Physiological Functions of the Cello-Oligosaccharides Binding CebE in the Pathogenic Streptomyces sp. AMCC400023
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. Bioinformatic Analysis
2.3. Protein Expression and Purification
2.4. MST Assays
2.5. Construction of CebE-Deficient Mutants
2.6. Plant Virulence Assays
2.7. Thaxtomin Quantification
2.8. Transcriptomic and RT-qPCR Analyses
3. Results
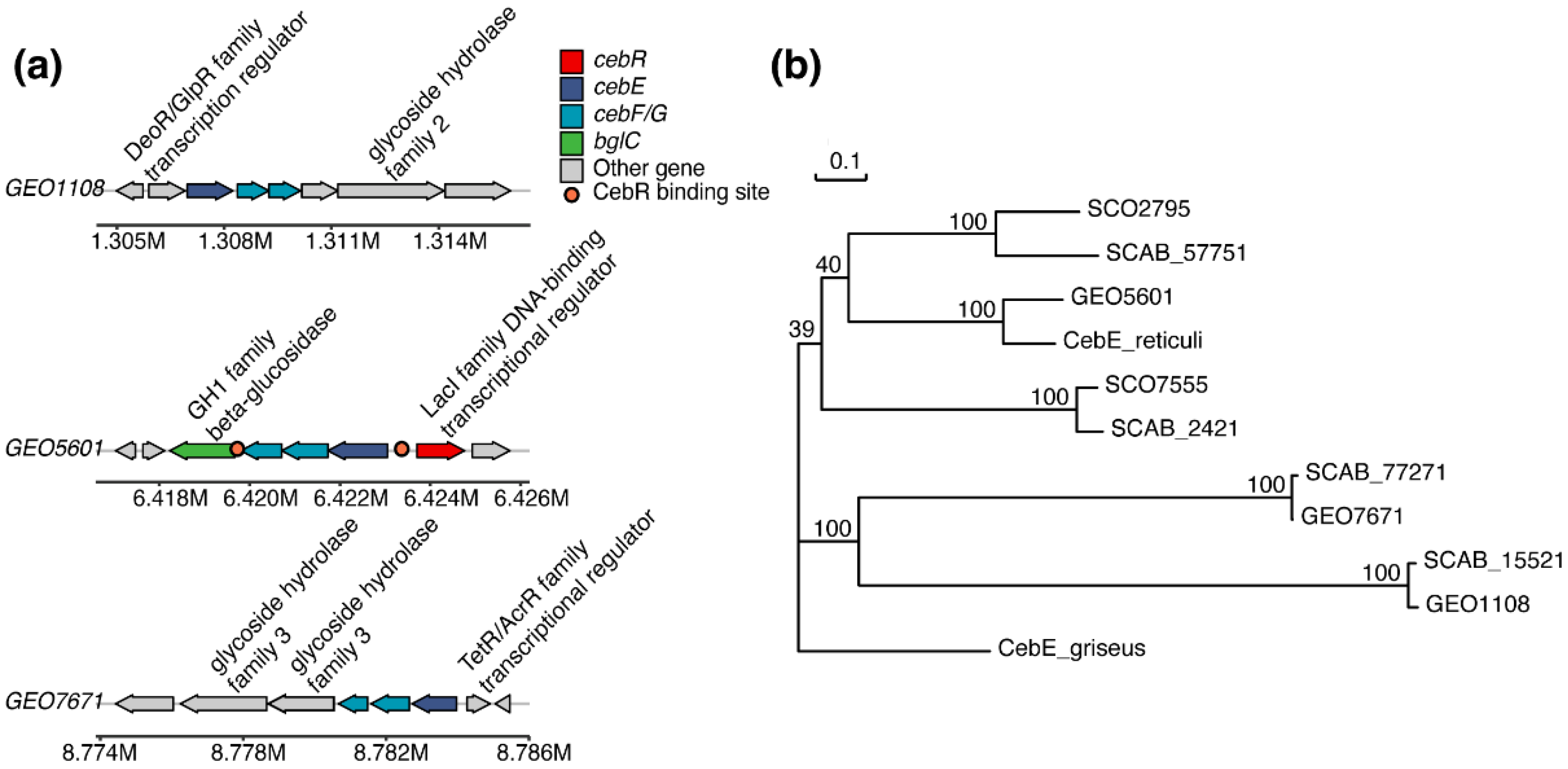
3.1. Bioinformatics Analysis of the Cello-Oligosaccharide Transporters
3.2. Cellobiose/Cellotriose Affinities of Three CebE Proteins
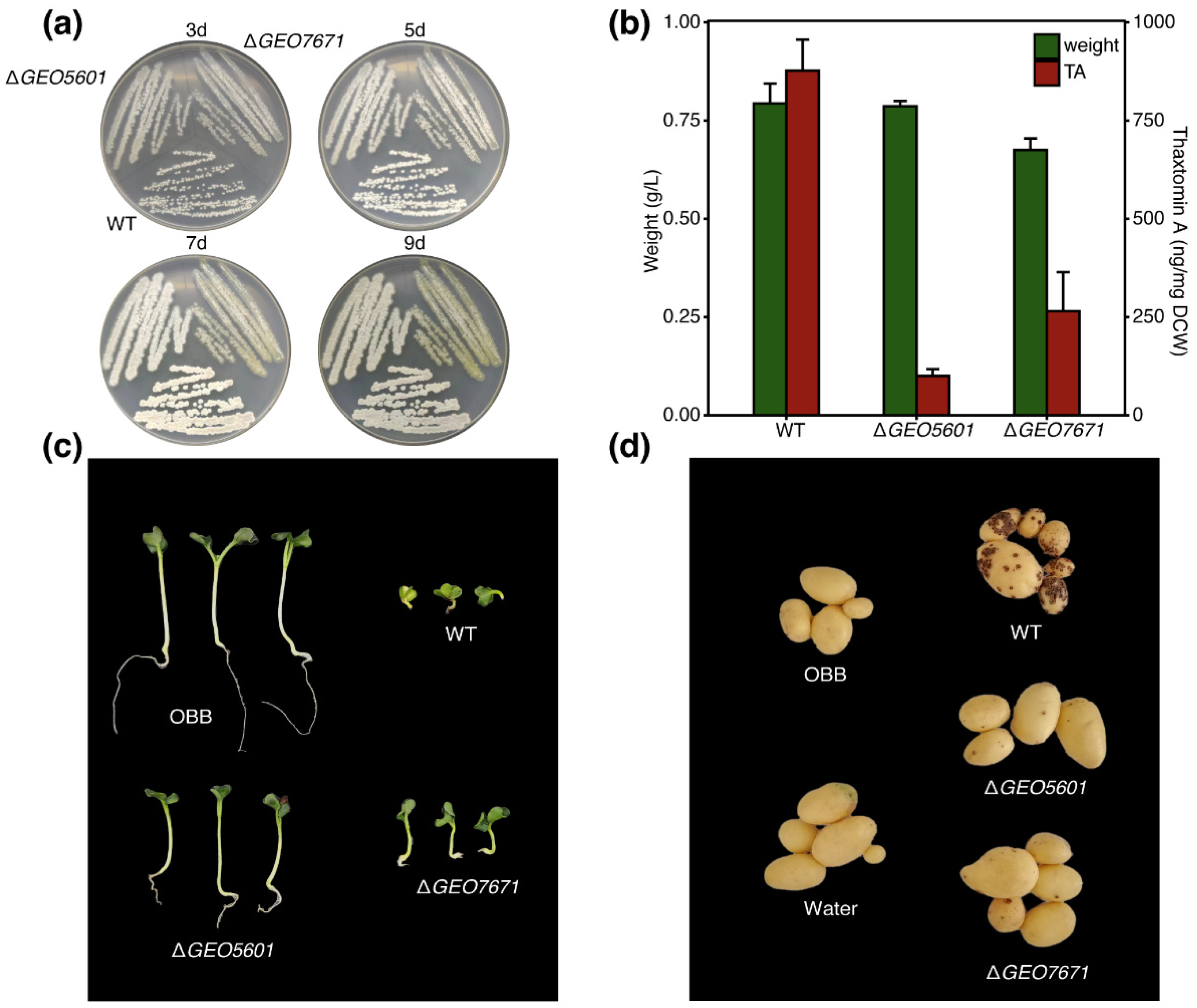
3.3. Pathogenicity Assay and Thaxtomin Production of CebE Mutants
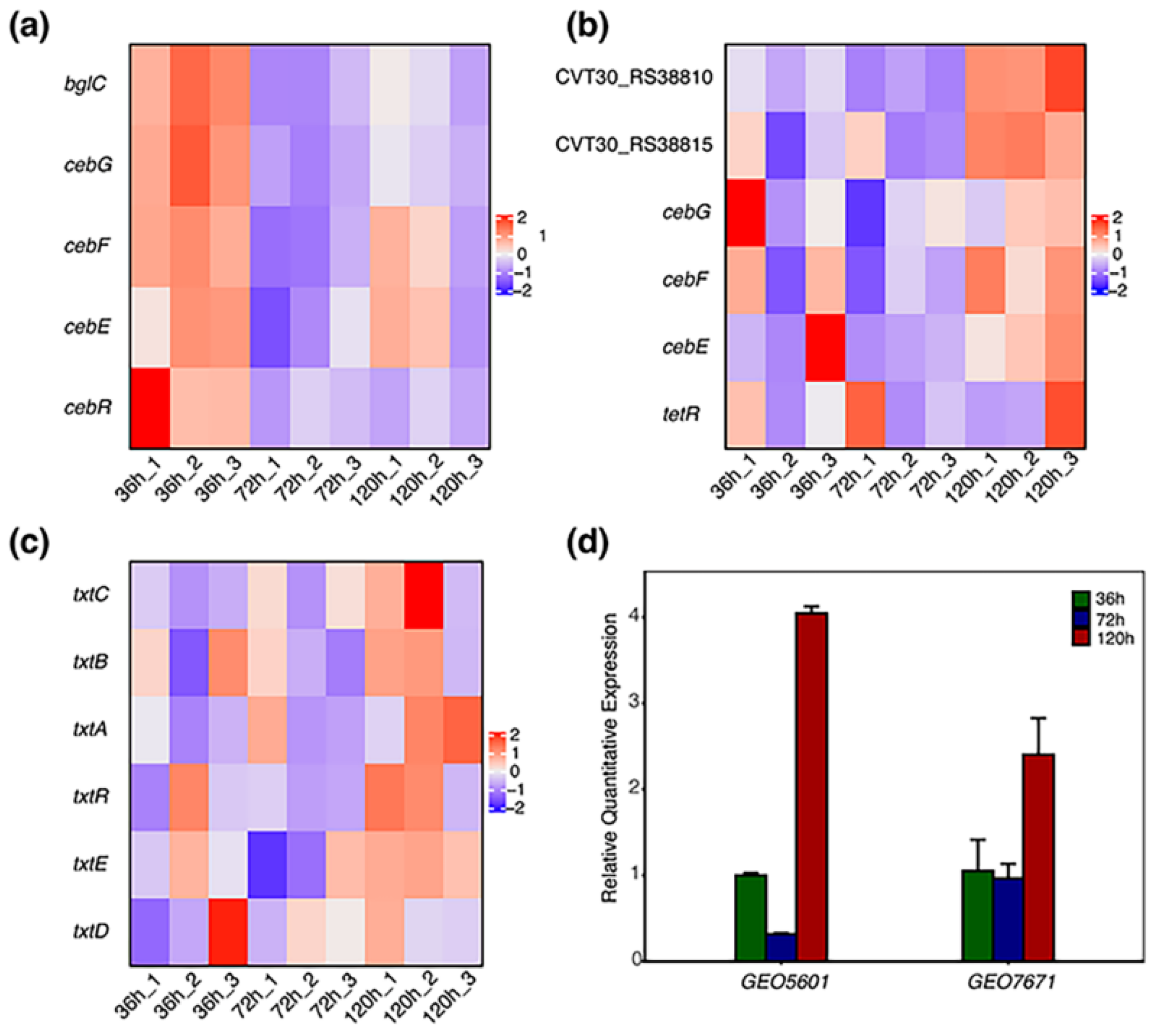
3.4. Transcriptional Variations of CebE Genes in the Presence of Cellobiose
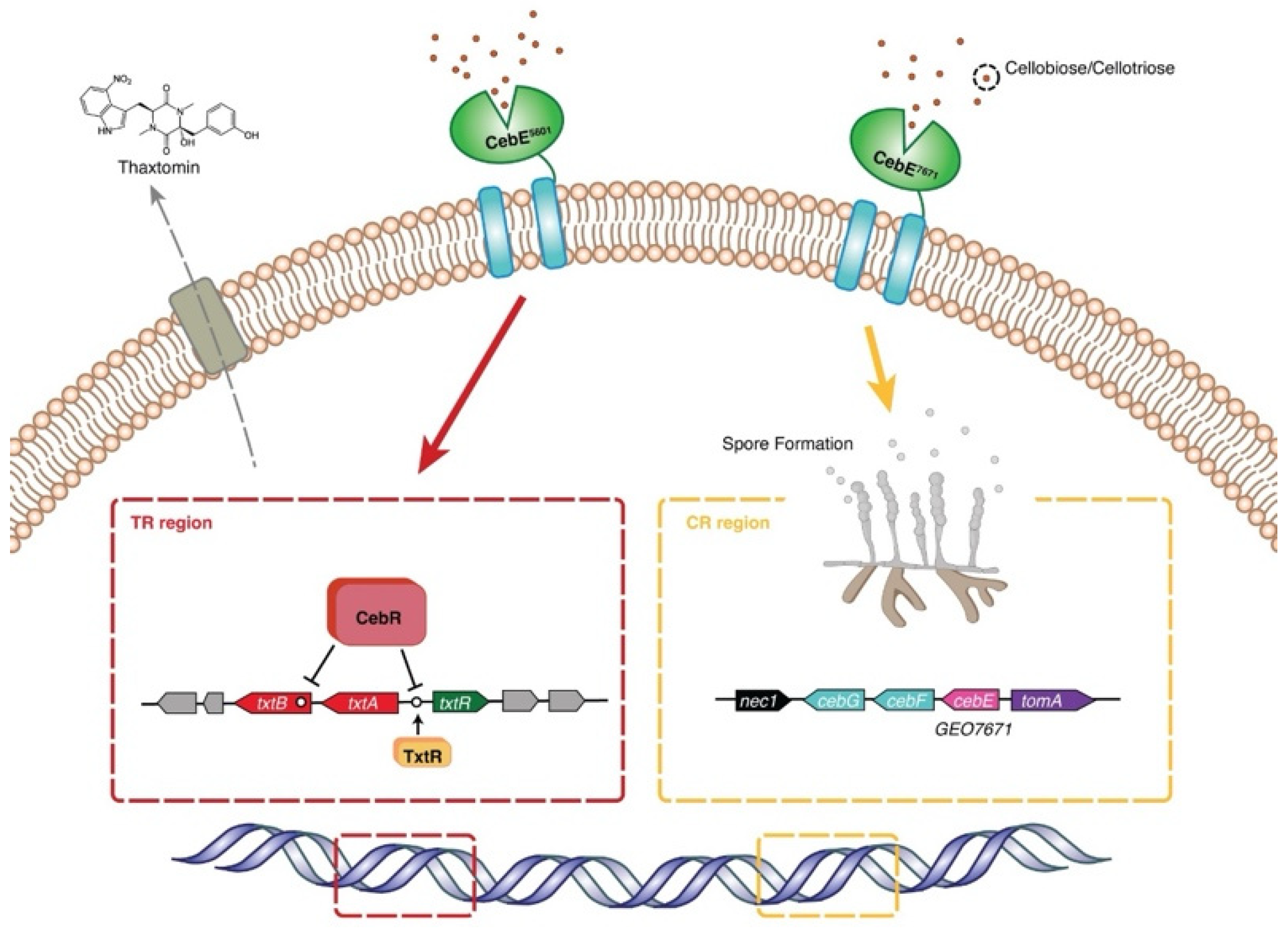
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a plant’s best friend. FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef]
- Braun, S.; Gevens, A.; Charkowski, A.; Allen, C.; Jansky, S. Potato common scab: A review of the causal pathogens, management practices, varietal resistance screening methods, and host resistance. Am. J. Potato Res. 2017, 94, 283–296. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Suomalainen, S.; Paulin, L.; Valkonen, J.P. Phylogenetic analysis of 16S rRNA genes and PCR analysis of the nec1 gene from Streptomyces spp. causing common scab, pitted scab, and netted scab in Finland. Phytopathology 1999, 89, 462–469. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Díaz-Cruz, G.; Cheng, Z.; Bignell, D.R.D. Virulence mechanisms of plant-pathogenic Streptomyces species: An updated review. Microbiology 2019, 165, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Lazarovits, G. A mail survey of growers to estimate potato common scab prevalence and economic loss in Canada. Can. J. Plant Pathol. 2005, 27, 46–52. [Google Scholar] [CrossRef]
- King, R.R.; Calhoun, L.A. The thaxtomin phytotoxins: Sources, synthesis, biosynthesis, biotransformation and biological activity. Phytochemistry 2009, 70, 833–841. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Fu, Y.; Huang, P.; Kong, D.; Niu, G. Engineered biosynthesis of thaxtomin phytotoxins. Crit. Rev. Biotechnol. 2020, 40, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.A.; Loria, R. Thaxtomin A: Evidence for a plant cell wall target. Physiol. Mol. Plant Pathol. 2002, 60, 1–8. [Google Scholar] [CrossRef]
- Bischoff, V.; Cookson, S.J.; Wu, S.; Scheible, W.R. Thaxtomin A affects CESA-complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J. Exp. Bot. 2009, 60, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Awwad, F.; Bertrand, G.; Grandbois, M.; Beaudoin, N. Auxin protects Arabidopsis thaliana cell suspension cultures from programmed cell death induced by the cellulose biosynthesis inhibitors thaxtomin A and isoxaben. BMC Plant Biol. 2019, 19, 512. [Google Scholar] [CrossRef]
- Joshi, M.; Rong, X.; Moll, S.; Kers, J.; Franco, C.; Loria, R. Streptomyces turgidiscabies secretes a novel virulence protein, Nec1, which facilitates infection. Mol. Plant-Microbe Interact. 2007, 20, 599–608. [Google Scholar] [CrossRef]
- Seipke, R.F.; Loria, R. Streptomyces scabies 87-22 possesses a functional tomatinase. J. Bacteriol. 2008, 190, 7684–7692. [Google Scholar] [CrossRef]
- Kers, J.A.; Cameron, K.D.; Joshi, M.V.; Bukhalid, R.A.; Morello, J.E.; Wach, M.J.; Gibson, D.M.; Loria, R. A large, mobile pathogenicity island confers plant pathogenicity on Streptomyces species. Mol. Microbiol. 2005, 55, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, G.; Ding, Y.; Loria, R. Genetic background affects pathogenicity island function and pathogen emergence in Streptomyces. Mol. Plant Pathol. 2018, 19, 1733–1741. [Google Scholar] [CrossRef]
- Book, A.J.; Lewin, G.R.; McDonald, B.R.; Takasuka, T.E.; Wendt-Pienkowski, E.; Doering, D.T.; Suh, S.; Raffa, K.F.; Fox, B.G.; Currie, C.R. Evolution of high cellulolytic activity in symbiotic Streptomyces through selection of expanded gene content and coordinated gene expression. PLoS Biol. 2016, 14, e1002475. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.G.; Joshi, M.V.; Gibson, D.M.; Loria, R. Cello-oligosaccharides released from host plants induce pathogenicity in scab-causing streptomyces species. Physiol. Mol. Plant Pathol. 2007, 71, 18–25. [Google Scholar] [CrossRef]
- Benoit, D.; Nudzejma, S.; Sören, P.; Sinaeda, A.; Bonometti, B.; Karim, L.; Coppieters, W.; Devreese, B.; Rigali, S. The virulome of Streptomyces scabiei in response to cello-oligosaccharide elicitors. Microb. Genom. 2022, 8, 000760. [Google Scholar]
- Schlösser, A.; Jantos, J.; Hackmann, K.; Schrempf, H. Characterization of the binding protein-dependent cellobiose and cellotriose transport system of the cellulose degrader Streptomyces reticuli. Appl. Environ. Microbiol. 1999, 65, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Marushima, K.; Ohnishi, Y.; Horinouchi, S. CebR as a master regulator for cellulose/cellooligosaccharide catabolism affects morphological development in Streptomyces griseus. J. Bacteriol. 2009, 191, 5930–5940. [Google Scholar] [CrossRef]
- Schlösser, A.; Schrempf, H. A lipid-anchored binding protein is a component of an ATP-dependent cellobiose/cellotriose-transport system from the cellulose degrader Streptomyces reticuli. Eur. J. Biochem. 1996, 242, 332–338. [Google Scholar] [CrossRef]
- Eitinger, T.; Rodionov, D.A.; Grote, M.; Schneider, E. Canonical and ECF-type ATP-binding cassette importers in prokaryotes: Diversity in modular organization and cellular functions. FEMS Microbiol. Rev. 2011, 35, 3–67. [Google Scholar] [CrossRef]
- Joshi, M.V.; Bignell, D.R.; Johnson, E.G.; Sparks, J.P.; Gibson, D.M.; Loria, R. The AraC/XylS regulator TxtR modulates thaxtomin biosynthesis and virulence in Streptomyces scabies. Mol. Microbiol. 2007, 66, 633–642. [Google Scholar] [CrossRef]
- Sören, P.; Jourdan, S.; Francis, I.M.; Benoit, D.; Rigali, S.; Devreese, B. Proteomic response to thaxtomin phytotoxin elicitor cellobiose and to deletion of cellulose utilization regulator CebR in Streptomyces scabies. J. Proteome Res. 2018, 17, 3837–3852. [Google Scholar]
- Francis, I.M.; Jourdan, S.; Fanara, S.; Loria, R.; Rigali, S. The cellobiose sensor CebR is the gatekeeper of Streptomyces scabies pathogenicity. mBio 2015, 6, e02018. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, S.; Francis, I.M.; Kim, M.J.; Salazar, J.J.; Planckaert, S.; Frère, J.M.; Matagne, A.; Kerff, F.; Devreese, B.; Loria, R.; et al. The CebE/MsiK Transporter is a doorway to the cello-oligosaccharide-mediated induction of Streptomyces scabies pathogenicity. Sci. Rep. 2016, 6, 27144. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.M.; Bergin, D.; Deflandre, B.; Gupta, S.; Salazar, C.; Villagrana, R.; Stulanovic, N.; Monteiro, S.R.; Kerff, F.; Loria, R.; et al. Role of alternative elicitor transporters in the onset of plant host colonization by Streptomyces scabiei 87-22. Biology 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lin, R.; Yao, Y.; Xiao, Y.; Zhang, M.; Shi, C.; He, X.; Zhou, B.; Wang, B. Systematic identification of pathogenic Streptomyces sp. AMCC400023 that causes common scab and genomic analysis of its pathogenicity island. Phytopathology 2019, 109, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, Y.; He, H.; Feng, J.; Zhang, X.; Han, L. Optimization of medium compositions to improve a novel glycoprotein production by Streptomyces kanasenisi ZX01. AMB Express 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, W.; Zong, Y.; Bai, C.; Guo, X.; Zhu, H.; Lou, C. Novel switchable ECF sigma factor transcription system for improving thaxtomin A production in Streptomyces. Synth. Syst. Biotechnol. 2022, 7, 972–981. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rebets, Y.; Tokovenko, B.; Brötz, E.; Luzhetskyy, A. Identification of butenolide regulatory system controlling secondary metabolism in Streptomyces albus J1074. Sci. Rep. 2017, 7, 9784. [Google Scholar] [CrossRef]
- Padilla-Reynaud, R.; Simao-Beaunoir, A.M.; Lerat, S.; Bernards, M.A.; Beaulieu, C. Suberin regulates the production of cellulolytic enzymes in Streptomyces scabiei, the causal agent of potato common scab. Microbes Environ. 2015, 30, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lerat, S.; Simao-Beaunoir, A.M.; Wu, R.; Beaudoin, N.; Beaulieu, C. Involvement of the plant polymer suberin and the disaccharide cellobiose in triggering thaxtomin A biosynthesis, a phytotoxin produced by the pathogenic agent Streptomyces scabies. Phytopathology 2010, 100, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hillerich, B.; Westpheling, J. A New TetR family transcriptional regulator required for morphogenesis in Streptomyces coelicolor. J. Bacteriol. 2008, 190, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Seghezzi, N.; Esnault, C.; Virolle, M.J. Repression of antibiotic production and sporulation in Streptomyces coelicolor by overexpression of a TetR family transcriptional regulator. Appl. Environ. Microbio. 2010, 76, 7741–7753. [Google Scholar] [CrossRef]



| Descriptions | |
|---|---|
| Plasmids | |
| pJTU1278 | Shuttle plasmid for DNA transfer (Km, Amp, Cml) |
| pEASY-Blunt | Cloning plasmid (Amp) |
| pET28a | Expression plasmid used to produce N-terminal His-tagged protein (Km) |
| Strains | |
| E. coli DH5α | General cloning host |
| E. coli BL21(C43) | Host for protein expression |
| E. coli ET12567 | Host for transfer of DNA into Streptomyces spp. |
| Streptomyces sp. AMCC400023 | Wild type strain |
| ΔGEO5601 | AMCC400023 derivative with GEO5601 deficient |
| ΔGEO7671 | AMCC400023 derivative with GEO7671 deficient |
| Treatment | Incidence Rate (%) | Disease Index (%) |
|---|---|---|
| CK1-Water | - | - |
| CK2-OBB | - | - |
| T1-WT | 97.06 | 63.53 |
| T2-ΔGEO5601 | 37.50 | 11.25 |
| T2-ΔGEO7671 | 70.37 | 16.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chang, J.; Lv, P.; Li, J.; Duan, Y.; Tian, D.; Ge, F.; Su, G.; Nie, F.; Gao, Z.; et al. Physiological Functions of the Cello-Oligosaccharides Binding CebE in the Pathogenic Streptomyces sp. AMCC400023. Microorganisms 2024, 12, 499. https://doi.org/10.3390/microorganisms12030499
Li Q, Chang J, Lv P, Li J, Duan Y, Tian D, Ge F, Su G, Nie F, Gao Z, et al. Physiological Functions of the Cello-Oligosaccharides Binding CebE in the Pathogenic Streptomyces sp. AMCC400023. Microorganisms. 2024; 12(3):499. https://doi.org/10.3390/microorganisms12030499
Chicago/Turabian StyleLi, Qiuyue, Jiawen Chang, Peiwen Lv, Junxia Li, Yuxia Duan, Dandan Tian, Fei Ge, Gaoya Su, Fengjie Nie, Zheng Gao, and et al. 2024. "Physiological Functions of the Cello-Oligosaccharides Binding CebE in the Pathogenic Streptomyces sp. AMCC400023" Microorganisms 12, no. 3: 499. https://doi.org/10.3390/microorganisms12030499
APA StyleLi, Q., Chang, J., Lv, P., Li, J., Duan, Y., Tian, D., Ge, F., Su, G., Nie, F., Gao, Z., Yang, C., & Zhou, B. (2024). Physiological Functions of the Cello-Oligosaccharides Binding CebE in the Pathogenic Streptomyces sp. AMCC400023. Microorganisms, 12(3), 499. https://doi.org/10.3390/microorganisms12030499






