Soil Microbes and Plant-Associated Microbes in Response to Radioactive Pollution May Indirectly Affect Plants and Insect Herbivores: Evidence for Indirect Field Effects from Chernobyl and Fukushima
Abstract
:1. Introduction
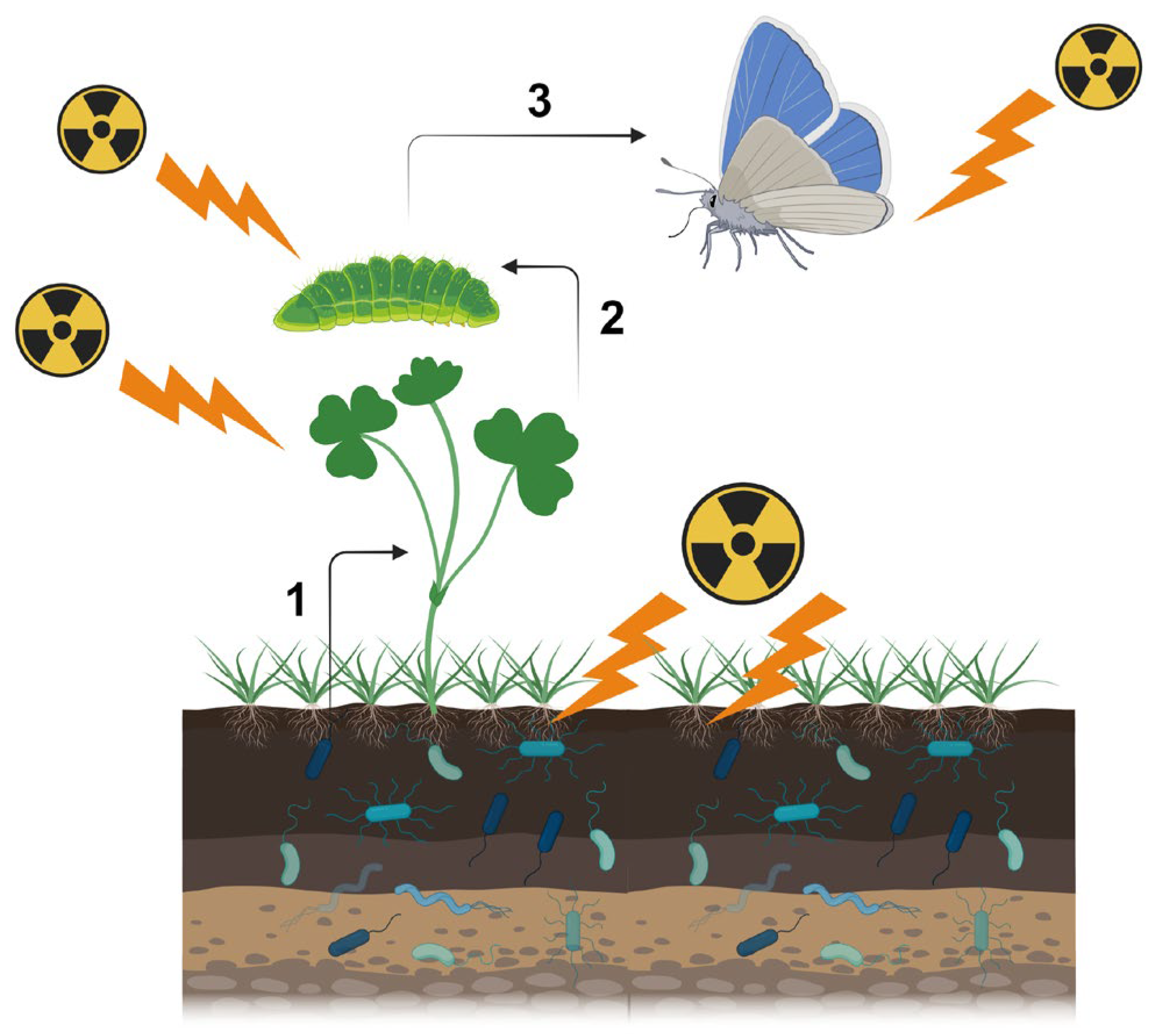
2. Multiple Pathways for Biological Effects
3. Soil Microbes and Soil Invertebrates
3.1. Chernobyl Studies
3.2. Fukushima Studies
3.3. Commonalities between Chernobyl and Fukushima
4. Plant-Associated Microbes
4.1. Chernobyl Studies
4.2. Fukushima Studies
4.3. Commonalities between Chernobyl and Fukushima
5. Plants and Insect Herbivores
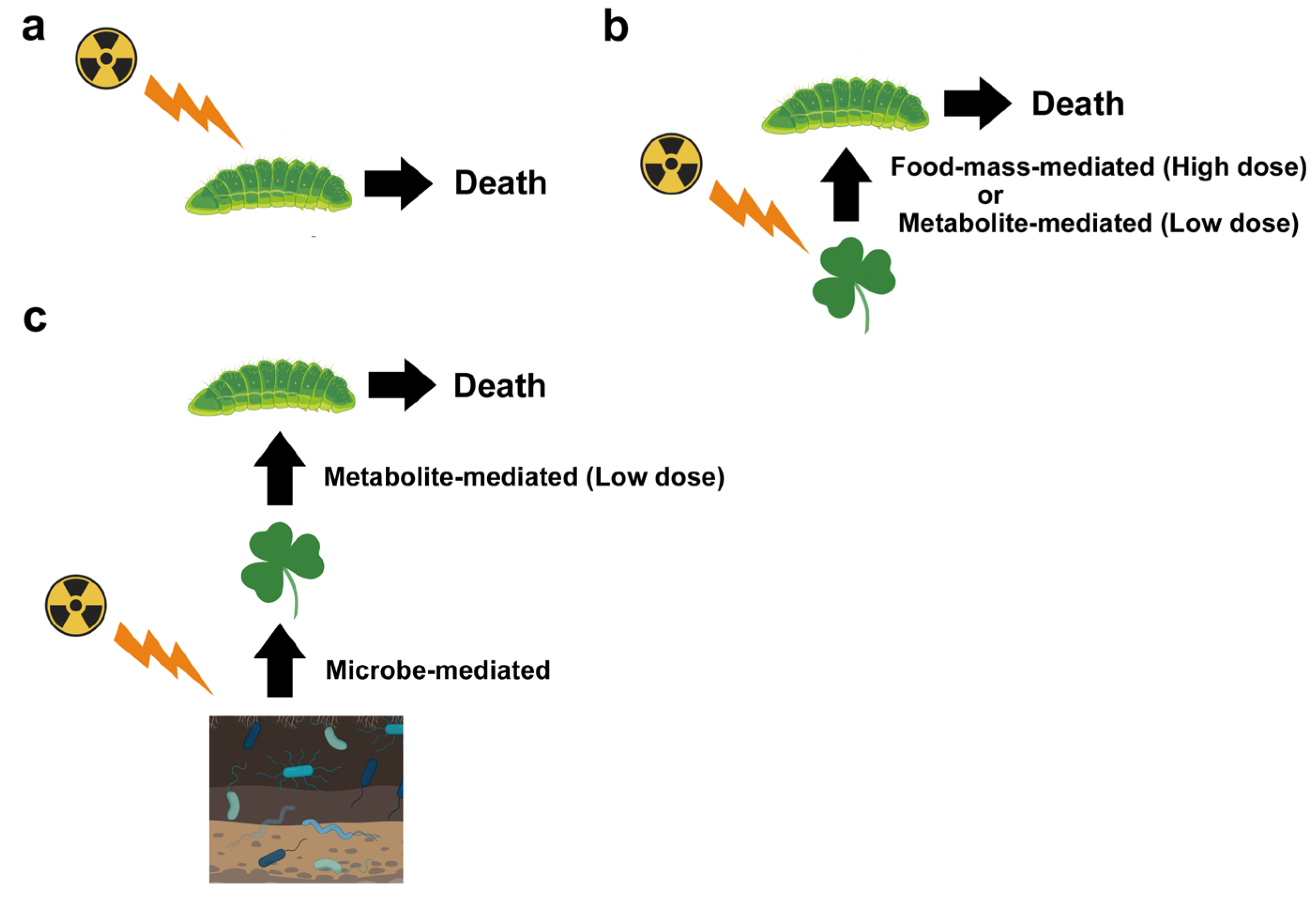
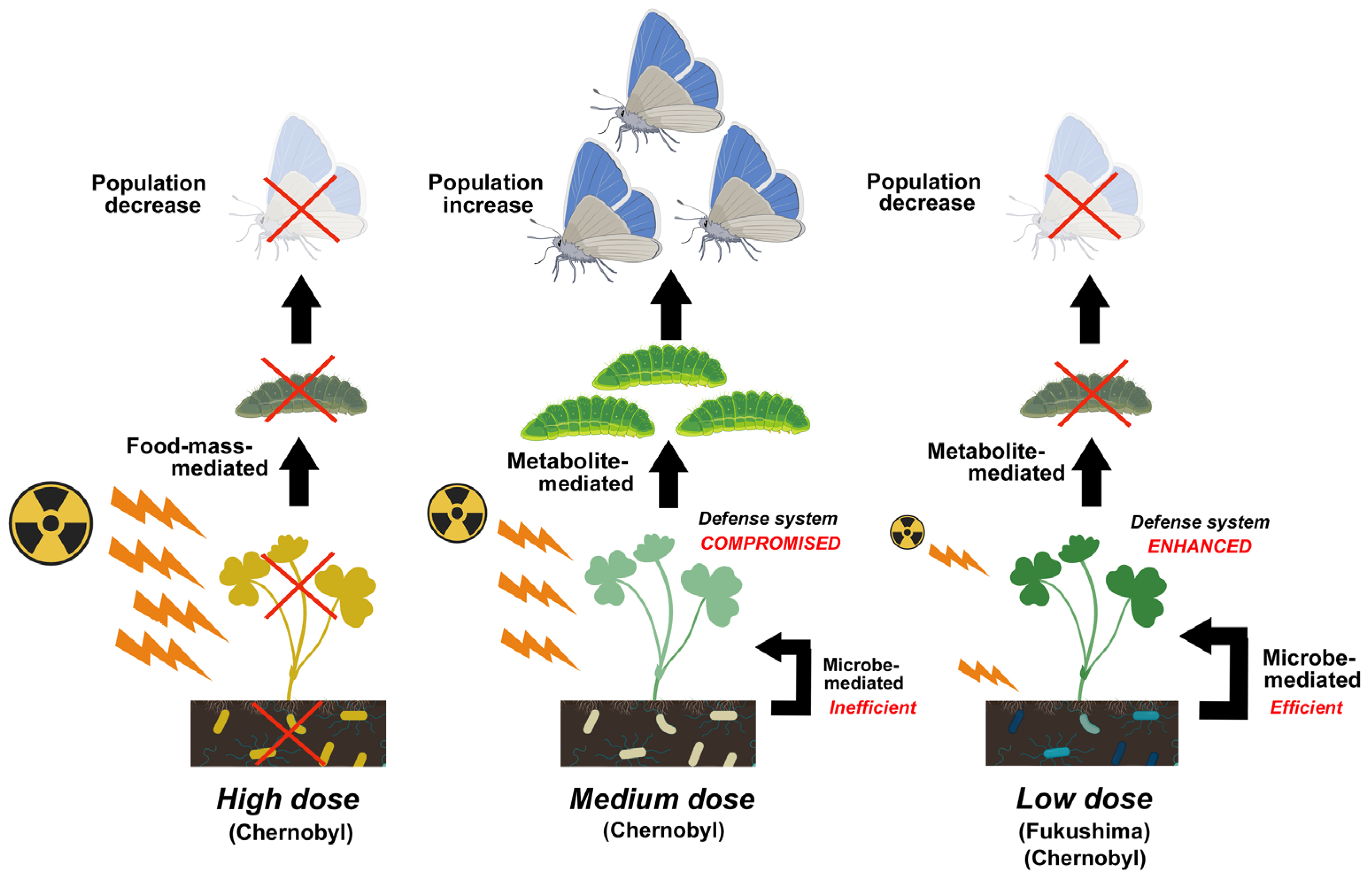
5.1. Food-Mass-Mediated Indirect Effects
5.2. Pollen-Mediated Indirect Effects
5.3. Metabolite-Mediated Indirect Effect
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagner, H.N., Jr. Atoms for peace (and health). J. Nucl. Med. 2004, 45, 24N. [Google Scholar] [PubMed]
- Babic, R.R.; Babic, G.S.; Babic, S.R.; Babic, N.R. 120 years since the discovery of x-rays. Med. Pregl. 2016, 69, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Nüsslin, F. Wilhelm Conrad Röntgen: The scientist and his discovery. Phys. Med. 2020, 79, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, A.; Kathren, R.L.; Willis, C.A. History of the medical uses of radiation: Regulatory and voluntary standards of protection. Health Phys. 1995, 69, 783–823. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.B. History of radiation use in medicine. J. Vasc. Surg. 2011, 53 (Suppl. S1), 3S–5S. [Google Scholar] [CrossRef] [PubMed]
- Scatliff, J.H.; Morris, P.J. From Röntgen to magnetic resonance imaging: The history of medical imaging. N. C. Med. J. 2014, 75, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kong, F.; Sun, K.; Wang, T.; Guo, T. From classical radiation to modern radiation: Past, present, and future of radiation mutation breeding. Front. Public Health 2021, 9, 768071. [Google Scholar] [CrossRef]
- Riviello-Flores, M.d.l.L.; Cadena-Iñiguez, J.; Ruiz-Posadas, L.d.M.; Arévalo-Galarza, M.d.L.; Castillo-Juárez, I.; Hernández, M.S.; Castillo-Martínez, C.R. Use of gamma radiation for the genetic improvement of underutilized plant varieties. Plants 2022, 11, 1161. [Google Scholar] [CrossRef]
- Whitmore, G.F. One hundred years of X rays in biological research. Radiat. Res. 1995, 144, 148–159. [Google Scholar] [CrossRef]
- D’Mello, J.P.F. Preface. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; pp. xxv–xxxvi. [Google Scholar]
- Geras’kin, S.A.; Fesenko, S.V.; Alexakhin, R.M. Effects of non-human species irradiation after the Chernobyl NPP accident. Environ. Int. 2008, 34, 880–897. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kanda, J. Geochemistry. Tracking the Fukushima radionuclides. Science 2012, 336, 1115–1116. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Environmental Consequences of the Chernobyl Accident and their Remediation: Twenty Years of Experience; Radiological Assessment Reports Series No. 8; IAEA: Vienna, Austria, 2006. [Google Scholar]
- Aliyu, A.S.; Evangeliou, N.; Mousseau, T.A.; Wu, J.; Ramli, A.T. An overview of current knowledge concerning the health and environmental consequences of the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. Environ. Int. 2015, 85, 213–228. [Google Scholar] [CrossRef]
- Ministry of the Environment (Government of Japan). Chapter 4. Concept of radiological protection. In BOOKLET to Provide Basic Information Regarding Health Effects of Radiation, 3rd ed.; Ministry of the Environment (Government of Japan): Tokyo, Japan, 2016; Available online: https://www.env.go.jp/en/chemi/rhm/basic-info/1st/04.html (accessed on 13 January 2024).
- International Atomic Energy Agency (IAEA). Effects of Ionizing Radiation on Plants and Animals at Levels Implied by Current Radiation Protection Standards; Technical Reports Series No. 332; IAEA: Vienna, Austria, 1992. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation UNSCEAR 1996 Report to the General Assembly, with Scientific Annex; United Nations: New York, NY, USA, 1996. [Google Scholar]
- European Commission. Protection of the Environment; European Union: Luxembourg, 2014; Available online: https://energy.ec.europa.eu/system/files/2014-11/177_0.pdf (accessed on 13 January 2024).
- Møller, A.P.; Mousseau, T.A. Biological consequences of Chernobyl: 20 years on. Trends Ecol. Evol. 2006, 21, 200–207. [Google Scholar] [CrossRef]
- Cannon, G.; Kiang, J.G. A review of the impact on the ecosystem after ionizing irradiation: Wildlife population. Int. J. Radiat. Biol. 2022, 98, 1054–1062. [Google Scholar] [CrossRef]
- Hiyama, A.; Nohara, C.; Taira, W.; Kinjo, S.; Iwata, M.; Otaki, J.M. The Fukushima nuclear accident and the pale grass blue butterfly: Evaluating biological effects of long-term low-dose exposures. BMC Evol. Biol. 2013, 13, 168. [Google Scholar] [CrossRef]
- Garnier-Laplace, J.; Geras’kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Understanding low-dose exposure and field effects to resolve the field-laboratory paradox: Multifaceted biological effects from the Fukushima nuclear accident. In New Trends in Nuclear Science; Awwad, N.S., AlFaify, S.A., Eds.; InTechOpen: London, UK, 2018; pp. 49–71. [Google Scholar] [CrossRef]
- Otaki, J.M.; Sakauchi, K.; Taira, W. The second decade of the blue butterfly in Fukushima: Untangling the ecological field effects after the Fukushima nuclear accident. Integr. Environ. Assess. Manag. 2022, 18, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Beaugelin-Seiller, K.; Della-Vedova, C.; Garnier-Laplace, J. Is non-human species radiosensitivity in the lab a good indicator of that in the field? Making the comparison more robust. J. Environ. Radioact. 2020, 211, 105870. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef] [PubMed]
- Nohara, C.; Hiyama, A.; Taira, W.; Tanahara, A.; Otaki, J.M. The biological impacts of ingested radioactive materials on the pale grass blue butterfly. Sci. Rep. 2014, 4, 4946. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.D.; Taira, W.; Sakauchi, K.; Iwata, M.; Hiyama, A.; Otaki, J.M. Tolerance of high oral doses of nonradioactive and radioactive caesium chloride in the pale grass blue butterfly Zizeeria maha. Insects 2019, 10, 290. [Google Scholar] [CrossRef]
- Hancock, S.; Vo, N.T.K.; Omar-Nazir, L.; Batlle, J.V.I.; Otaki, J.M.; Hiyama, A.; Byun, S.H.; Seymour, C.B.; Mothersill, C. Transgenerational effects of historic radiation dose in pale grass blue butterflies around Fukushima following the Fukushima Dai-ichi Nuclear Power Plant meltdown accident. Environ. Res. 2019, 168, 230–240. [Google Scholar] [CrossRef]
- Sakauchi, K.; Taira, W.; Hiyama, A.; Imanaka, T.; Otaki, J.M. The pale grass blue butterfly in ex-evacuation zones 5.5 years after the Fukushima nuclear accident: Contributions of initial high-dose exposure to transgenerational effects. J. Asia-Pac. Entomol. 2020, 23, 242–252. [Google Scholar] [CrossRef]
- Nohara, C.; Taira, W.; Hiyama, A.; Tanahara, A.; Takatsuji, T.; Otaki, J.M. Ingestion of radioactively contaminated diets for two generations in the pale grass blue butterfly. BMC Evol. Biol. 2014, 14, 193. [Google Scholar] [CrossRef]
- Taira, W.; Hiyama, A.; Nohara, C.; Sakauchi, K.; Otaki, J.M. Ingestional and transgenerational effects of the Fukushima nuclear accident on the pale grass blue butterfly. J. Radiat. Res. 2015, 56 (Suppl. S1), i2–i18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kinouchi, T.; Fujii, T.; Imanaka, T.; Takahashi, T.; Fukutani, S.; Maki, D.; Nohtomi, A.; Takahashi, S. Observation of morphological abnormalities in silkworm pupae after feeding 137CsCl-supplemented diet to evaluate the effects of low dose-rate exposure. Sci. Rep. 2020, 10, 16055. [Google Scholar] [CrossRef] [PubMed]
- Hesse-Honegger, C.; Wallimann, P. Malformation of true bug (Heteroptera): A phenotype field study on the possible influence of artificial low-level radioactivity. Chem. Biodivers. 2008, 5, 499–539. [Google Scholar] [CrossRef] [PubMed]
- Körblein, A.; Hesse-Honegger, C. Morphological abnormalities in true bugs (Heteroptera) near Swiss nuclear power stations. Chem. Biodivers. 2018, 15, e1800099. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, S. Morphological abnormalities in gall-forming aphids in a radiation-contaminated area near Fukushima Daiichi: Selective impact of fallout? Ecol. Evol. 2014, 4, 355–369. [Google Scholar] [CrossRef]
- Akimoto, S.I.; Li, Y.; Imanaka, T.; Sato, H.; Ishida, K. Effects of radiation from contaminated soil and moss in Fukushima on embryogenesis and egg hatching of the aphid Prociphilus oriens. J. Hered. 2018, 109, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Beresford, N.A.; Scott, E.M.; Copplestone, D. Field effects studies in the Chernobyl Exclusion Zone: Lessons to be learnt. J. Environ. Radioact. 2020, 211, 105893. [Google Scholar] [CrossRef]
- Woodwell, G.M.; Sparrow, A.H. Predicted and observed effects of chronic gamma radiation on a near-climax forest ecosystem. Radiat. Botany 1963, 3, 231–237. [Google Scholar] [CrossRef]
- Caffrey, E.; Leonard, M.; Napier, J.; Neville, D.; Higley, K. Radioecology: Why Bother? J. Environ. Prot. 2014, 5, 181–192. [Google Scholar] [CrossRef]
- Bradshaw, C.; Kapustka, L.; Barnthouse, L.; Brown, J.; Ciffroy, P.; Forbes, V.; Geras’kin, S.; Kautsky, U.; Bréchignac., F. Using an Ecosystem Approach to complement protection schemes based on organism-level endpoints. J. Environ. Radioact. 2014, 136, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Brèchignac, F.; Doi, M. Challenging the current strategy of radiological protection of the environment: Arguments for an ecosystem approach. J. Environ. Radioact. 2009, 100, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Woodwell, G.M. Ecological Effects of Nuclear War; Brookhaven National Laboratory: Upton, NY, USA, 1963. [Google Scholar]
- Bréchignac, F.; Alexakhin, R.; Godoy, J.M.; Oughton, D.; Sheppard, S.; Strand, P. Integrating environment protection, a new challenge: Strategy of the International Union of Radioecology. Radioprotection 2008, 43, 339–356. [Google Scholar] [CrossRef]
- Dalkvist, T.; Topping, C.J.; Forbes, V.E. Population-level impacts of pesticide-induced chronic effects on individuals depend more on ecology than toxicology. Ecotoxicol. Environ. Saf. 2009, 72, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.H.; Rohr, J.R. Community responses to contaminants: Using basic ecological principles to predict ecotoxicological effects. Environ. Toxicol. Chem. 2009, 28, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Communication of ionising radiation signals—A tale of two fish. Int. J. Radiat. Biol. 2009, 85, 909–919. [Google Scholar] [CrossRef]
- Otaki, J.M. Fukushima’s lessons from the blue butterfly: A risk assessment of the human living environment in the post-Fukushima era. Integr. Environ. Assess. Manag. 2016, 12, 667–672. [Google Scholar] [CrossRef]
- Otaki, J.M. Fukushima Nuclear Accident: Potential health effects inferred from butterfly and human cases. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2019; pp. 497–514. [Google Scholar]
- Brisbin, I.L., Jr. Avian radioecology. In Current Ornithology, Volume 8; Power, D.M., Ed.; Springer: New York, NY, USA, 1991; pp. 69–140. [Google Scholar]
- Matsui, S. Radiation ecology: Dynamics of radioactive materials in ecosystems and effects on birds. Anim. Husb. 2013, 67, 11–16. (In Japanese) [Google Scholar]
- Jonsson, B.; Forseth, T.; Ugedal, O. Chernobyl radioactivity persists in fish. Nature 1999, 400, 417. [Google Scholar] [CrossRef]
- Otaki, J.M.; Taira, W. Current status of the blue butterfly in Fukushima research. J. Hered. 2018, 109, 178–187. [Google Scholar] [CrossRef]
- Møller, A.P.; Nishiumi, I.; Suzuki, H.; Ueda, K.; Mousseau, T.A. Differences in effects of radiation on abundance of animals in Fukushima and Chernobyl. Ecol. Indic. 2013, 24, 75–81. [Google Scholar] [CrossRef]
- Møller, A.P.; Mousseau, T.A. Reduced abundance of insects and spiders linked to radiation at Chernobyl 20 years after the accident. Biol. Lett. 2009, 5, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Mousseau, T.A. Efficiency of bio-indicators for low-level radiation under field conditions. Ecol. Indic. 2011, 11, 424–430. [Google Scholar] [CrossRef]
- Turner, F.B. Effects of continuous irradiation on animal populations. Adv. Radiat. Biol. 1975, 5, 83–144. [Google Scholar] [CrossRef]
- Lecomte-Pradines, C.; Bonzom, J.M.; Della-Vedova, C.; Beaugelin-Seiller, K.; Villenave, C.; Gaschak, S.; Coppin, F.; Dubourg, N.; Maksimenko, A.; Adam-Guillermin, C.; et al. Soil nematode assemblages as bioindicators of radiation impact in the Chernobyl Exclusion Zone. Sci. Total Environ. 2014, 490, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Mousseau, T.A. Species richness and abundance of forest birds in relation to radiation at Chernobyl. Biol. Lett. 2007, 3, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, N.N.; Vasilevskaia, A.I.; Artyshkova, L.V.; Gavriliuk, V.I.; Lashko, T.N.; Sadovnikov, I.S. Kompleksy pochvennykh mikrokitsetov v zone vliianiia Chernobyl’skoĭ AES [Complexes of soil micromycetes in the area of the influence of the Chernobyl Atomic Electric Power Station]. Mikrobiol. Zh. 1991, 53, 3–9. (In Russian) [Google Scholar]
- Yablokov, A.V.; Nesterenko, V.B.; Nesterenko, A.V. Consequences of the Chernobyl catastrophe for public health and the environment 23 years later. Ann. N. Y. Acad. Sci. 2009, 1181, 318–326. [Google Scholar] [CrossRef]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Dadachova, E.; Bryan, R.A.; Huang, X.; Moadel, T.; Schweitzer, A.D.; Aisen, P.; Nosanchuk, J.D.; Casadevall, A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2007, 2, e457. [Google Scholar] [CrossRef]
- Dadachova, E.; Casadevall, A. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008, 11, 525–531. [Google Scholar] [CrossRef]
- Romanovskaia, V.A.; Stoliar, S.M.; Malashenko, I.R.; Shatokhina, E.S. Vliianie radiatsii dlitel’nogo deĭstviia na raznoobrazie geterotrofnykh bakteriĭ v pochvakh 10-kilometrovoĭ zony Chernobyl’skoĭ atomnoĭ élektrostantsii [The effect of long-acting radiation on the diversity of heterotrophic bacteria in the soils of a 10-kilometer area around the Chernobyl Atomic Electric Power Station]. Mikrobiol. Z. 1996, 58, 3–12. (In Russian) [Google Scholar]
- Romanovskaia, V.A.; Sokolov, I.G.; Rokitko, P.V.; Chernaia, N.A. Ekologicheskie posledstviia radioaktivnogo zagriazneniia dlia pochvennykh bakteriĭ v 10-km zone ChAES [Ecological consequences of radioactive pollution for soil bacteria within the 10-km region around the Chernobyl Atomic Energy Station]. Mikrobiologiia 1998, 67, 274–280. (In Russian) [Google Scholar]
- Krivolutzkii, D.A.; Pokarzhevskii, A.D. Effects of radioactive fallout on soil animal populations in the 30 km zone of the Chernobyl atomic power station. Sci. Total Environ. 1992, 112, 69–77. [Google Scholar] [CrossRef]
- Suzuki, J.; Egami, N. Mortality of the earthworms, Eisenia foetida, after γ-irradiation at different stages of their life history. J. Radiat. Res. 1983, 24, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Hertel-Aas, T.; Oughton, D.H.; Jaworska, A.; Bjerke, H.; Salbu, B.; Brunborg, G. Effects of chronic gamma irradiation on reproduction in the earthworm Eisenia fetida (Oligochaeta). Radiat. Res. 2007, 168, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Volkova, P.Y.; Geras’kin, S.A. ‘Omic’ technologies as a helpful tool in radioecological research. J. Environ. Radioact. 2018, 189, 156–167. [Google Scholar] [CrossRef]
- Beresford, N.A.; Wood, M.D.; Gashchak, S.; Barnett, C.L. Current ionising radiation doses in the Chernobyl Exclusion Zone do not directly impact on soil biological activity. PLoS ONE 2022, 17, e0263600. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Milinevsky, G.; Kenney-Hunt, J.; Møller, A.P. Highly reduced mass loss rates and increased litter layer in radioactively contaminated areas. Oecologia 2014, 175, 429–437. [Google Scholar] [CrossRef]
- Bonzom, J.M.; Hättenschwiler, S.; Lecomte-Pradines, C.; Chauvet, E.; Gaschak, S.; Beaugelin-Seiller, K.; Della-Vedova, C.; Dubourg, N.; Maksimenko, A.; Garnier-Laplace, J.; et al. Effects of radionuclide contamination on leaf litter decomposition in the Chernobyl exclusion zone. Sci. Total Environ. 2016, 562, 596–603. [Google Scholar] [CrossRef]
- Videvall, E.; Burraco, P.; Orizaola, G. Impact of ionizing radiation on the environmental microbiomes of Chornobyl wetlands. Environ. Pollut. 2023, 330, 121774. [Google Scholar] [CrossRef] [PubMed]
- Chapon, V.; Piette, L.; Vesvres, M.H.; Coppin, F.; Marrec, C.L.; Christen, R.; Theodorakopoulos, N.; Février, L.; Levchuk, S.; Martin-Garin, A.; et al. Microbial diversity in contaminated soils along the T22 trench of the Chernobyl experimental platform. Appl. Geochem. 2012, 27, 1375–1383. [Google Scholar] [CrossRef]
- Bugai, D.; Kashparov, V.; Dewiére, L.; Khomutinin, Y.; Levchuk, S.; Yoschenko, V. Characterization of subsurface geometry and radioactivity distribution in the trench containing Chernobyl clean-up wastes. Environ. Geol. 2005, 47, 869–881. [Google Scholar] [CrossRef]
- Ruban, Y.V.; Shavanova, K.E.; Samofalova, D.O.; Nikonov, S.B.; Pareniuk, O.Y. Comparison of microbiomes of two different ecotypes of the Chernobyl exclusion zone: Points of temporary localization of radioactive waste (PTLRW) and contaminated ecosystems. Hyg. Ecol. 2020, 3, 83–88. [Google Scholar] [CrossRef]
- Shuryak, I.; Dadachova, E. Quantitative modeling of microbial population responses to chronic irradiation combined with other stressors. PLoS ONE 2016, 11, e0147696. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, N.N.; Tugay, T.; Dighton, J.; Zheltonozhsky, V.; McDermott, P. Ionizing radiation attracts soil fungi. Mycol. Res. 2004, 108, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, Y.V.; Redchitz, T.I.; Zheltonozhsky, V.A.; Dighton, J.; Zhdanova, N.N. Comparative responses of microscopic fungi to ionizing radiation and light. Folia Microbiol. 2006, 51, 45–49. [Google Scholar] [CrossRef]
- Zavilgelsky, G.B.; Abilev, S.K.; Sukhodolets, V.V.; Ahmad, S.I. Isolation and analysis of UV and radio-resistant bacteria from Chernobyl. J. Photochem. Photobiol. B 1998, 43, 152–157. [Google Scholar] [CrossRef]
- Narumi, I. Unlocking radiation resistance mechanisms: Still a long way to go. Trends Microbiol. 2003, 11, 422–425. [Google Scholar] [CrossRef]
- Zhdanova, N.N.; Zakharchenko, V.A.; Vember, V.V.; Nakonechnaya, L.T. Fungi from Chernobyl: Mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol. Res. 2000, 104, 1421–1426. [Google Scholar] [CrossRef]
- Averesch, N.J.H.; Shunk, G.K.; Kern, C. Cultivation of the dematiaceous fungus Cladosporium sphaerospermum aboard the International Space Station and effects of ionizing radiation. Front. Microbiol. 2022, 13, 877625. [Google Scholar] [CrossRef]
- Byrne, A.R. Radioactivity in fungi in Slovenia, Yugoslavia, following the Chernobyl accident. J. Environ. Radioact. 1988, 6, 177–183. [Google Scholar] [CrossRef]
- Haselwandter, K.; Berreck, M.; Brunner, P. Fungi as bioindicators of radiocaesium contamination: Pre- and post-Chernobyl activities. Trans. British Mycol. Soc. 1988, 90, 171–174. [Google Scholar] [CrossRef]
- Steine, M.; Linkov, I.; Yoshida, S. The role of fungi in the transfer and cycling of radionuclides in forest ecosystems. J. Environ. Radioact. 2002, 58, 217–241. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Takenaka, C. Upward mobilization of 137Cs in surface soils of Chamaecyparis obtusa Sieb. et Zucc. (hinoki) plantation in Japan. Sci. Total Environ. 2004, 318, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, B.; Dawson, D.E.; Kliashtorin, A.L. Decomposition in two pine forests: The mobilisation of 137Cs and K from forest litter. Soil Biol. Biochem. 1997, 29, 1673–1681. [Google Scholar] [CrossRef]
- Wada, T. Radiocesium contamination of marine and freshwater fish after the Fukushima Dai-ichi Nuclear Power Plant accident. Geochemistry 2021, 55, 159–175. [Google Scholar] [CrossRef]
- Ihara, H.; Kumagai, A.; Hori, T.; Nanba, K.; Aoyagi, T.; Takasaki, M.; Katayama, Y. Direct comparison of bacterial communities in soils contaminated with different levels of radioactive cesium from the first Fukushima nuclear power plant accident. Sci. Total Environ. 2021, 756, 143844. [Google Scholar] [CrossRef]
- Higo, M.; Kang, D.J.; Isobe, K. First report of community dynamics of arbuscular mycorrhizal fungi in radiocesium degradation lands after the Fukushima-Daiichi Nuclear disaster in Japan. Sci. Rep. 2019, 9, 8240. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Richardson, S.C.; Lawrence, B.M.; Holmes, J.; Watson, M. Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol. Lett. 2009, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Barto, E.K.; Hilker, M.; Müller, F.; Mohney, B.K.; Weidenhamer, J.D.; Rillig, M.C. The fungal fast lane: Common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS ONE 2011, 6, e27195. [Google Scholar] [CrossRef]
- Huang, Y.; Kaneko, Y.; Nakamori, T.; Miura, T.; Tanaka, Y.; Nonaka, M.; Takenaka, C. Radiocesium immobilization to leaf litter by fungi during first-year decomposition in a deciduous forest in Fukushima. J. Environ. Radioact. 2016, 152, 28–34. [Google Scholar] [CrossRef]
- Garnier-Laplace, J.; Beaugelin-Seiller, K.; Hinton, T.G. Fukushima wildlife dose reconstruction signals ecological consequences. Environ. Sci. Technol. 2011, 45, 5077–5078. [Google Scholar] [CrossRef]
- Saito, M.; Yamada, A.; Matsuda, Y.; Yamato, M. Absorption and accumulation of radioactive cesium by fungi. Chem. Biol. 2013, 5, 748–751. (In Japanese) [Google Scholar]
- Yamada, T.; Murakawa, I.; Saito, T.; Omura, K.; Takatoku, K.; Saiki, M.; Iguchi, K.; Inoue, M.; Saito, H.; Tsuji, K.; et al. Radiocaesium accumulation in wild mushrooms from low-level contaminated area due to the Fukushima Daiichi Nuclear Power Plant Accident-A case study in the University of Tokyo forests. Radioisotopes 2013, 62, 141–147. [Google Scholar] [CrossRef]
- Arrange, A.A.; Phelps, T.J.; Benoit, R.E.; Palumbo, A.V.; White, D.C. Bacterial sensitivity to UV light as a model for ionizing radiation resistance. J. Microbiol. Methods 1993, 18, 127–136. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Shibuya, M.; Kinoshita, I.; Yatabe, J.; Narumi, I.; Shibata, H.; Hayashi, R.; Fujiwara, D.; Murano, Y.; Hashimoto, H.; et al. DNA damage and survival time course of deinococcal cell pellets during 3 years of exposure to outer space. Front. Microbiol. 2020, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Musilova, M.; Wright, G.; Ward, J.M.; Dartnell, L.R. Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance. Astrobiology 2015, 15, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Barescut, J.; Lariviere, D.; Stocki, T.; Alexakhin, R.; Geras’kin, S. 25 years after the accident at the Chernobyl nuclear power plant: Radioecological lessons. Radioprotection 2011, 46, S595–S600. [Google Scholar] [CrossRef]
- Cheng, F.; Huang, X.; Qin, Q.; Chen, Z.; Li, F.; Song, W. The effect of aboveground long-term low-dose ionizing radiation on soil microbial diversity and structure. Front. Ecol. Evol. 2023, 11, 1184582. [Google Scholar] [CrossRef]
- Geras’kin, S.A.; Dikarev, V.G.; Zyablitskaya, Y.Y.; Oudalova, A.A.; Spirin, Y.V. Cytogenetic effects of radiation on agricultural plants observed in the Chernobyl region during the first years after the accident. KURRI-KR 2002, 79, 287–296. [Google Scholar]
- Boratyński, Z.; Arias, J.; Garcia, C.; Mappes, T.; Mousseau, T.A.; Møller, A.P.; Pajares, A.J.M.; Piwczyński, M.; Tukalenko, E. Ionizing radiation from Chernobyl affects development of wild carrot plants. Sci. Rep. 2016, 6, 39282. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Zhang, Z.D.; Tang, Q.Y.; Gu, M.Y.; Zhang, L.J.; Hou, M.; Sharon, A.; Yuan, H.L. Effect of ionizing radiation on the bacterial and fungal endophytes of the halophytic plant Kalidium schrenkianum. Microorganisms 2021, 9, 1050. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth-promoting Rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Dias, M.P.; Bastos, M.S.; Xavier, V.B.; Cassel, E.; Astarita, L.V.; Santarém, E.R. Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol. Biochem. 2017, 118, 479–493. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Kim, Y.C.; Leveau, J.; McSpadden Gardener, B.B.; Pierson, E.A.; Pierson, L.S., III; Ryu, C.M. The multifactorial basis for plant health promotion by plant-associated bacteria. Appl. Environ. Microbiol. 2011, 77, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.; Guscha, N.; Dyachenko, A. Effects of low dose radiation on plant-pathogen interactions in Chernobyl Zone. Radioprotection 2008, 43, 148. [Google Scholar] [CrossRef]
- Hinton, T.G.; Alexakhin, R.; Balonov, M.; Gentner, N.; Hendry, J.; Prister, B.; Strand, P.; Woodhead, D. Radiation-induced effects on plants and animals: Findings of the United Nations Chernobyl Forum. Health Phys. 2007, 93, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Adam-Guillermin, C.; Hertal-Aas, T.; Oughton, D.; Blanchard, L.; Alonzo, F.; Armant, O.; Horemans, N. Radiosensitivity and transgenerational effects in non-human species. Ann. ICRP 2018, 47, 327–341. [Google Scholar] [CrossRef]
- Duarte, G.T.; Volkova, P.Y.; Perez, F.F.; Horemans, N. Chronic ionizing radiation of plants: An evolutionary factor from direct damage to non-target effects. Plants 2023, 12, 1178. [Google Scholar] [CrossRef] [PubMed]
- Volkova, P.Y.; Geras’kin, S.A.; Kazakova, E.A. Radiation exposure in the remote period after the Chernobyl accident caused oxidative stress and genetic effects in Scots pine populations. Sci. Rep. 2017, 7, 43009. [Google Scholar] [CrossRef]
- Volkova, P.Y.; Bondarenko, E.V.; Kazakova, E.A. Radiation hormesis in plants. Curr. Opin. Toxicol. 2022, 30, 100334. [Google Scholar] [CrossRef]
- Gudkov, I.N.; Gaychenko, V.A.; Pareniuk, O.Y.; Grodzinsky, D.M. Changes in biocenoses in the Chernobyl NPP accident zone. Nucl. Phys. Atomic Energy 2011, 12, 362–374. [Google Scholar]
- Branco, S.; Schauster, A.; Liao, H.L.; Ruytinx, J. Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol. 2022, 235, 2158–2175. [Google Scholar] [CrossRef] [PubMed]
- Kothamasi, D.; Wannijn, J.; van Hees, M.; Nauts, R.; van Gompel, A.; Vanhoudt, N.; Vandenhove, H. Exposure to ionizing radiation affects the growth of ectomycorrhizal fungi and induces increased melanin production and increased capacities of reactive oxygen species scavenging enzymes. J. Environ. Radioact. 2019, 197, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dixit, S.K.; Senthil-Kumar, M. Drought stress predominantly endures Arabidopsis thaliana to Pseudomonas syringae infection. Front. Plant Sci. 2016, 7, 808. [Google Scholar] [CrossRef] [PubMed]
- Sakauchi, K.; Taira, W.; Otaki, J.M. Metabolomic profiles of the creeping wood sorrel Oxalis corniculata in radioactively contaminated fields in Fukushima: Dose-dependent changes in key metabolites. Life 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Liu, J.; Gao, Y.; Chen, Z. Distribution of endophytic bacteria in Alopecurus aequalis Sobol and Oxalis corniculata L. from soils contaminated by polycyclic aromatic hydrocarbons. PLoS ONE 2013, 8, e83054. [Google Scholar] [CrossRef] [PubMed]
- Mufti, R.; AmnaRafique, M.; Haq, F.; Munis, M.F.H.; Masood, S.; Mumtaz, A.S.; Chaudhary, H.J. Genetic diversity and metal resistance assessment of endophytes isolated from Oxalis corniculata. Soil Environ. 2015, 34, 89–99. [Google Scholar]
- Sakauchi, K.; Taira, W.; Otaki, J.M. Metabolomic response of the creeping wood sorrel Oxalis corniculata to low-dose radiation exposure from Fukushima’s contaminated soil. Life 2021, 11, 990. [Google Scholar] [CrossRef]
- Aung, H.P.; Djedidi, S.; Oo, A.Z.; Aye, Y.S.; Yokoyama, T.; Suzuki, S.; Sekimoto, H.; Bellingrath-Kimura, S.D. Growth and 137Cs uptake of four Brassica species influenced by inoculation with a plant growth-promoting rhizobacterium Bacillus pumilus in three contaminated farmlands in Fukushima prefecture, Japan. Sci. Total Environ. 2015, 521–522, 261–269. [Google Scholar] [CrossRef]
- Aqvist, J.; Luzhkov, V. Ion permeation mechanism of the potassium channel. Nature 2000, 404, 881–884. [Google Scholar] [CrossRef]
- Hayashi, G.; Shibato, J.; Imanaka, T.; Cho, K.; Kubo, A.; Kikuchi, S.; Satoh, K.; Kimura, S.; Ozawa, S.; Fukutani, S.; et al. Unraveling low-level gamma radiation-responsive changes in expression of early and late genes in leaves of rice seedlings at Iitate Village, Fukushima. J. Hered. 2014, 105, 723–738. [Google Scholar] [CrossRef]
- Rakwal, R.; Hayashi, G.; Shibato, J.; Deepak, S.A.; Gundimeda, S.; Simha, U.; Padmanaban, A.; Gupta, R.; Han, S.I.; Kim, S.T.; et al. Progress toward rice seed OMICS in low-level gamma radiation environment in Iitate Village, Fukushima. J. Hered. 2018, 109, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ichikawa, S.; Kubota, M.; Hoshino, J.; Kubota, Y.; Maruyama, K.; Fuma, S.; Kawaguchi, I.; Yoschenko, V.I.; Yoshida, S. Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci. Rep. 2015, 5, 13232. [Google Scholar] [CrossRef] [PubMed]
- Yoschenko, V.; Nanba, K.; Yoshida, S.; Watanabe, Y.; Takase, T.; Sato, N.; Keitoku, K. Morphological abnormalities in Japanese red pine (Pinus densiflora) at the territories contaminated as a result of the accident at Fukushima Dai-Ichi Nuclear Power Plant. J. Environ. Radioact. 2016, 165, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M. Endophytic actinomycetes: Biocontrol agents and growth promoters. In Bacteria in Agrobiology: Pant Growth Responses; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 201–220. [Google Scholar] [CrossRef]
- Martino, E.; Perotto, S. Mineral Transformations by Mycorrhizal Fungi. Geomicrobiol. J. 2010, 27, 609–623. [Google Scholar] [CrossRef]
- Clasen, B.E.; Silveira, A.D.O.; Baldoni, D.B.; Montagner, D.F.; Jacques, R.J.S.; Antoniolli, Z.I. Characterization of Ectomycorrhizal species through molecular biology tools and morphotyping. Sci. Agric. 2018, 75, 246–254. [Google Scholar] [CrossRef]
- Li, F.; Deng, X.; Chen, H.; Lu, H.; Guo, W.; Song, W.; Ge, L. Long-term gamma radiation effect on functional traits of Tradescantia Flumnensis L. Pol. J. Environ. Stud. 2022, 31, 1153–1160. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.C.; Guzmán, J.P.S.; Shay, J.E. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Hofman, R.W.; Campbell, B.D.; McNabb, W.C.; Hunt, D.Y. Population differences in Trifolium repens L. response to ultraviolet-B radiation: Foliar chemistry and consequences for two lepidopteran herbivores. Oecologia 2000, 122, 20–28. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.D.; Arnold, A.E. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.; Allen, M. What is the role of arbuscular mycorrhizal fungi in plant-to-ecosystem responses to elevated atmospheric CO2? Mycorrhiza 1999, 9, 1–8. [Google Scholar] [CrossRef]
- Sanders, I.R.; Streitwolf-Engel, R.; van der Heijden, M.G.; Boller, T.; Wiemken, A. Increased allocation to external hyphae of arbuscular mycorrhizal fungi under CO2 enrichment. Oecologia 1998, 117, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Michiels, K.; Vanderleyden, J.; Van Gool, A. Azospirillum—Plant root associations: A review. Biol. Fert. Soils 1989, 8, 356–368. [Google Scholar] [CrossRef]
- Taskaev, A.I.; Frolova, N.P.; Popova, O.N.; Shevchenko, V.A. The monitoring of herbaceous seeds in the 30-km zone of the Chernobyl nuclear accident. Sci. Total Environ. 1992, 112, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Suvorova, L.I.; Spirin, D.A.; Martyushov, V.Z.; Smirnov, E.G.; Tarasov, O.V.; Shein, G.P. Assessment of biological and ecological consequences of radioactive contamination of biogeocenoses. In Radiation Aspects of the Chernobyl Accident, Volume 2; Izrael, Y.A., Ed.; Hydrometeoizdat: St. Petersburg, Russia, 1993; pp. 321–325. (In Russian) [Google Scholar]
- Alexakhin, R.M.; Buldakov, L.A.; Gubanov, V.A.; Drozhko, Y.G.; Ilyin, L.A.; Kryshev, I.I.; Linge, I.I.; Romanov, G.N.; Savki, M.N. Large Radiation Accidents: Consequences and Protective Counter-Measures; IzdAT Publisher: Moscow, Russia, 2004. [Google Scholar]
- Grodzinsky, D.M.; Gudkov, I.N. Radiation injury of the plant in the zone of influence of the accident on Chernobyl Nuclear Power Plant. Radiat. Biol. Radioecol. 2006, 46, 189–199. (In Russian) [Google Scholar]
- Møller, A.P.; Shyu, J.C.; Mousseau, T.A. Ionizing radiation from Chernobyl and the fraction of viable pollen. Int. J. Plant Sci. 2016, 177, 727–735. [Google Scholar] [CrossRef]
- Kim, D.R.; Cho, G.; Jeon, C.W.; Weller, D.M.; Thomashow, L.S.; Paulitz, T.C.; Kwak, Y.S. A mutualistic interaction between Streptomyces bacteria, strawberry plants and pollinating bees. Nat. Commun. 2019, 10, 4802. [Google Scholar] [CrossRef]
- Liu, H.; Macdonald, C.A.; Cook, J.; Anderson, I.C.; Singh, B.K. An Ecological Loop: Host Microbiomes across Multitrophic Interactions. Trends Ecol. Evol. 2019, 34, 1118–1130. [Google Scholar] [CrossRef]
- Møller, A.P.; Barnier, F.; Mousseau, T.A. Ecosystems effects 25 years after Chernobyl: Pollinators, fruit set and recruitment. Oecologia 2012, 170, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, T. Chernobyl and Fukushima: Comparison of accident process and radioactive contamination. Kagaku 2016, 86, 0252–0257. (In Japanese) [Google Scholar]
- Ludovici, G.M.; Chierici, A.; de Souza, S.O.; d’Errico, F.; Iannotti, A.; Malizia, A. Effects of ionizing radiation on flora ten years after the Fukushima Dai-ichi disaster. Plants 2022, 11, 222. [Google Scholar] [CrossRef]
- Yoshioka, A.; Mishima, Y.; Fukasawa, K. Pollinators and other flying insects inside and outside the Fukushima evacuation zone. PLoS ONE 2015, 10, e0140957. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Nohara, C.; Iwasaki, M.; Kinjo, S.; Iwata, M.; Otaki, J.M. Spatiotemporal abnormality dynamics of the pale grass blue butterfly: Three years of monitoring (2011–2013) after the Fukushima nuclear accident. BMC Evol. Biol. 2015, 15, 15. [Google Scholar] [CrossRef]
- Møller, A.P.; Nishiumi, I.; Mousseau, T.A. Cumulative effects of radioactivity from Fukushima on the abundance and biodiversity of birds. J. Ornithol. 2015, 156, 297–305. [Google Scholar] [CrossRef]
- Møller, A.P.; Hagiwara, A.; Matsui, S.; Kasahara, S.; Kawatsu, K.; Nishiumi, I.; Suzuki, H.; Ueda, K.; Mousseau, T.A. Abundance of birds in Fukushima as judged from Chernobyl. Environ. Pollut. 2012, 164, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Bonisoli-Alquati, A.; Koyama, K.; Tedeschi, D.J.; Kitamura, W.; Sukuzi, H.; Ostermiller, S.; Arai, E.; Møller, A.P.; Mousseau, T.A. Abundance and genetic damage of barn swallows from Fukushima. Sci. Rep. 2015, 5, 9432. [Google Scholar] [CrossRef] [PubMed]
- Murase, K.; Murase, J.; Horie, R.; Endo, K. Effects of the Fukushima Daiichi nuclear accident on goshawk reproduction. Sci. Rep. 2015, 5, 9405. [Google Scholar] [CrossRef] [PubMed]
- Gange, A.C.; Eschen, R.; Wearn, J.A.; Thawer, A.; Sutton, B.C. Differential effects of foliar endophytic fungi on insect herbivores attacking a herbaceous plant. Oecologia 2012, 168, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F. Phytotoxins. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; pp. 3–18. [Google Scholar]
- Zagrobelny, M.; de Castro, É.C.P.; Møller, B.L.; Bak, S. Cyanogenesis in arthropods: From chemical warfare to nuptial gifts. Insects 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Clay, K. Fungal endophytes of grasses: A defensive mutualism between plants and fungi. Ecology 1988, 69, 10–16. [Google Scholar] [CrossRef]
- Morita, A.; Sakauchi, K.; Taira, W.; Otaki, J.M. Ingestional toxicity of radiation-dependent metabolites of the host plant for the pale grass blue butterfly: A mechanism of field effects of radioactive pollution in Fukushima. Life 2022, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Jomon, K.; Kuroda, Y.; Ajisaka, M.; Sakai, H. A new antibiotic, ikarugamycin. J. Antibiot. 1972, 25, 271–280. [Google Scholar] [CrossRef]
- Lacret, R.; Oves-Costales, D.; Gómez, C.; Díaz, C.; de la Cruz, M.; Pérez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs 2014, 13, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.I.; Aklilu, E.; Mohammedsalih, K.M.; Adekola, A.A.; Mergani, A.E.; Mohamad, M.; Kamaruzzaman, N.F. Antibacterial activity of ikarugamycin against intracellular Staphylococcus aureus in bovine mammary epithelial cells in vitro infection model. Biology 2021, 10, 958. [Google Scholar] [CrossRef]
- Krivolutskii, D.A. Problems of sustainable development and ecological indication in radioactively contaminated areas. Russ. J. Ecol. 2000, 31, 233–237. [Google Scholar] [CrossRef]
- Woodwell, G.M.; Brower, J.H. An aphid population explosion induced by chronic gamma irradiation of a forest. Ecology 1967, 48, 680–683. [Google Scholar] [CrossRef]
- Ochiai, K.; Hayama, S.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Uno, T.; Kato, T.; Konno, F.; Kawamoto, Y.; Tsuchida, S.; et al. Low blood cell counts in wild Japanese monkeys after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2014, 4, 5793. [Google Scholar] [CrossRef]
- Urushihara, Y.; Suzuki, T.; Shimizu, Y.; Ohtaki, M.; Kuwahara, Y.; Suzuki, M.; Uno, T.; Fujita, S.; Saito, A.; Yamashiro, H.; et al. Haematological analysis of Japanese macaques (Macaca fuscata) in the area affected by the Fukushima Daiichi Nuclear Power Plant accident. Sci. Rep. 2018, 8, 16748. [Google Scholar] [CrossRef]
- Hayama, S.I.; Tsuchiya, M.; Ochiai, K.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Kato, T.; Tanaka, A.; Konno, F.; Kawamoto, Y.; et al. Small head size and delayed body weight growth in wild Japanese monkey fetuses after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2017, 7, 3528. [Google Scholar] [CrossRef]
- Hayama, S.I.; Nakanishi, S.; Tanaka, A.; Konno, F.; Kawamoto, Y.; Omi, T. Influence of radiation exposure to delayed fetal growth in wild Japanese monkeys after the Fukushima accident. Front. Vet. Sci. 2023, 10, 1151361. [Google Scholar] [CrossRef] [PubMed]
- Nohara, C.; Hiyama, A.; Taira, W.; Otaki, J.M. Robustness and radiation resistance of the pale grass blue butterfly from radioactively contaminated areas: A possible case of adaptive evolution. J. Hered. 2018, 109, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Horemans, N.; Spurgeon, D.J.; Lecomte-Pradines, C.; Saenen, E.; Bradshaw, C.; Oughton, D.; Rasnaca, I.; Kamstra, J.H.; Adam-Guillermin, C. Current evidence for a role of epigenetic mechanisms in response to ionizing radiation in an ecotoxicological context. Environ. Pollut. 2019, 251, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Gombeau, K.; Bonzom, J.M.; Cavalié, I.; Camilleri, V.; Orjollet, D.; Dubourg, N.; Beaugelin-Seiller, K.; Bourdineaud, J.P.; Lengagne, T.; Armant, O.; et al. Dose-dependent genomic DNA hypermethylation and mitochondrial DNA damage in Japanese tree frogs sampled in the Fukushima Daiichi area. J. Environ. Radioact. 2020, 225, 106429. [Google Scholar] [CrossRef]
- Hancock, S.; Vo, N.T.K.; Goncharova, R.I.; Seymour, C.B.; Byun, S.H.; Mothersill, C.E. One-Decade-Spanning transgenerational effects of historic radiation dose in wild populations of bank voles exposed to radioactive contamination following the chernobyl nuclear disaster. Environ. Res. 2020, 80, 108816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakauchi, K.; Otaki, J.M. Soil Microbes and Plant-Associated Microbes in Response to Radioactive Pollution May Indirectly Affect Plants and Insect Herbivores: Evidence for Indirect Field Effects from Chernobyl and Fukushima. Microorganisms 2024, 12, 364. https://doi.org/10.3390/microorganisms12020364
Sakauchi K, Otaki JM. Soil Microbes and Plant-Associated Microbes in Response to Radioactive Pollution May Indirectly Affect Plants and Insect Herbivores: Evidence for Indirect Field Effects from Chernobyl and Fukushima. Microorganisms. 2024; 12(2):364. https://doi.org/10.3390/microorganisms12020364
Chicago/Turabian StyleSakauchi, Ko, and Joji M. Otaki. 2024. "Soil Microbes and Plant-Associated Microbes in Response to Radioactive Pollution May Indirectly Affect Plants and Insect Herbivores: Evidence for Indirect Field Effects from Chernobyl and Fukushima" Microorganisms 12, no. 2: 364. https://doi.org/10.3390/microorganisms12020364
APA StyleSakauchi, K., & Otaki, J. M. (2024). Soil Microbes and Plant-Associated Microbes in Response to Radioactive Pollution May Indirectly Affect Plants and Insect Herbivores: Evidence for Indirect Field Effects from Chernobyl and Fukushima. Microorganisms, 12(2), 364. https://doi.org/10.3390/microorganisms12020364





