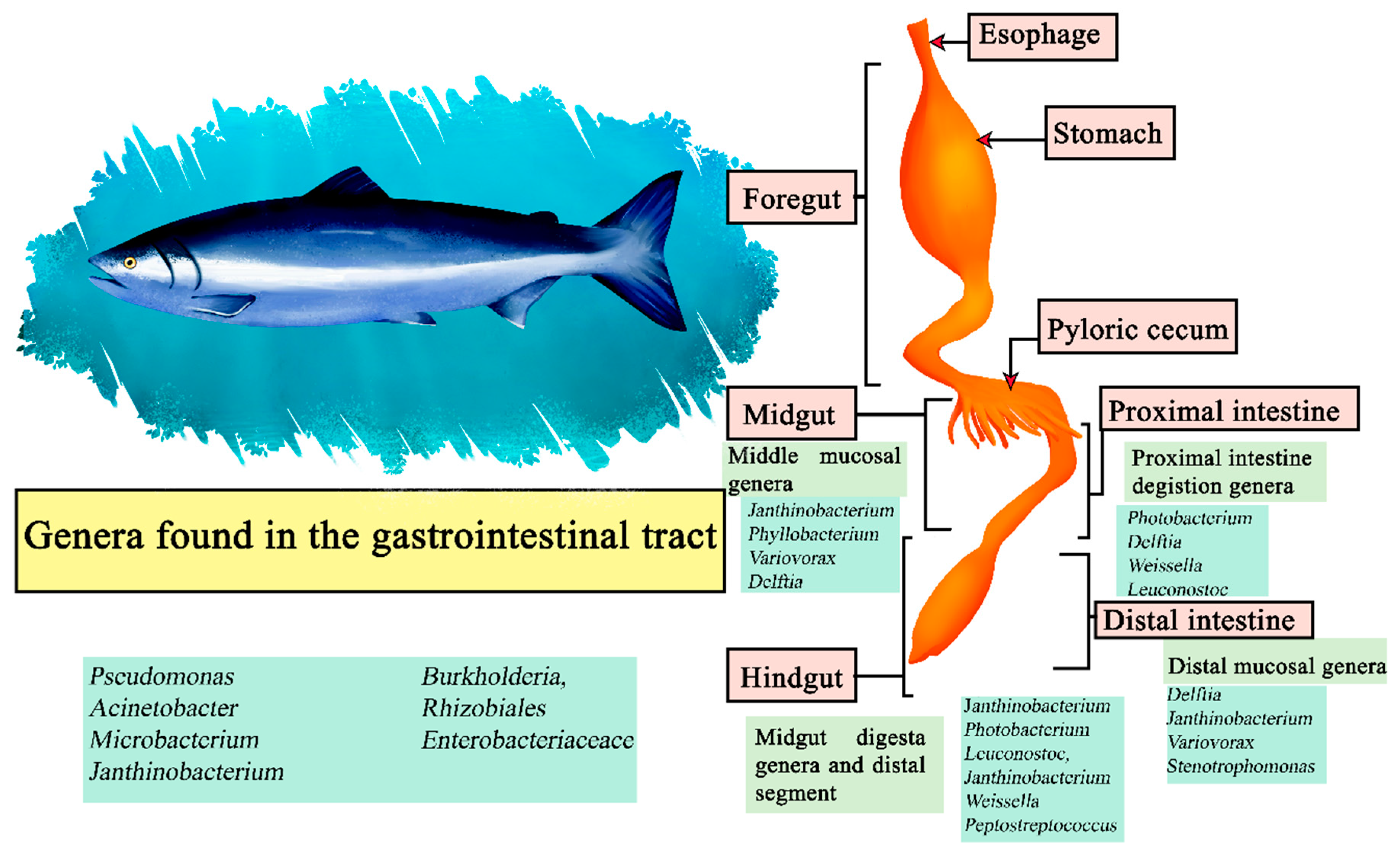
The Atlantic Salmon (Salmo salar) is carnivorous in nature, with Norway and Chile being its main producers [126]. Its gastrointestinal system is composed of an esophagus, and a U-shaped stomach and intestine with the presence of pyloric caecae attached to it [127]. Although it is a carnivorous fish, it has been proposed to use diets from terrestrial plant sources to improve economic and environmental sustainability, causing farmed Atlantic salmon to change their diet. This has a direct impact on their microbiome, which exhibits variations based on whether their diet is carnivorous or vegetarian [128]. In samples of the digesta in the proximal intestine the most abundant genera were Photobacterium, Delftia, Weissella, and Leuconostoc. The genera Janthinobacterium, Photobacterium, Leuconostoc, Janthinobacterium, Weissella, and Peptostreptococcus were the most abundant in the digesta of the midgut and distal segment, and in the middle mucosa Janthinobacterium, Phyllobacterium, Variovorax, and Delftia, while Delftia, Janthinobacterium, Variovorax and Stenotrophomonas had a higher relative abundance in the distal mucosa. Represented as central microbiota of the salmon were the genera Pseudomonas, Acinetobacter, Microbacterium, Janthinobacterium, Burkholderia, members of Rhizobiales, and Enterobacteriaceaceace (Figure 6) [129].
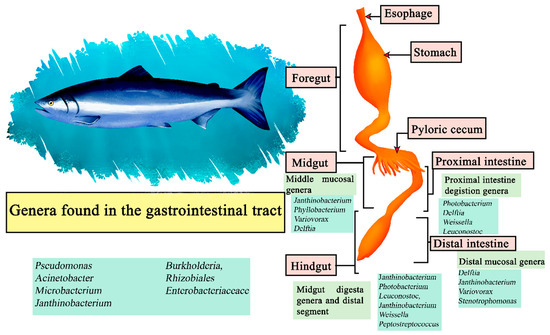
Figure 6.
Digestive tract of Atlantic Salmon. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
The use of alternative foods such as insects are proposed as potential ingredients for the manufacture of sustainable diets [130]. An experiment developed by Y. Li et al., 2021 used in Salmo salar two diets; a commercially relevant reference diet (REF) and produced from black soldier fly (BSF) larvae, which is an important source of chitin. The BSF diet increased the relative abundance of Actinomyces, Bacillus, Brevibacterium, Corynebacterium, and Enterococcus, highlighting that the microbiota is modified by feed and/or feed composition to the salmon. In addition, the study points out that chitin can favor Bacillus bacteria, as they can produce chitinase [131], this being beneficial for the probiotic role of Bacillus bacteria against pathogens.
Soybean meal (SBM) has been widely used in fish diets as a vegetable meal to replace fish meal. However, it has limitations for inclusion in carnivorous fish, as it has been shown that inclusion of plant-based feeds can have negative effects on weight gain in salmonids due to anti-nutritional factors [132]. A study conducted in salmon (Salmo salar) included two types of diets, the first based on soybean meal (30% SBM) and the second on fermented soybean meal (30% FSBM). The results showed that the Paracoccus genus was found in the SMB diet and the Acinetobacter, Shewanella, and Altererythrobacter genera in the FSBM diet. In addition, a higher amount of lactic acid bacteria (LAB), including Lactobacillus, Lactococcus, and Pediococcus of the phylum Firmicutes, was reported for both intestinal samples [133].
An experiment conducted by Villasante et al. (2019) used salmon with two experimental diets: a moderate carbohydrate and protein diet (MC/MP; with 15% wheat starch); and a high carbohydrate and protein diet (HC/LP; with 30% wheat starch). The highest to the lowest proportion of phyla found were Firmicutes, Actinobacteria, and Proteobacteria, with 78.6% and 71.8% established for Firmicutes in MC/MP and HC/LP diets, respectively. On the other hand, the most relevant genera found were Planctomycetes and Lactococcus which obtained a significant increase in relative abundance in fish fed the HC/LP diet. This study was able to prove that the ingestion of a diet rich in carbohydrates affects the microbial communities of salmon and, consequently, would benefit carbohydrate-metabolizing bacteria. It is also important to note that the HC/LP diet reported lower weight gain and a daily growth coefficient compared to fish fed the MC/MP diet [134].
5.2. Rainbow Trout
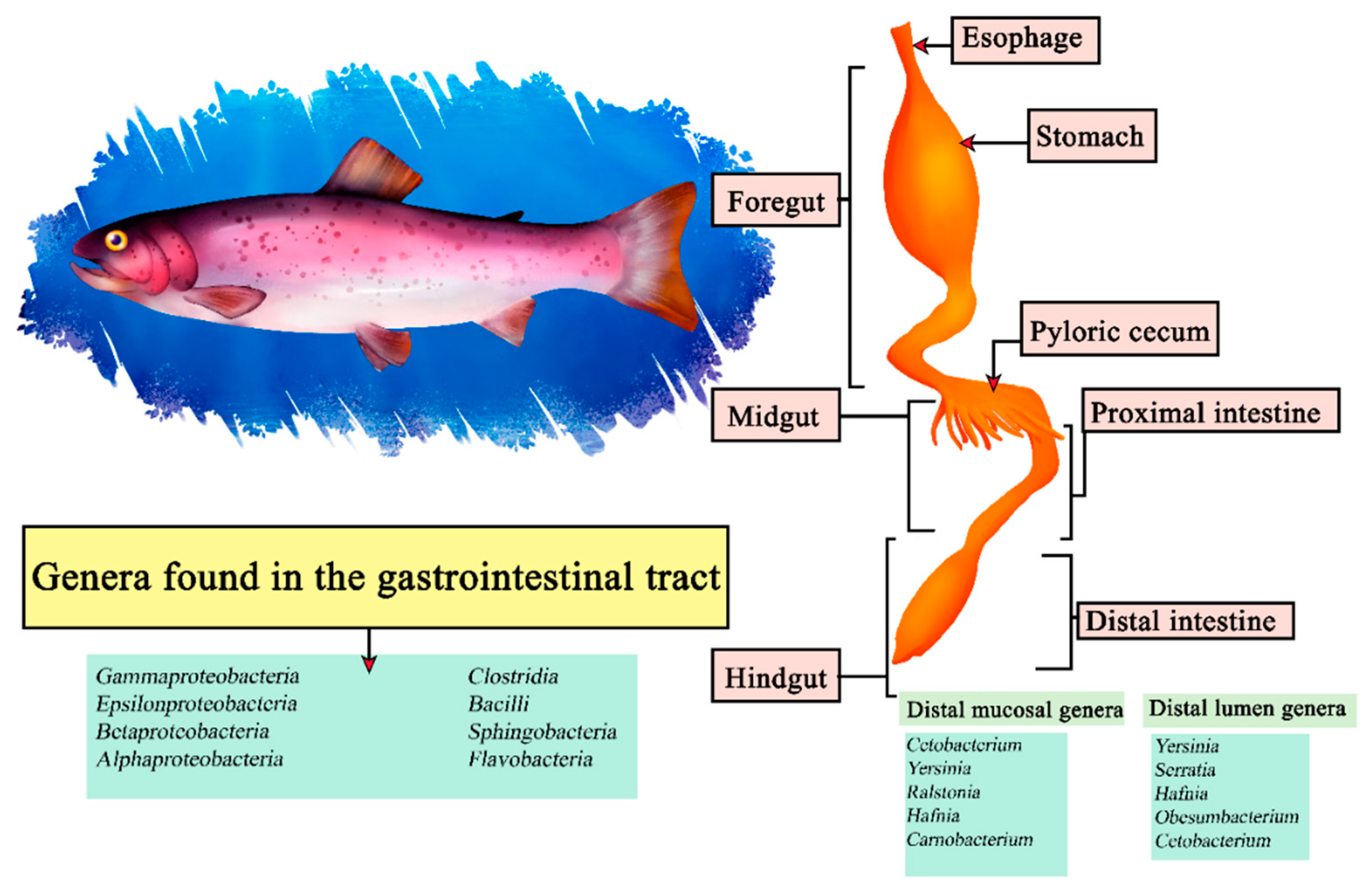
The rainbow trout (Oncorhynchus mykiss) is a carnivorous fish of the freshwater Salmonidae family, noted for its reproductive efficiency and intestinal tract adaptable to feeding conditions [135]. It belongs to the Teleosts, so it shares characteristics in its intestinal morphology with other fish, presenting a stomach consisting of cardiac, fundic and pyloric regions, forming a J-shape, which by surface extension benefits enzymatic digestion [136]. It has been reported through sequencing that Cetobacterium, Yersinia, Ralstonia, Hafnia and Carnobacterium are the most abundant within the distal intestinal mucosa and Yersinia, Serratia, Hafnia, Obesumbacterium and Cetobacterium in the distal lumen [137]. In turn, other studies indicate that under a diet with insect protein directly related genera are Oceanobacillus, Bacillus, Paenibacillus and Cetobacterium within the intestines [138]. In the case of a central microbiota in Oncorhynchus mykiss it is difficult to describe since they encompass around 52 taxa in some studies, from among them we can highlight the classes Gammaproteobacteria, Epsilonproteobacteria, Betaproteobacteria, Alphaproteobacteria, Clostridia, Bacilli, Sphingobacteria and Flavobacteria (Figure 7) [139].
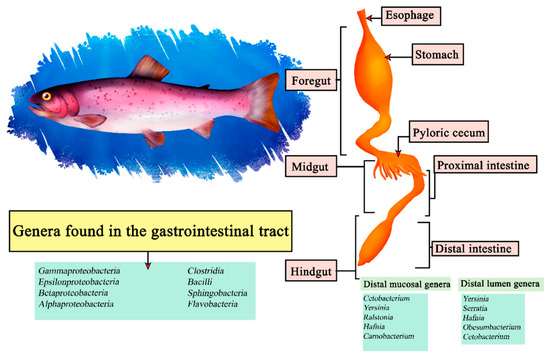
Figure 7.
Digestive tract of rainbow trout. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
Several studies have used insects as dietary components in farmed fish due to the nutritional richness they provide in terms of proteins, amino acids, fatty acids, vitamins, and minerals [140]. A study by Terova et al., 2019 also employed the species Hermetia illucens, known as black soldier fly, to study how the intake of Hermetia illucens (Hi) meal in different proportions: (0% (Hi 0); 10% (Hi 10); 20% (Hi 20); and 30% (Hi 30) can modulate the gut microbiota of rainbow trout (Oncorhynchus mykiss). The results indicated the presence of the phyla Actinobacteria, Firmicutes, and Proteobacteria, of which the most influenced by diet were Actinobacteria and Proteobacteria, which responded better to a higher proportion of Hi, except for Proteobacteria, which was decreased in the 20% and 30% treatments. In addition, the order of Lactobacillales, which at the genus level includes Facklamia, Enterococcus, Lactobacillus, and Pediococcus known as lactic acid bacteria (LAB), was favored in the Hi-containing diets. The genera Actinomyces, Brevibacterium, Corynebacterium, Leucobacter, and Staphylococcus were also reported. Finally, Corynebacterium variabile, Lactobacillus plantarum, Lactobacillus zeae, Weissella cibaria, Clostridium butyricum, and Clostridium fimentarium were named at the species level in the diets with Hi. Consequently, the experimental diets increased BAL bacteria and butyrate-producing bacteria, and fermentable chitin was stated to be an element acting as a prebiotic [141].
On the other hand, to replace fishmeal (FM) to achieve sustainable aquaculture. Mikołajczak et al. (2020) proposed using insects equally in sea trout (Salmo trutta) with: a control diet (CON); 10% hydrolyzed meal of mealworm (Tenebrio molitor) (TMD); and a third treatment using the previous proportion in hydrolyzed meal of super worm (Zophobas morio) (ZMD). The results indicated that the diet with TMD significantly reduced the concentration of the Lactobacillus group and Carnobacterium spp. In contrast, the ZMD diet reduced the populations of Aeromonas spp., Enterococcus spp., and Carnobacterium spp. It was concluded that diets with TMD and ZMD play a key role in the health of the microbiota [142].
Under the same previous objective, a method to replace the use of animal proteins from the sea, the use of vegetable proteins was proposed. However, vegetable proteins (VM) have nutritional disadvantages, as they contain non-digestible complex carbohydrates, and a lack of essential amino acids and n-3 polyunsaturated fatty acids (n-3 PUFA). Inclusion of plant protein in fish has presented challenges in providing essential amino acids, also relying on anti-nutritional factors that decrease feed efficiency [143]. Under this context, a study used rainbow trout (Oncorhynchus mykiss) with treatments that included: vegetable protein as the control (CV); a fish base diet (CF); and diets containing protein from Hermetia illucens (H), known as black soldier fly, where 10% (H10), 30 (H30) and 60% (H60) of the vegetable protein was replaced by C. Also, poultry by-product meal (P) diets were used as another source of animal protein. That description is elaborated from parts extracted from slaughtered poultry being an economical feed, rich in nutrients and essential amino acids, which has been tested in species such as Sparus aurata [144]. P was used at 30% (P30) and 60% (P60) of the CV diet. Finally, a combination of 10% and 50% of H and P, respectively, (H10P50) was used. In all diets at the phylum level the populations present were Proteobacteria, Firmicutes, and Tenericutes. With respect to the control, the diets with insect meal had a higher amount of Gammaproteobacteria, Enterococcus, and Actinomyces followed by a decrease in Pseudomonas. In addition, the occurrence of Erysipelothrix was reported compared to diets with CV or P. Therefore, it is concluded in this study that none of the diets had any negative effect. Diets that included insect meal helped to increase the diversity of intestinal microorganisms, with chitin being the prebiotic agent provided [145]. In summary, diets based on insect meal were beneficial and serve as a replacement for other types of animal protein, obtaining similar nutritional benefits in addition to providing a healthier microbiome.
As for medicinal herbs, Chinese yam (Dioscorea oppositifolia L.) is an important traditional Chinese medicinal herb widely cultivated in China and is used in vertebrates to help promote digestion, immunomodulation, antioxidant defense, and as an anti-inflammatory [146]. Its use in rainbow trout diets has not been studied and that is why the team of Wang et al., 2020, designed diets for trout containing: yam extract; a control (CON); and the other 0.1% (O1); 0.2% (O2); and 0.4% (O3) of extract. At the phylum level, mainly Bacteroidetes, Tenericutes, Firmicutes, and Actinobacteria were found; for the O1 treatment the population of Actinobacteria and Firmicutes decreased compared to the control. The opposite happened to the populations of Patescibacteria, which increased in the O3 treatment. At the genus level, Anaeroplasma, Candidatus_Saccharimonas, Ruminococcaceae, Lactobacillus, Rikenella, Lachnospiraceae, Bifidobacterium and Marvinbryantia were mentioned for a total of 10 phyla. Taking into account the changes between phylum populations, it was determined that Ruminococcoccaceae_UCG, Lactobacillus, Rikenella, Lachnospiraceae _NK4A136_group, Eubacterium, Bifidobacterium and Marvinbryantia had a lower relative population for the treatment with 0.1% yam extract, while the Lachnospiraceae _NK4A136_group and the [Eubacterium]_coprostanoligenes_group were reduced in the treatment with 0.2% extract. On the contrary, in the treatment with 0.4 extract Bifidobacterium, Marvinbryantia, and Candidatus_Saccharimonas increase. It is concluded that at the phylum level the treatment that produced more significant changes was O1 with 0.1% of yam extract, which greatly reduced the microbial diversity, fortunately this was recovered by increasing the concentration in the treatment with 0.4% of extract where at the genus level Bifidobacterium, Marvinbryantia, and Candidatus_Saccharimonas were favored and increased their population. Therefore, Bifidobacterium, Marvinbryantia, and Candidatus_Saccharimonas are beneficial bacteria that regulate homeostasis in the microflora, regulate metabolism and fulfill bactericidal function, treatment with 0.4% of yam extract being the recommended dose [147].
5.3. Tilapia
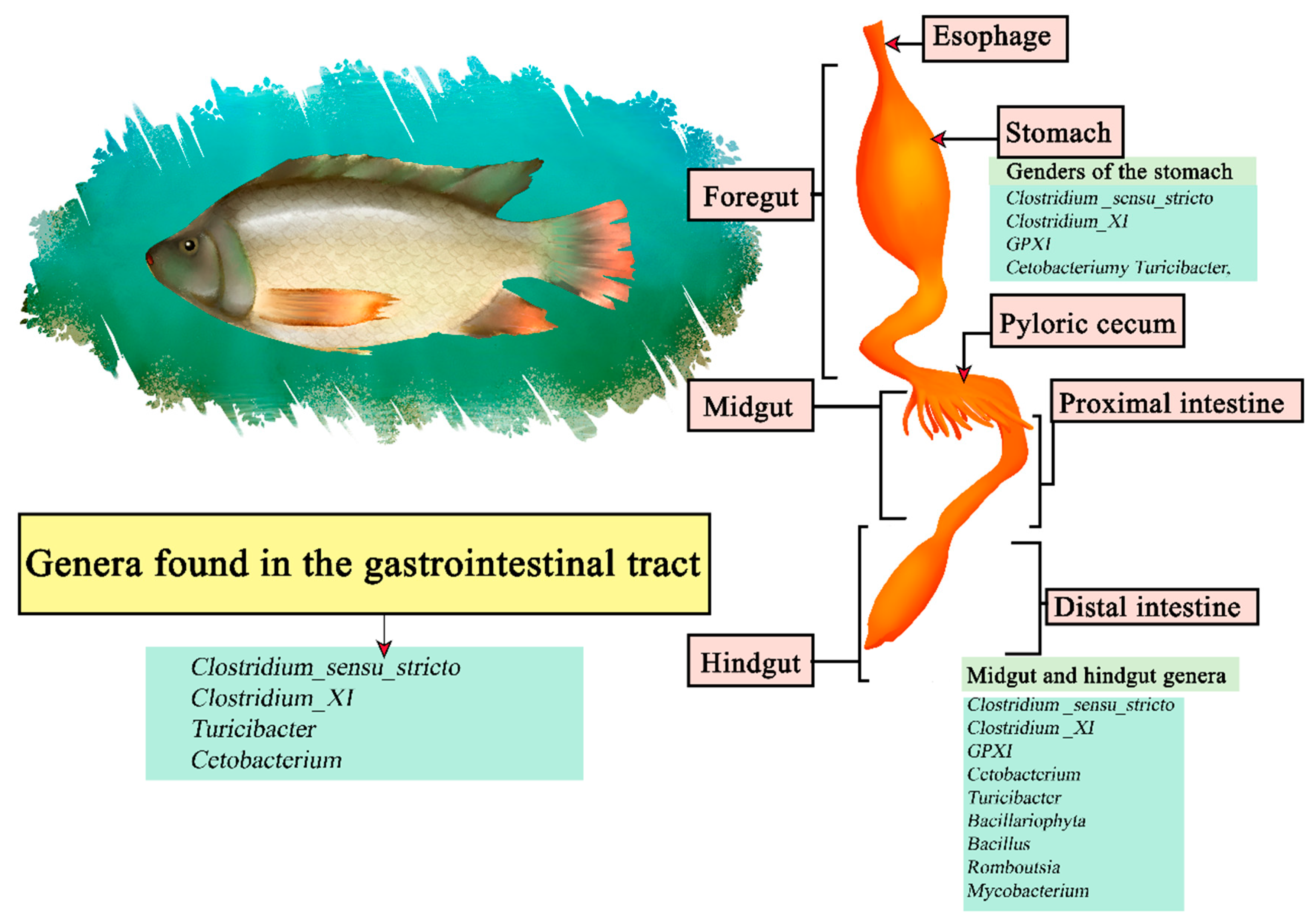
Nile tilapia (Oreochromis niloticus) is a freshwater fish native to Africa and the Middle East, being the second most cultured fish in the world [148]. Is omnivorous and with high plasticity focusing on phytoplankton, zooplankton detritus, and macrophytes [149]. It is part of the teleosts, consisting of a gastrointestinal gut consisting of the esophagus, Y-shaped stomach, and intestine [150]. It has been noted that Proteobacteria and Fusobacteria were the central phyla of intestinal microorganisms in tilapia, the most common genus being Cetobacterium within the inner, mid and hindgut [151]. Other studies focused on studying the stomach, midgut and hindgut segments, with sequenced luminal and mucosal samples, determining that the stomach section contains the genera Clostridium _sensu_stricto, Clostridium_XI, GPXI, Cetobacterium, and Turicibacter. The midgut region Clostridium _sensu_stricto, Clostridium _XI, GPXI, Cetobacterium, Turicibacterium, Cetobacterium, Cetobacterium, and Turicibacter, Cetobacterium, Turicibacter, Bacillariophyta, Bacillus, Romboutsia, and Mycobacterium. The hindgut Clostridium _sensu_stricto, Clostridium _XI, GPXI, Cetobacterium, Turicibacter, Bacillariophyta, Bacillus, Romboutsia, and Mycobacterium. The results indicate that Clostridium_sensu_stricto, Clostridium_XI, Turicibacter, and Cetobacterium are the core genera (Figure 8) [152].
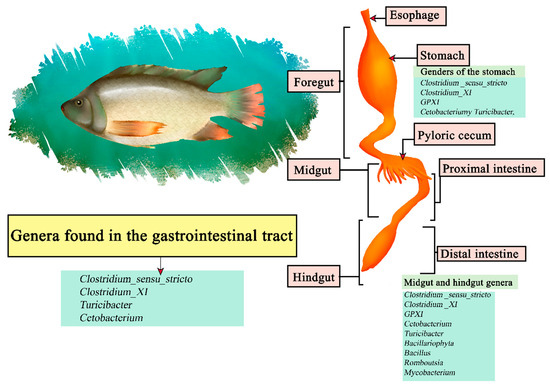
Figure 8.
Digestive tract of Nile tilapia. Microorganisms frequently found in different sections of the gastrointestinal tract compartments.
Garlic (Allium sativum) possesses antimicrobial properties, and it is reported that its use in fish can help improve growth, survival and provide better resistance to infections (Chen, J. et al., 2021). Garlic has been applied in several studies showing favorable results for Perca fluviatilis and Salmo caspius species [153,154]. Frequent streptococcal infection in fish leads to economic losses in the aquaculture industry, which is why there is a desire to find solutions to this infection [155]. Tilapia (Oreochromis niloticus) is reported to be the fish most susceptible to Streptococcus iniae (S. iniae) infection. In an investigation by Foysal et al., 2019, diets containing 0.5 g and 1.0 g/100 g of commercial garlic powder (TG1 and TG2, respectively) where fed. Fish were used that had been previously injected with bacterial suspension and placed in eight different aquaria: two for control without S. iniae (C1); and two for control with S. iniae, but without garlic supplementation (C2S). The last four were used for supplementation of the treatments with garlic and S. iniae in the mentioned proportions. In the control diets without bacteria, the following populations were found at the phylum level: Fusobacteria, Fusobacteriia, Fusobacteriales, Leptotrichiaceae, and Hypnocyclus. For the infected control treatments, Firmicutes, Clostridia, Proteobacteria, Alphaproteobacteria, Gammaproteobacteria, and Aeromonadales were found. The most remarkable result included the decrease in OTU counts for Vibrio (pathogen) with the 1.0 g garlic diet. Therefore, it was concluded that supplementation with 1.0 g garlic (TG2) was the most effective against S. iniae infection by improving the health status of fish [156].
A study by Li et al. (2019) focused on the use of probiotics, a necessary additive for the challenges faced in current aquaculture and the frequent use of antibiotics that induce resistance. The probiotic used was Clostridium butyricum, a typical butyric acid bacterium, which can help inhibit the proliferation of pathogenic bacteria. The diets were prepared at different concentrations of the bacterial suspension together with the basal feed: for the control group its concentration was 0 and the other treatments were at concentrations 1 × 104, 1 × 105, 1 × 106 and 1 × 107 CFU g−1 of diet (indicated as CG, CB1, CB2, CB3 and CB4, respectively). The diet was applied in tilapia (Oreochromis niloticus) and the results indicated 49 phyla, 108 classes, 210 orders, 394 families and 704 genera. At the phylum level, the relative abundance of Bacteroidetes, Firmicutes, Candidate-division-SR1, Chloroflexi, Chlorobi, Acidobacteria, Spirochaetae, Nitrospirae, Parcubacteria, Planctomycetes, and WCHB1-60 increased with respect to the control. Highlighted at the genus level was an increase in the relative abundance of Cetobacterium, CKC4, Aeromonas, and Gammaproteobacteria, in contrast to the CG group. The most interesting results show that the group with the most significant changes was CB2 showing at the phylum level a decrease in relative abundance of Fusobacterium and CKC4, and at the genus level Candidate_division_SR1_norank, Bacteroidetes_vadinHA17_norank, Dechloromonas, Zoogloea, Oligoflexales_noran, Comamonadaceae_unclassified, Draconibacteriaceae_uncultured, Saprospiraceae_uncultured, Nitrospira, Bacillus, and WCHB1-69_norank were more abundant. Finally, the authors pointed out that the diet with the most optimal concentration is CB2 (1 × 105 CFU g−1), which improves immunity by favoring higher diversity in microbiota and achieves better growth performance [157].
To improve the efficiency of fish feeding, Hassaan et al., 2020, proposed to use inert clays as a supplement for sustainable aquaculture. Sericite is described as a fine white powder of muscovite form, which like other clay materials was used in the practice of geophagy as a gastrointestinal lining against inflammations, and ascribed an antimicrobial role [158]. Nile tilapia were used with a total of five isonitrogenous (315.65 g/kg crude protein) and isocaloric (18.7 MJ/kg crude energy) experimental diets. One control diet was used without sericite supplementation and the other four were fed with different concentrations of sericite (2.5, 5, 7.5, and 10 g/kg). Experimental feeding in tilapia had an effect on the linear growth of bacteria, E. coli and Enterobacteriaceae in the stomach and intestine as sericite supplementation increased, implying that sericite administration may have bactericidal effects to buffer the aqueous pH and oxidation state. At a general level the experimental diet improved growth performance, digestive enzymes, and hematological and immunological parameters in fish [159].
6. An Approach to the Functions of Microorganisms
Within the microbiome of production animals, the microorganisms with the highest participation evaluated in this review and assessed by its authors involve at the phylum level the Bacteroidetes, which are found in large numbers and represent more than half of the intestinal microbiome of animals. These are in greater numbers in the distal intestine, performing fermentation processes in polysaccharides and producing short-chain fatty acids (SCFA), which can contribute up to 10% of daily calories when the diet is rich in fiber [160]. As ruminants are similar both morphologically and functionally, many of the microorganisms found are coincident in their digestive tract. One genus frequently observed in studies was Prevotella belonging to the phylum Bacteroidetes. It has been shown that it can prevent rumen acidosis in cattle by preventing the proliferation of acid-producing bacteria that disrupt digestive processes in cattle [161]. On the other hand, its colonization has been shown to decrease the relative abundance of Firmicutes such as the Ruminococcaceae family known for their fibrolytic activity [162,163]. Although Prevotella is present in many animals, its abundance has been increased in diets containing carbohydrates with a high level of dietary fiber as in studies in ruminants [164]. such as Holstein dairy cows and Hu lambs, as proposed by Wang et al. (2020) and Du et al. (2022), respectively. However, the diet proposed by Cremonesi et al. (2018) presented a decrease in the Prevotella genus, in the face of a diet with flaxseed as a dietary lipid supplement in alpine goats. This has been observed in diets that integrate a high content of polyunsaturated fatty acids (PUFA), impairing the colonization of the phylum Bacteroidetes in ruminants [165].
The phylum that continues in abundance is Firmicutes, gram-negative bacteria associated with the phylum Bacteroidetes, found in similar proportions. Moreover, their interaction is closely related to the performance of anaerobic digestion processes [166]. Firmicutes bacteria have been found to be associated with protein and fat metabolic activity. Therefore, a diet with a high amount of protein and fat increases the proportion of Firmicutes bacteria [167]. In ruminant animals, bacteria of the species Ruminococcus flavefaciens belonging to the phylum Firmicutes are cellulolytic bacteria involved in the breakdown of cellulose in the rumen. This gram-positive bacterium is able to form a cellulosome, i.e., a multienzyme complex composed of four protein components (ScaA, ScaB ScaC and ScaE) and cellulose-substrate-degrading enzymes [168,169]. Another cellulolytic bacterium that hydrolyzes polysaccharides in ruminants is Ruminococcus albus [21]. As expected, both Ruminococcus flavefaciens and Ruminococcus albus were found in diets containing fiber in studies described by Zhu et al., 2017 and Yusuf et al., 2017. Now, their decrease in the diet that included Piper sarmentosum described by Zhou et al., 2020, hints that bioactive compounds inhibit bacteria of the genus Ruminococcus, which has also been described by other authors who describe compounds such as tannins and essential oils exerting an antimicrobial role on gram-positive bacteria [170]. The bacterium Butyrivibrio fibrisolvens, present in the gastrointestinal tract of ruminants [171], showed an increase in its population under a diet containing flaxseed oil and vitamin E, described by Yoshimura et al., 2018, due to its role in the biohydrogenation process and the increase in compounds involved in biohydrogenation such as stearic acid (C18:0). As for the genus Bifidobacterium, of the phylum Actinobacteria, the diet that employed grape pomace in lambs [65], a food with high antioxidant power, presented an increase in its population, a result that agrees with other diets that have implemented antioxidants and that have presented an improvement in meat quality in terms of fatty acid content in lambs [172]. Bacteria of the genus Lactococcus, belonging to the order Lactobacillales, are considered within the group of lactic acid producing bacteria (LAB) [173]. Their presence was detected in Holstein Friesian cows in the diet presenting Ascophyllum nodosum [151]. The increase triggered in Lactococcus lactis and Lactococcus garvieae populations could be attributed to the fact that Ascophyllum nodosum, brown algae composed of polysaccharides, peptides, and nutritional compounds, can be used by the genus Lactococcus for the production of lactic acid.
Other phyla, such as proteobacteria, have been found in lesser proportion in the investigations. They are generally gram-negative and are involved in the microbiota as facultative anaerobes, unlike the other microorganisms considered strict anaerobes. In ruminants, in the phylum of proteobacteria is the genus Succinivibrio, which has shown that it can produce propionate in the rumen in the same way as the genus Prevotella [174]. Its presence has been attributed to higher feed efficiency, higher milk production and its protein quality in dairy cows [175]. In diets proposed by Cremonesi et al. (2018) that integrated flaxseed in alpine goats, the genus Succinivibrio was increased, as was also seen before the integration of essential oils in diets [176].
In the studies evaluated, the presence of the phylum actinobacteria, a group of Gram-positive bacteria with genera such as Micrococcus, Mycobacterium, Amycolatopsis, Frankia, and Streptomyces, was also described. These bacteria are mainly known for generating bioactive natural products, which have played a role in the prevention of gastrointestinal and systemic diseases, giving them therapeutic use in pharmacological industries and as probiotics [177,178].
Additionally, other phyla were found in a smaller proportion, such as Fibrobacteres, Fusobacterium, and Euryarchaeota. In ruminants, bacteria belonging to the phylum Fibrobacteres are cellulolytic bacteria capable of degrading polysaccharides and are considered one of the major fiber degraders at the rumen level. The best-known species is the species Fibrobacter succinogenes [179]. In the diet proposed by Zhu et al. (2017), which used the fungus Saccharomyces cerevisiae, a positive effect on increasing Fibrobacter succinogenes was detected, as has also been seen with other cellulolytic bacteria in diets incorporating the fungus [180]. Similarly, diets used in goats [80,81], in treatments including Andrographis paniculata and Piper sarmentosum, respectively, showed a population increase in Fibrobacter succinogenes. In the former, possibly due to the increase in ruminal pH caused by ruminal bacteria, which subsequently provides benefits [181]. On the other hand, the decrease in Fibrobacter succinogenes populations after the inclusion of Piper sarmentosum suggests that the inhibitory causes of bioactive compounds, such as tannins, affect other gram-positive bacteria, as well as essential oils, which have been shown to inhibit the relative abundance of Fibrobacter succinogenes as with other metagenomic bacteria in the rumen [176].
The animals with only one true stomach have a varied morphology being of very different species from each other and even from different habitats. Starting with chickens, we found the genus Lactobacillus, belonging to the order Lactobacillales, as well as bacteria of the genus Lactococcus; therefore, they are proteolytic and fermenters of sugars for the production of lactic acid, and used as probiotics. The diet used by Abu Hafsa & Ibrahim (2018) that includes grape seeds promoted the colonization of Lactobacillus in chicks; the same result occurs in other studies using grapes, benefiting the population increase in Lactobacillus [182]. The cause is due to the antioxidant power of polyphenols which promotes the growth of lactic acid bacteria such as Lactobacillus and Bifidobacterium (Pozuelo et al., 2012). Similar results are observed in diets proposed by Kwak et al., 2021, which used a diet that included dietary soropholipids in chicks, and J. H. Park & Kim, 2020, which used an Achyranthes japonica in chicks where it increased the population of Lactobacillus.
In animals such as swine, Prevotella have been found to be associated with positive traits in feed intake, feed efficiency and weight gain, being able to ferment complex dietary polysaccharides [183]. Diets used by Sun et al., 2020, that used sodium butyrate, showed an increase in the Prevotella genus in the cecum, where it is attributed that sodium butyrate could positively modify the mucosal walls of the small intestine decreasing the entry of carbohydrates and proteins to the cecum and increasing the population of bacteroidetes including the Prevotella genus. This genus has been described as helping the maturation of the intestinal mucosa by the production of acetate [184]. Similar result has also been seen in chickens upon the inclusion of sodium butyrate (800 mg/kg) benefiting bacteroidetes proliferation [185]. The study by C. Wang et al., 2019, used a basal diet with tributyrin which increased the population of Lactobacillus, while other studies report that the use of tributyrin decreases Lactobacillus populations by reducing intestinal pH due to the production of butyric acid in the hydrolysis of tributyrin [186].
Studies conducted by Villasante et al. (2019) investigated the effects of different carbohydrates levels in the diet of fish species such as Salmo salar, Oncorhynchus mykiss and Oreochromis niloticus. These studies suggest that higher levels of digestible carbohydrates promote and increase in the relative abundance of Lactococcus populations. These finding have been replicated in subsequent studies as well [187].
Terova et al., 2019 used a diet based on black soldier fly in rainbow trout which increased the population of bacteria belonging to the genus Lactobacillus and butyrate-producing bacteria such as Clostridium. Similar results have been seen in chickens [188] and pigs [189] with diets involving Hermetia illucens. In addition, it should be considered that the chitin present in insect meal reduces the abundance of Proteobacteria as it is a type of insoluble fiber analogous to cellulose found in vegetables [190].
Finally, in cases where the Vibrio genus and Streptococcus iniae, a bacterial pathogen known to be a threat against marine species [191], were reduced in their population in the diet incorporating garlic (Allium sativum) by Foysal et al., 2019, in tilapia, this is attributed to the beneficial bioactive qualities found in garlic improving the immune response [192].
7. Conclusions
The study of the microbiome is multifactorial in nature. The events that cause variations in the population of microorganisms in the intestinal tract of production animals are diverse and are produced mainly by the type of diet and the chain of reactions it generates. However, factors such as the animal’s life stage, external conditions (climatic or captivity), social behavior, and microorganisms native to the intestinal tract can also affect microbial diversity. From the above studies, it is clear that the proportion of microorganisms varies among the different types of feed and that there is a clear difference between species. Ruminants are the most similar, since their microbial communities are related to the degradation of fiber and carbohydrates in ruminal fermentation, including the presence of methanogenic archaea. Similarly, the other species described have their own microbial populations with variations according to their type of diet, which changes as there is a greater relationship between carbohydrates, fat, and proteins, among others. It is emphasized that a diet that includes antioxidants or probiotics helps to promote microbes that benefit health and inhibit the increase in pathogenic strains. In addition, it has been suggested that certain microbial genera are related to positive aspects for production, as in lambs where the presence of Succiniclasticum correlates with the final weight. However, there is a lack of information to explain the role of certain microorganisms in a diet, such as the Cetobacterium genus that is inhibited in tilapia by the use of probiotics. In general, many of the studies reviewed do not consider aspects such as pH in the different sections of the gastrointestinal tract, morphological and histological aspects that could be key to a better understanding of how microorganisms interact with each other and with the gastrointestinal environment.
8. Future Perspectives
Microbiome-based studies will become more relevant as more knowledge is gained about its functions and existing variables. So far, the main focus of microbiome research in production animals has been to understand its changes under defined feeding strategies and how this influences performance. Understanding how diet components and various supplements affect microbial populations in the gastrointestinal tract of production animals could be very useful, since their modulation would be related to obtaining better quality livestock products, as well as better feed efficiency and resource optimization. The use of insects and sea products as potential foods of nutritional value and as replacements for commonly used dietary components of vegetable origin is highlighted. It is hoped that future studies can enhance their nutritional characteristics, taking into account environmental care, especially in the aquaculture area.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/microorganisms11092219/s1, Table S1: Summary of experimental diets in production animals.
Author Contributions
Conceptualization, R.H., E.A.P., J.Q. and R.D.; Data curation, R.H., L.V. and C.V.; Formal analysis, R.H.; Investigation, R.H. and C.V.; Methodology, J.Q.; Resources, J.Q. and R.D.; Supervision, D.T., G.S. and N.S.; Writing—original draft, R.H., C.V., L.V. and D.T.; Writing—review and editing, E.A.P., D.C., R.D., D.T. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Project PP22-0017. VRIP-UFRO. The authors would like to thank Fondecyt Project: Initiation in Research N°11220471 (J.Q.). The authors would like to thank Fondecyt Project: Initiation in Research N°11190621 (R.D.).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The Future of Food and Agriculture Trends and Challenges. 2017. Available online: www.fao.org/publications (accessed on 3 March 2023).
- Lederberg, J.; Mccray, A.T. Ome SweetOmics—A Genealogical Treasury of Words. Scientist 2001, 15, 8. Available online: https://go.gale.com/ps/i.do?id=GALE%7CA73535513&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=08903670&p=AONE&sw=w (accessed on 12 July 2022).
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V. Microbiome: Impact of Gender on Function & Characteristics of Gut Microbiome. In Principles of Gender-Specific Medicine: Gender in the Genomic Era, 3rd ed.; Academic Press: San Diego, CA, USA, 2017; pp. 569–583. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.S.; Harkins, D.M.; Nelson, K.E. Advances in Microbiome Research for Animal Health. Annu. Rev. Anim. Biosci. 2021, 9, 289–311. [Google Scholar] [CrossRef]
- Sirisinha, S. The potential impact of gut microbiota on your health: Current status and future challenges. Asian Pac. J. Allergy Immunol. 2016, 34, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in Ruminant Nutrition and Their Effects on Reproduction. Antioxidants 2022, 11, 970. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Tretola, M.; Silacci, P.; Sousa, R.; Colombo, F.; Panseri, S.; Ottoboni, M.; Pinotti, L.; Bee, G. Chestnut extracts decrease the in-vitro digestibility and polyphenol bioavailability of soy-based nutrients but protect the epithelial barrier function of pig jejunum segments after digestion. Anim. Feed. Sci. Technol. 2022, 294, 115501. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Bik, H.M. Microbial Metazoa Are Microbes Too. Msystems 2019, 4, e00109-19. [Google Scholar] [CrossRef] [PubMed]
- Téllez, G.; Lauková, A.; Latorre, J.D.; Hernandez-Velasco, X.; Hargis, B.M.; Callaway, T. Food-producing animals and their health in relation to human health. Microb. Ecol. Health Dis. 2015, 26, 25876. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; Brugman, S.; Warden, C.H.; Rebel, J.M.J.; Folkerts, G.; Pieterse, C.M.J. How Can We Define “Optimal Microbiota?”: A Comparative Review of Structure and Functions of Microbiota of Animals, Fish, and Plants in Agriculture. Front. Nutr. 2018, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Vorste, R.V.; Sarremejane, R.; Datry, T. Intermittent Rivers and Ephemeral Streams: A Unique Biome with Important Contributions to Biodiversity and Ecosystem Services. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar] [CrossRef]
- Na Shi, N.; Na Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Lange, M.; Lee, H.; Dallas, D.; Le Parc, A.; Bell, J.d.M.; Barile, D. Determining Functional Properties and Sources of Recently Identified Bioactive Food Components: Oligosaccharides, Glycolipids, Glycoproteins, and Peptides. Encycl. Agric. Food Syst. 2014, 441–461. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Semenova, E.V.; Manzhurina, O.A.; Parkhomenko, Y.S. Review article: Key aspects of mammal microbiome development. Veter.-Sci. Today 2021, 1, 68–71. [Google Scholar] [CrossRef]
- Yeoman, C.J.; White, B.A. Gastrointestinal Tract Microbiota and Probiotics in Production Animals. Annu. Rev. Anim. Biosci. 2014, 2, 469–486. [Google Scholar] [CrossRef]
- Arshad, M.A.; Hassan, F.-U.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut microbiome colonization and development in neonatal ruminants: Strategies, prospects, and opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef]
- Loor, J.J.; Elolimy, A.A. Immunometabolism in livestock: Triggers and physiological role of transcription regulators, nutrients, and microbiota. Anim. Front. 2022, 12, 13–22. [Google Scholar] [CrossRef]
- Sarkar, A.; Harty, S.; Johnson, K.V.-A.; Moeller, A.H.; Archie, E.A.; Schell, L.D.; Carmody, R.N.; Clutton-Brock, T.H.; Dunbar, R.I.M.; Burnet, P.W.J. Microbial transmission in animal social networks and the social microbiome. Nat. Ecol. Evol. 2020, 4, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Reese, A.T. Possibilities and limits for using the gut microbiome to improve captive animal health. Anim. Microbiome 2021, 3, 89. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, V.J.; Song, S.J.; Delsuc, F.; Prest, T.L.; Oliverio, A.M.; Korpita, T.M.; Alexiev, A.; Amato, K.R.; Metcalf, J.L.; Kowalewski, M.; et al. The Effects of Captivity on the Mammalian Gut Microbiome. Integr. Comp. Biol. 2017, 57, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Attwood, G.T.; Wakelin, S.A.; Leahy, S.C.; Rowe, S.; Clarke, S.; Chapman, D.F.; Muirhead, R.; Jacobs, J.M.E. Applications of the Soil, Plant and Rumen Microbiomes in Pastoral Agriculture. Front. Nutr. 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Chen, H.; Luo, X.; Guan, J.; He, Y.; Zhao, X. Microbial diversity in the rumen, reticulum, omasum, and abomasum of yak on a rapid fattening regime in an agro-pastoral transition zone. J. Microbiol. 2018, 56, 734–743. [Google Scholar] [CrossRef]
- Swanson, K.C. Small Intestinal Anatomy, Physiology, and Digestion in Ruminants. Ref. Modul. Food Sci. 2019, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhury, P.K.; Carro, M.D.; Griffith, G.W.; Dagar, S.S.; Puniya, M.; Calabro, S.; Ravella, S.R.; Dhewa, T.; Upadhyay, R.C.; et al. New aspects and strategies for methane mitigation from ruminants. Appl. Microbiol. Biotechnol. 2014, 98, 31–44. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Kittelmann, S.; Pinares-Patiño, C.S.; Seedorf, H.; Kirk, M.R.; Ganesh, S.; McEwan, J.C.; Janssen, P.H. Two Different Bacterial Community Types Are Linked with the Low-Methane Emission Trait in Sheep. PLoS ONE 2014, 9, e103171. [Google Scholar] [CrossRef]
- Ramayo-Caldas, Y.; Zingaretti, L.; Popova, M.; Estellé, J.; Bernard, A.; Pons, N.; Bellot, P.; Mach, N.; Rau, A.; Roume, H.; et al. Identification of rumen microbial biomarkers linked to methane emission in Holstein dairy cows. J. Anim. Breed. Genet. 2020, 137, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Króliczewska, B.; Pecka-Kiełb, E.; Bujok, J. Strategies Used to Reduce Methane Emissions from Ruminants: Controversies and Issues. Agriculture 2023, 13, 602. [Google Scholar] [CrossRef]
- Millen, D.D.; Pacheco, R.D.L.; da Silva Cabral, L.; Cursino, L.L.; Watanabe, D.H.M.; Rigueiro, A.L.N. Ruminal acidosis. In Rmenology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 127–156. [Google Scholar] [CrossRef]
- Valente, T.N.P.; Lima, E.S.; Santos, W.B.R.; Cesário, A.S.; Tavares, C.J.; Fernandes, Ã.L.; Freitas, M.A.M. Ruminal microorganism consideration and protein used in the metabolism of the ruminants: A review. Afr. J. Microbiol. Res. 2016, 10, 456–464. [Google Scholar] [CrossRef]
- Rinke, C.; Chuvochina, M.; Mussig, A.J.; Chaumeil, P.-A.; Davín, A.A.; Waite, D.W.; Whitman, W.B.; Parks, D.H.; Hugenholtz, P. A standardized archaeal taxonomy for the Genome Taxonomy Database. Nat. Microbiol. 2021, 6, 946–959. [Google Scholar] [CrossRef]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef]
- Choudhury, P.K.; Jena, R.; Tomar, S.K.; Puniya, A.K. Reducing Enteric Methanogenesis through Alternate Hydrogen Sinks in the Rumen. Methane 2022, 1, 320–341. [Google Scholar] [CrossRef]
- Williams, C.; Thomas, B.J.; McEwan, N.R.; Stevens, P.R.; Creevey, C.; Huws, S.A. Rumen Protozoa Play a Significant Role in Fungal Predation and Plant Carbohydrate Breakdown. Front. Microbiol. 2020, 11, 720. [Google Scholar] [CrossRef]
- Elghandour, M.M.; Khusro, A.; Adegbeye, M.J.; Tan, Z.; Abu Hafsa, S.H.; Greiner, R.; Ugbogu, E.A.; Anele, U.Y.; Salem, A.Z.M. Dynamic role of single-celled fungi in ruminal microbial ecology and activities. J. Appl. Microbiol. 2020, 128, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Gotoh, T.; Greenwood, P.L. Current situation and future prospects for global beef production: Overview of special issue. Asian-Australas. J. Anim. Sci. 2018, 31, 927–932. [Google Scholar] [CrossRef]
- Livestock and Poultry: World Markets and Trade|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/livestock-and-poultry-world-markets-and-trade (accessed on 14 July 2022).
- Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- USDA. Dairy Production and Trade Developments. 2021. Available online: https://usda.library.cornell.edu/concern/publications/5t34sj56t?locale=en (accessed on 9 September 2022).
- Britt, J.H.; Cushman, R.A.; Dechow, C.D.; Dobson, H.; Humblot, P.; Hutjens, M.F.; Jones, G.A.; Ruegg, P.S.; Sheldon, I.M.; Stevenson, J.S. Invited review: Learning from the future—A vision for dairy farms and cows in 2067. J. Dairy Sci. 2018, 101, 3722–3741. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: Membership and potential function. Sci. Rep. 2015, 5, 16116. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yin, J.; Liu, X.; Jia, B.; Chang, Z.; Lu, H.; Jiang, N.; Chen, Q. First insights into the microbial diversity in the omasum and reticulum of bovine using Illumina sequencing. J. Appl. Genet. 2015, 56, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhang, Y.; Wang, L. The Effects of Different Concentrate-to-Forage Ratio Diets on Rumen Bacterial Microbiota and the Structures of Holstein Cows during the Feeding Cycle. Animals 2020, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Chaves Lopez, C.; Serio, A.; Rossi, C.; Mazzarrino, G.; Marchetti, S.; Castellani, F.; Grotta, L.; Fiorentino, F.P.; Paparella, A.; Martino, G. Effect of diet supplementation with Ascophyllum nodosum on cow milk composition and microbiota. J. Dairy Sci. 2016, 99, 6285–6297. [Google Scholar] [CrossRef] [PubMed]
- Farid, F.; Sideeq, O.; Khan, F.; Niaz, K. Saccharomyces cerevisiae. In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 501–508. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Biobutanol from lignocellulosic biomass and microalgae: Scope, technology, and economics. In Sustainable Biofuels; Academic Press: San Diego, CA, USA, 2021; pp. 163–223. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Xu, N.; Yang, F.; Yoon, I.; Chung, Y.; Liu, J.; Wang, J. Effects of Saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J. Anim. Sci. Biotechnol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in animals: Metabolism and effects in livestock and occurrence in feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef]
- Kasparovska, J.; Pecinkova, M.; Dadakova, K.; Krizova, L.; Hadrova, S.; Lexa, M.; Lochman, J.; Kasparovsky, T. Effects of Isoflavone-Enriched Feed on the Rumen Microbiota in Dairy Cows. PLoS ONE 2016, 11, e0154642. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Guo, W.; Zhao, N.; Chen, F.; Fan, Q.; Zhang, W.; Huang, S.; Zhou, G.; Fu, T.; Wei, J. Effects of diets with various levels of forage rape (Brassi canapus) on growth performance, carcass traits, meat quality and rumen microbiota of Hu lambs. J. Sci. Food Agric. 2022, 102, 1281–1291. [Google Scholar] [CrossRef]
- Yoshimura, E.; Santos, N.; Machado, E.; Agustinho, B.; Pereira, L.; de Aguiar, S.; Franzolin, R.; Gasparino, E.; dos Santos, G.; Zeoula, L. Effects of dairy cow diets supplied with flaxseed oil and propolis extract, with or without vitamin E, on the ruminal microbiota, biohydrogenation, and digestion. Anim. Feed. Sci. Technol. 2018, 241, 163–172. [Google Scholar] [CrossRef]
- Morris, S.T. Overview of sheep production systems. In Advances in Sheep Welfare; Woodhead Publishing: Sawston, UK, 2017; pp. 19–35. [Google Scholar] [CrossRef]
- Wang, J.; Fan, H.; Han, Y.; Zhao, J.; Zhou, Z. Characterization of the microbial communities along the gastrointestinal tract of sheep by 454 pyrosequencing analysis. Asian-Australas. J. Anim. Sci. 2017, 30, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zeng, D.; Ni, X.; Zhu, H.; Jian, P.; Zhou, Y.; Xu, S.; Lin, Y.; Li, Y.; Yin, Z.; et al. Microbial community compositions in the gastrointestinal tract of Chinese Mongolian sheep using Illumina MiSeq sequencing revealed high microbial diversity. AMB Express 2017, 7, 75. [Google Scholar] [CrossRef]
- Du, S.; You, S.; Sun, L.; Wang, X.; Jia, Y.; Zhou, Y. Effects of Replacing Alfalfa Hay With Native Grass Hay in Pelleted Total Mixed Ration on Physicochemical Parameters, Fatty Acid Profile, and Rumen Microbiota in Lamb. Front. Microbiol. 2022, 13, 861025. [Google Scholar] [CrossRef] [PubMed]
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica Napus Rapeseed. In The Brassica Napus Genome; Springer: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Zhao, J.X.; Li, Q.; Zhang, R.X.; Liu, W.Z.; Ren, Y.S.; Zhang, C.X.; Zhang, J.X. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim. Feed Sci. Technol. 2018, 236, 76–85. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos, K.; Stagos, D.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2017, 101, e108–e121. [Google Scholar] [CrossRef]
- Park, J.; Kim, I. Effects of dietary Achyranthes japonica extract supplementation on the growth performance, total tract digestibility, cecal microflora, excreta noxious gas emission, and meat quality of broiler chickens. Poult. Sci. 2020, 99, 463–470. [Google Scholar] [CrossRef]
- Lv, X.; Cui, K.; Qi, M.; Wang, S.; Diao, Q.; Zhang, N. Ruminal Microbiota and Fermentation in Response to Dietary Protein and Energy Levels in Weaned Lambs. Animals 2020, 10, 109. [Google Scholar] [CrossRef]
- Greenhalgh, S.; Chrystal, P.V.; Selle, P.H.; Liu, S.Y. Reduced-crude protein diets in chicken-meat production: Justification for an imperative. World Poult. Sci. J. 2020, 76, 537–548. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef]
- Gebeyew, K.; Yang, C.; He, Z.; Tan, Z. Low-protein diets supplemented with methionine and lysine alter the gut microbiota composition and improve the immune status of growing lambs. Appl. Microbiol. Biotechnol. 2021, 105, 8393–8410. [Google Scholar] [CrossRef]
- Webb, E. Goat meat production, composition, and quality. Anim. Front. 2014, 4, 33–37. [Google Scholar] [CrossRef]
- Mazhangara, I.R.; Chivandi, E.; Mupangwa, J.F.; Muchenje, V. The Potential of Goat Meat in the Red Meat Industry. Sustainability 2019, 11, 3671. [Google Scholar] [CrossRef]
- Guerra, V.; Tiago, I.; Aires, A.; Coelho, C.; Nunes, J.; Martins, L.O.; Veríssimo, A. The gastrointestinal microbiome of browsing goats (Capra hircus). PLoS ONE 2022, 17, e0276262. [Google Scholar] [CrossRef]
- Wang, L.; Jin, L.; Xue, B.; Wang, Z.; Peng, Q. Characterizing the bacterial community across the gastrointestinal tract of goats: Composition and potential function. Microbiologyopen 2019, 8, e00820. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, K.; Guo, M.; Li, G.; Li, C.; Li, B.; Yang, Y.; Chen, Y.; Wang, X. Exploring the Spatial-Temporal Microbiota of Compound Stomachs in a Pre-Weaned Goat Model. Front. Microbiol. 2018, 9, 1846. [Google Scholar] [CrossRef]
- Lock, A.L.; O’Donnell-Megaro, A.M.; Bauman, D.E. Conjugated Linoleic Acid. In Encyclopedia of Dairy Sciences; Academic Press: San Diego, CA, USA, 2021; pp. 798–802. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Cremonesi, P.; Conte, G.; Severgnini, M.; Turri, F.; Monni, A.; Capra, E.; Rapetti, L.; Colombini, S.; Chessa, S.; Battelli, G.; et al. Evaluation of the effects of different diets on microbiome diversity and fatty acid composition of rumen liquor in dairy goat. Animal 2018, 12, 1856–1866. [Google Scholar] [CrossRef]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and Its Bioactive Diterpenoids against Inflammation and Oxidative Stress in Keratinocytes. Antioxidants 2020, 9, 530. [Google Scholar] [CrossRef]
- Yusuf, A.L.; Adeyemi, K.D.; Samsudin, A.A.; Goh, Y.M.; Alimon, A.R.; Sazili, A.Q. Effects of dietary supplementation of leaves and whole plant of Andrographis paniculata on rumen fermentation, fatty acid composition and microbiota in goats. BMC Veter.-Res. 2017, 13, 349. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, D.; Hu, H.; Zhou, H. Effects of Piper sarmentosum extract supplementation on growth performances and rumen fermentation and microflora characteristics in goats. J. Anim. Physiol. Anim. Nutr. 2020, 104, 431–438. [Google Scholar] [CrossRef]
- Wang, K.; Peng, X.; Lv, F.; Zheng, M.; Long, D.; Mao, H.; Si, H.; Zhang, P. Microbiome-Metabolites Analysis Reveals Unhealthy Alterations in the Gut Microbiota but Improved Meat Quality with a High-Rice Diet Challenge in a Small Ruminant Model. Animals 2021, 11, 2306. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Al-Sagheer, A.; Noreldin, A.E.; El-Hack, M.E.A.; Khafaga, A.F.; Abdel-Latif, M.A.; Swelum, A.A.; Arif, M.; Salem, A.Z.M. Beneficial effects of rumen-protected methionine on nitrogen-use efficiency, histological parameters, productivity and reproductive performance of ruminants. Anim. Biotechnol. 2019, 32, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yan, J.; Shen, W.; Song, Y.; Lan, X.; Yi, K.; Muhammad, A.U.R. Effect of inclusion of HMBi in the ration of goats on feed intake, nutrient digestibility, rumen bacteria community and blood serum parameters. J. Anim. Physiol. Anim. Nutr. 2020, 104, 987–997. [Google Scholar] [CrossRef]
- Kobayashi, R.; Nagaoka, K.; Nishimura, N.; Koike, S.; Takahashi, E.; Niimi, K.; Murase, H.; Kinjo, T.; Tsukahara, T.; Inoue, R. Comparison of the fecal microbiota of two monogastric herbivorous and five omnivorous mammals. Anim. Sci. J. 2020, 91, e13366. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Maltecca, C.; Tiezzi, F. Potential Use of Gut Microbiota Composition as a Biomarker of Heat Stress in Monogastric Species: A Review. Animals 2021, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, S.; Yan, C. Gut Microbiota Implications for Health and Welfare in Farm Animals: A Review. Animals 2021, 12, 93. [Google Scholar] [CrossRef]
- López-Andrés, J.J.; Aguilar-Lasserre, A.A.; Morales-Mendoza, L.F.; Azzaro-Pantel, C.; Pérez-Gallardo, J.R.; Rico-Contreras, J.O. Environmental impact assessment of chicken meat production via an integrated methodology based on LCA, simulation and genetic algorithms. J. Clean. Prod. 2018, 174, 477–491. [Google Scholar] [CrossRef]
- Svihus, B. Function of the digestive system. J. Appl. Poult. Res. 2014, 23, 306–314. [Google Scholar] [CrossRef]
- Kırkpınar, F.; Açıkgöz, Z. Feeding. In Animal Husbandry and Nutrition; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Denbow, D.M. Gastrointestinal Anatomy and Physiology. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 337–366. [Google Scholar] [CrossRef]
- Zhou, Q.; Lan, F.; Li, X.; Yan, W.; Sun, C.; Li, J.; Yang, N.; Wen, C. The Spatial and Temporal Characterization of Gut Microbiota in Broilers. Front. Veter.-Sci. 2021, 8, 712226. [Google Scholar] [CrossRef]
- Stamilla, A.; Ruiz-Ruiz, S.; Artacho, A.; Pons, J.; Messina, A.; Randazzo, C.L.; Caggia, C.; Lanza, M.; Moya, A. Analysis of the Microbial Intestinal Tract in Broiler Chickens during the Rearing Period. Biology 2021, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, S.I.; Kim, I.H. Achyranthes japonica extracts supplementation to growing pigs positively influences growth performance, nutrient digestibility, fecal microbial shedding, and fecal gas emission. Anim. Biosci. 2021, 34, 427–433. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Ibrahim, S.A. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2017, 102, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.L.; Patience, J.F. Xylanase supplementation in corn-based swine diets: A review with emphasis on potential mechanisms of action. J. Anim. Sci. 2020, 98, skaa318. [Google Scholar] [CrossRef] [PubMed]
- Kubiś, M.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Sassek, M.; Konieczka, P.; Górka, P.; Flaga, J.; Katarzyńska-Banasik, D.; Hejdysz, M.; Wiśniewska, Z.; et al. Emulsifier and Xylanase Can Modulate the Gut Microbiota Activity of Broiler Chickens. Animals 2020, 10, 2197. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, E.I.; Van Geem, K.M.; Stevens, C.V.; Van Bogaert, I.N. Sophorolipid Modification: The Power of Yeasts and Enzymes. In Lipid Modification by Enzymes and Engineered Microbes; AOCS Press: Champaign, IL, USA, 2018; pp. 315–341. [Google Scholar] [CrossRef]
- Kwak, M.-J.; Park, M.-Y.; Choi, Y.-S.; Cho, J.; Pathiraja, D.; Kim, J.; Lee, H.; Choi, I.-G.; Whang, K.-Y. Dietary sophorolipid accelerates growth by modulation of gut microbiota population and intestinal environments in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Porcine models of digestive disease: The future of large animal translational research. Transl. Res. 2015, 166, 12–27. [Google Scholar] [CrossRef]
- Hu, J.; Chen, L.; Tang, Y.; Xie, C.; Xu, B.; Shi, M.; Zheng, W.; Zhou, S.; Wang, X.; Liu, L.; et al. Standardized Preparation for Fecal Microbiota Transplantation in Pigs. Front. Microbiol. 2018, 9, 1328. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.R.; Friendship, R.M. Digestive System. In Diseases of Swine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 234–263. [Google Scholar] [CrossRef]
- Holman, D.; Brunelle, B.W.; Trachsel, J.; Allen, H.K. Meta-analysis To Define a Core Microbiota in the Swine Gut. Msystems 2017, 2, e00004-17. [Google Scholar] [CrossRef]
- Jones, D.L.; Cross, P.; Withers, P.J.A.; DeLuca, T.H.; Robinson, D.A.; Quilliam, R.S.; Harris, I.M.; Chadwick, D.R.; Edwards-Jones, G. REVIEW: Nutrient stripping: The global disparity between food security and soil nutrient stocks. J. Appl. Ecol. 2013, 50, 851–862. [Google Scholar] [CrossRef]
- Pinotti, L.; Giromini, C.; Ottoboni, M.; Tretola, M.; Marchis, D. Review: Insects and former foodstuffs for upgrading food waste biomasses/streams to feed ingredients for farm animals. Animal 2019, 13, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Tretola, M.; Luciano, A.; Ottoboni, M.; Baldi, A.; Pinotti, L. Influence of Traditional vs. Alternative Dietary Carbohydrates Sources on the Large Intestinal Microbiota in Post-Weaning Piglets. Animals 2019, 9, 516. [Google Scholar] [CrossRef]
- Grosu, I.A.; Pistol, G.C.; Taranu, I.; Marin, D.E. The Impact of Dietary Grape Seed Meal on Healthy and Aflatoxin B1 Afflicted Microbiota of Pigs after Weaning. Toxins 2019, 11, 25. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Shen, Z.; Hong, Q.; Feng, J.; Peng, Y.; Hu, C. Effects of dietary tributyrin on intestinal mucosa development, mitochondrial function and AMPK-mTOR pathway in weaned pigs. J. Anim. Sci. Biotechnol. 2019, 10, 93. [Google Scholar] [CrossRef]
- Hu, Q.; Yin, F.; Li, B.; Guo, Y.; Yin, Y. Dietary Tributyrin Administration Improves Intestinal Morphology and Selected Bacterial and Short-Chain Fatty Acid Profiles in Broilers Under an Isocaloric Feeding Regime. Front. Microbiol. 2021, 12, 715712. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, J.; Li, M.; Xu, Q.; Zhang, X.; Tang, Z.; Chen, J.; Zhen, J.; Sun, Z. The effects of dietary sodium butyrate supplementation on the growth performance, carcass traits and intestinal microbiota of growing-finishing pigs. J. Appl. Microbiol. 2020, 128, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Z.; Cao, S.; Zhang, Q.; Peng, Y.; Hong, Q.; Feng, J.; Hu, C. Effects of tributyrin on growth performance, intestinal microflora and barrier function of weaned pigs. Anim. Feed. Sci. Technol. 2019, 258, 114311. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus coagulans in the Food Industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Kiarie, E.G.; Leung, H.; Kakhki, R.A.M.; Patterson, R.; Barta, J.R. Utility of Feed Enzymes and Yeast Derivatives in Ameliorating Deleterious Effects of Coccidiosis on Intestinal Health and Function in Broiler Chickens. Front. Veter.-Sci. 2019, 6, 473. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Yu, B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2019, 5, 366–372. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Duan, H.; Zhao, R.; Xiao, Y.; Guo, M.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. The Protection of Lactiplantibacillus plantarum CCFM8661 Against Benzopyrene-Induced Toxicity via Regulation of the Gut Microbiota. Front. Immunol. 2021, 12, 736129. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.-Y.; Choi, Y.-J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [PubMed]
- Maulu, S.; Nawanzi, K.; Abdel-Tawwab, M.; Khalil, H.S. Fish Nutritional Value as an Approach to Children’s Nutrition. Front. Nutr. 2021, 8, 1090. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2020; FAO-Food & Agriculture Organization of the United Nation: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Rodiles, A. 10–The fish microbiome and its interactions with mucosal tissues. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 273–295. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The Gut Microbiota of Marine Fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ding, L.; Huang, Z.; Xu, H.; Xu, Z. Commensal bacteria-immunity crosstalk shapes mucosal homeostasis in teleost fish. Rev. Aquac. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]
- Optimism Persists in Farmed Salmon Sector Despite Price Lull|GLOBEFISH|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/in-action/globefish/marketreports/resource-detail/es/c/1263849/ (accessed on 20 July 2022).
- Navarrete, P.; Espejo, R.T.; Romero, J. Molecular Analysis of Microbiota along the Digestive Tract of Juvenile Atlantic Salmon (Salmo salar L.). Microb. Ecol. 2009, 57, 550–561. [Google Scholar] [CrossRef]
- Schmidt, V.; Amaral-Zettler, L.; Davidson, J.; Summerfelt, S.; Good, C. Influence of Fishmeal-Free Diets on Microbial Communities in Atlantic Salmon (Salmo salar) Recirculation Aquaculture Systems. Appl. Environ. Microbiol. 2016, 82, 4470–4481. [Google Scholar] [CrossRef] [PubMed]
- Gajardo, K.; Rodiles, A.; Kortner, T.M.; Krogdahl, Å.; Bakke, A.M.; Merrifield, D.L.; Sørum, H. A high-resolution map of the gut microbiota in Atlantic salmon (Salmo salar): A basis for comparative gut microbial research. Sci. Rep. 2016, 6, 30893. [Google Scholar] [CrossRef] [PubMed]
- Onwezen, M.C.; van den Puttelaar, J.; Verain, M.C.D.; Veldkamp, T. Consumer acceptance of insects as food and feed: The relevance of affective factors. Food Qual. Prefer. 2019, 77, 51–63. [Google Scholar] [CrossRef]
- Li, Y.; Bruni, L.; Jaramillo-Torres, A.; Gajardo, K.; Kortner, T.M.; Krogdahl, Å. Differential response of digesta- and mucosa-associated intestinal microbiota to dietary insect meal during the seawater phase of Atlantic salmon. Anim. Microbiome 2021, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Catalán, N.; Villasante, A.; Wacyk, J.; Ramírez, C.; Romero, J. Fermented Soybean Meal Increases Lactic Acid Bacteria in Gut Microbiota of Atlantic Salmon (Salmo salar). Probiotics Antimicrob. Proteins 2018, 10, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Villasante, A.; Ramírez, C.; Catalán, N.; Opazo, R.; Dantagnan, P.; Romero, J. Effect of Dietary Carbohydrate-to-Protein Ratio on Gut Microbiota in Atlantic Salmon (Salmo salar). Animals 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Verdile, N.; Pasquariello, R.; Scolari, M.; Scirè, G.; Brevini, T.A.L.; Gandolfi, F. A Detailed Study of Rainbow Trout (Onchorhynchus mykiss) Intestine Revealed That Digestive and Absorptive Functions Are Not Linearly Distributed along Its Length. Animals 2020, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- de Felice, E.; Palladino, A.; Tardella, F.M.; Giaquinto, D.; Barone, C.M.A.; Crasto, A.; Scocco, P. A morphological, glycohistochemical and ultrastructural study on the stomach of adult Rainbow trout Oncorhynchus mykiss. Eur. Zool. J. 2021, 88, 269–278. [Google Scholar] [CrossRef]
- Lyons, P.P.; Turnbull, J.; Dawson, K.A.; Crumlish, M. Exploring the microbial diversity of the distal intestinal lumen and mucosa of farmed rainbow troutOncorhynchus mykiss(Walbaum) using next generation sequencing (NGS). Aquac. Res. 2015, 48, 77–91. [Google Scholar] [CrossRef]
- Rimoldi, S.; Antonini, M.; Gasco, L.; Moroni, F.; Terova, G. Intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) may be improved by feeding a Hermetia illucens meal/low-fishmeal diet. Fish Physiol. Biochem. 2021, 47, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Waldrop, T.; Summerfelt, S.; Davidson, J.; Barrows, F.; Kenney, P.B.; Welch, T.; Wiens, G.D.; Snekvik, K.; Rawls, J.F.; et al. Aquacultured Rainbow Trout (Oncorhynchus mykiss) Possess a Large Core Intestinal Microbiota That Is Resistant to Variation in Diet and Rearing Density. Appl. Environ. Microbiol. 2013, 79, 4974–4984. [Google Scholar] [CrossRef]
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals Fed Insect-Based Diets: State-of-the-Art on Digestibility, Performance and Product Quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- Mikołajczak, Z.; Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. The Effect of Hydrolyzed Insect Meals in Sea Trout Fingerling (Salmo trutta m. trutta) Diets on Growth Performance, Microbiota and Biochemical Blood Parameters. Animals 2020, 10, 1031. [Google Scholar] [CrossRef]
- Antoniussen, C.S.; Rasmussen, H.H.; Holst, M.; Lauridsen, C. Reducing Disease Activity of Inflammatory Bowel Disease by Consumption of Plant-Based Foods and Nutrients. Front. Nutr. 2021, 8, 733433. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Psofakis, P.; Mente, E.; Malandrakis, E.; Golomazou, E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac. Nutr. 2019, 25, 3–14. [Google Scholar] [CrossRef]
- Gaudioso, G.; Marzorati, G.; Faccenda, F.; Weil, T.; Lunelli, F.; Cardinaletti, G.; Marino, G.; Olivotto, I.; Parisi, G.; Tibaldi, E.; et al. Processed Animal Proteins from Insect and Poultry By-Products in a Fish Meal-Free Diet for Rainbow Trout: Impact on Intestinal Microbiota and Inflammatory Markers. Int. J. Mol. Sci. 2021, 22, 5454. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, F.; Wang, X.; Chen, W.; Meng, X. Transcriptomic responses to yam (Dioscorea oppositifolia L.) extract dietary supplementation in rainbow trout (Oncorhynchus mykiss) liver. Aquac. Res. 2019, 51, 932–945. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Liu, F.; Chen, W. Effects of Chinese yam (Dioscorea oppositifolia L.) dietary supplementation on intestinal microflora, digestive enzyme activity and immunity in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2020, 51, 4698–4712. [Google Scholar] [CrossRef]
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Tsegay, T.; Natarajan, P.; Zelealem, T. Analysis of Diet and Biochemical Composition of Nile Tilapia (O. niloticus) from Tekeze Reservoir and Lake Hashenge, Ethiopia. J. Fish. Livest. Prod. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Phrompanya, P.; Saenphet, K.; Saenphet, S. Comparative histochemical study of the gastrointestinal tracts of the Nile tilapia (Oreochromis niloticus) and the hybrid catfish (Clarias batrachus × Clarias gariepinus). Acta Histochem. 2019, 121, 261–267. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z.; Yi, M.; Liu, Z.; Ke, X.; Gao, F.; Cao, J.; Wang, M.; Chen, G.; Lu, M. Characterization of the core gut microbiota of Nile tilapia (Oreochromis niloticus): Indication of a putative novel Cetobacterium species and analysis of its potential function on nutrition. Arch. Microbiol. 2022, 204, 690. [Google Scholar] [CrossRef]
- Bereded, N.K.; Curto, M.; Domig, K.J.; Abebe, G.B.; Fanta, S.W.; Waidbacher, H.; Meimberg, H. Metabarcoding Analyses of Gut Microbiota of Nile Tilapia (Oreochromis niloticus) from Lake Awassa and Lake Chamo, Ethiopia. Microorganisms 2020, 8, 1040. [Google Scholar] [CrossRef] [PubMed]
- Zaefarian, A.; Yeganeh, S.; Adhami, B. Dietary effects of garlic powder (Allium sativum) on growth, blood indices, carcass composition, and lysozyme activity in brown trout (Salmo caspius) and resistance against Yersinia ruckeri infection. Aquac. Int. 2017, 25, 1987–1996. [Google Scholar] [CrossRef]
- Zare, M.; Tran, H.Q.; Prokešová, M.; Stejskal, V. Effects of Garlic Allium sativum Powder on Nutrient Digestibility, Haematology, and Immune and Stress Responses in Eurasian Perch Perca fluviatilis Juveniles. Animals 2021, 11, 2735. [Google Scholar] [CrossRef]
- Osman, K.M.; Al-Maary, K.S.; Mubarak, A.S.; Dawoud, T.M.; Moussa, I.M.I.; Ibrahim, M.D.S.; Hessain, A.M.; Orabi, A.; Fawzy, N.M. Characterization and susceptibility of streptococci and enterococci isolated from Nile tilapia (Oreochromis niloticus) showing septicaemia in aquaculture and wild sites in Egypt. BMC Veter.-Res. 2017, 13, 357. [Google Scholar] [CrossRef]
- Foysal, J.; Alam, M.; Momtaz, F.; Chaklader, R.; Siddik, M.A.B.; Cole, A.; Fotedar, R.; Rahman, M. Dietary supplementation of garlic (Allium sativum) modulates gut microbiota and health status of tilapia (Oreochromis niloticus) against Streptococcus iniae infection. Aquac. Res. 2019, 50, 2107–2116. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Ling, H.; Luo, L.; Qi, D.; Feng, L. The effect of dietary supplementation with Clostridium butyricum on the growth performance, immunity, intestinal microbiota and disease resistance of tilapia (Oreochromis niloticus). PLoS ONE 2019, 14, e0223428. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Skorochod, I. Microbial interaction with clay minerals and its environmental and biotechnological implications. Minerals 2020, 10, 861. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Palma, J.; Shawer, E.E.; El-Haroun, E. The effect of dietary sericite on growth performance, digestive enzymes activity, gut microbiota and haematological parameters of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Anim. Feed. Sci. Technol. 2020, 262, 114400. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Margolis, E.; Fredricks, D.N. Bacterial Vaginosis-Associated Bacteria. In Molecular Medical Microbiology, 2nd ed.; Academic Press: San Diego, CA, USA, 2015; pp. 1487–1496. [Google Scholar] [CrossRef]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Pacífico, C.; Petri, R.M.; Ricci, S.; Mickdam, E.; Wetzels, S.U.; Neubauer, V.; Zebeli, Q. Unveiling the Bovine Epimural Microbiota Composition and Putative Function. Microorganisms 2021, 9, 342. [Google Scholar] [CrossRef]
- Wu, X.; Elekwachi, C.O.; Bai, S.; Luo, Y.; Zhang, K.; Forster, R.J. Characterizing the Alteration in Rumen Microbiome and Carbohydrate-Active Enzymes Profile with Forage of Muskoxen Rumen through Comparative Metatranscriptomics. Microorganisms 2022, 10, 71. [Google Scholar] [CrossRef]
- Cancino-Padilla, N.; Catalán, N.; Siu-Ting, K.; Creevey, C.J.; Huws, S.A.; Romero, J.; Vargas-Bello-Pérez, E. Long-Term Effects of Dietary Supplementation with Olive Oil and Hydrogenated Vegetable Oil on the Rumen Microbiome of Dairy Cows. Microorganisms 2021, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheng, H.; Wyckoff, K.N.; He, Q. Linkages of Firmicutes and Bacteroidetes populations to methanogenic process performance. J. Ind. Microbiol. Biotechnol. 2016, 43, 771–781. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, D.; Kim, K.-W.; Lee, S.-H.; Jang, A. Comparative analysis of the gut microbiota of mice fed a diet supplemented with raw and cooked beef loin powder. Sci. Rep. 2021, 11, 11489. [Google Scholar] [CrossRef] [PubMed]
- Artzi, L.; Bayer, E.A.; Morais, S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Genet. 2016, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Brulc, J.M.; Yeoman, C.J.; Wilson, M.K.; Miller, M.E.B.; Jeraldo, P.; Jindou, S.; Goldenfeld, N.; Flint, H.J.; Lamed, R.; Borovok, I.; et al. Cellulosomics, a Gene-Centric Approach to Investigating the Intraspecific Diversity and Adaptation of Ruminococcus flavefaciens within the Rumen. PLoS ONE 2011, 6, e25329. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Palevich, N.; Kelly, W.J.; Leahy, S.C.; Denman, S.; Altermann, E.; Rakonjac, J.; Attwood, G.T. Comparative genomics of rumen Butyrivibrio spp. uncovers a continuum of polysaccharide-degrading capabilities. Appl. Environ. Microbiol. 2020, 86, e01993-19. [Google Scholar] [CrossRef]
- Wang, B.; Luo, H. Effects of mulberry leaf silage on antioxidant and immunomodulatory activity and rumen bacterial community of lambs. BMC Microbiol. 2021, 21, 250. [Google Scholar] [CrossRef] [PubMed]
- Uwamahoro, H.P.; Li, F.; Timilsina, A.; Liu, B.; Wang, X.; Tian, Y. An Assessment of the Lactic Acid-Producing Potential of Bacterial Strains Isolated from Food Waste. Microbiol. Res. 2022, 13, 278–291. [Google Scholar] [CrossRef]
- Liu, C.; Meng, Q.; Chen, Y.; Xu, M.; Shen, M.; Gao, R.; Gan, S. Role of Age-Related Shifts in Rumen Bacteria and Methanogens in Methane Production in Cattle. Front. Microbiol. 2017, 8, 1563. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Liu, Y.; Wang, S.; Zhang, Y.; Wang, W.; Yang, H.; Lu, N.; Li, S. Nutrient Digestibility, Microbial Fermentation, and Response in Bacterial Composition to Methionine Dipeptide: An In Vitro Study. Biology 2022, 11, 93. [Google Scholar] [CrossRef]
- Dorantes-Iturbide, G.; Orzuna-Orzuna, J.F.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Lee-Rangel, H.A. Essential Oils as a Dietary Additive for Small Ruminants: A Meta-Analysis on Performance, Rumen Parameters, Serum Metabolites, and Product Quality. Veter. Sci. 2022, 9, 475. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- van Bergeijk, D.A.; Terlouw, B.R.; Medema, M.H.; van Wezel, G.P. Ecology and genomics of Actinobacteria: New concepts for natural product discovery. Nat. Rev. Genet. 2020, 18, 546–558. [Google Scholar] [CrossRef]
- Moraïs, S.; Mizrahi, I. The Road Not Taken: The Rumen Microbiome, Functional Groups, and Community States. Trends Microbiol. 2019, 27, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Alugongo, G.M.; Ji, S.; Wu, Z.; Dong, S.; Li, S.; Yoon, I.; Chung, R.; Cao, Z. Effects of Saccharomyces Cerevisiae Fermentation Products on the Microbial Community throughout the Gastrointestinal Tract of Calves. Animals 2018, 9, 4. [Google Scholar] [CrossRef]
- Priyoatmojo, D.; Maharani, Y.; Ansori, D.; Hardani, S.N.W.; Trinugraha, A.C.; Handayani, T.; Sasongko, W.T.; Wahyono, T. Effects of Harvesting Time on Tannin Biological Activity in Sambiloto (Andrographis paniculata) Leaves and in vitro Diet Digestibility Supplemented with Sambiloto Leaves. J. Anim. Health Prod. 2021, 9, 425–434. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of raw and fermented grape seed on growth performance, antioxidant capacity, and cecal microflora in broiler chickens. Animal 2021, 15, 100194. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Lantz, H.; Munyaka, P.M.; Willing, B.P. Prevotella in Pigs: The Positive and Negative Associations with Production and Health. Microorganisms 2020, 8, 1584. [Google Scholar] [CrossRef]
- Bernad-Roche, M.; Bellés, A.; Grasa, L.; Casanova-Higes, A.; Mainar-Jaime, R.C. Effects of Dietary Supplementation with Protected Sodium Butyrate on Gut Microbiota in Growing-Finishing Pigs. Animals 2021, 11, 2137. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Z.; An, W.; Dong, Y.; Zhang, B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef]
- Sotira, S.; Dell’anno, M.; Caprarulo, V.; Hejna, M.; Pirrone, F.; Callegari, M.L.; Tucci, T.V.; Rossi, L. Effects of Tributyrin Supplementation on Growth Performance, Insulin, Blood Metabolites and Gut Microbiota in Weaned Piglets. Animals 2020, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Villasante, A.; Ramírez, C.; Rodríguez, H.; Dantagnan, P.; Hernández, A.; Figueroa, E.; Romero, J. Dietary carbohydrate-to-protein ratio influences growth performance, hepatic health and dynamic of gut microbiota in atlantic salmon (Salmo salar). Anim. Nutr. 2022, 10, 261–279. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lei, J.; Yao, Y.; Qu, X.; Chen, J.; Xie, K.; Wang, X.; Yi, Q.; Xiao, B.; Guo, S.; et al. Black Soldier Fly (Hermetia illucens) Larvae Meal Modulates Intestinal Morphology and Microbiota in Xuefeng Black-Bone Chickens. Front. Microbiol. 2021, 12, 706424. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 50. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gini, E.; Iannini, F.; Gasco, L.; Terova, G. The Effects of Dietary Insect Meal from Hermetia illucens Prepupae on Autochthonous Gut Microbiota of Rainbow Trout (Oncorhynchus mykiss). Animals 2019, 9, 143. [Google Scholar] [CrossRef]
- Farris, N.W.; Hamidoghli, A.; Bae, J.; Won, S.; Choi, W.; Biró, J.; Lee, S.; Bai, S.C. Dietary Supplementation with γ-Aminobutyric Acid Improves Growth, Digestive Enzyme Activity, Non-Specific Immunity and Disease Resistance against Streptococcus iniae in Juvenile Olive Flounder, Paralichthys olivaceus. Animals 2022, 12, 248. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Lee, C.-H.; Kim, K.-D.; Lim, H.J.; Kim, H.S. Effects of diet supplementation with plant juice processing by-products on juvenile black rockfish (Sebastes schlegelii) growth performance, feed utilization, non-specific immunity, and disease resistance against Vibrio harveyi. Aquac. Rep. 2021, 21, 100831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).