Plant-Based Films and Hydrogels for Wound Healing
Abstract
:1. Introduction
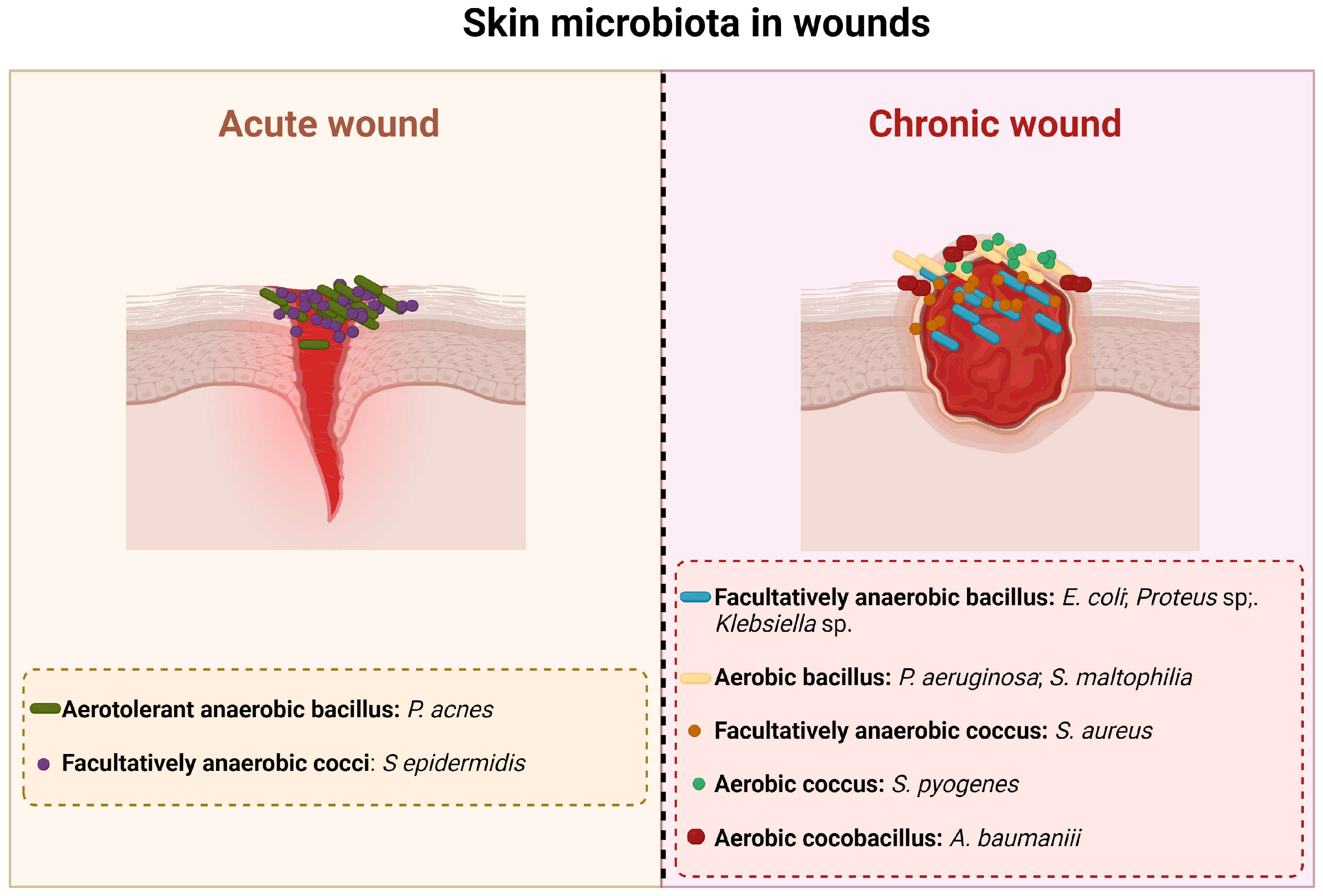
2. Injured Skin: Microbiology
3. Wound Healing
4. Wound Healing: A Brief History and Current Treatments
| Type of Dressing | Formulation | Advantages | Disadvantages | Commercially Available Products | References |
|---|---|---|---|---|---|
| Inert/passive | Gauzes |
|
| Curity, Vaseline Gauze, Xeroform, Multisorb, Urgotul SSD/S.Ag | [15,32] |
| Bioactive | Hydrocolloids |
|
| DouDERM, Granuflex, Comfeel, Tegasorb | [8,15] |
| Alginates |
|
| Algisite, Kaltostat, Sorbsan, Tegagen, SeaSorb, PolyMem | [15,32] | |
| Collagens |
|
| Puracol Plus, Triple Helix Collagen, Cutimed Epiona Sterile, BIOSTEP | [18,33] | |
| Hydrofibers |
|
| Aquacel | [15,34] | |
| Interactive | Hydrogels |
|
| Carrasyn, Curagel, Nu-Gel, Purilon, Restore, SAF-gel, XCell | [15,29,32,34] |
| Semi-permeable films |
|
| Opsite, Tegaderm, Biooclusive, Polyskin, Blisterfilm, Cutifilm, Flexigrid | [15,23,32,34] | |
| Semi-permeable foams |
|
| Allevyn, Lyofoam, Tielle, Curafoam, Mepilex, Permafoam, Tegafoam, | [15,23,34] | |
| Skin substitutes |
|
| Epicel®, Laserskin®, TransCyte®, Dermagraft®, AlloDerm®, Strattice®, Biobrane®, Integra® Dermal Regeneration Template, Apligraft®, Graftskin®, OrCell®, Graftjacket®, PermaDerm® | [35] |
5. The Role of Essential Oils and Plant Extracts in the Wound Healing Process
| Minimum Inhibitory Concentrations (%) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Essential oils | Acinetobacter sp. | E. coli | K. pneumoniae | P. aeruginosa | P. vulgaris | S. aureus | S. epidermidis | ||
| Arborvitae sp. | 0.125 | 0.25 | 0.125 | [54] | |||||
| Cassia sp. | 0.125 | 0.125 | 0.125 | [54] | |||||
| Cinnamomum zeylanicum (Cinnamon) | 0.8 | 0.2 | 0.8 | 0.05 | 0.1 | [55] | |||
| Cymbopogan citratus (Lemongrass) | 0.06 | 0.25 | 0.25 | 0.25 | [54,55] | ||||
| Eucalyptus sp. (eucalyptus) | 1.25 | 2.5 | 1.25 | [54] | |||||
| Lavandula officinalis (Lavender) | 0.2 | 0.1 | [55] | ||||||
| Melaleuca alternifolia (Tea tree) | 0.125 | 0.5 | 0.125 | [54] | |||||
| Pimenta dioica (Jamaica pepper) | 0.8 | 0.4 | 0.1 | 0.1 | [55] | ||||
| Piper betle (Betel) | 0.8 | 0.4 | 0.4 | 0.05 | 0.05 | [55] | |||
| Psiadia arguta | 1.6 | 0.05 | 0.025 | [55] | |||||
| Psiadia terebinthina | 1.6 | 0.4 | 0.8 | 0.05 | 0.025 | [55] | |||
| Origanum vulgarae (Oregano) | 0.115 | 0.05 | 0.125 | 0.05 | 0.029 | [54,56] | |||
| Rosmarinus officinalis (Rosemary) | 0.256 | [57] | |||||||
| Salvia officinalis (Sage) | >0.256 | [57] | |||||||
| Satureja montana (Winter savory) | 2.33 | [55] | |||||||
| Syzygium aromaticum (Clove) | 0.125 | 0.5 | 0.125 | [54] | |||||
| Thymus vulgaris (Thyme) | 0.064 | 0.05 | 0.125 | 0.05 | [54,57] | ||||
| Plant extracts | Acacia nilotica 1 | 0.312 | [47] | ||||||
| Bauhinia kockiana 2 | 0.00625 | [48] | |||||||
| Cistus salviifolius 3 | 0.00807 | [49] | |||||||
| Cytinus hypocistis 1 | >0.05 | >0.05 | 0.0125 | 0.025 | [53] | ||||
| Cytinus ruber 1 | >0.05 | >0.05 | 0.0125 | 0.025 | [53] | ||||
| Phaseolus vulgaris 4 | 0.0512 | 0.0512 | 0.0512 | [58] | |||||
| Punica granatum 3 | 0.005167 | [49] | |||||||
| Quercus variabilis 1 | 0.0625 | [50] | |||||||
| Smilax china 1 | 0.0195 | 0.0195 | [51] | ||||||
| Theobroma cacao 4 | 0.0064 | 0.0064 | 0.1024 | [58] | |||||
| Triumfetta welwitschii 1 | 0.01 | 0.01 | [39] | ||||||
6. Formulations Incorporated with EOs and PEs
6.1. Films
6.2. Hydrogels
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S.F. Carrier Oils in Dermatology. Arch. Dermatol. Res. 2019, 311, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic Polymeric Biomaterials for Wound Healing: A Review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing From Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noël, A.; Jérome, C.; Nusgens, B.; et al. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Guterres, S.; Balducci, A.; Colombo, P.; Sonvico, F. Polymeric Films Loaded with Vitamin E and Aloe vera for Topical Application in the Treatment of Burn Wounds. BioMed Res. Int. 2014, 2014, 641590. [Google Scholar] [CrossRef]
- Das, U.; Behera, S.S.; Singh, S.; Rizvi, S.I.; Singh, A.K. Progress in the Development and Applicability of Potential Medicinal Plant Extract-Conjugated Polymeric Constructs for Wound Healing and Tissue Regeneration. Phyther. Res. 2016, 30, 1895–1904. [Google Scholar] [CrossRef]
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in Exploring and Manipulating the Human Skin Microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Grice, E.A. Microbial Ecology of the Skin in the Era of Metagenomics and Molecular Microbiology. Cold Spring Harb. Perspect. Med. 2013, 3, a015362. [Google Scholar] [CrossRef]
- Pereira, S.G.; Moura, J.; Carvalho, E.; Empadinhas, N. Microbiota of Chronic Diabetic Wounds: Ecology, Impact, and Potential for Innovative Treatment Strategies. Front. Microbiol. 2017, 8, 1791. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Okur, M.; Karantas, I.; Ay, Z.; Üstündağ Okur, N.; Siafaka, P. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Harding, K.G. Bacteria and Wound Healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef]
- Cardona, A.F.; Wilson, S.E. Skin and Soft-Tissue Infections: A Critical Review and the Role of Telavancin in Their Treatment. Clin. Infect. Dis. 2015, 61, S69–S78. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Ashworth, J.J.; Ashcroft, G.S. The Hormonal Regulation of Cutaneous Wound Healing. Clin. Dermatol. 2007, 25, 56–62. [Google Scholar] [CrossRef]
- Wilson, J.; Clark, J. Obesity: Impediment to Postsurgical Wound Healing. Adv. Skin Wound Care 2004, 17, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Vickery, K.; Deva, A.K.; Hu, H. Efficacy of Surgical/Wound Washes against Bacteria: Effect of Different In Vitro Models. Materials 2022, 15, 3630. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Alven, S.; Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds. Pharmaceutics 2021, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.B. The History of Wound Care. J. Am. Col. Certif. Wound Spec. 2011, 3, 65–66. [Google Scholar] [CrossRef]
- Daunton, C.; Kothari, S.; Smith, L.E.; Steele, D. A History of Materials and Practices for Wound Management. Wound Pract. Res. 2012, 20, 174–186. [Google Scholar]
- Fu, X.; Wang, Z.; Sheng, Z. Advances in Wound Healing Research in China: From Antiquity to the Present. Wound Repair Regen. 2001, 9, 2–10. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound Healing and Antimicrobial Effect of Active Secondary Metabolites in Chitosan-Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel Wound Dressings for Bioactive Treatment of Acute and Chronic Wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Taghipour, N.; Deravi, N.; Rahimi, M. Chitosan-Based Scaffolds, Suitable Structures for Wound Healing Dressing: A Short Review. J. Regen. Reconstr. Restor. (Triple R) 2020, 5, e11. [Google Scholar] [CrossRef]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.; Vasconez, H. Wound Healing: Biologics, Skin Substitutes, Biomembranes and Scaffolds. Healthcare 2014, 2, 356–400. [Google Scholar] [CrossRef]
- Freitas, I.R.; Cattelan, M.G. Chapter 15—Antimicrobial and Antioxidant Properties of Essential Oils in Food Systems—An Overview. In Handbook of Food Bioengineering: Volume 10: Microbial Contamination and Food Degradation; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; pp. 443–470. ISBN 978-0-12-811515-2. [Google Scholar]
- Bashir, A.; Jabeen, S.; Gull, N.; Islam, A.; Sultan, M.; Ghaffar, A.; Khan, S.; Sagar, S.; Jamil, T. Co-Concentration Effect of Silane with Natural Extract on Biodegradable Polymeric Films for Food Packaging. Int. J. Biol. Macromol. 2018, 106, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, L.; Gong, X.; Chi, F.; Ma, Z. Fabrication and Comparison of Active Films from Chitosan Incorporating Different Spice Extracts for Shelf Life Extension of Refrigerated Pork. LWT 2021, 135, 110181. [Google Scholar] [CrossRef]
- Mombeshora, M.; Mukanganyama, S. Antibacterial Activities, Proposed Mode of Action and Cytotoxicity of Leaf Extracts from Triumfetta welwitschii against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2019, 19, 315. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.; Canhoto, J.; Salgueiro, L. Antidermatophytic Activity of Essential Oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; A. Méndez-Vilas, Ed.; FORMATEX: Badajoz, Spain, 2011; pp. 1167–1178. [Google Scholar]
- Acosta, S.; Chiralt, A.; Santamarina Siurana, M.; Roselló, J.; Gonzalez-Martinez, C.; Cháfer, M. Antifungal Films Based on Starch-Gelatin Blend, Containing Essential Oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Burt, S.; Van der Zee, R.; Koets, A.; Graaff, A.; Knapen, F.; Gaastra, W.; Haagsman, H.; Veldhuizen, E. Carvacrol Induces Heat Shock Protein 60 and Inhibits Synthesis of Flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum Sensing and Phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Tarning, J.; Cho, T.Z.A.; Anal, A.K. Activities and Possible Modes of Action of Acacia nilotica (L.) Del. against Multidrug-Resistant Escherichia coli and Salmonella. Molecules 2017, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.L.; Mahadi, A.M.; Wong, K.M.; Goh, J.K. Anti-Methicillin-Resistance Staphylococcus aureus (MRSA) Compounds from Bauhinia kockiana Korth. and Their Mechanism of Antibacterial Activity. BMC Complement. Altern. Med. 2018, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The Antimicrobial Capacity of Cistus salviifolius and Punica granatum Plant Extracts against Clinical Pathogens Is Related to Their Polyphenolic Composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Z.-H.; Wang, D.-M.; Li, D.-W.; Yang, L.-N.; Wang, W. Chemical Composition, Antibacterial Activity and Related Mechanism of Valonia and Shell from Quercus variabilis Blume (Fagaceae) against Salmonella paratyphi a and Staphylococcus aureus. BMC Complement. Altern. Med. 2019, 19, 271. [Google Scholar] [CrossRef]
- Xu, M.; Xue, H.; Li, X.; Zhao, Y.; Lin, L.; Yang, L.; Zheng, G. Chemical Composition, Antibacterial Properties, and Mechanism of Smilax china L. Polyphenols. Appl. Microbiol. Biotechnol. 2019, 103, 9013–9022. [Google Scholar] [CrossRef]
- Roshan, N.; Riley, T.V.; Knight, D.R.; Steer, J.H.; Hammer, K.A. Natural Products Show Diverse Mechanisms of Action against Clostridium difficile. J. Appl. Microbiol. 2019, 126, 468–479. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin Profile, Antioxidant Properties, and Antimicrobial Activity of Extracts from Two Mediterranean Species of Parasitic Plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; White, A.W.; Laird, K. Characterisation and Screening of Antimicrobial Essential Oil Components against Clinically Important Antibiotic-resistant Bacteria Using Thin Layer Chromatography-direct Bioautography Hyphenated with GC-MS, LC-MS and NMR. Phytochem. Anal. 2019, 30, 121–131. [Google Scholar] [CrossRef]
- Ivanovic, J.; Misic, D.; Zizovic, I.; Ristic, M. In Vitro Control of Multiplication of Some Food-Associated Bacteria by Thyme, Rosemary and Sage Isolates. Food Control 2012, 25, 110–116. [Google Scholar] [CrossRef]
- Nayim, P.; Mbaveng, A.T.; Wamba, B.E.N.; Fankam, A.G.; Dzotam, J.K.; Kuete, V. Antibacterial and Antibiotic-Potentiating Activities of Thirteen Cameroonian Edible Plants against Gram-Negative Resistant Phenotypes. Sci. World J. 2018, 2018, 4020294. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial Hydrogels: Promising Materials for Medical Application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Ziora, Z.M. Wound Dressings from Naturally-Occurring Polymers: A Review on Homopolysaccharide-Based Composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of Advances in Polymeric Wound Dressing Films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Palou, E.; López-Malo, A. Chapter 15—Essential Oils Added to Edible Films. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; ISBN 978-0-12-416641-7. [Google Scholar]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef]
- Pérez-Recalde, M.; Arias, I.E.R.; Hermida, É.B. Could Essential Oils Enhance Biopolymers Performance for Wound Healing? A Systematic Review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Augusto, A.; Dias, J.R.; Campos, M.J.; Alves, N.M.; Pedrosa, R.; Silva, S.F.J. Influence of Codium tomentosum Extract in the Properties of Alginate and Chitosan Edible Films. Foods 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-Containing Essential Oils Loaded onto Chitosan/Polyvinyl Alcohol Blended Films and Their Ability to Eradicate Staphylococcus aureus or Pseudomonas aeruginosa from Infected Microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum Perforatum Incorporated Chitosan Films as Potential Bioactive Wound Dressing Material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Scurr, D.; Pompa, P.; Athanassiou, A. All-Natural Composite Wound Dressing Films of Essential Oils Encapsulated in Sodium Alginate with Antimicrobial Properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, Antioxidant and Antimicrobial Properties of Chitosan Films Containing Eucalyptus globulus Essential Oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic Antimicrobial Activities of Natural Essential Oils with Chitosan Films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef]
- Ge, Y.; Ge, M. Sustained Broad-Spectrum Antimicrobial and Haemostatic Chitosan-Based Film with Immerged Tea Tree Oil Droplets. Fibers Polym. 2015, 16, 308–318. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, Antibacterial and Antioxidant Properties of Chitosan Films Incorporated with Thyme Oil for Potential Wound Healing Applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef]
- Dos Santos, E.P.; Nicácio, P.H.M.; Barbosa, F.C.; da Silva, H.N.; Andrade, A.L.S.; Fook, M.V.L.; de Silva, S.M.; Lima, I.F.L. Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [PubMed]
- Râpă, M.; Zaharescu, T.; Stefan, L.M.; Gaidău, C.; Stănculescu, I.; Constantinescu, R.R.; Stanca, M. Bioactivity and Thermal Stability of Collagen–Chitosan Containing Lemongrass Essential Oil for Potential Medical Applications. Polymers 2022, 14, 3884. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Gómez, L.E.; Martel-Estrada, S.A.; Vargas-Requena, C.; Rivera-Armenta, J.L.; Alba-Baena, N.; Rodríguez-González, C.; Olivas-Armendáriz, I. Chitosan/Mimosa tenuiflora Films as Potential Cellular Patch for Skin Regeneration. Int. J. Biol. Macromol. 2016, 93, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Gómez, L.; Martel-Estrada, S.-A.; Vargas-Requena, C.-L.; Acevedo-Fernández, J.-J.; Rodriguez, C.; Hernández-Paz, J.-F.; Santos-Rodríguez, E.; Olivas-Armendariz, I. Characterization and Evaluation of a Novel O-Carboxymethyl Chitosan Films with Mimosa tenuiflora Extract for Skin Regeneration and Wound Healing. J. Bioact. Compat. Polym. 2019, 35, 39–56. [Google Scholar] [CrossRef]
- Ghafoor, B.; Ali, M.N.; Ansari, U.; Bhatti, M.; Akhtar, H.; Mir, M.; Darkhsan, F. New Biofunctional Loading of Natural Antimicrobial Agent in Biodegradable Polymeric Films for Biomedical Applications. Int. J. Biomater. 2016, 2016, 6964938. [Google Scholar] [CrossRef]
- Costa, N.N.; de Faria Lopes, L.; Ferreira, D.F.; de Prado, E.M.L.; Severi, J.A.; Resende, J.A.; de Paula Careta, F.; Ferreira, M.C.P.; Carreira, L.G.; de Souza, S.O.L.; et al. Polymeric Films Containing Pomegranate Peel Extract Based on PVA/Starch/PAA Blends for Use as Wound Dressing: In Vitro Analysis and Physicochemical Evaluation. Mater. Sci. Eng. C 2020, 109, 110643. [Google Scholar] [CrossRef]
- Muthukumar, T.; Senthil, R.; Sastry, T.P. Synthesis and Characterization of Biosheet Impregnated with Macrotyloma uniflorum Extract for Burn/Wound Dressings. Colloids Surf. B Biointerfaces 2013, 102, 694–699. [Google Scholar] [CrossRef]
- Agarwal, R.; Alam, M.S.; Gupta, B. Preparation of Curcumin Loaded Poly(vinyl alcohol)-Poly(ethylene oxide)-carboxymethyl Cellulose Membranes for Wound Care Application. J. Biomater. Tissue Eng. 2013, 3, 273–283. [Google Scholar] [CrossRef]
- Mutlu, B.; Erci, F.; Çakır Koç, R. Production of Alginate Films Containing Hypericum perforatum Extract as an Antibacterial and Antioxidant Wound Dressing Material. J. Bioact. Compat. Polym. 2022, 37, 134–148. [Google Scholar] [CrossRef]
- Cruz Sánchez, E.; García, M.T.; Pereira, J.; Oliveira, F.; Craveiro, R.; Paiva, A.; Gracia, I.; García-Vargas, J.M.; Duarte, A.R.C. Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing. Molecules 2023, 28, 3689. [Google Scholar] [CrossRef]
- Sathuvan, M.; Thangam, R.; Cheong, K.-L.; Kang, H.; Liu, Y. κ-Carrageenan-Essential Oil Loaded Composite Biomaterial Film Facilitates Mechanosensing and Tissue Regenerative Wound Healing. Int. J. Biol. Macromol. 2023, 241, 124490. [Google Scholar] [CrossRef]
- Harrison, I.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef]
- Weller, C.; Weller, C.; Team, V. 4—Interactive Dressings and Their Role in Moist Wound Management. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2019; pp. 105–134. ISBN 978-0-08-102192-7. [Google Scholar]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for Delivery of Bioactive Agents: A Historical Perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a Historical Perspective: From Simple Networks to Smart Materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Chummun, I.; Bekah, D.; Goonoo, N.; Bhaw-Luximon, A. Assessing the Mechanisms of Action of Natural Molecules/Extracts for Phase-Directed Wound Healing in Hydrogel Scaffolds. RSC Med. Chem. 2021, 12, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.; Ullah Khan, I.; Asif, M.; Khan, R.; Asghar, S.; Khalid, I.; Syed, H.; Irfan, M.; Rehman, F.; Shahzad, Y.; et al. In Vitro and In Vivo Evaluation of Gellan Gum Hydrogel Films: Assessing the Co Impact of Therapeutic Oils and Ofloxacin on Wound Healing. Int. J. Biol. Macromol. 2021, 166, 483–495. [Google Scholar] [CrossRef]
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.-K.; Achim, M.; Simona, M. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial Polysaccharide-Based Hydrogel Dressing Containing Plant Essential Oil for Burn Wound Healing. Burns Trauma 2021, 9, tkab041. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Liu, Y.; Yue, Y.; Liu, Y.; Guo, F. Essential Oil Nanoemulsion Hydrogel with Anti-Biofilm Activity for the Treatment of Infected Wounds. Polymers 2023, 15, 1376. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chimnoi, N.; Reuk-Ngam, N.; Khlaychan, P.; Makarasen, A.; Wetprasit, N.; Dechtrirat, D.; Supaphol, P.; Techasakul, S. Development of Gelatin Hydrogel Pads Incorporated with Eupatorium adenophorum Essential Oil as Antibacterial Wound Dressing. Polym. Bull. 2019, 76, 701–724. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to Be Used in Wound Dressing Applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Singh, P.; Verma, C.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Preparation of Thyme Oil Loaded κ-Carrageenan-Polyethylene Glycol Hydrogel Membranes as Wound Care System. Int. J. Pharm. 2022, 618, 121661. [Google Scholar] [CrossRef]
- Kavoosi, G.; Bordbar, Z.; Dadfar, S.M.; Dadfar, S.M.M. Preparation and Characterization of a Novel Gelatin–Poly(vinyl alcohol) Hydrogel Film Loaded with Zataria multiflora Essential Oil for Antibacterial–Antioxidant Wound-Dressing Applications. J. Appl. Polym. Sci. 2017, 134, 45351. [Google Scholar] [CrossRef]
- Sami, D.G.; Abdellatif, A.; Azzazy, H.M.E. Turmeric/Oregano Formulations for Treatment of Diabetic Ulcer Wounds. Drug Dev. Ind. Pharm. 2020, 46, 1613–1621. [Google Scholar] [CrossRef]
- Annapoorna, M.; Kumar, P.T.; Lakshman, L.R.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Biochemical Properties of Hemigraphis Alternata Incorporated Chitosan Hydrogel Scaffold. Carbohydr. Polym. 2013, 92, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmałek, T.; Kaprón, B.; Plech, T.; Szymanowska, D.; Karazniewicz-Łada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel Delivery System Containing Calendulae flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics 2020, 12, 634. [Google Scholar] [CrossRef]
- Salehi, M.; Zamiri, S.; Samadian, H.; Ai, J.; Foroutani, L.; Ai, A.; Khanmohammadi, M. Chitosan Hydrogel Loaded with Aloe vera Gel and Tetrasodium Ethylenediaminetetraacetic Acid (EDTA) as the Wound Healing Material: In Vitro and in Vivo Study. J. Appl. Polym. Sci. 2021, 138, e50225. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Khatoon, F.; Rizvi, M.A.; Zafaryab, M. Ethyl Acetate Salix alba Leaves Extract-Loaded Chitosan-Based Hydrogel Film for Wound Dressing Applications. J. Biomater. Sci. Polym. Ed. 2015, 26, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Pelin, I.M.; Silion, M.; Popescu, I.; Rîmbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/Poly(vinyl alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications. Pharmaceutics 2023, 15, 1674. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zagórska-Dziok, M.; Perużyńska, M.; Cybulska, K.; Kucharska, E.; Ossowicz-Rupniewska, P.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł.; Sulikowski, T.; et al. Assessment of the Anti-Inflammatory, Antibacterial and Anti-Aging Properties and Possible Use on the Skin of Hydrogels Containing Epilobium angustifolium L. Extracts. Front. Pharmacol. 2022, 13, 896706. [Google Scholar] [CrossRef] [PubMed]
- Rathod, L.; Bhowmick, S.; Patel, P.; Sawant, K. Calendula Flower Extract Loaded PVA Hydrogel Sheet for Wound Management: Optimization, Characterization and In-Vivo Study. J. Drug Deliv. Sci. Technol. 2022, 68, 103035. [Google Scholar] [CrossRef]
- Alsakhawy, S.A.; Baghdadi, H.H.; El-Shenawy, M.A.; Sabra, S.A.; El-Hosseiny, L.S. Encapsulation of Thymus vulgaris Essential Oil in Caseinate/Gelatin Nanocomposite Hydrogel: In Vitro Antibacterial Activity and In Vivo Wound Healing Potential. Int. J. Pharm. 2022, 628, 122280. [Google Scholar] [CrossRef]
- Ali, A.; Garg, P.; Goyal, R.; Khan, A.; Negi, P.; Li, X.; Kulshrestha, S. An Efficient Wound Healing Hydrogel Based on a Hydroalcoholic Extract of MORINGA OLEIFERA Seeds. S. Afr. J. Bot. 2022, 145, 192–198. [Google Scholar] [CrossRef]
- Casado-Diaz, A.; Moreno-Rojas, J.M.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Tunez, I.; La Torre, M.; Berenguer Pérez, M.; Priego-Capote, F.; Pereira-Caro, G. Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract. Pharmaceutics 2022, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Khan, A.; Khan, M.K.; Braga, V.A. Preparation and Properties of High Sheared Poly(vinyl alcohol)/Chitosan Blended Hydrogels Films with Lawsonia inermis Extract as Wound Dressing. J. Drug Deliv. Sci. Technol. 2021, 61, 102227. [Google Scholar] [CrossRef]
- Antonescu, I.A.; Antonescu, A.; Miere, F.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; et al. Evaluation of Wound Healing Potential of Novel Hydrogel Based on Ocimum basilicum and Trifolium pratense Extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]



| (a) Films Prepared by Solvent Casting Incorporated with EOs | |||||||
| Film Characteristics | Antibacterial Activity | ||||||
| Polymer | Plasticizer | Essential Oil | Experimental Method | Studied Species | Antibacterial Activity Values | Intended Application | Reference |
| Alginate | Glycerol | Cinnamon | Disc diffusion assay | E. coli | 12 mm | Wound dressing | [72] |
| Lavender | Disc diffusion assay | E. coli | 2 mm | Wound dressing | [72] | ||
| Tea tree | Disc diffusion assay | E. coli | 2 mm | Wound dressing | [72] | ||
| Peppermint | Disc diffusion assay | E. coli | 2 mm | Wound dressing | [72] | ||
| Lemongrass | Disc diffusion assay | E. coli | 3 mm | Wound dressing | [72] | ||
| Chitosan | Poly-vinyl alcohol | Cinnamon | Time-kill kinetics | S. aureus P. aeruginosa | Inhibition at 6 h No inhibition | -------------- | [70] |
| Clove | Time-kill kinetics | S. aureus P. aeruginosa | Inhibition at 24 h No inhibition | -------------- | [70] | ||
| Glycerol | Eucalyptus | Disc diffusion assay | E. coli S. aureus P. aeruginosa | 153.37 mm2 61.35 mm2 118.29 mm2 | -------------- | [73] | |
| Clove bud | Disc diffusion assay | E. coli S. aureus | 5 mm 20 mm | -------------- | [74] | ||
| Cinnamon | Disc diffusion assay | E. coli S. aureus | 10 mm 30 mm | -------------- | [74] | ||
| Tea tree | Turbidimetric method | E. coli S. aureus | No inhibition A significant difference in optical density | Wound healing | [75] | ||
| Thyme | Agar diffusion assay | E. coli K. pneumxoniae S. aureus P. aeruginosa | 17 mm 19 mm 16 mm 16 mm | Wound healing | [76] | ||
| Clove | Disc diffusion assay | E. coli S. aureus | 8 mm 9 mm | Wound dressing | [77] | ||
| Tea tree | Disc diffusion assay | E. coli S. aureus | 9 mm 6 mm | Wound dressing | [77] | ||
| Hypericum perforatum | Agar diffusion assay | E. coli S. aureus | 2.9 ± 0.1 cm 1.97 ± 0.05 cm | Wound dressing | [71] | ||
| Chitosan/collagen | Lemongrass | Colony count method (inhibition percentage) | E. coli S. aureus | 99.8% 99.9% | -------------- | [78] | |
| (b) Films Prepared by Solvent Casting Incorporated with PEs | |||||||
| Antibacterial Activity | |||||||
| Polymer(s) | Plasticizer | Plant Extract | Experimental Method | Studied Species | Antibacterial Activity Values | Intended Application | Reference |
| Chitosan | Glycerol | Mimosa tenuiflora | Turbidimetry assay | E. coli Micrococcus luteus | 24% 95% | Skin regeneration | [79] |
| O-carboxymethyl chitosan | Glycerol | Mimosa tenuiflora | Turbidimetry assay | E. coli Micrococcus luteus | 22% 17% | Skin regeneration | [80] |
| Poly-vinyl alcohol | Aloe vera | Disc diffusion assay | E. coli P. aeruginosa | 16 mm 16 mm | Coating of surgical sutures | [81] | |
| Poly-vinyl alcohol/starch/polyacrylic acid | Glycerin | Punica granatum | Disc diffusion assay | S. epidermidis MRSA | 23 mm 20 mm | Wound healing | [82] |
| Collagen/fibrin | Ethylene glycol | Macrotyloma uniflorum | Agar well diffusion assay | B. subtilis S. aureus P. vulgaris E. coli | Presents antibacterial activity against all studied species | Burn Wound dressing | [83] |
| Poly (Vinyl Alcohol)-Poly (Ethylene Oxide)-Carboxymethyl Cellulose | Curcuma longa (curcumin) | Colony count method (inhibition percentage) | E. coli S. aureus | 74.7% 96% | Wound dressing | [84] | |
| Alginate | Hypericum perforatum | Viable cell count | E. coli S. aureus | Presents antibacterial activity against all studied species. | Wound dressing | [85] | |
| (a) HGs Incorporated with EOs | |||||||
| Hydrogel Constitution | Essential Oil | Preparation Method | Antibacterial Activity | Intended Application | Reference | ||
| Experimental Method | Studied Species | Antibacterial Activity Values | |||||
| Chitosan/carboner 940 | Eucalyptus | Simple blending | Turbidimetric analysis | E. coli S. aureus | 46.26% 63.05% | Burn wound | [97] |
| Chitosan/carboner 940 | Ginger | Simple blending | Turbidimetric analysis | E. coli S. aureus | 18.21% 38.41% | Burn wound | [97] |
| Chitosan/carboner 940 | Cumin | Simple blending | Turbidimetric analysis | E. coli S. aureus | 22.90% 53.67% | Burn wound | [97] |
| Carbomer 940/chitosan | Eucalyptus | Simple blending | Turbidimetric analysis | S. aureus | Greater than 50% | Wound healing | [98] |
| Gellan gum/propylene glycol/calcium chloride | Lavender | Solvent casting ionotropic gelation | Agar well diffusion method | E. coli S. aureus | 20 mm 21 mm | Wound healing | [95] |
| Gellan gum/propylene glycol/calcium chloride | Tea tree | Solvent casting ionotropic gelation | Agar well diffusion method | E. coli S. aureus | 30 mm 31 mm | Wound healing | [95] |
| Gelatin/glutaraldehyde | Eupatorium adenophorum | Solvent casting ionotropic gelation | Disc diffusion assay | E. coli S. aureus S. epidermidis B. cereus | 23 mm (circa) 29 mm (circa) 26 mm (circa) 26 mm (circa) | Wound healing | [99] |
| Starch/poly-vinyl alcohol/glycerin | Oregano | Solution casting | Disc diffusion assay | E. coli S. aureus | 31 mm 34 mm | Wound dressing | [100] |
| Starch/poly-vinyl alcohol/glycerin | Tea tree | Solution casting | Disc diffusion assay | E. coli S. aureus | 32 mm 35 mm | Wound dressing | [100] |
| Starch/poly-vinyl alcohol/glycerin | Clove | Solution casting | Disc diffusion assay | E. coli S. aureus | 37 mm 39 mm | Wound dressing | [100] |
| κ-Carrageenan/polyethylene glycol | Thyme | Solution casting | Disc diffusion assay | E. coli S. aureus | 13.8 mm 14.9 mm | Wound dressing | [101] |
| Gelatin/poly-vinyl alcohol/glycerol/glutaraldehyde | Zataria multiflora | Simple blending | Microdilution method | P. aeruginosa E. coli S. aureus B. subtilis | 400 µg/mL 200 µg/mL 200 µg/mL 100 µg/mL | Wound dressing | [102] |
| Polyvinyl alcohol | Oregano | Freeze-thawing | Serial dilutions method | E. coli S. aureus | Complete inhibition of both bacteria | Diabetic ulcers | [103] |
| (b) HGs Incorporated with PEs | |||||||
| Hydrogel Constitution | Plant Extract | Preparation Method | Antibacterial Activity | Intended Application | Reference | ||
| Experimental Method | Studied Species | Antibacterial Activity Values | |||||
| Chitosan | Hemigraphis alternata | Freeze-drying | Viable cell method (after 24 h) | E. coli S. aureus | 0.5 × 1010 CFU 1 × 1010 CFU | -------------- | [104] |
| Chitosan/cellulose | Calendula offcinalis | Simple blending | Agar well diffusion method | S. aureus E. coli P. acnes | 4 mm 2 mm 2 mm | Chronic wound | [105] |
| Chitosan/EDTA/β-glycerol phosphate | Aloe vera | Simple blending | Time-kill assay | P. aeruginosa S. aureus | Antibacterial activity after 24 h | Full-thickness excisional wound | [106] |
| Chitosan/poly (vinyl pyrrolidone)/poly (N-isopropyl acrylamide) | Salix alba | Simple blending | Disk diffusion method | S. aureus E. coli P. aeruginosa | 5 mm 4 mm 4 mm | Wound dressing | [107] |
| Carbopol 980NF/polyethylene glycol | Rosmarini herba | Simple blending | Disk diffusion method | S. aureus P. aeruginosa | 10 mm 10 mm | Wound dressing | [96] |
| Polyvinyl alcohol/pullulan | Calendula officinalis | Freeze-thawing | Disk diffusion method | S. aureus E. coli P. aeruginosa | 13 ± 0.35 mm 12 ± 0.5 mm 15 ± 0.1 mm | Wound healing | [108] |
| Cellulose/propylene glycol | Epilobium angustifolium | Simple blending | Agar well diffusion method | S. aureus E. coli S. epidermidis | 7 ± 0.5 mm 15 ± 0.5 mm 8.5 ± 0.5 mm | ----------------- | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.I.; Pintado, M.M.; Tavaria, F.K. Plant-Based Films and Hydrogels for Wound Healing. Microorganisms 2024, 12, 438. https://doi.org/10.3390/microorganisms12030438
Lopes AI, Pintado MM, Tavaria FK. Plant-Based Films and Hydrogels for Wound Healing. Microorganisms. 2024; 12(3):438. https://doi.org/10.3390/microorganisms12030438
Chicago/Turabian StyleLopes, Ana I., Maria M. Pintado, and Freni K. Tavaria. 2024. "Plant-Based Films and Hydrogels for Wound Healing" Microorganisms 12, no. 3: 438. https://doi.org/10.3390/microorganisms12030438





