Characterization of a Novel Hyperthermophilic GH1 β-Glucosidase from Acidilobus sp. and Its Application in the Hydrolysis of Soybean Isoflavone Glycosides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Gene Sequence Analysis and Cloning of BglAc from Acidilobus sp.
2.3. Expression and Purification of Recombinant Protein BglAc
2.4. Enzyme Activity Assay
2.5. Optimum pH and Temperature of BglAc
2.6. Thermostability Determination
2.7. Effects of Metal Ions and Chemical Reagents on Enzyme Activity
2.8. Effect of Glucose on Enzyme Activity
2.9. Substrate Specificity and Kinetic Parameters
2.10. HPLC Analysis of Soybean Isoflavone Glycosides Hydrolyzed by BglAc
2.11. Enzyme Characteristics of Mutant BglAcN
3. Results
3.1. Gene Sequence Analysis
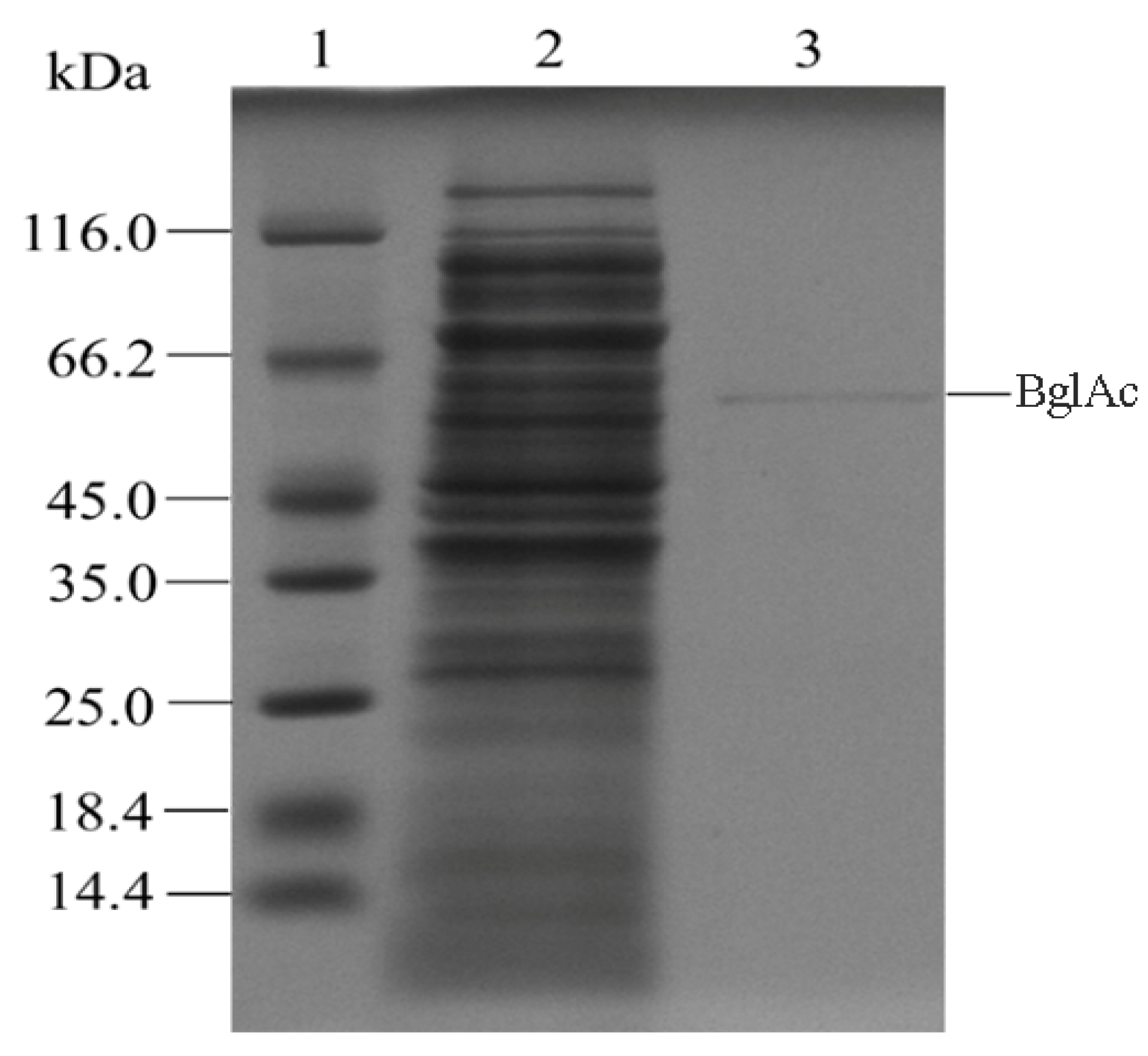
3.2. Enzyme Expression and Purification
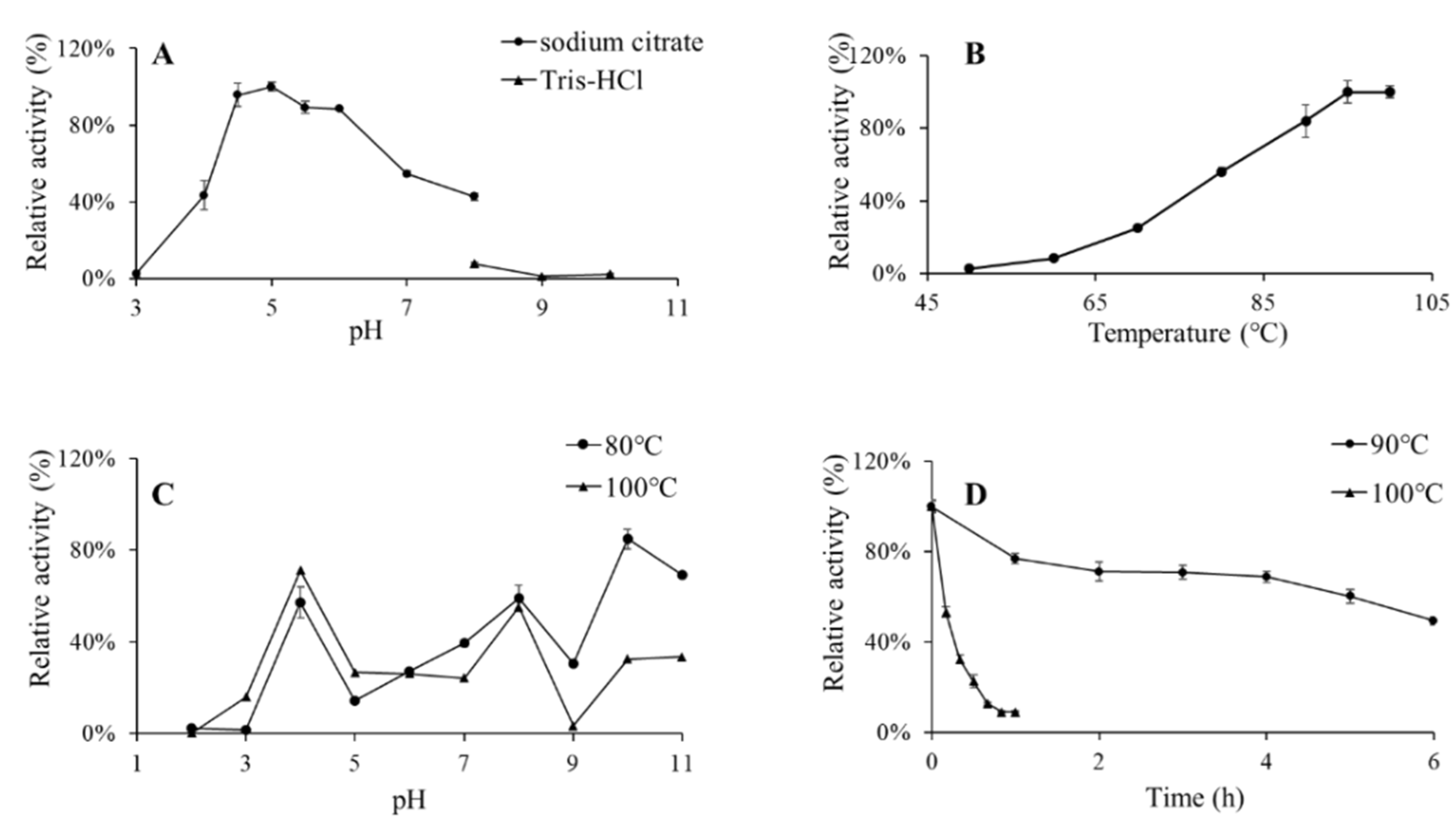
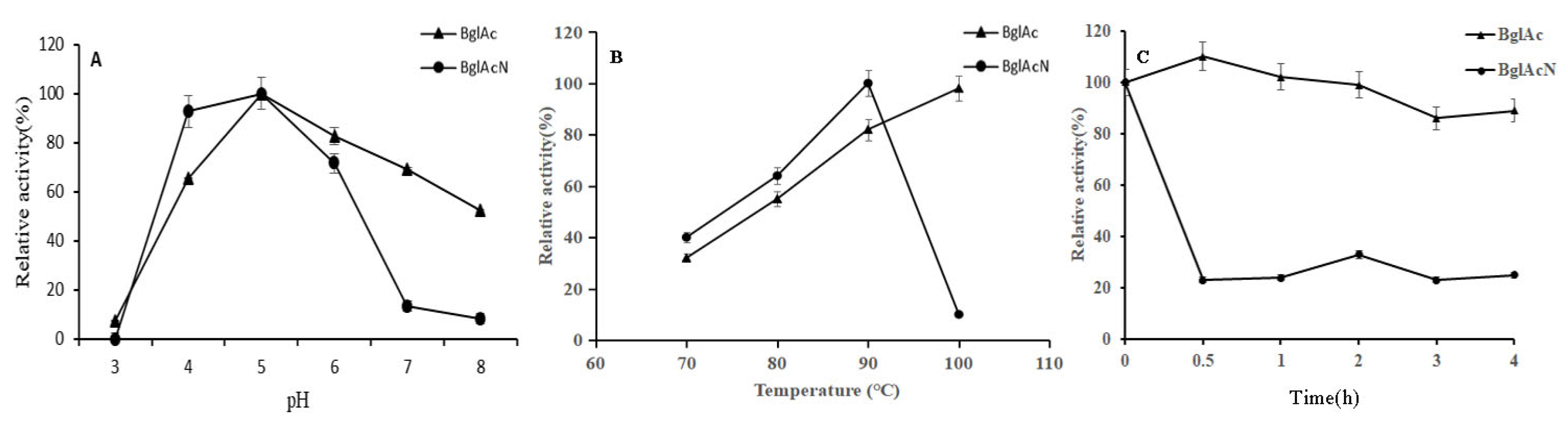
3.3. Effects of pH and Temperature on BglAc and Thermostability Evaluation
3.4. Effects of Metal Ions and Chemical Reagents on BglAc
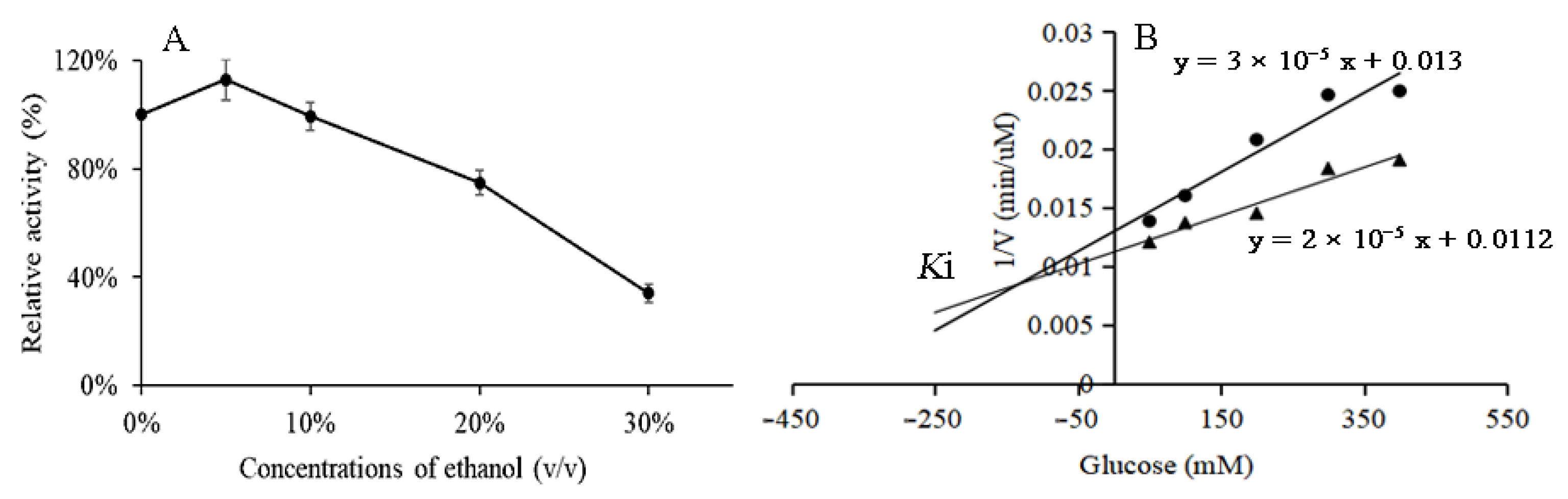
3.5. Ethanol and Glucose Tolerance of BglAc
3.6. Substrate Specificity and Kinetic Parameters of BglAc
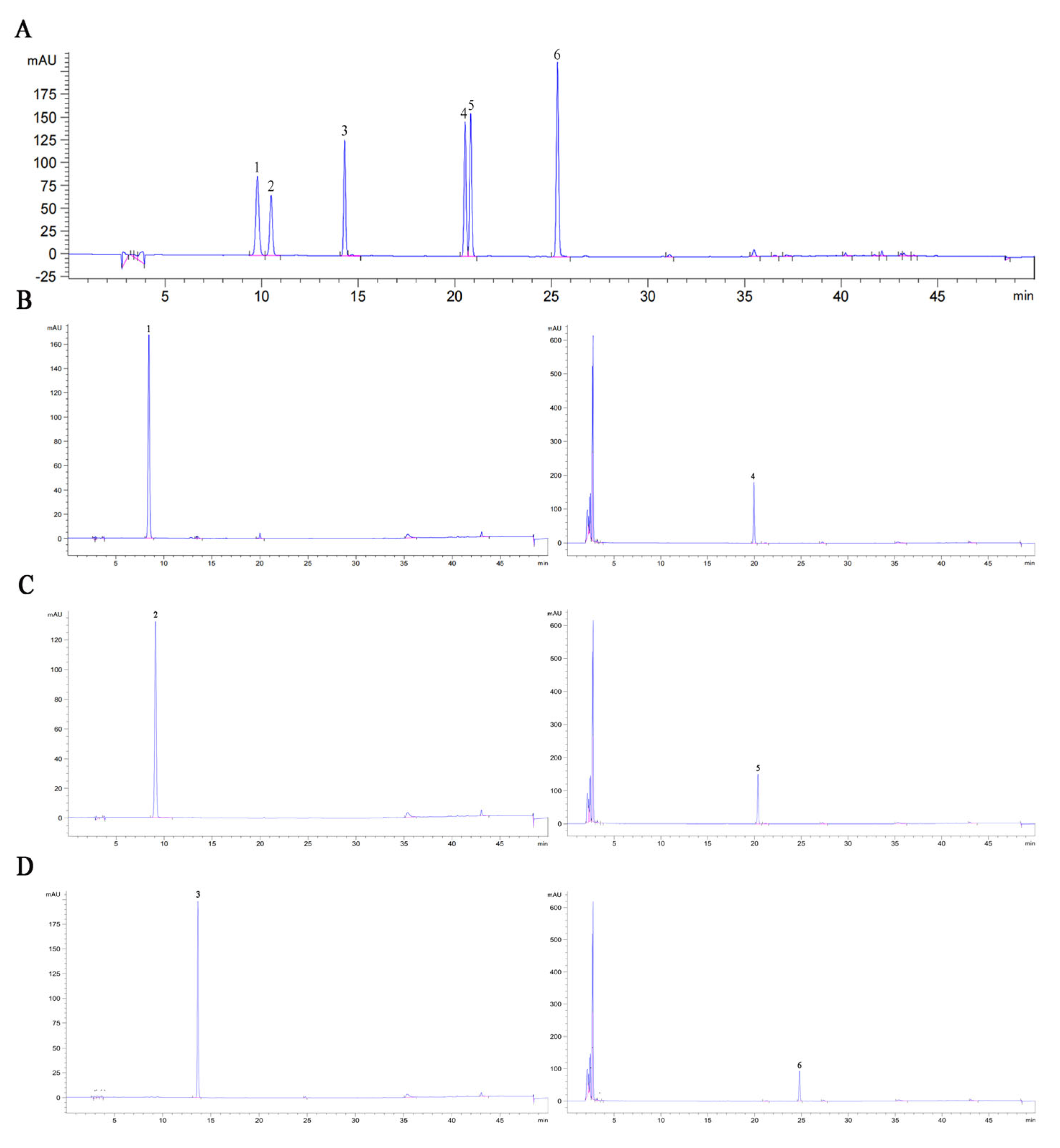
3.7. Hydrolysis Products of Soybean Isoflavone Glycosides Generated by BglAc
3.8. The Optimal Temperature, pH, and Thermostability of Mutant Enzyme BglAcN
4. Discussion
| Organisms | Enzyme | Optimum Temperature (°C) | Optimum pH | T 1/2 | Reference |
|---|---|---|---|---|---|
| Alicyclobacillus sp. | AsBG1 | 100 | 6.5 | 1 h (50 °C) | [16] |
| P. furiosus | / | 100 | 5.0 | 85 h (100 °C) | [19] |
| S. solfataricus | Sβ-gly | 85 | 5.5 | 48 h (85 °C) | [20] |
| P. kodakaraensis | Pk-gly | 100 | 6.5 | 18 h (90 °C) | [21] |
| T. acidophilum | TaBglA | 90 | 6.0 | 0.4 h (90 °C) | [22] |
| S. acidocaldarius | bgaS | 90 | 5.5 | 0.2 h (90 °C) | [23] |
| A. saccharovorans | Asac_1390 | 93 | 6.0 | 7 h (90 °C) | [24] |
| T. naphthophila | BglA | 95 | 7.0 | 8 h (80 °C) | [25] |
| T. kodakarensis | vul_bgl1A | 105 | 7.0 | 0.25 h (90 °C) | [26] |
| Hot spring | bglM | 70 | 5.0 | 32 h (60 °C) | [36] |
| J. malaysiensis | BglD5 | 65 | 7.0 | 0.6 h (65 °C) | [37] |
| B. cellulosilyticus | BcBgl1A | 40 | 7.0 | 24 h (40 °C) | [38] |
| E. antarcticum B7 | EaBgl1A | 30 | 7.0 | 48 h (30 °C) | [39] |
| Thermococcus sp. | O08324 | 78 | 6.5 | 14.3 h (78 °C) | [40] |
| Acidilobus sp. | BglAc | 100 | 5.0 | 6 h (90 °C) | This study |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godse, R.; Bawane, H.; Tripathi, J.; Kulkarni, R. Unconventional β-Glucosidases: A Promising Biocatalyst for Industrial Biotechnology. Appl. Biochem. Biotechnol. 2021, 193, 2993–3016. [Google Scholar] [CrossRef]
- Kannan, P.; Shafreen, M.M.; Achudhan, A.B.; Gupta, A.; Saleena, L.M. A review on applications of β-glucosidase in food, brewery, pharmaceutical and cosmetic industries. Carbohydr. Res. 2023, 530, 108855. [Google Scholar] [CrossRef]
- Abdella, A.; El-Baz, A.F.; Ibrahim, I.A.; Mahrous, E.E.; Yang, S.T. Biotransformation of soy flour isoflavones by Aspergillus niger NRRL 3122 β-glucosidase enzyme. Nat. Prod. Res. 2018, 32, 2382–2391. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, X.M.; Yan, H.J.; Liu, X.Y.; Zhou, Q.; Luo, Z.Y.; Tan, X.N.; Zhang, B.L. Highly Selective Production of Compound K from Ginsenoside Rd by Hydrolyzing Glucose at C-3 Glycoside Using β-Glucosidase of Bifidobacterium breve ATCC 15700. J. Microbiol. Biotechnol. 2019, 29, 410–418. [Google Scholar] [CrossRef]
- Tran, T.N.A.; Son, J.S.; Awais, M.; Ko, J.H.; Yang, D.C.; Jung, S.K. β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng. Bioengineering 2023, 10, 484. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, R.; Huang, Z.; Zhou, J. Progress in the Conversion of Ginsenoside Rb1 into Minor Ginsenosides Using β-Glucosidases. Foods 2023, 12, 397. [Google Scholar] [CrossRef]
- Mu, Y.; Meng, F.; Ju, X.; Li, L. Inactivation and process intensification of β-glucosidase in biomass utilization. Appl. Microbiol. Biotechnol. 2023, 107, 3191–3204. [Google Scholar] [CrossRef]
- Bader, A.N.; Sanchez Rizza, L.; Consolo, V.F.; Curatti, L. Bioprospecting for fungal enzymes for applications in microalgal biomass biorefineries. Appl. Microbiol. Biotechnol. 2023, 107, 591–607. [Google Scholar] [CrossRef]
- Srivastava, N.; Rathour, R.; Jha, S.; Pandey, K.; Srivastava, M.; Thakur, V.K.; Sengar, R.S.; Gupta, V.K.; Mazumder, P.B.; Khan, A.F.; et al. Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application. Biomolecules 2019, 9, 220. [Google Scholar] [CrossRef]
- Li, M.; Jiang, B.; Wu, W.; Wu, S.; Yang, Y.; Song, J.; Ahmad, M.; Jin, Y. Current understanding and optimization strategies for efficient lignin-enzyme interaction: A review. Int. J. Biol. Macromol. 2022, 195, 274–286. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, J.; Song, X. Hydrolysis of soy isoflavone glycosides by recombinant beta-glucosidase from hyperthermophile Thermotoga maritima. J. Ind. Microbiol. Biotechnol. 2009, 36, 1401–1408. [Google Scholar] [CrossRef]
- Song, X.; Xue, Y.; Wang, Q.; Wu, X. Comparison of three thermostable beta-glucosidases for application in the hydrolysis of soybean isoflavone glycosides. J. Agric. Food Chem. 2011, 59, 1954–1961. [Google Scholar] [CrossRef]
- Yeom, S.J.; Kim, B.N.; Kim, Y.S.; Oh, D.K. Hydrolysis of isoflavone glycosides by a thermostable beta-glucosidase from Pyrococcus furiosus. J. Agric. Food Chem. 2012, 60, 1535–1541. [Google Scholar] [CrossRef]
- Yang, X.; Ma, R.; Shi, P.; Huang, H.; Bai, Y.; Wang, Y.; Yang, P.; Fan, Y.; Yao, B. Molecular characterization of a highly-active thermophilic β-glucosidase from Neosartorya fischeri P1 and its application in the hydrolysis of soybean isoflavone glycosides. PLoS ONE 2014, 9, e106785. [Google Scholar] [CrossRef]
- Li, X.; Xia, W.; Bai, Y.; Ma, R.; Yang, H.; Luo, H.; Shi, P. A Novel Thermostable GH3 β-Glucosidase from Talaromyce leycettanus with Broad Substrate Specificity and Significant Soybean Isoflavone Glycosides-Hydrolyzing Capability. Biomed. Res. Int. 2018, 2018, 4794690. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Y.; Shi, P.; Ma, R.; Yang, H.; Xia, W.; Cui, Y.; Luo, H.; Bai, Y.; Yao, B. A highly glucose-tolerant GH1 beta-glucosidase with greater conversion rate of soybean isoflavones in monogastric animals. J. Ind. Microbiol. Biotechnol. 2018, 45, 369–378. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Ouyang, B.; Wang, G.; Zhang, N.; Zuo, J.; Huang, Y.; Zhao, X. Recent Advances in β-Glucosidase Sequence and Structure Engineering: A Brief Review. Molecules 2023, 28, 4990. [Google Scholar] [CrossRef]
- Kengen, S.W.; Luesink, E.J.; Stams, A.J.; Zehnder, A.J. Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur. J. Biochem. 1993, 213, 305–312. [Google Scholar] [CrossRef]
- Moracci, M.; Nucci, R.; Febbraio, F.; Vaccaro, C.; Vespa, N.; La Cara, F.; Rossi, M. Expression and extensive characterization of a beta-glycosidase from the extreme thermoacidophilic archaeon Sulfolobus solfataricus in Escherichia coli: Authenticity of the recombinant enzyme. Enzyme Microb. Technol. 1995, 17, 992–997. [Google Scholar] [CrossRef]
- Ezaki, S.; Miyaoku, K.; Nishi, K.; Tanaka, T.; Fujiwara, S.; Takagi, M.; Atomi, H.; Imanaka, T. Gene analysis and enzymatic properties of thermostable beta-glycosidase from Pyrococcus kodakaraensis KOD1. J. Biosci. Bioeng. 1999, 88, 130–135. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, A.R.; Lee, J.K.; Oh, D.K. Characterization of an acid-labile, thermostable beta-glycosidase from Thermoplasma acidophilum. Biotechnol. Lett. 2009, 31, 1457–1462. [Google Scholar] [CrossRef]
- Park, A.R.; Kim, H.J.; Lee, J.K.; Oh, D.K. Hydrolysis and transglycosylation activity of a thermostable recombinant beta-glycosidase from Sulfolobus acidocaldarius. Appl. Biochem. Biotechnol. 2010, 160, 2236–2247. [Google Scholar] [CrossRef]
- Gumerov, V.M.; Rakitin, A.L.; Mardanov, A.V.; Ravin, N.V. A Novel Highly Thermostable Multifunctional Beta-Glycosidase from Crenarchaeon Acidilobus saccharovorans. Archaea 2015, 2015, 978632. [Google Scholar] [CrossRef]
- Akram, F.; ul Haq, I.; Khan, M.A.; Hussain, Z.; Mukhtar, H.; Iqbal, K. Cloning with kinetic and thermodynamic insight of a novel hyperthermostable beta-glucosidase from Thermotoga naphthophila rku-10(t) with excellent glucose tolerance. J. Mol. Catal. B-Enzym. 2016, 124, 92–104. [Google Scholar] [CrossRef]
- Schröder, C.; Eixenberger, D.; Suleiman, M.; Schäfers, C.; Antranikian, G. Characterization of an extremely thermo-active archaeal β-glucosidase and its activity towards glucan and mannan in concert with an endoglucanase. Appl. Microbiol. Biotechnol. 2019, 103, 9505–9514. [Google Scholar] [CrossRef]
- Payne, D.; Dunham, E.C.; Mohr, E.; Miller, I.; Arnold, A.; Erickson, R.; Fones, E.M.; Lindsay, M.R.; Colman, D.R.; Boyd, E.S. Geologic legacy spanning >90 years explains unique Yellowstone hot spring geochemistry and biodiversity. Environ. Microbiol. 2019, 21, 4180–4195. [Google Scholar] [CrossRef]
- Craven, G.R.; Steers Jr, E.; Anfinsen, C.B. Purification, composition, and molecular weight of the β-galactosidase of Escherichia coli K12. J. Biol. Chem. 1965, 240, 2468–2477. [Google Scholar] [CrossRef]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef]
- Fang, W.; Song, R.; Zhang, X.; Zhang, X.; Zhang, X.; Wang, X.; Fang, Z.; Xiao, Y. Characterization of a novel β-glucosidase from Gongronella sp. W5 and its application in the hydrolysis of soybean isoflavone glycosides. J. Agric. Food Chem. 2014, 62, 11688–11695. [Google Scholar] [CrossRef]
- Park, D.J.; Lee, Y.S.; Choi, Y.L. Characterization of a cold-active β-glucosidase from Paenibacillus xylanilyticus KJ-03 capable of hydrolyzing isoflavones daidzin and genistin. Protein J. 2013, 32, 579–584. [Google Scholar] [CrossRef]
- Mai, Z.; Wang, L.; Zeng, Q. Characterization of a novel isoflavone glycoside-hydrolyzing β-glucosidase from mangrove soil metagenomic library. Biochem. Biophys. Res. Commun. 2021, 569, 61–65. [Google Scholar] [CrossRef]
- Chi, Y.I.; Martinez-Cruz, L.A.; Jancarik, J.; Swanson, R.V.; Robertson, D.E.; Kim, S.H. Crystal structure of the beta-glycosidase from the hyperthermophile Thermosphaera aggregans: Insights into its activity and thermostability. FEBS Lett. 1999, 445, 375–383. [Google Scholar] [CrossRef]
- Kado, Y.; Inoue, T.; Ishikawa, K. Structure of hyperthermophilic β-glucosidase from Pyrococcus furiosus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 1473–1479. [Google Scholar] [CrossRef]
- Nakabayashi, M.; Kataoka, M.; Mishima, Y.; Maeno, Y.; Ishikawa, K. Structural analysis of β-glucosidase mutants derived from a hyperthermophilic tetrameric structure. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 877–888. [Google Scholar] [CrossRef]
- Kaushal, G.; Rai, A.K.; Singh, S.P. A novel β-glucosidase from a hot-spring metagenome shows elevated thermal stability and tolerance to glucose and ethanol. Enzyme Microb. Technol. 2021, 145, 109764. [Google Scholar] [CrossRef]
- Liew, K.J.; Lim, L.; Woo, H.Y.; Chan, K.G.; Shamsir, M.S.; Goh, K.M. Purification and characterization of a novel GH1 beta-glucosidase from Jeotgalibacillus malaysiensis. Int. J. Biol. Macromol. 2018, 115, 1094–1102. [Google Scholar] [CrossRef]
- Wu, J.; Geng, A.; Xie, R.; Wang, H.; Sun, J. Characterization of cold adapted and ethanol tolerant β-glucosidase from Bacillus cellulosilyticus and its application for directed hydrolysis of cellobiose to ethanol. Int. J. Biol. Macromol. 2018, 109, 872–879. [Google Scholar] [CrossRef]
- Crespim, E.; Zanphorlin, L.M.; de Souza, F.H.; Diogo, J.A.; Gazolla, A.C.; Machado, C.B.; Figueiredo, F.; Sousa, A.S.; Nóbrega, F.; Pellizari, V.H.; et al. A novel cold-adapted and glucose-tolerant GH1 β-glucosidase from Exiguobacterium antarcticum B7. Int. J. Biol. Macromol. 2016, 82, 375–380. [Google Scholar] [CrossRef]
- Sinha, S.K.; Datta, S. β-Glucosidase from the hyperthermophilic archaeon Thermococcus sp. is a salt-tolerant enzyme that is stabilized by its reaction product glucose. Appl. Microbiol. Biotechnol. 2016, 100, 8399–8409. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Sukumaran, R.K.; Larroche, C.; Pandey, A. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour. Technol. 2013, 127, 500–507. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Y.; Fan, X.J.; Liu, Y.H. Molecular cloning and characterization of a novel β-glucosidase with high hydrolyzing ability for soybean isoflavone glycosides and glucose-tolerance from soil metagenomic library. Bioresour. Technol. 2012, 123, 15–22. [Google Scholar] [CrossRef]
- Li, H.; Yi, S.; Bell, E.W.; Huang, Q.; Zhao, X. Recombinant Penicillium oxalicum 16 β-Glucosidase 1 Displays Comprehensive Inhibitory Resistance to Several Lignocellulose Pretreatment Products, Ethanol, and Salt. Appl. Biochem. Biotechnol. 2020, 191, 772–784. [Google Scholar] [CrossRef]


| Reagent | Relativity Activity (%) | Reagent | Relativity Activity (%) |
|---|---|---|---|
| 5 mM | 5 mM | ||
| Control a | 100.0 ± 5.72 | Ni2+ | 103.5 ± 7.35 |
| K+ | 116.7 ± 0.72 | Li+ | 95.6 ± 9.56 |
| Na+ | 114.0 ± 9.65 | Co2+ | 104.7 + 8.97 |
| Cu2+ | 116.9 ± 4.20 | Ag+ | ND |
| Fe2+ | 85.0 ± 5.32 | Cd2+ | 20.0 ± 1.03 |
| Fe3+ | 121.7 ± 1.06 | SDS | ND |
| Mg2+ | 124.4 ± 6.35 | EDTA | 87.2 ± 5.31 |
| Zn2+ | 103.5 ± 9.42 | methanol | 89.1 ± 0.35 |
| Ca2+ | 118.6 ± 0.49 | isopropanol | 107.6 ± 1.6 |
| Pb2+ | 72.4 ± 4.24 | β-mercaptoethanol | 64.1 ± 3.96 |
| Substrate | Specific Activity (U mg−1) |
|---|---|
| Disaccharide | |
| Cellobiose | ND |
| Sophorise | 1.11 ± 0.22 |
| Gentiobiose | 14.93 ± 0.90 |
| Aryl β-glycoside | |
| pNPG | 357.62 ± 1.85 |
| pNPX | 2.93 ± 0.20 |
| pNPGal | ND |
| pNPAf | 2.90 ± 0.07 |
| pNPman | 1.78 ± 0.39 |
| oNPGal | ND |
| Enzyme | Specific Activity (U mg−1) | Km (mM) | Vmax (μmol min−1 mg−1) | Kcat (s−1) | Kcat/Km (s−1 mM−1) |
|---|---|---|---|---|---|
| BglAc (WT) | 357.62 | 3.41 | 474.0 | 417.9 | 122.7 |
| BglAcN (Mutant) | 52.44 | 8.58 | 287.87 | 259.66 | 30.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Li, Y.; Sun, X.; Zuo, D.; Wang, M.; Zheng, X.; Yu, P.; Shi, P. Characterization of a Novel Hyperthermophilic GH1 β-Glucosidase from Acidilobus sp. and Its Application in the Hydrolysis of Soybean Isoflavone Glycosides. Microorganisms 2024, 12, 533. https://doi.org/10.3390/microorganisms12030533
He J, Li Y, Sun X, Zuo D, Wang M, Zheng X, Yu P, Shi P. Characterization of a Novel Hyperthermophilic GH1 β-Glucosidase from Acidilobus sp. and Its Application in the Hydrolysis of Soybean Isoflavone Glycosides. Microorganisms. 2024; 12(3):533. https://doi.org/10.3390/microorganisms12030533
Chicago/Turabian StyleHe, Jinjian, Yuying Li, Xihang Sun, Dinghui Zuo, Mansheng Wang, Xia Zheng, Pinglian Yu, and Pengjun Shi. 2024. "Characterization of a Novel Hyperthermophilic GH1 β-Glucosidase from Acidilobus sp. and Its Application in the Hydrolysis of Soybean Isoflavone Glycosides" Microorganisms 12, no. 3: 533. https://doi.org/10.3390/microorganisms12030533










