Different Microeukaryotic Trophic Groups Show Different Latitudinal Spatial Scale Dependences in Assembly Processes across the Continental Shelves of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Molecular Analyses
2.3. Taxonomic and Trophic Compositions
2.4. Analysis of Microeukaryotic Community
2.5. Ecological Processes Analyses
3. Results
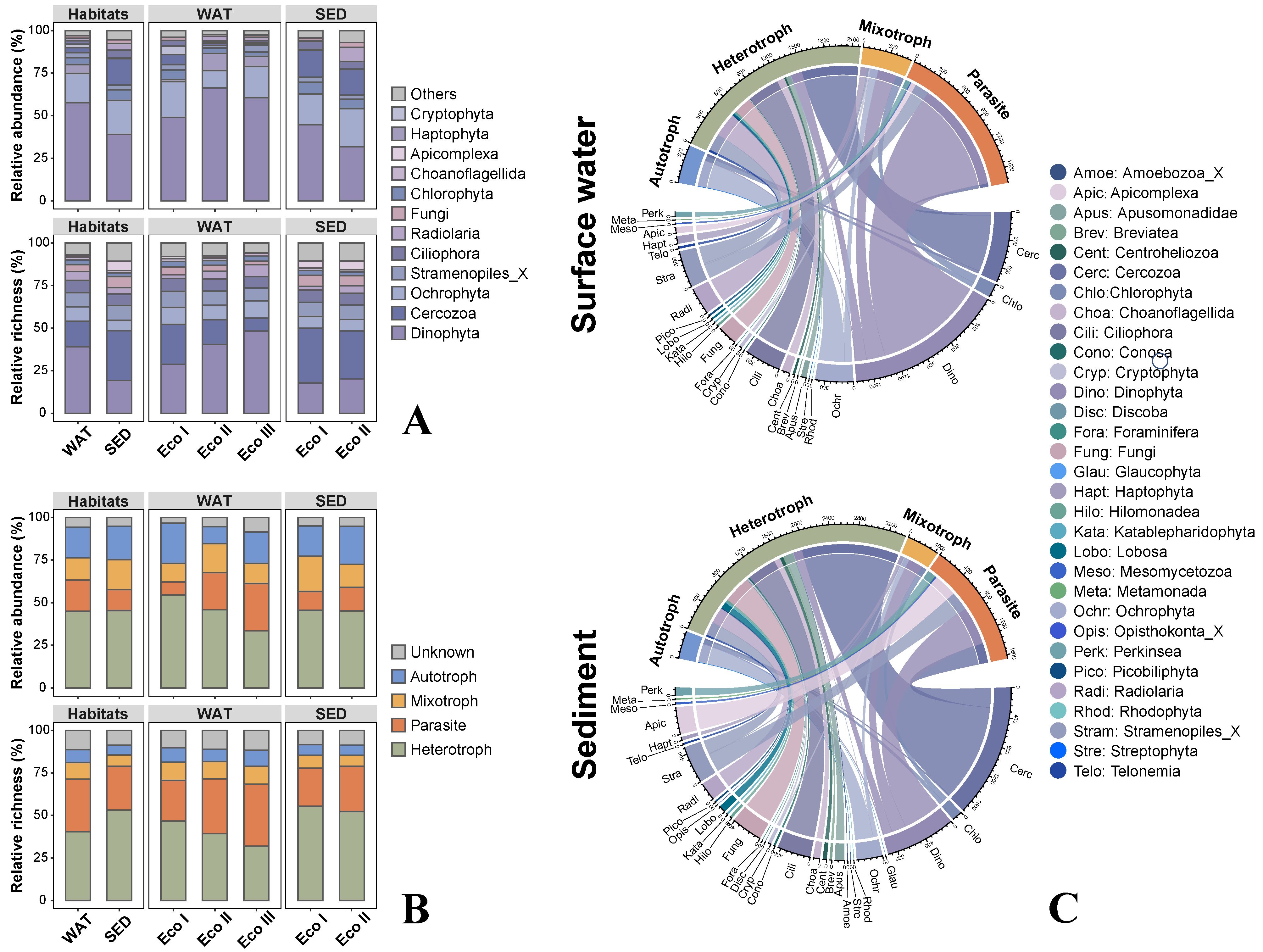
3.1. Microeukaryotic Community Composition
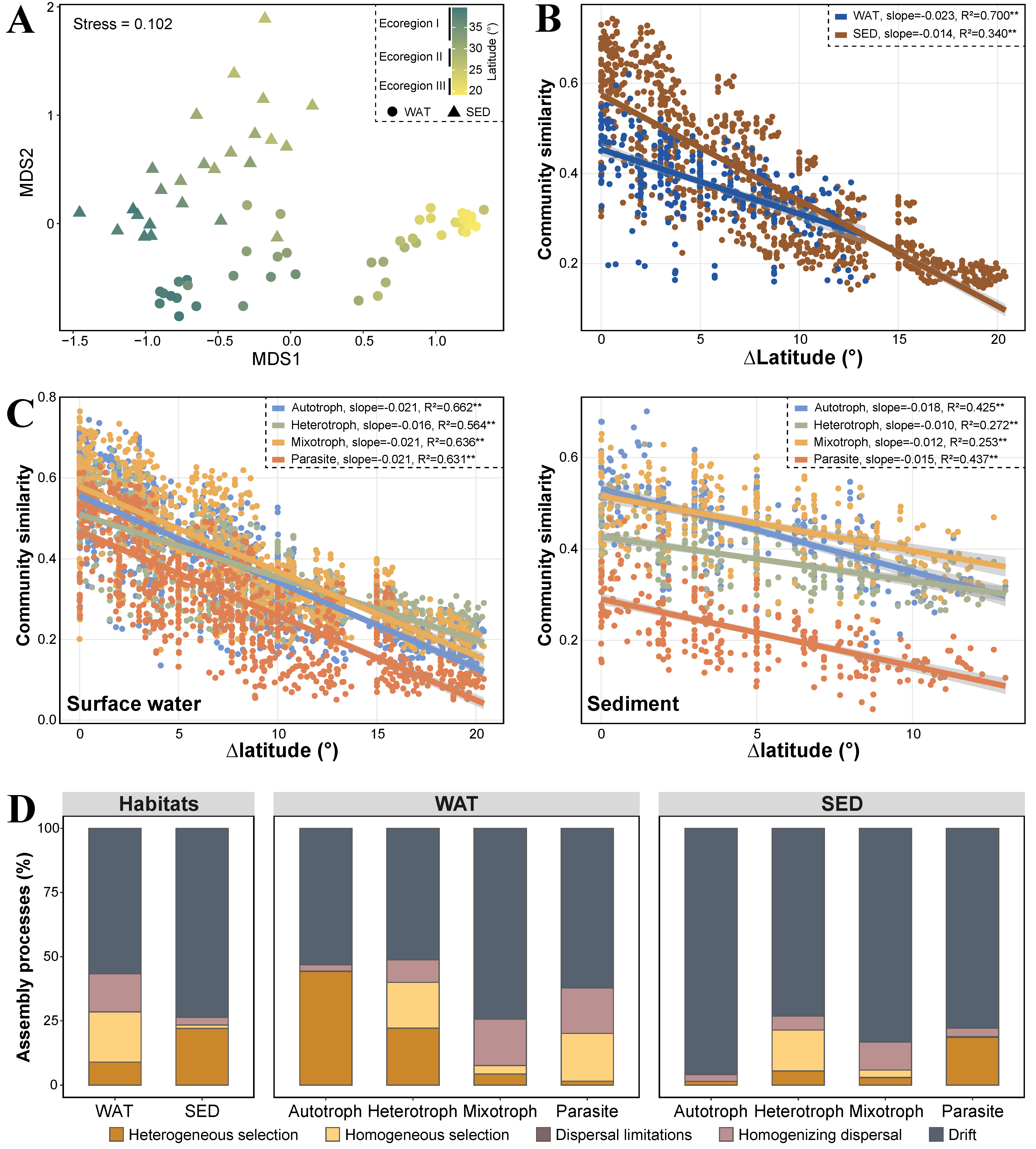
3.2. Spatial Distribution Patterns of Microeukaryotic Communities
3.3. Ecological Processes Governing Microeukaryotic Communities
3.4. Spatial Scale Dependence of Microeukaryotic Community Assembly
4. Discussion
4.1. Different Taxonomic but Similar Trophic Compositions between Planktonic and Sedimental Habitats
4.2. Similar Biogeographical Patterns with Distinct Assembly Mechanisms
4.3. Latitudinal Spatial Scale Dependence of Assembly Processes in Planktonic Communities Contrasting Sedimental Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| Community assembly process | The process by which species colonize and interact to establish and maintain local communities through repeated sequential immigration from the regional species pool. |
| Drift | Random changes of the community structure over time due the inherent stochastic processes of birth, death, and reproduction. |
| Heterogeneous selection | Selection under heterogeneous environmental conditions, which leads to more dissimilar structures in communities. |
| Homogeneous selection | Selection under homogeneous environmental conditions, which leads to more similar structures in communities. |
| Homogenizing dispersal | Very high rate of dispersal among communities, leading to more similar structures in communities. |
| Stochastic process | Neutral theory assumes that community structures are independent of species traits and governed by random processes of birth, death, colonization, extinction, and speciation. |
| Deterministic process | Major niche-based process that shapes community structure due to fitness differences among different organisms, including effects of abiotic conditions and biotic interactions. |
References
- Martiny, J.B.H.; Bohannan, B.J.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Nekola, J.C.; White, P.S. The distance decay of similarity in biogeography and ecology. J. Biogeogr. 1999, 26, 867–878. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, H.; Wang, L.; Zhou, Y.; Li, S.; Zhang, Z.; Feng, K.; Deng, Y. Environmental DNA metabarcoding reveals the influence of human activities on microeukaryotic plankton along the Chinese coastline. Water Res. 2023, 233, 119730. [Google Scholar] [CrossRef] [PubMed]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Vellend, M. The Theory of Ecological Communities; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Serra, M.; Ontiveros, V.J.; Cáliz, J.; Alonso, D.; Casamayor, E.O. Understanding stochastic and deterministic assembly processes in microbial communities along temporal, spatial and environmental scales. Mol. Ecol. 2023, 32, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, X.; Wang, Y.; Huang, B. Protistan-bacterial microbiota exhibit stronger species sorting and greater network connectivity offshore than nearshore across a coast-to-basin continuum. mSystems 2021, 6, e00100-21. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Xiang, X.; Sun, R.; Yang, T.; He, D.; Zhang, K.; Ni, Y.; Zhu, Y.-G.; Adams, J.M.; et al. Spatial scale affects the relative role of stochasticity versus determinism in soil bacterial communities in wheat fields across the North China Plain. Microbiome 2018, 6, 27. [Google Scholar] [CrossRef]
- Xu, Z.; Cheung, S.; Endo, H.; Xia, X.; Wu, W.; Chen, B.; Ho, N.H.E.; Suzuki, K.; Li, M.; Liu, H. Disentangling the ecological processes shaping the latitudinal pattern of phytoplankton communities in the Pacific Ocean. mSystems 2022, 7, e01203-21. [Google Scholar] [CrossRef]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef]
- Guidi, L.; Chaffron, S.; Bittner, L.; Eveillard, D.; Larhlimi, A.; Roux, S.; Darzi, Y.; Audic, S.; Berline, L.; Brum, J.R.; et al. Plankton networks driving carbon export in the oligotrophic ocean. Nature 2016, 532, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Logares, R.; Deutschmann, I.M.; Junger, P.C.; Giner, C.R.; Krabberød, A.K.; Schmidt, T.S.; Rubinat-Ripoll, L.; Mestre, M.; Salazar, G.; Ruiz-González, C. Disentangling the mechanisms shaping the surface ocean microbiota. Microbiome 2020, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.B.; Antunes, A.M.; Targueta, C.P.; Fernandes, J.G.; Soares, T.N.; Nabout, J.C. DNA metabarcoding reveals the responses of prokaryotes and eukaryotes microbiota to warming: Are the patterns similar between taxonomic and trophic groups? Ecol. Indic. 2020, 115, 106452. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Shen, W.; Du, X.; Li, S.; Wei, Z.; Zhang, Z.; Feng, K.; Deng, Y. The large-scale spatial patterns of ecological networks between phytoplankton and zooplankton in coastal marine ecosystems. Sci. Total Environ. 2022, 827, 154285. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Vogt, M.; Elizondo, U.H.; Righetti, D.; Zimmermann, N.E.; Gruber, N. Major restructuring of marine plankton assemblages under global warming. Nat. Commun. 2021, 12, 5226. [Google Scholar] [CrossRef]
- Ibarbalz, F.M.; Henry, N.; Brandão, M.C.; Martini, S.; Busseni, G.; Byrne, H.; Coelho, L.P.; Endo, H.; Gasol, J.M.; Gregory, A.C.; et al. Global trends in marine plankton diversity across kingdoms of life. Cell 2019, 179, 1084–1097. [Google Scholar] [CrossRef]
- Hobday, A.J.; Pecl, G.T. Identification of global marine hotspots: Sentinels for change and vanguards for adaptation action. Rev. Fish Biol. Fish. 2014, 24, 415–425. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems And Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Sun, P.; Wang, Y.; Laws, E.; Huang, B. Water mass-driven spatial effects and environmental heterogeneity shape microeukaryote biogeography in a subtropical, hydrographically complex ocean system—A case study of ciliates. Sci. Total Environ. 2020, 706, 135753. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Liu, X.; Yao, P.; Ge, T.; Zhang, X.-H. Spatiotemporal dynamics of the archaeal community in coastal sediments: Assembly process and co-occurrence relationship. ISME J. 2020, 14, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.K.; Au, C.H.; Chu, K.H.; Kwan, H.S.; Wong, C.K. Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J. 2010, 4, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2012, 41, D597–D604. [Google Scholar] [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef]
- Armeli Minicante, S.; Piredda, R.; Quero, G.M.; Finotto, S.; Bernardi Aubry, F.; Bastianini, M.; Pugnetti, A.; Zingone, A. Habitat heterogeneity and connectivity: Effects on the planktonic protist community structure at two adjacent coastal sites (the Lagoon and the Gulf of Venice, Northern Adriatic Sea, Italy) revealed by metabarcoding. Front. Microbiol. 2019, 10, 2736. [Google Scholar] [CrossRef] [PubMed]
- Dumack, K.; Fiore-Donno, A.M.; Bass, D.; Bonkowski, M. Making sense of environmental sequencing data: Ecologically important functional traits of the protistan groups Cercozoa and Endomyxa (Rhizaria). Mol. Ecol. Resour. 2020, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-10. 2013. R Package 2015. Available online: http://CRAN.R-project.org/package=vegan (accessed on 1 September 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 September 2022).
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pan, Y.; Yu, L.; Yang, J.; Zhang, W. Patterns and processes in marine microeukaryotic community biogeography from Xiamen coastal waters and intertidal sediments, southeast China. Front. Microbiol. 2017, 8, 1912. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Wang, Y.; Warren, A.; Huang, B.; Sun, P. Diversity distribution and assembly mechanisms of planktonic and benthic microeukaryote communities in intertidal zones of southeast Fujian, China. Front. Microbiol. 2019, 10, 2640. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Zinger, L.; Amaral-Zettler, L.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Huse, S.M.; Welch, D.B.M.; Martiny, J.B.; Sogin, M.; Boetius, A.; Ramette, A. Global patterns of bacterial beta-diversity in seafloor and seawater ecosystems. PLoS ONE 2011, 6, e24570. [Google Scholar] [CrossRef]
- Mestre, M.; Ruiz-González, C.; Logares, R.; Duarte, C.M.; Gasol, J.M.; Sala, M.M. Sinking particles promote vertical connectivity in the ocean microbiome. Proc. Natl. Acad. Sci. USA 2018, 115, E6799–E6807. [Google Scholar] [CrossRef]
- Massana, R.; Gobet, A.; Audic, S.; Bass, D.; Bittner, L.; Boutte, C.; Chambouvet, A.; Christen, R.; Claverie, J.M.; Decelle, J.; et al. Marine protist diversity in European coastal waters and sediments as revealed by high-throughput sequencing. Environ. Microbiol. 2015, 17, 4035–4049. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Li, M.; Wei, G.; Liu, J.; Gao, Z. Distribution patterns of microeukaryotic community between sediment and water of the Yellow River estuary. Curr. Microbiol. 2020, 77, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Brannock, P.M.; Ortmann, A.C.; Moss, A.G.; Halanych, K.M. Metabarcoding reveals environmental factors influencing spatio-temporal variation in pelagic micro-eukaryotes. Mol. Ecol. 2016, 25, 3593–3604. [Google Scholar] [CrossRef]
- Wu, W.; Huang, B. Protist diversity and community assembly in surface sediments of the South China Sea. MicrobiologyOpen 2019, 8, e891. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F. A quantitative review of the lifestyle, habitat and trophic diversity of dinoflagellates (Dinoflagellata, Alveolata). Syst. Biodivers. 2012, 10, 267–275. [Google Scholar] [CrossRef]
- Burki, F.; Sandin, M.M.; Jamy, M. Diversity and ecology of protists revealed by metabarcoding. Curr. Biol. 2021, 31, R1267–R1280. [Google Scholar] [CrossRef] [PubMed]
- de Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef]
- Singer, D.; Seppey, C.V.; Lentendu, G.; Dunthorn, M.; Bass, D.; Belbahri, L.; Blandenier, Q.; Debroas, D.; de Groot, G.A.; de Vargas, C.; et al. Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems. Environ. Int. 2020, 146, 106262. [Google Scholar] [CrossRef]
- Hünninghaus, M.; Koller, R.; Kramer, S.; Marhan, S.; Kandeler, E.; Bonkowski, M. Changes in bacterial community composition and soil respiration indicate rapid successions of protist grazers during mineralization of maize crop residues. Pedobiologia 2017, 62, 1–8. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Geisen, S.; Delgado-Baquerizo, M.; Maestre, F.T.; Turner, B.L.; Fierer, N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci. Adv. 2020, 6, eaax8787. [Google Scholar] [CrossRef]
- Hörstmann, C.; Buttigieg, P.L.; John, U.; Raes, E.J.; Wolf-Gladrow, D.; Bracher, A.; Waite, A.M. Microbial diversity through an oceanographic lens: Refining the concept of ocean provinces through trophic-level analysis and productivity-specific length scales. Environ. Microbiol. 2022, 24, 404–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, Z.; Li, J.; Hu, T.; Chen, C.; Lin, X. From river to ocean: Connectivity and heterogeneity of aquatic ecosystems depicted by planktonic microeukaryotes. Ecol. Indic. 2023, 148, 110136. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Zhang, J.; Soininen, J. Regional and local environment drive biogeographic patterns in intertidal microorganisms. J. Biogeogr. 2022, 49, 1576–1585. [Google Scholar] [CrossRef]
- Grossmann, L.; Jensen, M.; Heider, D.; Jost, S.; Glücksman, E.; Hartikainen, H.; Mahamdallie, S.S.; Gardner, M.; Hoffmann, D.; Bass, D.; et al. Protistan community analysis: Key findings of a large-scale molecular sampling. ISME J. 2016, 10, 2269–2279. [Google Scholar] [CrossRef]
- Gu, R.; Sun, P.; Wang, Y.; Yu, F.; Jiao, N.; Xu, D. Genetic diversity, community assembly, and shaping factors of benthic microbial eukaryotes in Dongshan Bay, Southeast China. Front. Microbiol. 2020, 11, 592489. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Boetius, A.; Ramette, A. Bacterial taxa-area and distance-decay relationships in marine environments. Mol. Ecol. 2014, 23, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.S.; Waite, D.; Lear, G.; Handley, K.M. Microbial river-to-sea continuum: Gradients in benthic and planktonic diversity, osmoregulation and nutrient cycling. Microbiome 2021, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, H.; Sun, Y.; Shao, K.; Wang, X.; Ma, X.; Hu, A.; Zhang, H.; Fan, J. How habitat heterogeneity shapes bacterial and protistan communities in temperate coastal areas near estuaries. Environ. Microbiol. 2022, 24, 1775–1789. [Google Scholar] [CrossRef]
- Mansfeldt, C.; Achermann, S.; Men, Y.; Walser, J.-C.; Villez, K.; Joss, A.; Johnson, D.R.; Fenner, K. Microbial residence time is a controlling parameter of the taxonomic composition and functional profile of microbial communities. ISME J. 2019, 13, 1589–1601. [Google Scholar] [CrossRef]
- Wu, W.; Lu, H.-P.; Sastri, A.; Yeh, Y.-C.; Gong, G.-C.; Chou, W.-C.; Hsieh, C.-H. Contrasting the relative importance of species sorting and dispersal limitation in shaping marine bacterial versus protist communities. ISME J. 2018, 12, 485–494. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, M.; Liu, X.; Xia, J.; Yin, H.; Li, X. Different responses of bacteria and microeukaryote to assembly processes and co-occurrence pattern in the coastal upwelling. Microb. Ecol. 2022, 86, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, V. Marine protist associations and environmental impacts across trophic levels in the twilight zone and below. Curr. Opin. Microbiol. 2016, 31, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Kohout, P.; Anslan, S.; Harend, H.; Abarenkov, K.; Tedersoo, L. Stochastic distribution of small soil eukaryotes resulting from high dispersal and drift in a local environment. ISME J. 2016, 10, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef] [PubMed]
- Milke, F.; Wagner-Doebler, I.; Wienhausen, G.; Simon, M. Selection, drift and community interactions shape microbial biogeographic patterns in the Pacific Ocean. ISME J. 2022, 16, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, Y.; Lin, T.; Xie, W.; Hu, J.; Hou, F.; Han, Q.; Zhu, X.; Zhang, D. Disentangling the mechanisms shaping the prokaryotic communities in a eutrophic bay. Microbiol. Spectr. 2022, 10, e01481-22. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Tripathi, B.M.; Shi, Y.; Adams, J.M.; Zhu, Y.G.; Chu, H. Interpreting distance-decay pattern of soil bacteria via quantifying the assembly processes at multiple spatial scales. MicrobiologyOpen 2019, 8, e00851. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef]
- Liu, C.; Wu, F.; Jiang, X.; Hu, Y.; Shao, K.; Tang, X.; Qin, B.; Gao, G. Salinity is a key determinant for the microeukaryotic community in lake ecosystems of the Inner Mongolia plateau, China. Front. Microbiol. 2022, 13, 841686. [Google Scholar] [CrossRef]
- Hanson, C.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Martiny, J.B. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qu, Z.; Zhang, K.; Li, J.; Lin, X. Different Microeukaryotic Trophic Groups Show Different Latitudinal Spatial Scale Dependences in Assembly Processes across the Continental Shelves of China. Microorganisms 2024, 12, 124. https://doi.org/10.3390/microorganisms12010124
Zhang Y, Qu Z, Zhang K, Li J, Lin X. Different Microeukaryotic Trophic Groups Show Different Latitudinal Spatial Scale Dependences in Assembly Processes across the Continental Shelves of China. Microorganisms. 2024; 12(1):124. https://doi.org/10.3390/microorganisms12010124
Chicago/Turabian StyleZhang, Yong, Zhishuai Qu, Kexin Zhang, Jiqiu Li, and Xiaofeng Lin. 2024. "Different Microeukaryotic Trophic Groups Show Different Latitudinal Spatial Scale Dependences in Assembly Processes across the Continental Shelves of China" Microorganisms 12, no. 1: 124. https://doi.org/10.3390/microorganisms12010124




