The Molecular Profile of Soil Microbial Communities Inhabiting a Cambrian Host Rock
Abstract
1. Introduction
2. Materials and Methods
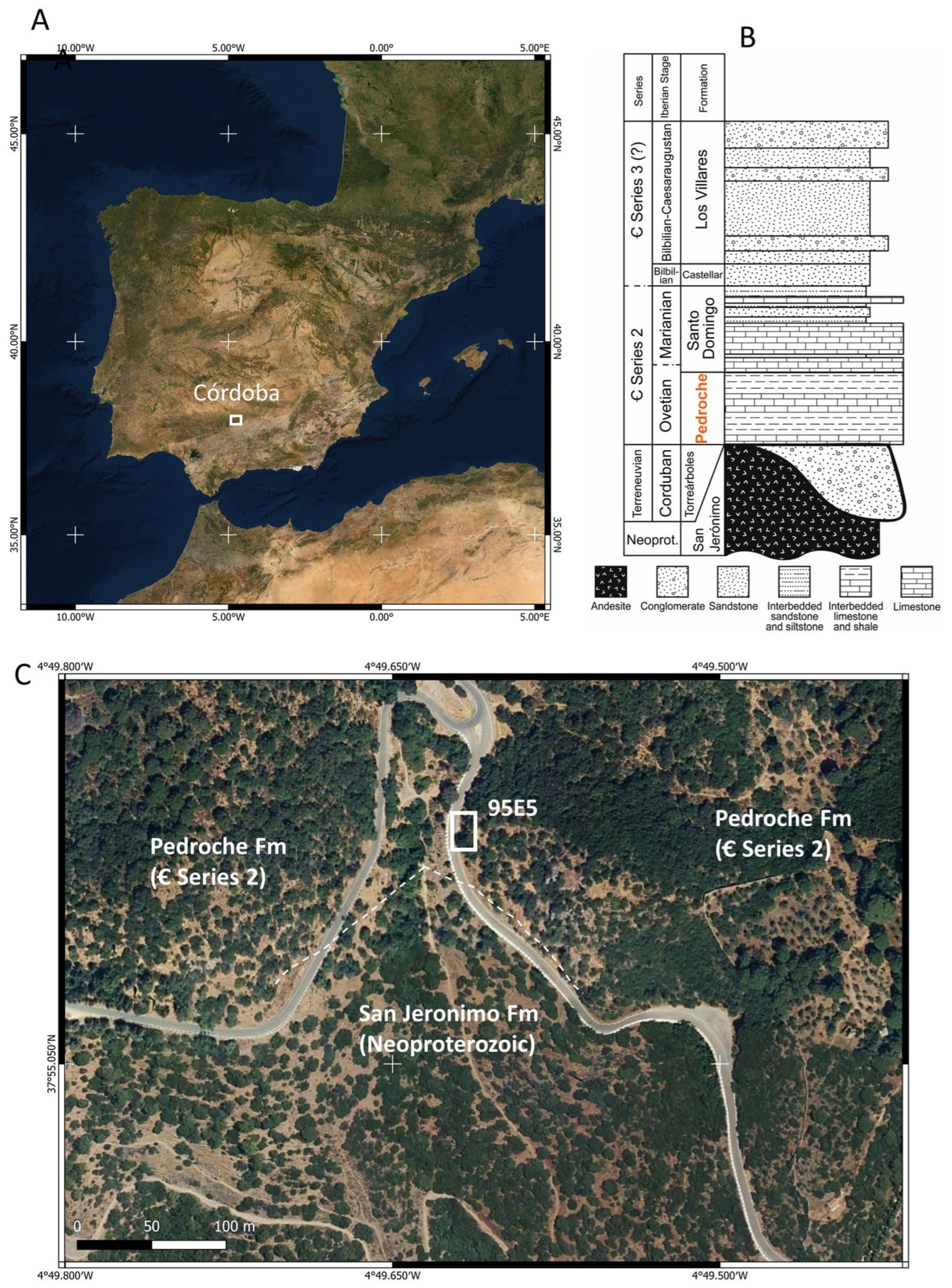
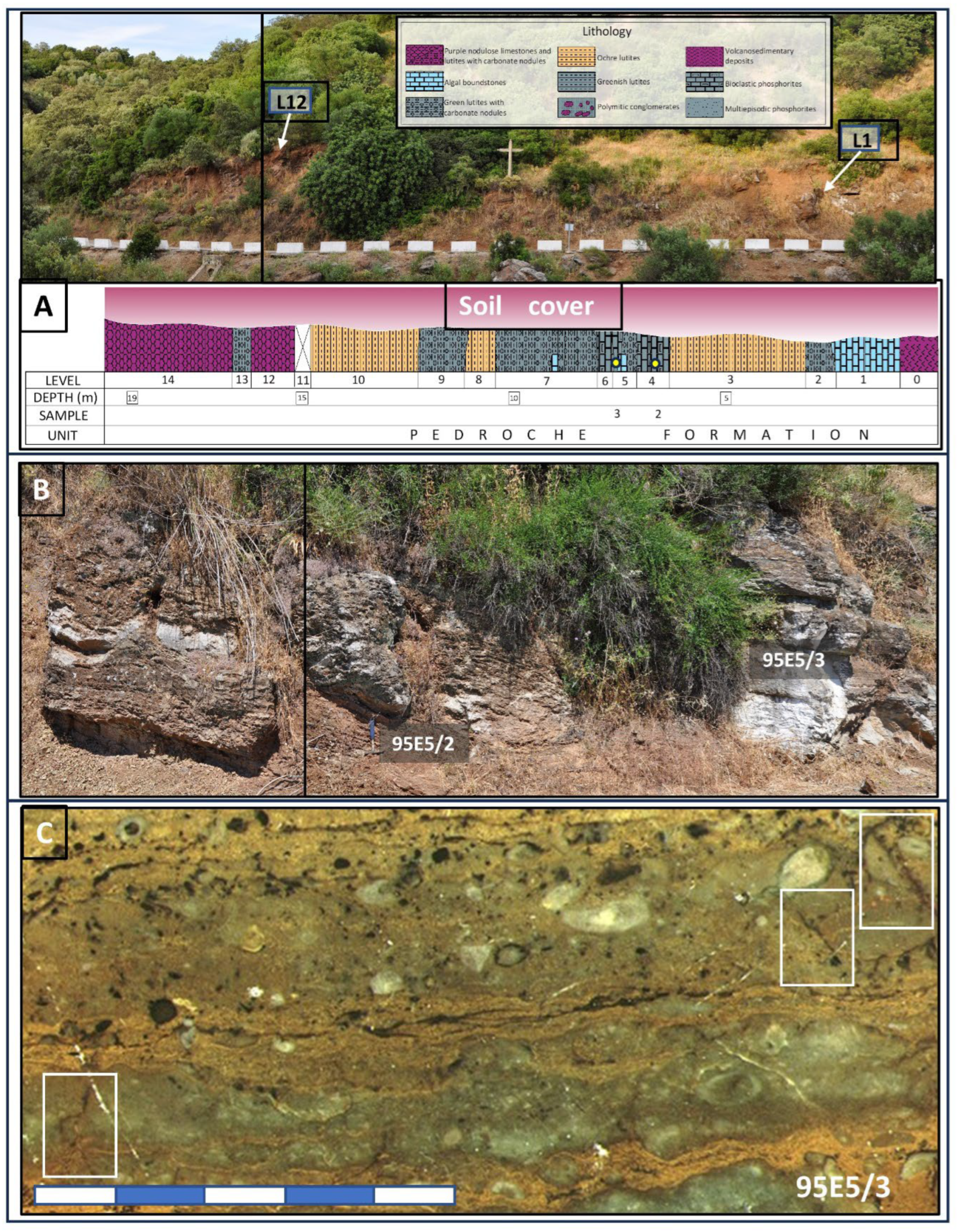
2.1. Geologic Background and Sampling
2.2. Methods
2.2.1. Organic Extraction, Fractionation, and Analysis under GC-MS
2.2.2. ToF-SIMS Analysis
3. Results
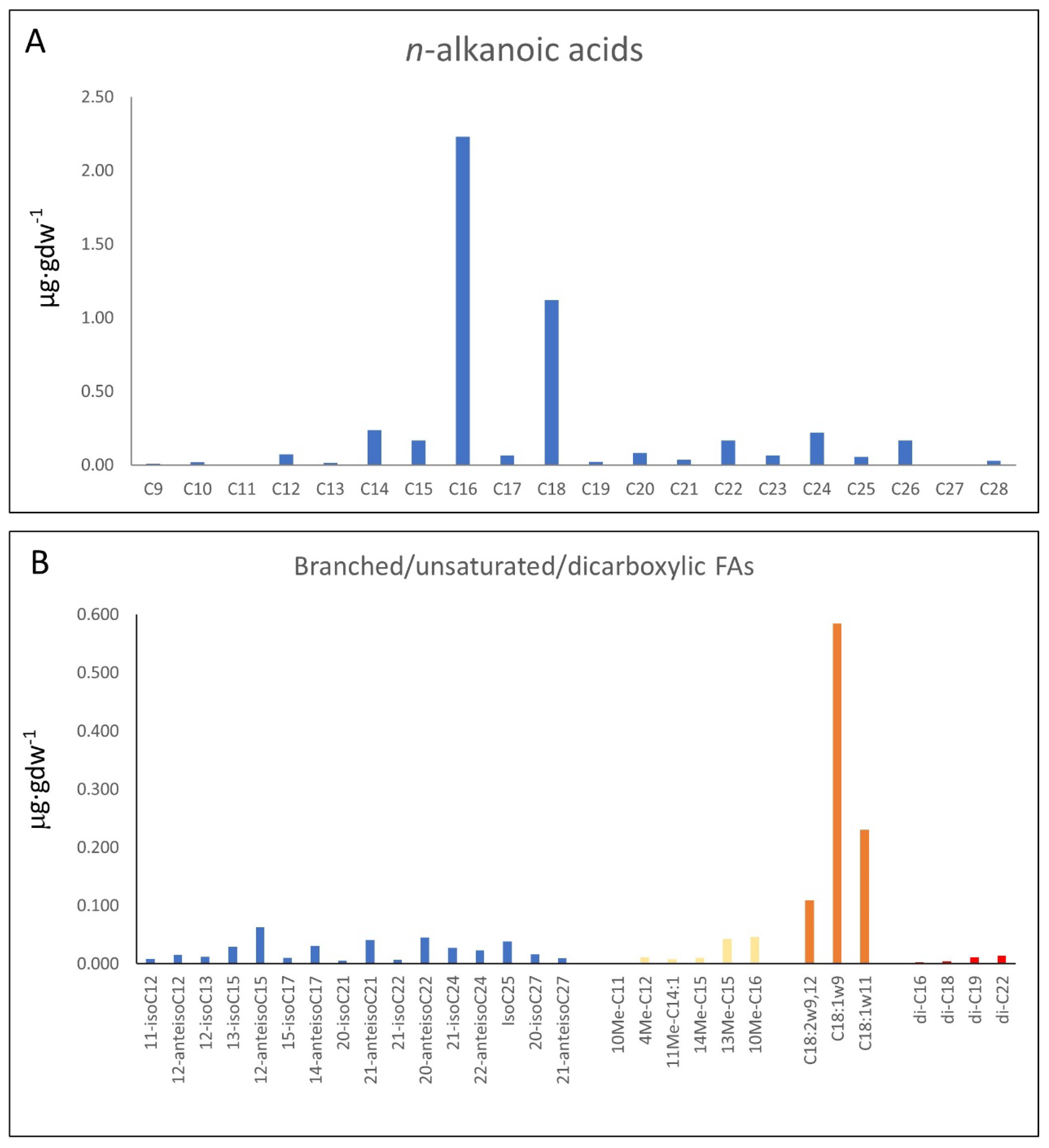
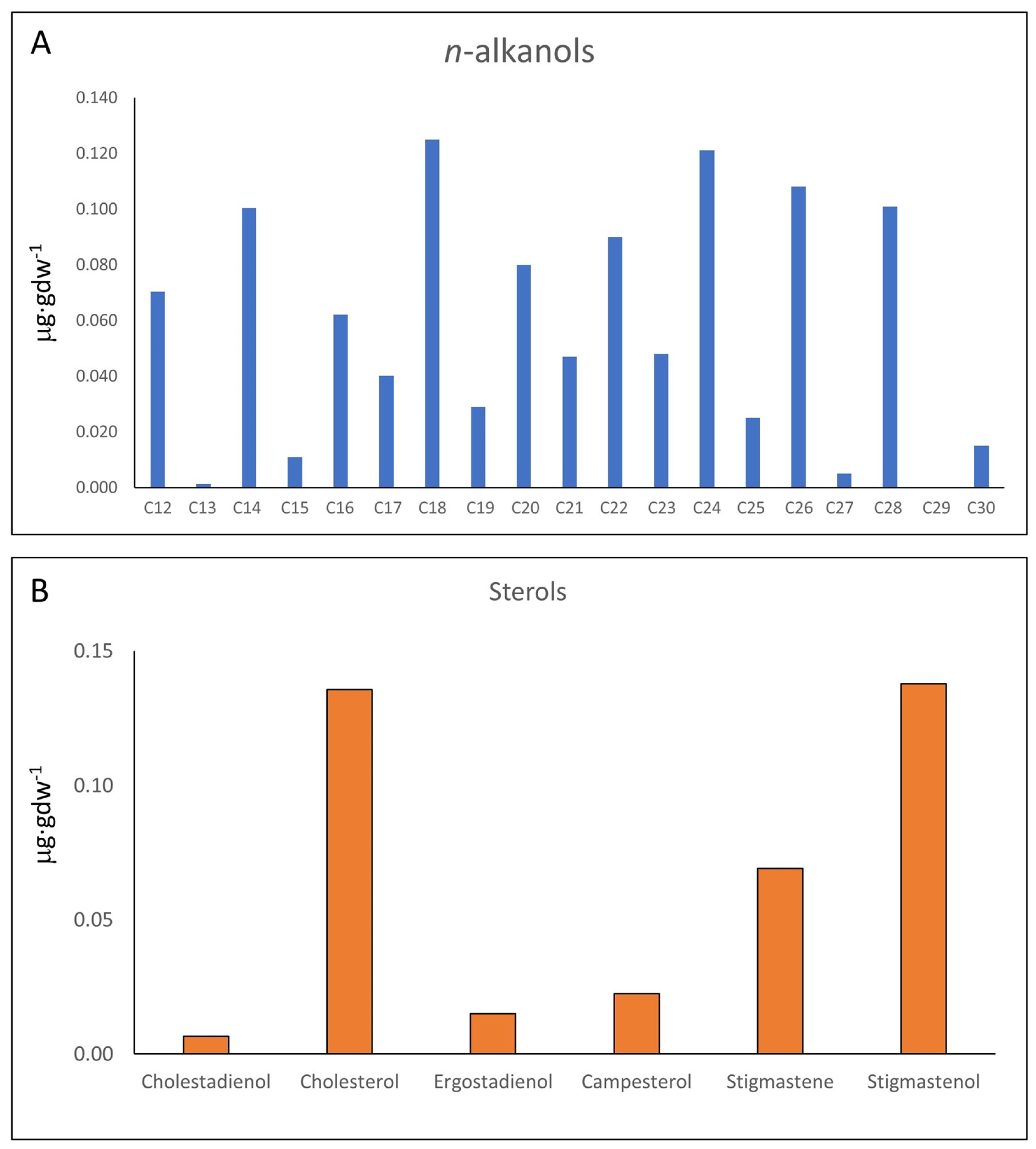
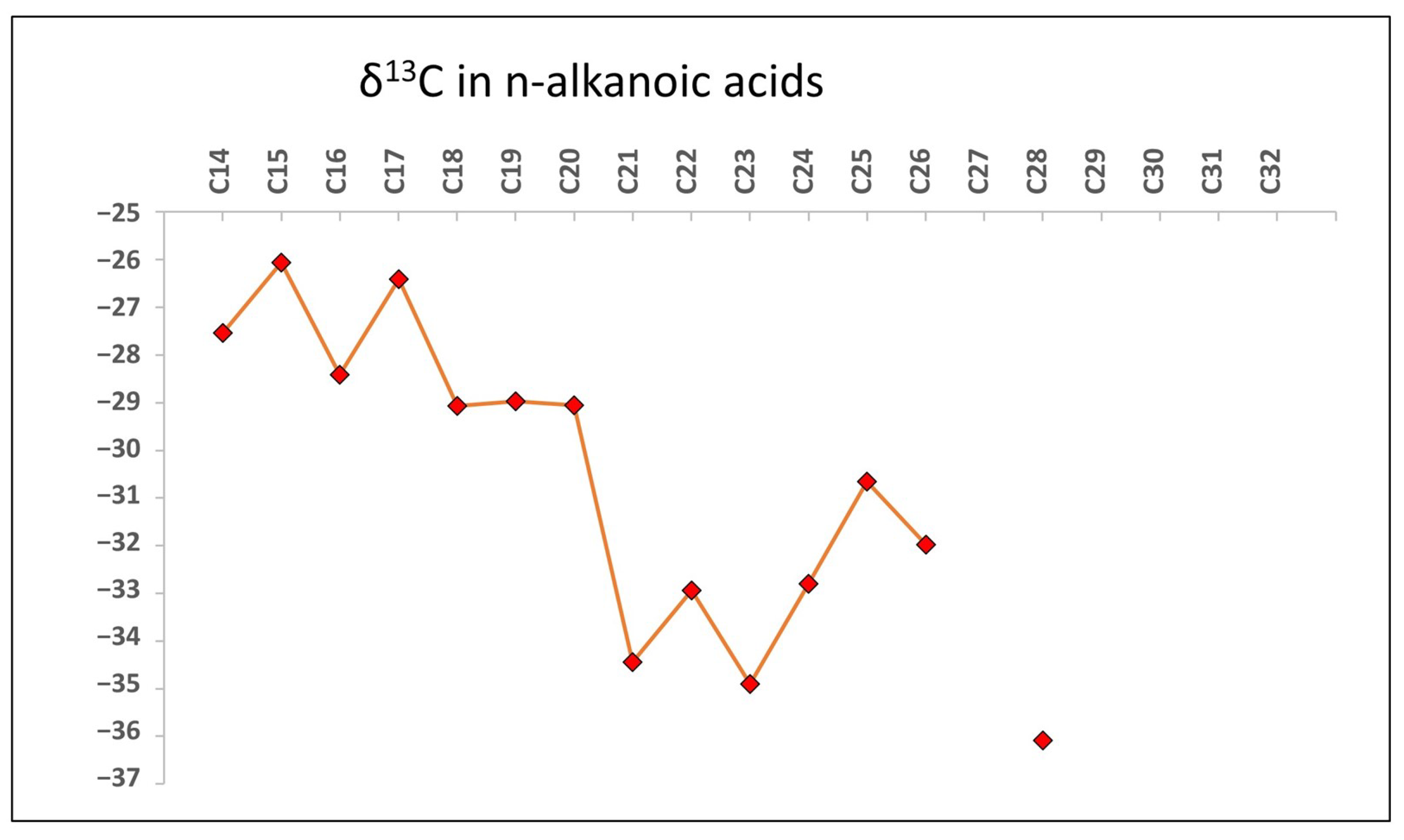
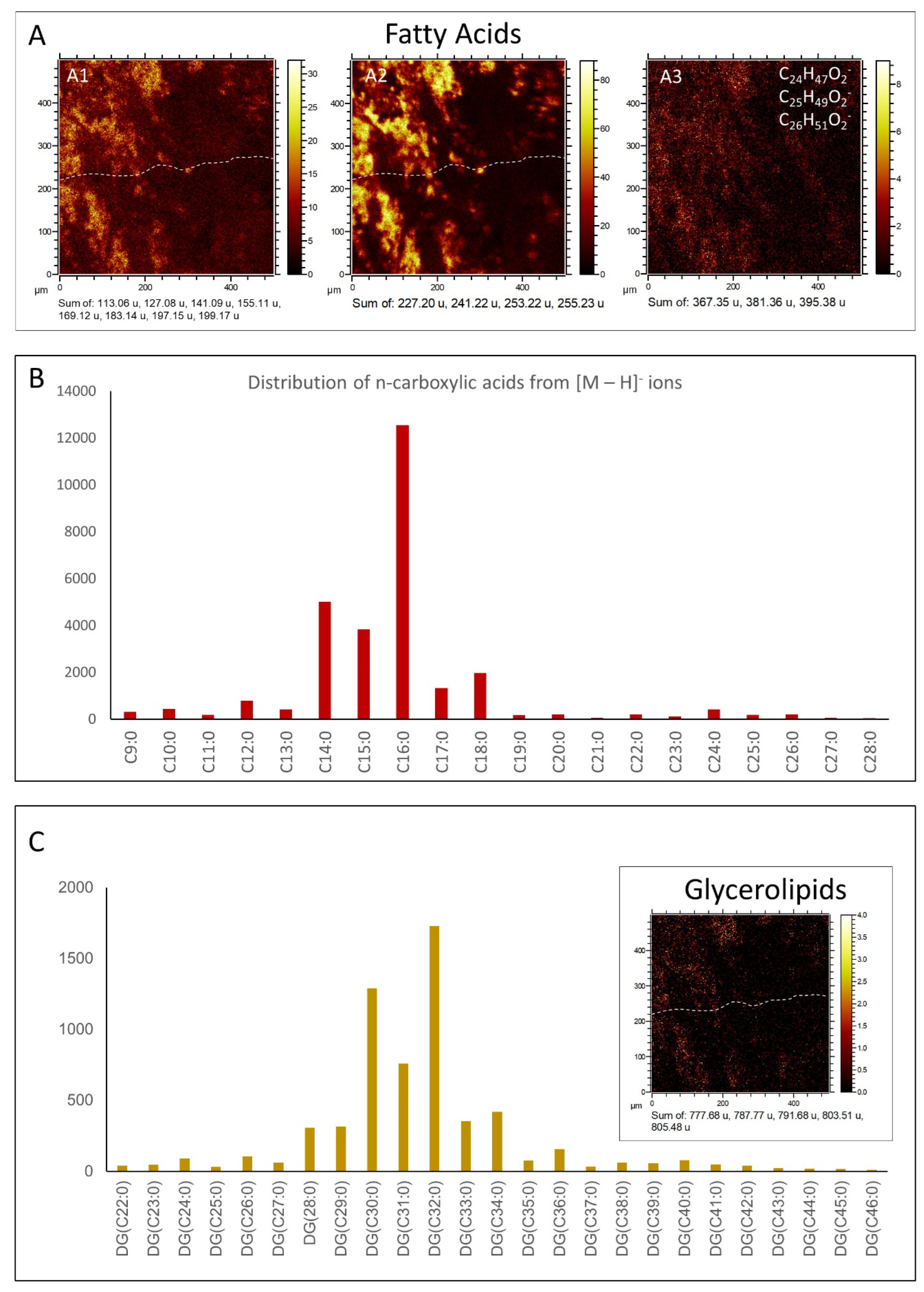
3.1. GC-MS Results
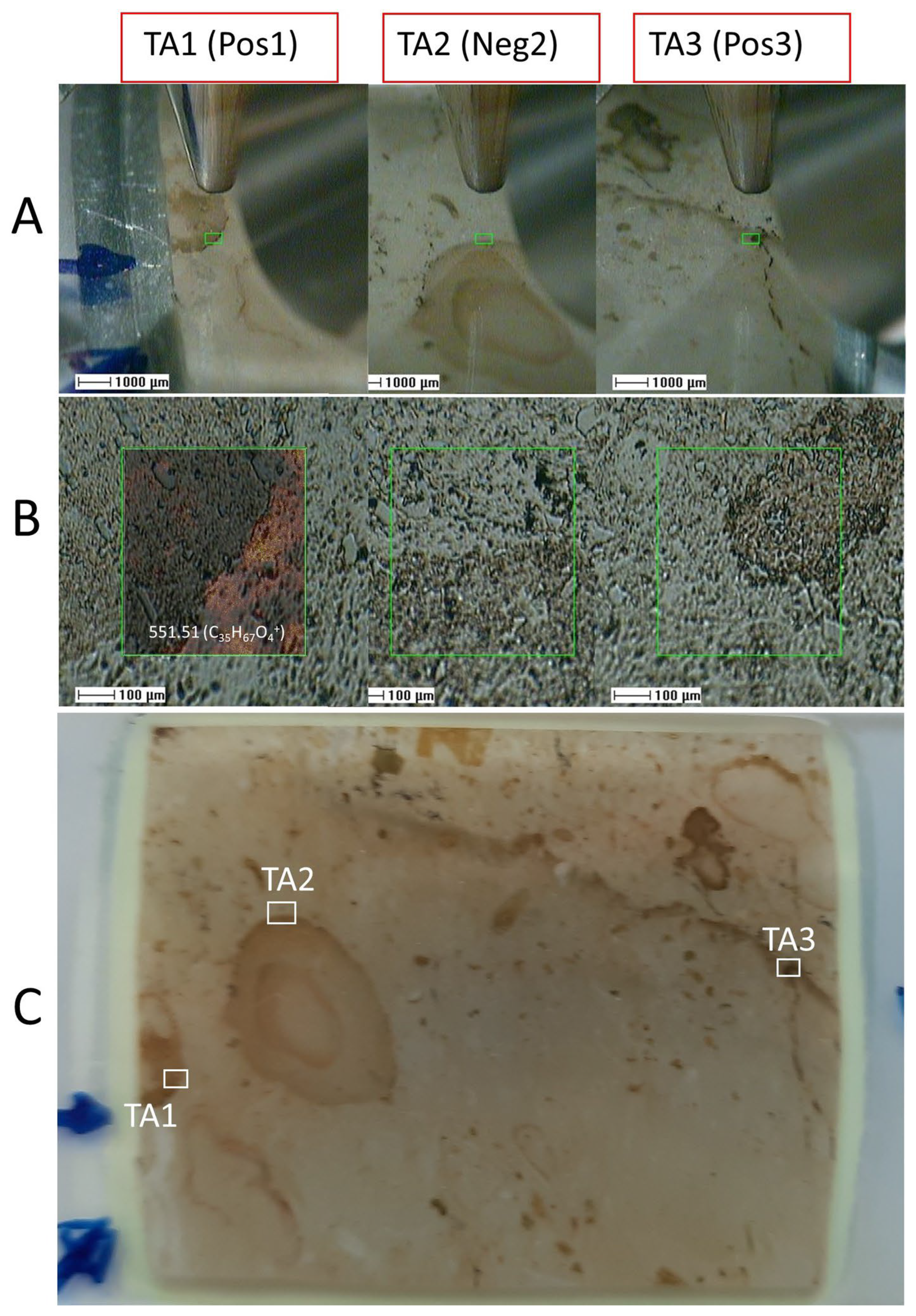
3.2. ToF-SIMS Results
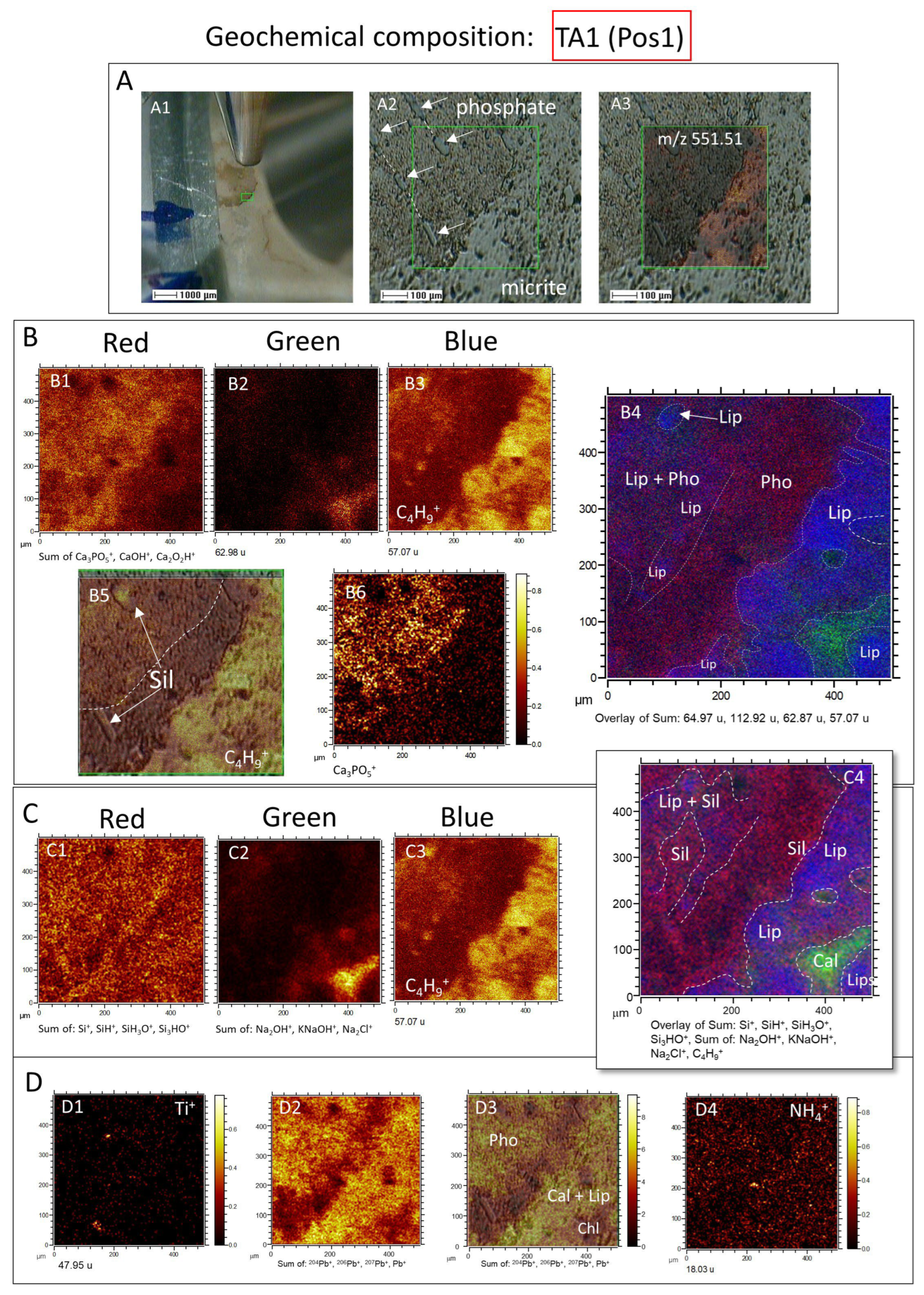
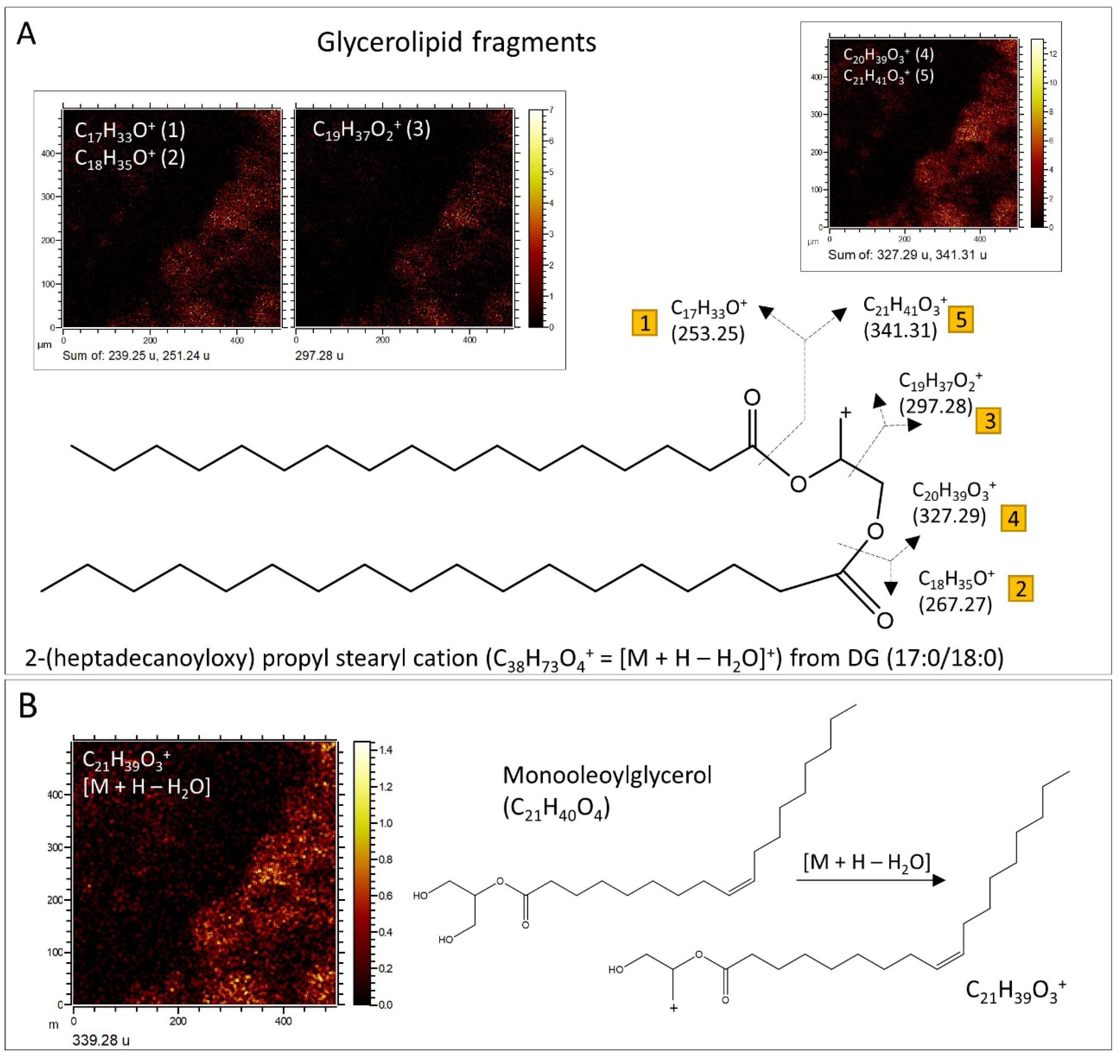
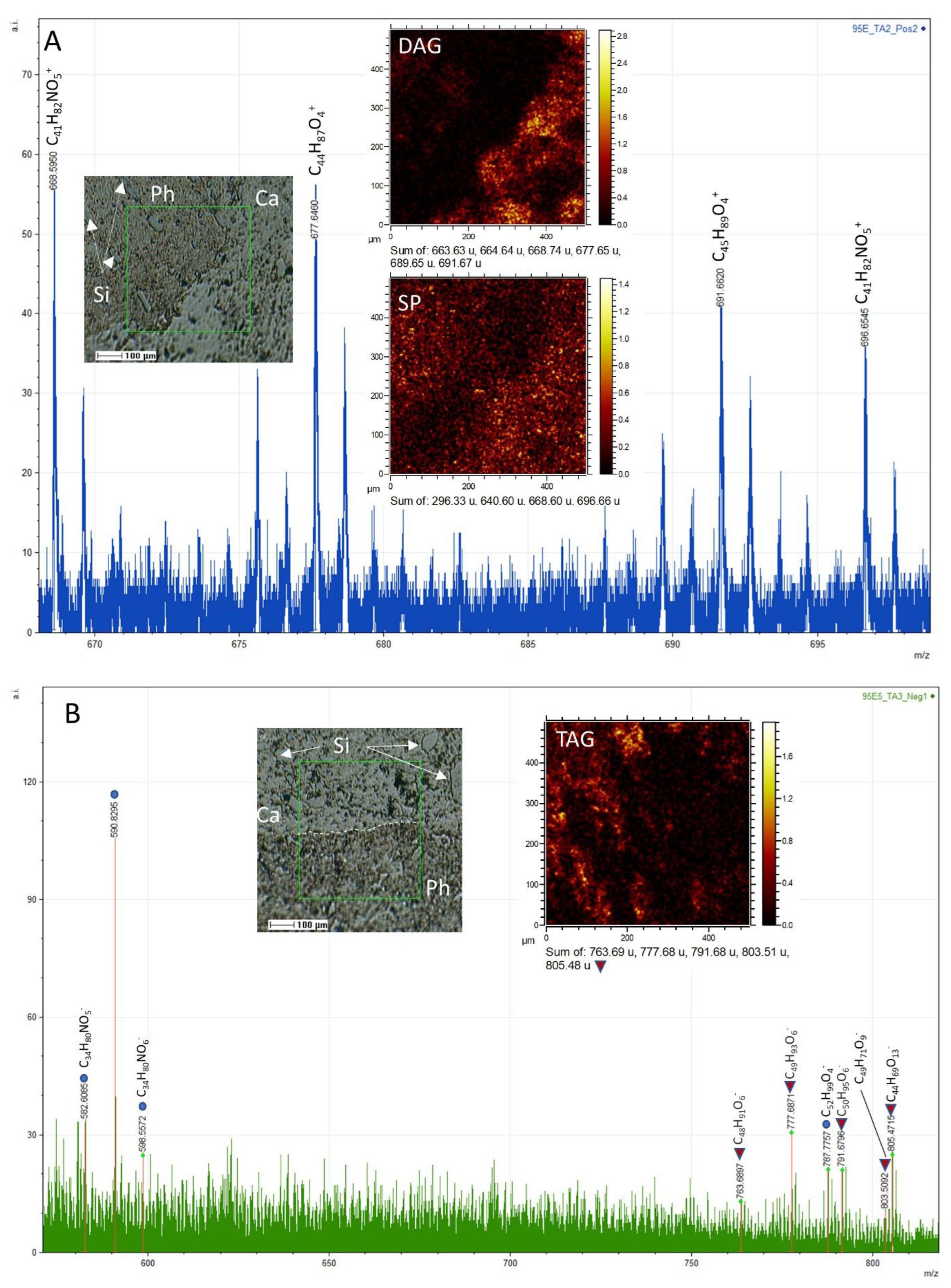
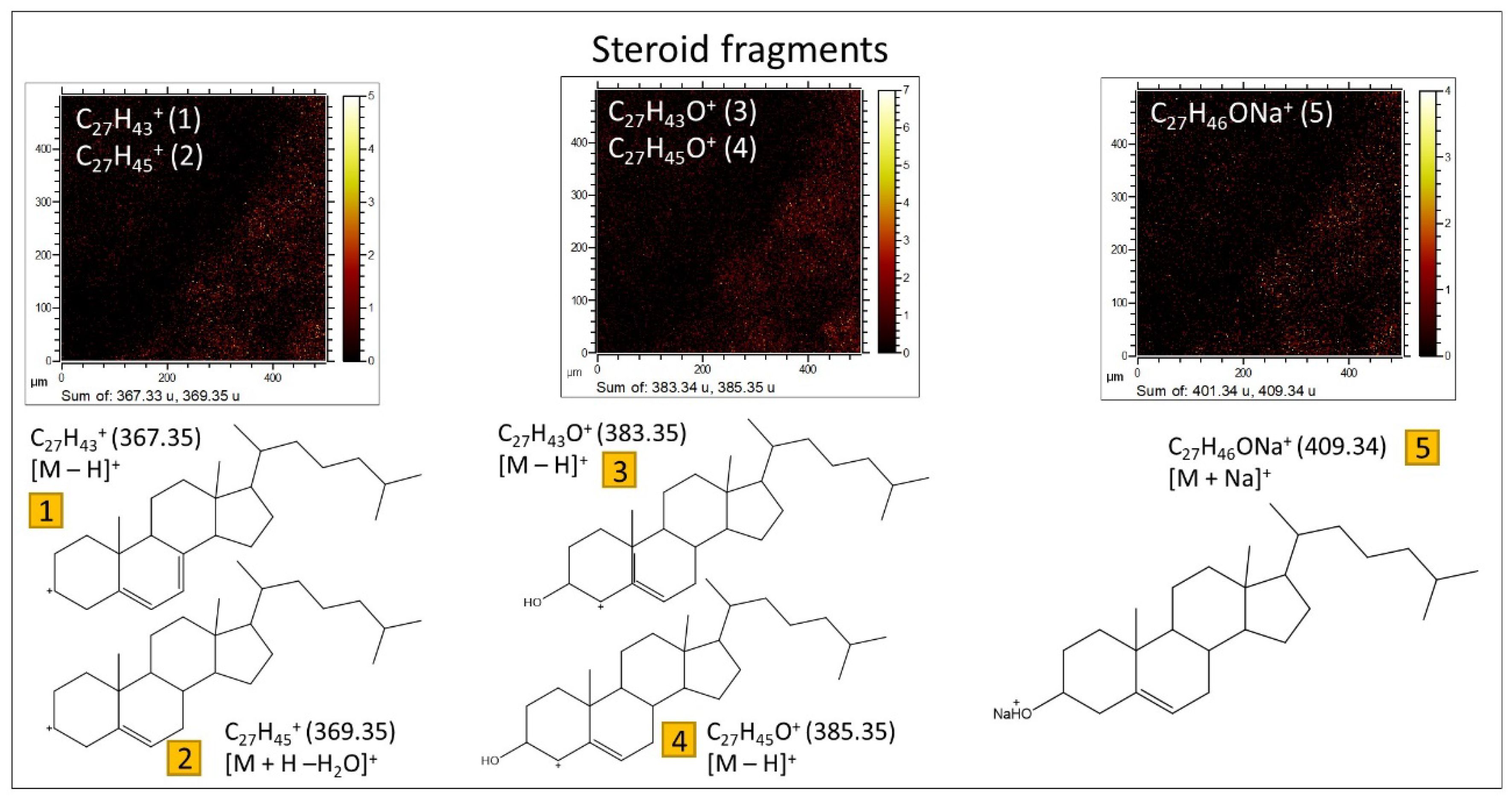
3.2.1. ToF-SIMS Molecular Mapping
3.2.2. ToF-SIMS Spectral Analysis: Compound Classification
4. Discussion
4.1. GC-MS Data Interpretation
4.2. ToF-SIMS Data Interpretation
4.3. Data Synthesis: Organic Matter Origin
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 2013, 10, 3983–3996. [Google Scholar] [CrossRef]
- Wu, L.; Jacobson, A.D.; Hausner, M. Characterization of elemental release during microbe–granite interactions at T = 28 °C. Geochim. Cosmochim. Acta 2008, 72, 1076–1095. [Google Scholar] [CrossRef]
- Mackenzie, F.T.; Garrels, R.M. Evolution of Sedimentary Rocks; Norton: New York, NY, USA, 1971. [Google Scholar]
- Dong, H. Clay–microbe interactions and implications for environmental mitigation. Elements 2012, 8, 113–118. [Google Scholar] [CrossRef]
- Fiore, S.; Dumontet, S.; Huertas, F.J.; Pasquale, V. Bacteria-induced crystallization of kaolinite. Appl. Clay Sci. 2011, 53, 566–571. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Lu, A.; Wang, H.; Zhu, Y.; Ding, H.; Wang, X. Effect of Bacillus mucilaginosus D4B1 on the structure and soil-conservation-related properties of montmorillonite. Appl. Clay Sci. 2016, 119, 141–145. [Google Scholar] [CrossRef]
- Balogh-Brunstad, Z.; Keller, C.K.; Dickinson, J.T.; Stevens, F.; Li, C.Y.; Bormann, B.T. Biotite weathering and nutrient uptake by ectomycorrhizal fungus, Suillus tomentosus, in liquid-culture experiments. Geochim. Cosmochim. Acta 2008, 72, 2601–2618. [Google Scholar] [CrossRef]
- Biswas, B.; Chakraborty, A.; Sarkar, B.; Naidu, R. Structural changes in smectite due to interaction with a biosurfactant-producing bacterium Pseudoxanthomonas kaohsiungensis. Appl. Clay Sci. 2017, 136, 51–57. [Google Scholar] [CrossRef]
- Kompantseva, E.I.; Naimark, E.B.; Boeva, N.M.; Zhukhlistov, A.P.; Novikov, V.M.; Nikitina, N.S. Interaction of anoxygenic phototrophic bacteria Rhodopseudomonas sp. with kaolinite. Microbiology 2013, 82, 316–326. [Google Scholar] [CrossRef]
- Ren, X.; Li, F.; Cai, Y.; Li, Y.; Jin, Z.; Li, X.; Chen, J. Paenibacillus sp. strain SB-6 induces weathering of Ca-montmorillonite: Illitization and formation of calcite. Geomicrobiol. J. 2017, 34, 1–10. [Google Scholar] [CrossRef]
- Huang, P.-M.; Wang, M.-K.; Chiu, C.-Y. Soil mineral–organic matter–microbe interactions: Impacts on biogeochemical processes and biodiversity in soils. Pedobiologia 2005, 49, 609–635. [Google Scholar] [CrossRef]
- Jin, C.; Sengupta, A. Microbes in porous environments: From active interactions to emergent feedback. arXiv 2023, arXiv:2311.11440. [Google Scholar]
- Uroz, S.; Kelly, L.C.; Turpault, M.-P.; Lepleux, C.; Frey-Klett, P. The Mineralosphere Concept: Mineralogical Control of the Distribution and Function of Mineral-associated Bacterial Communities. Trends Microbiol. 2015, 23, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; van den Hoogen, J.; Wang, Z.; Averill, C.; Routh, D.; Smith, G.R.; Drenovsky, R.E.; Scow, K.M.; Mo, F.; Waldrop, M.P.; et al. The biogeography of relative abundance of soil fungi versus bacteria in surface topsoil. Earth Syst. Sci. Data 2022, 14, 4339–4350. [Google Scholar] [CrossRef]
- Boer, W.d.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development⋆. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T. A Comprehensive Survey of Soil Rhizobiales Diversity Using High-Throughput DNA Sequencing. In Biological Nitrogen Fixation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 769–776. [Google Scholar]
- Nwachukwu, B.C.; Babalola, O.O. Metagenomics: A Tool for Exploring Key Microbiome with the Potentials for Improving Sustainable Agriculture. Front. Sustain. Food Syst. 2022, 6, 886987. [Google Scholar] [CrossRef]
- Hearing, T.W.; Harvey, T.H.P.; Williams, M.; Leng, M.J.; Lamb, A.L.; Wilby, P.R.; Gabbott, S.E.; Pohl, A.; Donnadieu, Y. An early Cambrian greenhouse climate. Sci. Adv. 2018, 4, eaar5690. [Google Scholar] [CrossRef]
- Dallmeyer, R.D.; Martínez García, E. Pre-Mesozoic Geology of Iberia; Springer: New York, NY, USA, 1990. [Google Scholar]
- Delgado-Quesada, M.; Liñan, E.; Pascual Martínez, E.; Pérez Lorente, F. Criterios para la diferenciación de dominios en Sierra Morena Central. Stud. Geol. 1977, 12, 75–90. [Google Scholar]
- Ábalos, B.; Carreras, J.; Druguet, E.; Viruete, J.E.; Pugnaire, M.T.G.; Alvarez, S.L.; Quesada, C.; Fernández, L.R.R.; Gil-Ibarguchi, J.I. Variscan and Pre-Variscan Tectonics. In The Geology of Spain; Gibbons, W., Moreno, T., Eds.; Geological Society of London: London, UK, 2002. [Google Scholar]
- Gozalo, R.; Liñan, E.; Palacios, T.; Gámez Vintaned, J.A.; Mayoral, E. The Cambrian of the Iberian Peninsula: An overview. Geol. Acta 2003, 1, 103–112. [Google Scholar]
- Liñán, E.; Gozalo, R.; Palacios, T.; Gámez Vintaned, J.A.; Ugidos, J.M.; Mayoral, E. Cambrian. In The Geology of Spain; Gibbons, W., Moreno, T., Eds.; Geological Society of London: London, UK, 2002. [Google Scholar]
- Álvaro, J.J.; Bellido, F.; Gasquet, D.; Pereira, M.F.; Quesada, C.; Sánchez-García, T. Diachronism in the late Neoproterozoic–Cambrian arc-rift transition of North Gondwana: A comparison of Morocco and the Iberian Ossa-Morena Zone. J. Afr. Earth Sci. 2014, 98, 113–132. [Google Scholar] [CrossRef]
- Liñan, E. Bioestratigrafia de la Sierra de Córdoba; Universidad de Granada: Granada, Spain, 1978. [Google Scholar]
- Liñán, E.; Perejón, A.; Sdzuy, K. The Lower—Middle Cambrian stages and stratotypes from the Iberian Peninsula: A revision. Geol. Mag. 1993, 130, 817–833. [Google Scholar] [CrossRef]
- Liñán, E.; Fernández-Carrasco, J. La Formación Torreárboles y la Paleogeografía del límite Precámbrico-Cámbrico en Ossa-Morena (flanco norte de la alineación Olivenza-Monesterio). Cad. Lab. Xeolóxico Laxe 1984, 8, 315–328. [Google Scholar]
- Liñán, E. Introducción al problema de la paleogeografía del Cámbrico de Ossa Morena. Cad. Lab. Xeolóxico Laxe 1984, 8, 282–314. [Google Scholar]
- Zamarreño, I.; Debrenne, F. Sédimentologie et biologie des constructions organogènes du Cambrien inférieur du Sud de l’Espagne. Bur. Rech. Géologiques Minières Mémoire 1977, 89, 49–61. [Google Scholar]
- Fernández-Remolar, D.C. The phosphoritic limestones of the Lower Ovetian from the Sierra de Córdoba, Spain. Boletín Real Soc. Española Hist. Nat. Sección Geológica 1999, 95, 15–45. [Google Scholar]
- Nuñez Granados, M.A.; Recio Espejo, J.M. Holocene pedoenvironmental situations in the eastern Sierra Morena region (Andújar, Spain). Quat. Int. 2002, 93–94, 191–195. [Google Scholar] [CrossRef]
- Parras-Alcántara, L.; Lozano-García, B.; Galán-Espejo, A. Soil organic carbon along an altitudinal gradient in the Despeñaperros Natural Park, southern Spain. Solid Earth 2015, 6, 125–134. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Carrizo, D.; Harir, M.; Huang, T.; Amils, R.; Schmitt-Kopplin, P.; Sánchez-García, L.; Gomez-Ortiz, D.; Malmberg, P. Unveiling microbial preservation under hyperacidic and oxidizing conditions in the Oligocene Rio Tinto deposit. Sci. Rep. 2021, 11, 21543. [Google Scholar] [CrossRef]
- Megevand, V.; Carrizo, D.; Lezcano, M.Á.; Moreno-Paz, M.; Cabrol, N.A.; Parro, V.; Sánchez-García, L. Lipid Profiles from Fresh Biofilms Along a Temperature Gradient on a Hydrothermal Stream at El Tatio (Chilean Andes), as a Proxy for the Interpretation of Past and Present Biomarkers Beyond Earth. Front. Microbiol. 2022, 13, 811904. [Google Scholar] [CrossRef]
- Carrizo, D.; Vignale, F.A.; Sánchez-García, L.; Farías, M.E. Ecological variability based on lipid biomarkers in astrobiologically interesting wetlands from the Argentinian central Andes. FEMS Microbiol. Ecol. 2022, 98, fiac049. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Gómez-Ortiz, D.; Malmberg, P.; Huang, T.; Shen, Y.; Anglés, A.; Amils, R. Preservation of Underground Microbial Diversity in Ancient Subsurface Deposits (>6 Ma) of the Rio Tinto Basement. Microorganisms 2021, 9, 1592. [Google Scholar] [CrossRef]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J.; Strohalm, M. mMass as a Software Tool for the Annotation of Cyclic Peptide Tandem Mass Spectra. PLoS ONE 2012, 7, e44913. [Google Scholar] [CrossRef]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, W606–W612. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, B.E.; Semiletov, I.; Weijers, J.W.H.; Gustafsson, Ö. Contrasting lipid biomarker composition of terrestrial organic matter exported from across the Eurasian Arctic by the five great Russian Arctic rivers. Glob. Biogeochem. Cycles 2008, 22, 2974. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Leierer, L.; Jambrina-Enríquez, M.; Connolly, R.; Mallol, C. Evaluating different methods for calculating the Carbon Preference Index (CPI): Implications for palaeoecological and archaeological research. Org. Geochem. 2020, 146, 104056. [Google Scholar] [CrossRef]
- Rielley, G.; Collier, R.J.; Jones, D.M.; Eglinton, G. The biogeochemistry of Ellesmere Lake, UK—I: Source correlation of leaf wax inputs to the sedimentary lipid record. Org. Geochem. 1991, 17, 901–912. [Google Scholar] [CrossRef]
- Grimalt, J.; Albaigés, J. Sources and occurrence of C12—C22n-alkane distributions with even carbon-number preference in sedimentary environments. Geochim. Cosmochim. Acta 1987, 51, 1379–1384. [Google Scholar] [CrossRef]
- Sánchez-García, L.; Vonk, J.E.; Charkin, A.N.; Kosmach, D.; Dudarev, O.V.; Semiletov, I.P.; Gustafsson, Ö. Characterisation of Three Regimes of Collapsing Arctic Ice Complex Deposits on the SE Laptev Sea Coast using Biomarkers and Dual Carbon Isotopes. Permafr. Periglac. Process. 2014, 25, 172–183. [Google Scholar] [CrossRef]
- Scholle, P.A.; Ulmer-Scholle, D.S. A Color Guide to the Petrography of Carbonate Rocks: Grains, Textures, Porosity, Diagenesis; American Association of Petroleum Geologists: Tulsa, OK, USA, 2003. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C. Estudio Paleontológico de Taxones Problemáticos del Cámbrico de Sierra Morena; Universidad Complutense de Madrid: Madrid, Spain, 1999. [Google Scholar]
- Chen, X.-G.; Lyu, S.-S.; Garbe-Schönberg, D.; Lebrato, M.; Li, X.; Zhang, H.-Y.; Zhang, P.-P.; Chen, C.-T.A.; Ye, Y. Heavy metals from Kueishantao shallow-sea hydrothermal vents, offshore northeast Taiwan. J. Mar. Syst. 2018, 180, 211–219. [Google Scholar] [CrossRef]
- Schulz, H.N.; Schulz, H.D. Large Sulfur Bacteria and the Formation of Phosphorite. Science 2005, 307, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Sjövall, P.; Lausmaa, J.; Leefmann, T.; Thiel, V. Spectral characterisation of eight glycerolipids and their detection in natural samples using time-of-flight secondary ion mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2741–2753. [Google Scholar] [CrossRef]
- Newell, C.L.; Vorng, J.L.; MacRae, J.I.; Gilmore, I.S.; Gould, A.P. Cryogenic OrbiSIMS localizes semi-volatile molecules in biological tissues. Angew. Chem. Int. Ed. 2020, 59, 18194–18200. [Google Scholar] [CrossRef]
- Siljeström, S.; Hode, T.; Lausmaa, J.; Sjövall, P.; Toporski, J.; Thiel, V. Detection of organic biomarkers in crude oils using ToF-SIMS. Org. Geochem. 2009, 40, 135–143. [Google Scholar] [CrossRef]
- Leefmann, T.; Heim, C.; Kryvenda, A.; Siljeström, S.; Sjövall, P.; Thiel, V. Biomarker imaging of single diatom cells in a microbial mat using time-of-flight secondary ion mass spectrometry (ToF-SIMS). Org. Geochem. 2013, 57, 23–33. [Google Scholar] [CrossRef]
- Leefmann, T.; Heim, C.; Siljeström, S.; Blumenberg, M.; Sjövall, P.; Thiel, V. Spectral characterization of ten cyclic lipids using time-of-flight secondary ion mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 565–581. [Google Scholar] [CrossRef]
- Siljeström, S.; Parenteau, M.N.; Jahnke, L.L.; Cady, S.L. A comparative ToF-SIMS and GC–MS analysis of phototrophic communities collected from an alkaline silica-depositing hot spring. Org. Geochem. 2017, 109, 14–30. [Google Scholar] [CrossRef]
- Aasen, A.J.; Hofstetter, H.H.; Iyengar, B.T.R.; Holman, R.T. Identification and analysis of wax esters by mass spectrometry. Lipids 1971, 6, 502–507. [Google Scholar] [CrossRef]
- Xu, M.-L.; Qi, L.; Cai, X.; Cao, T.; Tang, R.; Cao, K.; Lian, Y. Speculation of Sphingolipids in Capsanthin by Ultra-Performance Liquid Chromatography Coupled with Electrospray Ionization-Quadrupole—Time-of-Flight Mass Spectrometry. Molecules 2023, 28, 1010. [Google Scholar] [CrossRef]
- Brocks, J.J.; Summons, R.E. 10.3—Sedimentary Hydrocarbons, Biomarkers for Early Life. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 61–103. [Google Scholar]
- Kaneda, T. Iso-and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Mol. Biol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Burdige, D.J. The biogeochemistry of manganese and iron reduction in marine sediments. Earth Sci. Rev. 1993, 35, 249–284. [Google Scholar] [CrossRef]
- Burdige, D.J. Preservation of Organic Matter in Marine Sediments: Controls, Mechanisms, and an Imbalance in Sediment Organic Carbon Budgets? Chem. Rev. 2007, 107, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P. Cutin, suberin, and waxes. In Lipids: Structure and Function; Elsevier: Amsterdam, The Netherlands, 1980; pp. 571–645. [Google Scholar]
- Colombo, J.; Silverberg, N.; Gearing, J. Lipid biogeochemistry in the Laurentian Trough: I—Fatty acids, sterols and aliphatic hydrocarbons in rapidly settling particles. Org. Geochem. 1996, 25, 211–225. [Google Scholar] [CrossRef]
- Lebreton, B.; Richard, P.; Galois, R.; Radenac, G.; Pfléger, C.; Guillou, G.; Mornet, F.; Blanchard, G.F. Trophic importance of diatoms in an intertidal Zostera noltii seagrass bed: Evidence from stable isotope and fatty acid analyses. Estuar. Coast. Shelf Sci. 2011, 92, 140–153. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.-Z.; Xu, X.-P.; Fang, Y.-X.; Liu, J.-Z.; Wang, J.-H. Source Indication and Geochemical Significance of Sedimentary Organic Matters from the Xisha Area, the South China Sea. Molecules 2021, 26, 6808. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.; Cranwell, P.; Finlay, B.; Eglinton, G. Lipids of aquatic organisms as potential contributors to lacustrine sediments. Org. Geochem. 1984, 6, 143–152. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Blackburn, S.I. Eustigmatophyte microalgae are potential sources of C29 sterols, C22–C28 n-alcohols and C28–C32 n-alkyl diols in freshwater environments. Org. Geochem. 1999, 30, 307–318. [Google Scholar] [CrossRef]
- Eglinton, G.; Hamilton, R.J. Leaf Epicuticular Waxes: The waxy outer surfaces of most plants display a wide diversity of fine structure and chemical constituents. Science 1967, 156, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.; Falk-Petersen, S. Ecological investigations on the zooplankton community in Balsfjorden, Northern Norway: Lipids and fatty acids in Meganyctiphanes norvegica, Thysanoessa raschi and T. inermis during mid-winter. Mar. Biol. 1981, 62, 131–137. [Google Scholar] [CrossRef]
- Vestal, J.R.; White, D.C. Lipid Analysis in Microbial Ecology. BioScience 1989, 39, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.; Russell, N. Lipids in Plants and Microbes; George Allen and Unwin: London, UK, 1984. [Google Scholar]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.A.; McIntosh, A.C.; Norris, C.E.; Lloret, E.; Swallow, M.J.; Hannam, K. Extraction and analysis of microbial phospholipid fatty acids in soils. J. Vis. Exp. 2016, 114, e54360. [Google Scholar]
- Ruess, L.; Chamberlain, P.M. The fat that matters: Soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol. Biochem. 2010, 42, 1898–1910. [Google Scholar] [CrossRef]
- Kühn, J.; Schweitzer, K.; Ruess, L. Diversity and specificity of lipid patterns in basal soil food web resources. PLoS ONE 2019, 14, e0221102. [Google Scholar] [CrossRef]
- Cantrell, S.A.; Lodge, D.J.; Cruz, C.A.; García, L.M.; Pérez-Jiménez, J.R.; Molina, M. Differential abundance of microbial functional groups along the elevation gradient from the coast to the Luquillo Mountains. Ecol. Bull. 2013, 54, 87–100. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Volume 1, Biomarkers and Isotopes in the Environment and Human History; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Awad, A.; Majcherczyk, A.; Schall, P.; Schröter, K.; Schöning, I.; Schrumpf, M.; Ehbrecht, M.; Boch, S.; Kahl, T.; Bauhus, J. Ectomycorrhizal and saprotrophic soil fungal biomass are driven by different factors and vary among broadleaf and coniferous temperate forests. Soil Biol. Biochem. 2019, 131, 9–18. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, X.; Ji, W.; Hai, Z.; Hu, N.; Li, X.; Liu, G.; Yu, C.; Chen, Y.; Lian, B.; et al. Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. J. Fungi 2022, 8, 124. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the roles of plant sterols in development and stress responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Naafs, B.D.A.; Inglis, G.N.; Blewett, J.; McClymont, E.L.; Lauretano, V.; Xie, S.; Evershed, R.P.; Pancost, R.D. The potential of biomarker proxies to trace climate, vegetation, and biogeochemical processes in peat: A review. Glob. Planet. Chang. 2019, 179, 57–79. [Google Scholar] [CrossRef]
- Anglés, A.; He, Q.; Sánchez García, L.; Carrizo, D.; Rodriguez, N.; Huang, T.; Shen, Y.; Amils, R.; Fernández-Remolar, D.C. Biospeleothems Formed by Fungal Activity During the Early Holocene in the “Salar de Uyuni”. Front. Microbiol. 2022, 13, 913452. [Google Scholar] [CrossRef]
- Hayes, J.M. Fractionation of Carbon and Hydrogen Isotopes in Biosynthetic Processes*. Rev. Mineral. Geochem. 2001, 43, 225–277. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Naraoka, H. Carbon and hydrogen isotope variation of plant biomarkers in a plant–soil system. Chem. Geol. 2006, 231, 190–202. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Kalachova, G.S.; Makhutova, O.N. Stable Isotope Composition of Fatty Acids in Organisms of Different Trophic Levels in the Yenisei River. PLoS ONE 2012, 7, e34059. [Google Scholar] [CrossRef]
- Zocatelli, R.; Lavrieux, M.; Disnar, J.-R.; Le Milbeau, C.; Jacob, J.; Bréheret, J.G. Free fatty acids in Lake Aydat catchment soils (French Massif Central): Sources, distributions and potential use as sediment biomarkers. J. Soils Sediments 2012, 12, 734–748. [Google Scholar] [CrossRef][Green Version]
- Wiesenberg, G.L.B.; Dorodnikov, M.; Kuzyakov, Y. Source determination of lipids in bulk soil and soil density fractions after four years of wheat cropping. Geoderma 2010, 156, 267–277. [Google Scholar] [CrossRef]
- Hirave, P.; Wiesenberg, G.L.B.; Birkholz, A.; Alewell, C. Understanding the effects of early degradation on isotopic tracers: Implications for sediment source attribution using compound-specific isotope analysis (CSIA). Biogeosciences 2020, 17, 2169–2180. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants, 2nd ed.; John Wiley & Sons: Oxford, UK, 2015; p. 1264. [Google Scholar]
- Pruski, A.M.; Buscail, R.; Bourgeois, S.; Vétion, G.; Coston-Guarini, J.; Rabouille, C. Biogeochemistry of fatty acids in a river-dominated Mediterranean ecosystem (Rhône River prodelta, Gulf of Lions, France): Origins and diagenesis. Org. Geochem. 2015, 83–84, 227–240. [Google Scholar] [CrossRef]
- Werne, J.P.; Baas, M.; Sinninghe Damsté, J.S. Molecular isotopic tracing of carbon flow and trophic relationships in a methane-supported benthic microbial community. Limnol. Oceanogr. 2002, 47, 1694–1701. [Google Scholar] [CrossRef]
- Stadnitskaia, A.; Muyzer, G.; Abbas, B.; Coolen, M.J.L.; Hopmans, E.C.; Baas, M.; Van Weering, T.C.E.; Ivanov, M.K.; Poludetkina, E.; Damsté, J.S.S. Biomarker and 16S rDNA evidence for anaerobic oxidation of methane and related carbonate precipitation in deep-sea mud volcanoes of the Sorokin Trough, Black Sea. Mar. Geol. 2005, 217, 67–96. [Google Scholar] [CrossRef]
- Blumenberg, M.; Krüger, M.; Nauhaus, K.; Talbot, H.M.; Oppermann, B.I.; Seifert, R.; Pape, T.; Michaelis, W. Biosynthesis of hopanoids by sulfate-reducing bacteria (genus Desulfovibrio). Environ. Microbiol. 2006, 8, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A. Chemistry and morphology of plant epicuticular waxes. Plant Cuticle 1982, 139–166. [Google Scholar]
- Bianchi, G. Plant waxes. Waxes Chem. Mol. Biol. Funct. 1995, 6, 175–222. [Google Scholar]
- Kolattukudy, P.E. Chemistry and Biochemistry of Natural Waxes; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Barrett, S.M.; Volkman, J.K.; Dunstan, G.A.; LeRoi, J.M. Sterols of 14 species of marine diatoms (bacillariophyta) 1. J. Phycol. 1995, 31, 360–369. [Google Scholar] [CrossRef]
- Ruzicka, S.; Edgerton, D.; Norman, M.; Hill, T. The utility of ergosterol as a bioindicator of fungi in temperate soils. Soil Biol. Biochem. 2000, 32, 989–1005. [Google Scholar] [CrossRef]
- Sejal, P.; Dennis, R.N.; Allen, G.G. Chemical and physical analyses of wax ester properties. J. Insect Sci. 2001, 1, 4. [Google Scholar]
- Rontani, J.F. Production of Wax Esters by Bacteria. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 459–470. [Google Scholar]
- Fixter, L.M.; Nagi, M.N.; Mccormack, J.G.; Fewson, C.A. Structure, distribution and function of wax esters in Acinetobacter calcoaceticus. Microbiology 1986, 132, 3147–3157. [Google Scholar] [CrossRef][Green Version]
- Wältermann, M.; Hinz, A.; Robenek, H.; Troyer, D.; Reichelt, R.; Malkus, U.; Galla, H.J.; Kalscheuer, R.; Stöveken, T.; Von Landenberg, P. Mechanism of lipid-body formation in prokaryotes: How bacteria fatten up. Mol. Microbiol. 2005, 55, 750–763. [Google Scholar] [CrossRef]
- Ishige, T.; Tani, A.; Sakai, Y.; Kato, N. Wax ester production by bacteria. Curr. Opin. Microbiol. 2003, 6, 244–250. [Google Scholar] [CrossRef]
- Wältermann, M.; Steinbüchel, A. Neutral lipid bodies in prokaryotes: Recent insights into structure, formation, and relationship to eukaryotic lipid depots. J. Bacteriol. 2005, 187, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Knicker, H. Stabilization of N-compounds in soil and organic-matter-rich sediments—What is the difference? Mar. Chem. 2004, 92, 167–195. [Google Scholar] [CrossRef]
- Herbert, R.A. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol. Rev. 1999, 23, 563–590. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. The role of sterols in plant response to abiotic stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Volkman, J.K. A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Hartmann, M.-A. Plant sterols and the membrane environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Núñez, M.A.; Recio, J.M. Kaolinitic paleosols in the south west of the Iberian Peninsula (Sierra Morena region, Spain). Paleoenvironmental implications. Catena 2007, 70, 388–395. [Google Scholar] [CrossRef]
- Fernández-Caliani, J.C. Rare-earth element and stable isotope signatures of kaolin from a Pliocene lateritic weathering profile at mid-latitude region (Andalusia, Spain): Implications for paleoweathering and paleoclimatic reconstructions. Catena 2018, 167, 160–170. [Google Scholar] [CrossRef]
- Sánchez-García, L.; Aeppli, C.; Parro, V.; Fernández-Remolar, D.; García-Villadangos, M.; Chong-Diaz, G.; Blanco, Y.; Carrizo, D. Molecular biomarkers in the subsurface of the Salar Grande (Atacama, Chile) evaporitic deposits. Biogeochemistry 2018, 140, 31–52. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Gomez-Ortiz, D.; Huang, T.; Anglés, A.; Shen, Y.; Hu, Q.; Amils, R.; Rodríguez, N.; Escudero, C.; Banerjee, N.R. The Molecular Record of Metabolic Activity in the Subsurface of the Río Tinto Mars Analog. Astrobiology 2021, 21, 1387–1405. [Google Scholar] [CrossRef]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Stopnisek, N.; Zühlke, D.; Carlier, A.; Barberán, A.; Fierer, N.; Becher, D.; Riedel, K.; Eberl, L.; Weisskopf, L. Molecular mechanisms underlying the close association between soil Burkholderia and fungi. ISME J. 2016, 10, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Remolar, D.C.; Gómez, F.; Prieto-Ballesteros, O.; Schelble, R.T.; Rodríguez, N.; Amils, R. Some Ecological Mechanisms to Generate Habitability in Planetary Subsurface Areas by Chemolithotrophic Communities: The Río Tinto Subsurface Ecosystem as a Model System. Astrobiology 2008, 8, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Remolar, D.C.; Banerjee, N.; Gómez-Ortiz, D.; Izawa, M.; Amils, R. A mineralogical archive of the biogeochemical sulfur cycle preserved in the subsurface of the Río Tinto system. Am. Mineral. 2018, 103, 394–411. [Google Scholar] [CrossRef]
- Grayston, S.; Vaughan, D.; Jones, D. Rhizosphere carbon flow in trees, in comparison with annual plants: The importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 1997, 5, 29–56. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Prieto-Ballesteros, O.; Rodríguez, N.; Gómez, F.; Amils, R.; Gómez-Elvira, J.; Stoker, C.R. Underground Habitats in the Río Tinto Basin: A Model for Subsurface Life Habitats on Mars. Astrobiology 2008, 8, 1023–1047. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Carrizo, D.; Sánchez-García, L.; Hu, Q.; Anglés, A.; Gómez-Ortiz, D.; Yu, L.-L.; Fernández-Remolar, D.C. The Molecular Profile of Soil Microbial Communities Inhabiting a Cambrian Host Rock. Microorganisms 2024, 12, 513. https://doi.org/10.3390/microorganisms12030513
Huang T, Carrizo D, Sánchez-García L, Hu Q, Anglés A, Gómez-Ortiz D, Yu L-L, Fernández-Remolar DC. The Molecular Profile of Soil Microbial Communities Inhabiting a Cambrian Host Rock. Microorganisms. 2024; 12(3):513. https://doi.org/10.3390/microorganisms12030513
Chicago/Turabian StyleHuang, Ting, Daniel Carrizo, Laura Sánchez-García, Qitao Hu, Angélica Anglés, David Gómez-Ortiz, Liang-Liang Yu, and David C. Fernández-Remolar. 2024. "The Molecular Profile of Soil Microbial Communities Inhabiting a Cambrian Host Rock" Microorganisms 12, no. 3: 513. https://doi.org/10.3390/microorganisms12030513
APA StyleHuang, T., Carrizo, D., Sánchez-García, L., Hu, Q., Anglés, A., Gómez-Ortiz, D., Yu, L.-L., & Fernández-Remolar, D. C. (2024). The Molecular Profile of Soil Microbial Communities Inhabiting a Cambrian Host Rock. Microorganisms, 12(3), 513. https://doi.org/10.3390/microorganisms12030513






