Anti-Biofilm Strategies: A Focused Review on Innovative Approaches
Abstract
:1. Introduction
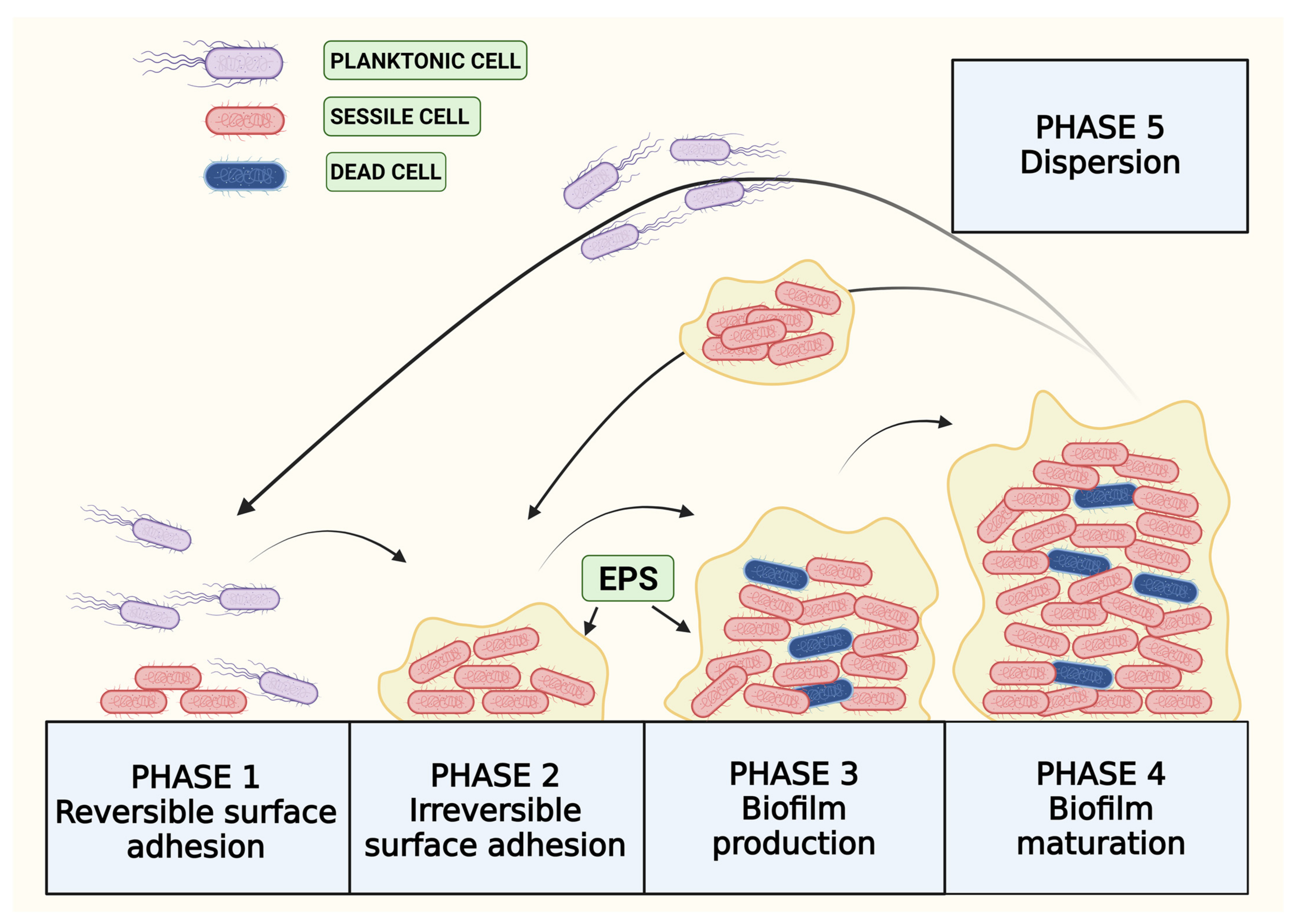
2. Life Cycle of Biofilm
2.1. Reversible Adhesion
2.2. Extracellular Polymeric Substances (EPSs)
2.3. Quorum Sensing in Biofilm Production
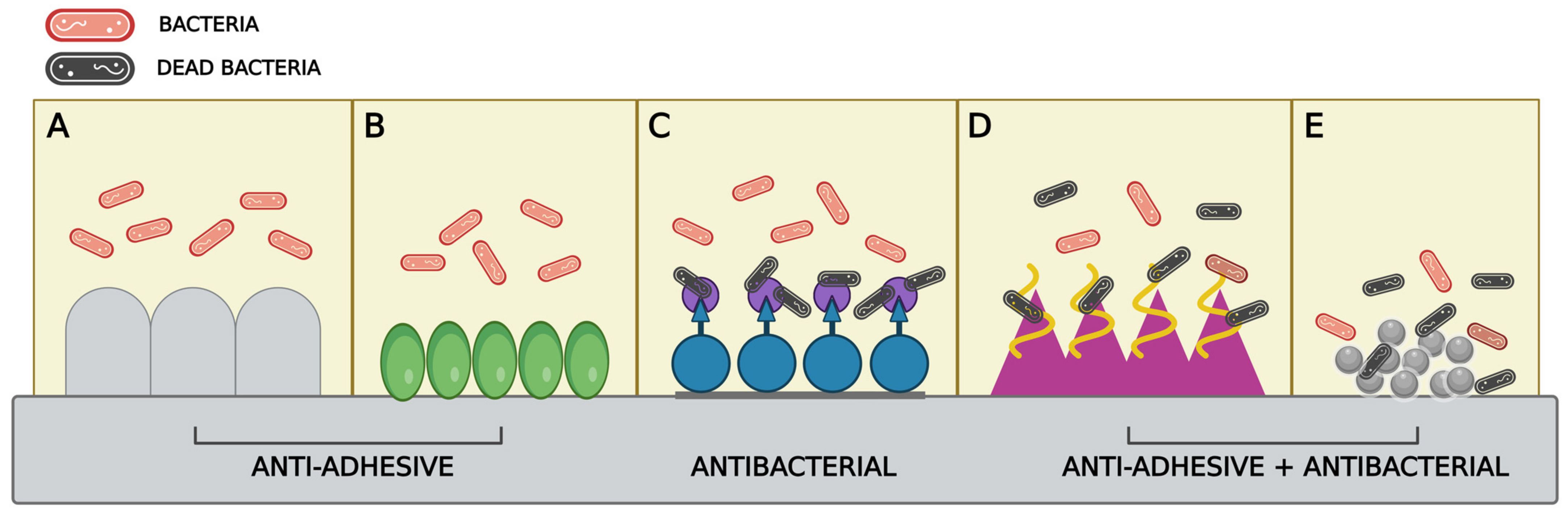
3. Anti-BF Strategies Acting on Bacterial Adhesion to Surfaces
3.1. Antiadhesive and Antibacterial Surface Modulation
3.2. Use of Nanoparticles (NPs) in Surface Modulation
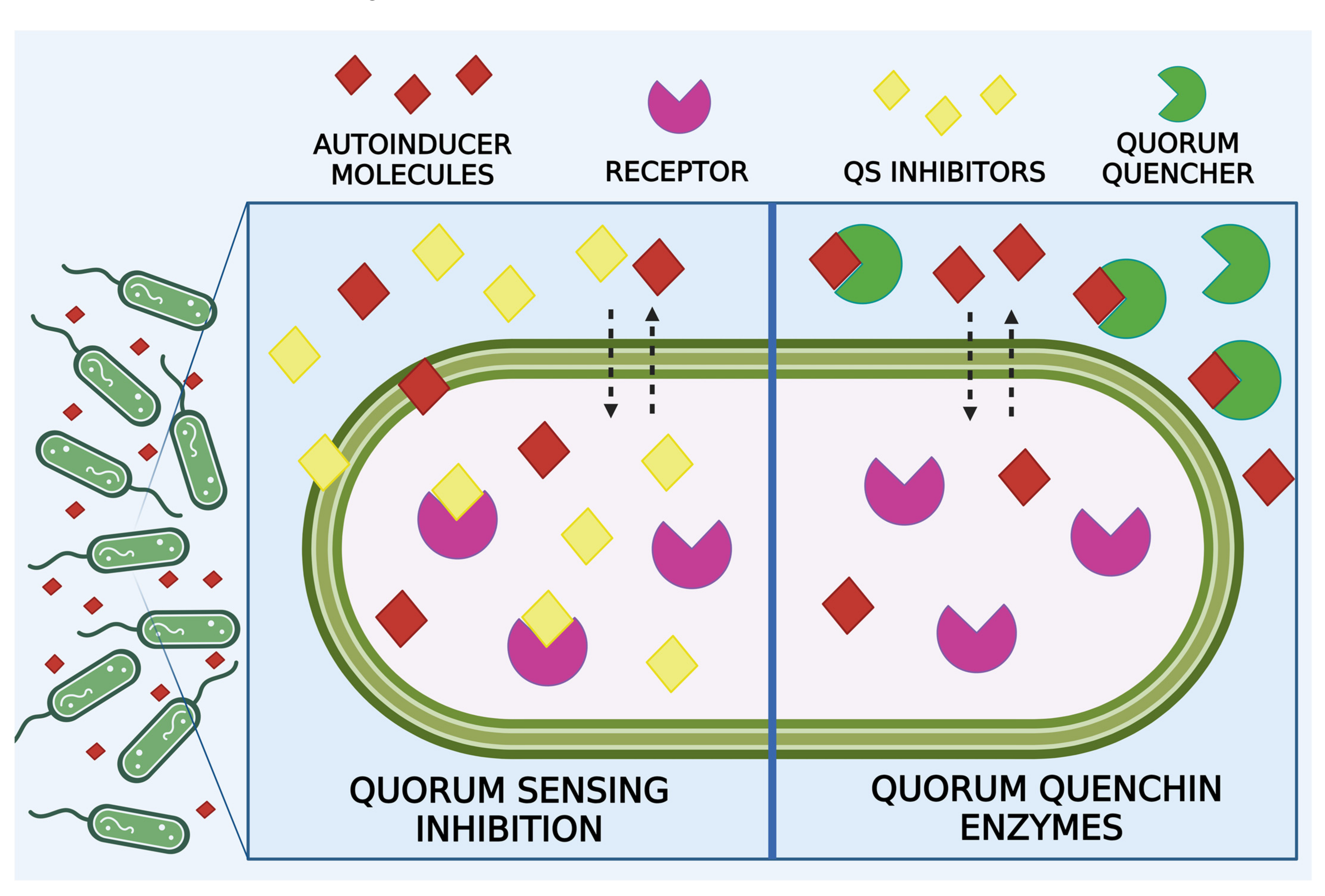
4. Anti-BF Strategies Acting on Quorum Sensing
4.1. Quorum Sensing Inhibitors
4.2. Metal Nanoparticles as QS Inhibitors
4.3. Quorum Quenching Enzymes
5. Anti-BF Strategies Acting on EPS
5.1. Enzymes That Act on EPS Components
5.2. EPS Disruption Mediated by Nanoparticles
5.3. Electrochemical Method to Deteriorate EPS
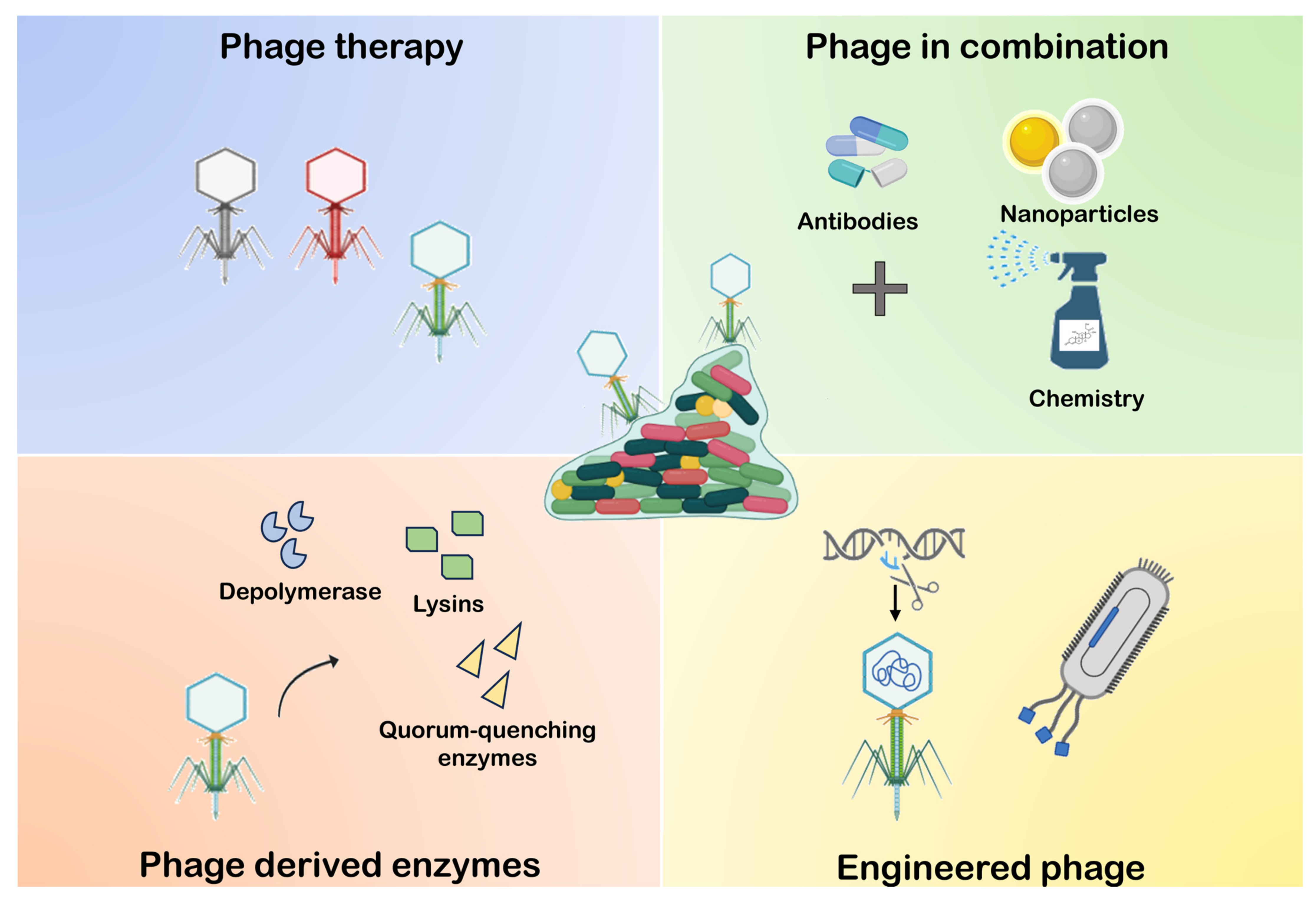
6. Anti-BF Strategies Mediated by Phage
6.1. Phage Therapy
6.2. Phage-Derived Enzyme
6.3. Phage Combination with Other Elements
6.4. Phage Engineering
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Tian, X.; Koseki, S.; Chen, S.; Ye, X.; Ding, T. Novel Antibacterial Modalities against Methicillin Resistant Staphylococcus aureus Derived from Plants. Crit. Rev. Food Sci. Nutr. 2019, 59, S153–S161. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking Biofilm Formation and Development: Recent Progress in In Vitro and In Vivo Biofilm Models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, J.; Liu, B.; Wang, Y. Advances in Research on Signal Molecules Regulating Biofilms. World J. Microbiol. Biotechnol. 2019, 35, 130. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.G.; Săndulescu, O. Communication Is the Key: Biofilms, Quorum Sensing, Formation and Prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Fang, K.; Park, O.-J.; Hong, S.H. Controlling Biofilms Using Synthetic Biology Approaches. Biotechnol. Adv. 2020, 40, 107518. [Google Scholar] [CrossRef]
- Anju, V.T.; Busi, S.; Imchen, M.; Kumavath, R.; Mohan, M.S.; Salim, S.A.; Subhaswaraj, P.; Dyavaiah, M. Polymicrobial Infections and Biofilms: Clinical Significance and Eradication Strategies. Antibiotics 2022, 11, 1731. [Google Scholar] [CrossRef]
- Tian, F.; Li, J.; Nazir, A.; Tong, Y. Bacteriophage—A Promising Alternative Measure for Bacterial Biofilm Control. Infect. Drug Resist. 2021, 14, 205–217. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Erschen, S.; Krause, R.; Müller, H.; Cernava, T.; Berg, G. Enhanced Survival of Multi-Species Biofilms under Stress Is Promoted by Low-Abundant but Antimicrobial-Resistant Keystone Species. J. Hazard. Mater. 2022, 422, 126836. [Google Scholar] [CrossRef]
- Khan, A.A.; Manzoor, K.N.; Sultan, A.; Saeed, M.; Rafique, M.; Noushad, S.; Talib, A.; Rentschler, S.; Deigner, H.-P. Pulling the Brakes on Fast and Furious Multiple Drug-Resistant (MDR) Bacteria. Int. J. Mol. Sci. 2021, 22, 859. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- CDC. The Biggest Antibiotic-Resistant Threats in the U.S. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 5 April 2022).
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by Bacterial Human Pathogens: Clinical Relevance—Development, Composition and Regulation—Therapeutical Strategies. Microb. Cell 2021, 8, 28. [Google Scholar] [CrossRef]
- Minkiewicz-Zochniak, A.; Jarzynka, S.; Iwańska, A.; Strom, K.; Iwańczyk, B.; Bartel, M.; Mazur, M.; Pietruczuk-Padzik, A.; Konieczna, M.; Augustynowicz-Kopeć, E.; et al. Biofilm Formation on Dental Implant Biomaterials by Staphylococcus aureus Strains Isolated from Patients with Cystic Fibrosis. Materials 2021, 14, 2030. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Adv. Sci. 2022, 9, 2203291. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2017, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Delik, E.; Eroğlu, B.; Çolak, Ç.Y.; Özçelik, A.T.; Tefon Öztürk, B.E. Alterations of Growth, Biofilm-Forming, and Gene Expression of Bordetella Pertussis by Antibiotics at Sub-Minimum Inhibitory Concentrations. Res. Microbiol. 2023, 174, 104058. [Google Scholar] [CrossRef]
- Rather, M.A.; Mandal, M. Attenuation of Biofilm and Quorum Sensing Regulated Virulence Factors of an Opportunistic Pathogen Pseudomonas aeruginosa by Phytofabricated Silver Nanoparticles. Microb. Pathog. 2023, 185, 106433. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Zhao, C.; Zhang, J.; He, W. Autoinducer-2 Quorum Sensing Regulates Biofilm Formation and Chain Elongation Metabolic Pathways to Enhance Caproate Synthesis in Microbial Electrochemical System. Chemosphere 2023, 344, 140384. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Ding, H.; Xiao, K.; Huang, X. Quorum Sensing-Fe Metabolism Interplay Affects Biofouling on Reverse Osmosis Membrane: Evidences from Microbial Shift and Structure Alteration. Desalination 2023, 551, 116416. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, Drug Resistance and Alternatives to Conventional Approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic Dispersion of Biofilms: An Emerging Biocatalytic Avenue to Combat Biofilm-Mediated Microbial Infections. J. Biol. Chem. 2022, 298, 102352. [Google Scholar] [CrossRef] [PubMed]
- Silpe, J.E.; Duddy, O.P.; Bassler, B.L. Natural and Synthetic Inhibitors of a Phage-Encoded Quorum-Sensing Receptor Affect Phage–Host Dynamics in Mixed Bacterial Communities. Proc. Natl. Acad. Sci. USA 2022, 119, e2217813119. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Ivanova, A.; Hoyo, J.; Pérez-Rafael, S.; Tzanov, T. Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 7632. [Google Scholar] [CrossRef]
- Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef]
- Sheridan, M.; Winters, C.; Zamboni, F.; Collins, M.N. Biomaterials: Antimicrobial Surfaces in Biomedical Engineering and Healthcare. Curr. Opin. Biomed. Eng. 2022, 22, 100373. [Google Scholar] [CrossRef]
- Beaudoin, C.M.; Cox, Z.; Dundore, T.; Thomas, T.; Kim, J.; Pillivant, D. Effect of Bench Press Load Knowledge on Repetitions, Rating of Perceived Exertion, and Attentional Focus. J. Strength Cond. Res. 2018, 32, 514. [Google Scholar] [CrossRef]
- Lopes, S.P.; Azevedo, N.F.; Pereira, M.O. Quantitative Assessment of Individual Populations within Polymicrobial Biofilms. Sci. Rep. 2018, 8, 9494. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm Dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Kragh, K.N.; Hutchison, J.B.; Melaugh, G.; Rodesney, C.; Roberts, A.E.L.; Irie, Y.; Jensen, P.Ø.; Diggle, S.P.; Allen, R.J.; Gordon, V.; et al. Role of Multicellular Aggregates in Biofilm Formation. mBio 2016, 7, e00237-16. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry from Initial Bacterial Adhesion to Surface-Programmed Biofilm Growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial Adhesion at the Single-Cell Level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Tang, W.S.; Si, T.; Tang, J.X. Influence of Physical Effects on the Swarming Motility of Pseudomonas aeruginosa. Biophys. J. 2017, 112, 1462–1471. [Google Scholar] [CrossRef]
- Berne, C. Sticky Decisions: The Multilayered Regulation of Adhesin Production by Bacteria. PLoS Genet. 2023, 19, e1010648. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial Exo-Polysaccharides in Biofilms: Role in Antimicrobial Resistance and Treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental Factors That Shape Biofilm Formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Hu, X.; Zhang, X.; Wang, W.; Sun, J.; Su, Z.; Zhu, C. Strategies to Prevent, Curb and Eliminate Biofilm Formation Based on the Characteristics of Various Periods in One Biofilm Life Cycle. Front. Cell. Infect. Microbiol. 2022, 12, 1003033. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J.D.P.; Straub, H.; Pietsch, F.; Lemare, M.; Ahrens, C.H.; Schreiber, F.; Webb, J.S.; van der Mei, H.C.; Ren, Q. Role of the Flagellar Hook in the Structural Development and Antibiotic Tolerance of Pseudomonas aeruginosa Biofilms. ISME J. 2022, 16, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Tian, W.; Chen, K.; Lan, L.; Kan, J.; Shi, H. Flagella-Mediated Adhesion of Escherichia coli O157:H7 to Surface of Stainless Steel, Glass and Fresh Produces during Sublethal Injury and Recovery. Food Microbiol. 2024, 117, 104383. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm Patterns in Gram-Positive and Gram-Negative Bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, Invasion and Evasion: The Many Functions of the Surface Proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Schniederberend, M.; Williams, J.F.; Shine, E.; Shen, C.; Jain, R.; Emonet, T.; Kazmierczak, B.I. Modulation of Flagellar Rotation in Surface-Attached Bacteria: A Pathway for Rapid Surface-Sensing after Flagellar Attachment. PLoS Pathog. 2019, 15, e1008149. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The Influence of Surface Chemistry on the Kinetics and Thermodynamics of Bacterial Adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Viljoen, A. Binding Strength of Gram-Positive Bacterial Adhesins. Front. Microbiol. 2020, 11, 1457. [Google Scholar] [CrossRef]
- Bucher, T.; Oppenheimer-Shaanan, Y.; Savidor, A.; Bloom-Ackermann, Z.; Kolodkin-Gal, I. Disturbance of the Bacterial Cell Wall Specifically Interferes with Biofilm Formation. Env. Microbiol. Rep. 2015, 7, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rhman, S.H. Role of Pseudomonas aeruginosa Lipopolysaccharides in Modulation of Biofilm and Virulence Factors of Enterobacteriaceae. Ann. Microbiol. 2019, 69, 299–305. [Google Scholar] [CrossRef]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the Structure and Contribution towards Bacterial Resistance in Antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and Their Role on Diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef]
- Li, S.; Duan, G.; Xi, Y.; Chu, Y.; Li, F.; Ho, S.-H. Insights into the Role of Extracellular Polymeric Substances (EPS) in the Spread of Antibiotic Resistance Genes. Env. Pollut. 2023, 343, 123285. [Google Scholar] [CrossRef]
- Molina-Santiago, C.; de Vicente, A.; Romero, D. Bacterial Extracellular Matrix as a Natural Source of Biotechnologically Multivalent Materials. Comput. Struct. Biotechnol. J. 2021, 19, 2796–2805. [Google Scholar] [CrossRef]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef]
- Latasa, C.; Solano, C.; Penadés, J.R.; Lasa, I. Biofilm-Associated Proteins. Comptes. Rendus. Biol. 2006, 329, 849–857. [Google Scholar] [CrossRef]
- Valle, J.; Fang, X.; Lasa, I. Revisiting Bap Multidomain Protein: More than Sticking Bacteria Together. Front. Microbiol. 2020, 11, 613581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, J.; Gao, Y.; Sun, Y.; Dai, J.; Wu, Y.; Qu, D.; Ma, G.; Fang, X. Staphylococcus epidermidis Small Basic Protein (Sbp) Forms Amyloid Fibrils, Consistent with Its Function as a Scaffolding Protein in Biofilms. J. Biol. Chem. 2018, 293, 14296–14311. [Google Scholar] [CrossRef] [PubMed]
- Bleem, A.; Christiansen, G.; Madsen, D.J.; Maric, H.; Strømgaard, K.; Bryers, J.D.; Daggett, V.; Meyer, R.L.; Otzen, D.E. Protein Engineering Reveals Mechanisms of Functional Amyloid Formation in Pseudomonas aeruginosa Biofilms. J. Mol. Biol. 2018, 430, 3751–3763. [Google Scholar] [CrossRef]
- França, A.; Gaio, V.; Lopes, N.; Melo, L.D.R. Virulence Factors in Coagulase-Negative Staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Eisha, S.; Park, S.; Morris, A.J.; Martin, I. How Three Self-Secreted Biofilm Exopolysaccharides of Pseudomonas aeruginosa, Psl, Pel, and Alginate, Can Each Be Exploited for Antibiotic Adjuvant Effects in Cystic Fibrosis Lung Infection. Int. J. Mol. Sci. 2023, 24, 8709. [Google Scholar] [CrossRef] [PubMed]
- Cordisco, E.; Zanor, M.I.; Moreno, D.M.; Serra, D.O. Selective Inhibition of the Amyloid Matrix of Escherichia coli Biofilms by a Bifunctional Microbial Metabolite. NPJ Biofilms Microbiomes 2023, 9, 81. [Google Scholar] [CrossRef]
- Ray, V.A.; Hill, P.J.; Stover, C.K.; Roy, S.; Sen, C.K.; Yu, L.; Wozniak, D.J.; DiGiandomenico, A. Anti-Psl Targeting of Pseudomonas aeruginosa Biofilms for Neutrophil-Mediated Disruption. Sci. Rep. 2017, 7, 16065. [Google Scholar] [CrossRef]
- Soliman, C.; Walduck, A.K.; Yuriev, E.; Richards, J.S.; Cywes-Bentley, C.; Pier, G.B.; Ramsland, P.A. Structural Basis for Antibody Targeting of the Broadly Expressed Microbial Polysaccharide Poly-N-Acetylglucosamine. J. Biol. Chem. 2018, 293, 5079–5089. [Google Scholar] [CrossRef]
- DeFrancesco, A.S.; Masloboeva, N.; Syed, A.K.; DeLoughery, A.; Bradshaw, N.; Li, G.-W.; Gilmore, M.S.; Walker, S.; Losick, R. Genome-Wide Screen for Genes Involved in eDNA Release during Biofilm Formation by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2017, 114, E5969–E5978. [Google Scholar] [CrossRef]
- Lee, A.R.; Park, S.B.; Kim, S.W.; Jung, J.W.; Chun, J.H.; Kim, J.; Kim, Y.R.; Lazarte, J.M.S.; Jang, H.B.; Thompson, K.D.; et al. Membrane Vesicles from Antibiotic-resistant Staphylococcus aureus Transfer Antibiotic-Resistance to Antibiotic-Susceptible Escherichia coli. J. Appl. Microbiol. 2022, 132, 2746–2759. [Google Scholar] [CrossRef]
- Wang, W.; Chanda, W.; Zhong, M. The Relationship between Biofilm and Outer Membrane Vesicles: A Novel Therapy Overview. FEMS Microbiol. Lett. 2015, 362, fnv117. [Google Scholar] [CrossRef] [PubMed]
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. 2022, 86, e00032-22. [Google Scholar] [CrossRef]
- Henke, J.M.; Bassler, B.L. Three Parallel Quorum-Sensing Systems Regulate Gene Expression in Vibrio Harveyi. J. Bacteriol. 2004, 186, 6902–6914. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.A.; Pomianek, M.E.; Kraml, C.M.; Taylor, R.K.; Semmelhack, M.F.; Bassler, B.L. The Major Vibrio Cholerae Autoinducer and Its Role in Virulence Factor Production. Nature 2007, 450, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luo, Y. Bacterial Quorum-Sensing Systems and Their Role in Intestinal Bacteria-Host Crosstalk. Front. Microbiol. 2021, 12, 611413. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bacterial Quorum Sensing in Complex and Dynamically Changing Environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- McBrayer, D.N.; Cameron, C.D.; Tal-Gan, Y. Development and Utilization of Peptide-Based Quorum Sensing Modulators in Gram-Positive Bacteria. Org. Biomol. Chem. 2020, 18, 7273–7290. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum Sensing Signal–Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Loughran, A.J.; Atwood, D.N.; Anthony, A.C.; Harik, N.S.; Spencer, H.J.; Beenken, K.E.; Smeltzer, M.S. Impact of Individual Extracellular Proteases on Staphylococcus aureus Biofilm Formation in Diverse Clinical Isolates and Their Isogenic sarA Mutants. MicrobiologyOpen 2014, 3, 897–909. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef]
- Coquant, G.; Grill, J.-P.; Seksik, P. Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity. Front. Immunol. 2020, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Jo, A.R.; Jang, M.C.; Nam, J.; Choi, H.J.; Choi, G.-W.; Sung, H.Y.; Bae, H.; Ku, Y.-G.; Chi, Y.-T. Analysis of Two Quorum Sensing-Deficient Isolates of Pseudomonas aeruginosa. Microb. Pathog. 2018, 119, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Mathur, P.; Sharma, C.; Chaturvedi, P. Chapter 9—Quorum Quenching Strategies of Endophytic Bacteria: Role in Plant Protection. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 149–166. ISBN 978-0-323-91876-3. [Google Scholar]
- Wang, Y.; Wang, Y.; Sun, L.; Grenier, D.; Yi, L. The LuxS/AI-2 System of Streptococcus Suis. Appl. Microbiol. Biotechnol. 2018, 102, 7231–7238. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-P.; Sun, Y.-C. New Insights into the Non-Orthodox Two Component Rcs Phosphorelay System. Front. Microbiol. 2017, 8, 2014. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- The Two-Component Signal Transduction System and Its Regulation in Candida albicans. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8274445/ (accessed on 18 November 2023).
- Ghilini, F.; Pissinis, D.E.; Miñán, A.; Schilardi, P.L.; Diaz, C. How Functionalized Surfaces Can Inhibit Bacterial Adhesion and Viability. ACS Biomater. Sci. Eng. 2019, 5, 4920–4936. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Reis, A.C.D. Bacterial Adhesion to Biomaterials: What Regulates This Attachment? A Review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Parrino, B.; Cascioferro, S.; Diana, P. Recent Developments in the Inhibition of Bacterial Adhesion as Promising Anti-Virulence Strategy. Int. J. Mol. Sci. 2023, 24, 4872. [Google Scholar] [CrossRef]
- Chug, M.K.; Brisbois, E.J. Recent Developments in Multifunctional Antimicrobial Surfaces and Applications toward Advanced Nitric Oxide-Based Biomaterials. ACS Mater. Au 2022, 2, 525–551. [Google Scholar] [CrossRef]
- Alves, D.; Pereira, M.O. Bio-Inspired Coating Strategies for the Immobilization of Polymyxins to Generate Contact-Killing Surfaces. Macromol. Biosci. 2016, 16, 1450–1460. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic Materials for Antifouling Membrane Surface Construction. Acta Biomaterialia 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines 2018, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Kalan, L.; Bradu, A.; Turner, R.J. Silver Oxynitrate, an Unexplored Silver Compound with Antimicrobial and Antibiofilm Activity. Antimicrob. Agents Chemother. 2015, 59, 4031–4039. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.R.; Pepin, D.M.; Ul-Haq, I.; Miller, S.B.; Hay, M.E.; Precht, R.J. Targeting Biofilms of Multidrug-Resistant Bacteria with Silver Oxynitrate. Int. J. Antimicrob. Agents 2017, 49, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Kalan, L.; Gugala, N.; Bradu, A.; Turner, R.J. Silver Oxynitrate—An Efficacious Compound for the Prevention and Eradication of Dual-Species Biofilms. Biofouling 2017, 33, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Toh, Y.S. Anti-Biofilm Agents: Recent Breakthrough against Multi-Drug Resistant Staphylococcus Aureus. Pathog. Dis. 2014, 70, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, G.; Ivanova, K.; Ivanov, I.; Tzanov, T. Nanomaterials and Coatings for Managing Antibiotic-Resistant Biofilms. Antibiotics 2023, 12, 310. [Google Scholar] [CrossRef]
- Xiang, J.; Zhu, R.; Lang, S.; Yan, H.; Liu, G.; Peng, B. Mussel-Inspired Immobilization of Zwitterionic Silver Nanoparticles toward Antibacterial Cotton Gauze for Promoting Wound Healing. Chem. Eng. J. 2021, 409, 128291. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yang, Y.; Cheng, G.; Yang, Z. Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef]
- Winkelströter, L.K.; Gomes, B.C.; Thomaz, M.R.S.; Souza, V.M.; De Martinis, E.C.P. Lactobacillus sakei 1 and Its Bacteriocin Influence Adhesion of Listeria monocytogenes on Stainless Steel Surface. Food Control 2011, 22, 1404–1407. [Google Scholar] [CrossRef]
- Pimentel-Filho, N.D.J.; Martins, M.C.D.F.; Nogueira, G.B.; Mantovani, H.C.; Vanetti, M.C.D. Bovicin HC5 and Nisin Reduce Staphylococcus aureus Adhesion to Polystyrene and Change the Hydrophobicity Profile and Gibbs Free Energy of Adhesion. Int. J. Food Microbiol. 2014, 190, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-Biofilm Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Combatting Persisted and Biofilm Antimicrobial Resistant Bacterial by Using Nanoparticles. Z. Naturforsch. C J. Biosci. 2022, 77, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Agirre, J.; Moroz, O.; Meier, S.; Brask, J.; Munch, A.; Hoff, T.; Andersen, C.; Wilson, K.S.; Davies, G.J. The Structure of the AliC GH13 α-Amylase from Alicyclobacillus sp. Reveals the Accommodation of Starch Branching Points in the α-Amylase Family. Acta Crystallogr. D Struct. Biol. 2019, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abeleda, H.E.P.; Javier, A.P.; Murillo, A.Q.M.; Baculi, R.Q. Alpha-Amylase Conjugated Biogenic Silver Nanoparticles as Innovative Strategy against Biofilm-Forming Multidrug Resistant Bacteria. Biocatal. Agric. Biotechnol. 2020, 29, 101784. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Perelshtein, I.; Gedanken, A.; Todorova, K.; Milcheva, R.; Dimitrov, P.; Popova, T.; Tzanov, T. Sonochemically Engineered Nano-Enabled Zinc Oxide/Amylase Coatings Prevent the Occurrence of Catheter-Associated Urinary Tract Infections. Mater. Sci. Eng. C 2021, 131, 112518. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Li, P.; Zhu, Y.; Shi, L.; Yuan, J.; Shen, J. Mussel-Inspired Surface Functionalization of PET with Zwitterions and Silver Nanoparticles for the Dual-Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1788–1797. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Islam, T.; Rahaman, M.M.; Mia, M.N.; Ara, I.; Islam, M.T.; Alam Riaz, T.; Araújo, A.C.J.; de Lima Silva, J.M.F.; de Lacerda, B.C.G.V.; de Andrade, E.M.; et al. Therapeutic Perspectives of Metal Nanoformulations. Drugs Drug Candidates 2023, 2, 232–278. [Google Scholar] [CrossRef]
- Chowdhury, N.; Kwan, B.W.; Wood, T.K. Persistence Increases in the Absence of the Alarmone Guanosine Tetraphosphate by Reducing Cell Growth. Sci. Rep. 2016, 6, 20519. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.R.; Slate, A.J.; Ryder, S.F.; Akram, M.; Iruzubieta, C.J.C.; Whitehead, K.A. Ionic Gold Demonstrates Antimicrobial Activity against Pseudomonas aeruginosa Strains Due to Cellular Ultrastructure Damage. Arch. Microbiol. 2021, 203, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Kang, M.-G.; Jo, D.-M.; Chandika, P.; Jung, W.-K.; Kang, H.W.; Kim, Y.-M. Phloroglucinol-Gold and -Zinc Oxide Nanoparticles: Antibiofilm and Antivirulence Activities towards Pseudomonasaeruginosa PAO1. Mar. Drugs 2021, 19, 601. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, Q.; Chen, L.; Liu, H.; Ding, M.; Dong, H.; Mou, Y. Therapeutic Applications of Antimicrobial Silver-Based Biomaterials in Dentistry. Int. J. Nanomed. 2022, 17, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria Viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic Antibacterial Effects of Curcumin Modified Silver Nanoparticles through ROS-Mediated Pathways. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 255–263. [Google Scholar] [CrossRef]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected Insights into Antibacterial Activity of Zinc Oxide Nanoparticles against Methicillin Resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Li, J.; Qin, G. New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition. Toxins 2023, 15, 570. [Google Scholar] [CrossRef]
- Raju, D.V.; Nagarajan, A.; Pandit, S.; Nag, M.; Lahiri, D.; Upadhye, V. Effect of Bacterial Quorum Sensing and Mechanism of Antimicrobial Resistance. Biocatal. Agric. Biotechnol. 2022, 43, 102409. [Google Scholar] [CrossRef]
- Vashistha, A.; Sharma, N.; Nanaji, Y.; Kumar, D.; Singh, G.; Barnwal, R.P.; Yadav, A.K. Quorum Sensing Inhibitors as Therapeutics: Bacterial Biofilm Inhibition. Bioorganic Chem. 2023, 136, 106551. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum Quenching: Role in Nature and Applied Developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Niu, C.; Afre, S.; Gilbert, E.S. Subinhibitory Concentrations of Cinnamaldehyde Interfere with Quorum Sensing. Lett. Appl. Microbiol. 2006, 43, 489–494. [Google Scholar] [CrossRef]
- Topa, S.H.; Palombo, E.A.; Kingshott, P.; Blackall, L.L. Activity of Cinnamaldehyde on Quorum Sensing and Biofilm Susceptibility to Antibiotics in Pseudomonas aeruginosa. Microorganisms 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, S.; Yang, Q.; Liu, Y.; Yang, Y.; Xu, N.; Ai, X.; Dong, J. Cinnamaldehyde Decreases the Pathogenesis of Aeromonas hydrophila by Inhibiting Quorum Sensing and Biofilm Formation. Fishes 2023, 8, 122. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus. Antibiotics 2022, 11, 1403. [Google Scholar] [CrossRef]
- Subhaswaraj, P.; Barik, S.; Macha, C.; Chiranjeevi, P.V.; Siddhardha, B. Anti Quorum Sensing and Anti Biofilm Efficacy of Cinnamaldehyde Encapsulated Chitosan Nanoparticles against Pseudomonas aeruginosa PAO1. LWT 2018, 97, 752–759. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Development of Gold Nanoparticles Coated with Silica Containing the Antibiofilm Drug Cinnamaldehyde and Their Effects on Pathogenic Bacteria. IJN 2017, 12, 2813–2828. [Google Scholar] [CrossRef]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.-D. Anti-Quorum Sensing Activity of Psidium guajava L. Flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol. Immunol. 2014, 58, 286–293. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids Suppress Pseudomonas aeruginosa Virulence through Allosteric Inhibition of Quorum-Sensing Receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Roshni, P.S.; Alexpandi, R.; Abirami, G.; Durgadevi, R.; Cai, Y.; Kumar, P.; Ravi, A.V. Hesperidin Methyl Chalcone, a Citrus Flavonoid, Inhibits Aeromonas hydrophila Infection Mediated by Quorum Sensing. Microb. Pathog. 2023, 177, 106029. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Rajamuthu, T.P.; Sarkar, A.; Natrajan, P.; Krishnan, N.; Sakthivelu, M.; Velusamy, P.; Ramasamy, P.; Gopinath, S.C.B. N-Acyl-Homoserine Lactone Mediated Virulence Factor(s) of Pseudomonas aeruginosa Inhibited by Flavonoids and Isoflavonoids. Process. Biochem. 2022, 116, 84–93. [Google Scholar] [CrossRef]
- Omwenga, E.O.; Hensel, A.; Shitandi, A.; Goycoolea, F.M. Chitosan Nanoencapsulation of Flavonoids Enhances Their Quorum Sensing and Biofilm Formation Inhibitory Activities against an E. coli Top 10 Biosensor. Colloids Surf. B Biointerfaces 2018, 164, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-T.; Hadinoto, K. A Potential Quorum-Sensing Inhibitor for Bronchiectasis Therapy: Quercetin-Chitosan Nanoparticle Complex Exhibiting Superior Inhibition of Biofilm Formation and Swimming Motility of Pseudomonas aeruginosa to the Native Quercetin. Int. J. Mol. Sci. 2021, 22, 1541. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.I.D.; Judan Cruz, K.G. Ethnobotanical Biosynthesis of Gold Nanoparticles and Its Downregulation of Quorum Sensing-Linked AhyR Gene in Aeromonas hydrophila. SN Appl. Sci. 2020, 2, 570. [Google Scholar] [CrossRef]
- Ilk, S.; Tan, G.; Emül, E.; Sağlam, N. Investigation the Potential Use of Silver Nanoparticles Synthesized by Propolis Extract as N-Acyl-Homoserine Lactone-Mediated Quorum Sensing Systems Inhibitor. Turk. J. Med. Sci. 2020, 50, 1147–1156. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Ha, A.J.; Park, S.H.; Ha, S.-D. Antibacterial and Antibiofilm Mechanism of Eugenol against Antibiotic Resistance Vibrio Parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef]
- Fekrirad, Z.; Gattali, B.; Kashef, N. Quorum Sensing-Regulated Functions of Serratia Marcescens Are Reduced by Eugenol. Iran. J. Microbiol. 2020, 12, 451–459. [Google Scholar] [CrossRef]
- Nabawy, A.; Makabenta, J.M.; Schmidt-Malan, S.; Park, J.; Li, C.-H.; Huang, R.; Fedeli, S.; Chattopadhyay, A.N.; Patel, R.; Rotello, V.M. Dual Antimicrobial-Loaded Biodegradable Nanoemulsions for Synergistic Treatment of Wound Biofilms. J. Control. Release 2022, 347, 379–388. [Google Scholar] [CrossRef]
- Sethuram, L.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Eugenol Micro-Emulsion Reinforced with Silver Nanocomposite Electrospun Mats for Wound Dressing Strategies. Mater. Adv. 2021, 2, 2971–2988. [Google Scholar] [CrossRef]
- Lou, Z.; Letsididi, K.S.; Yu, F.; Pei, Z.; Wang, H.; Letsididi, R. Inhibitive Effect of Eugenol and Its Nanoemulsion on Quorum Sensing–Mediated Virulence Factors and Biofilm Formation by Pseudomonas aeruginosa. J. Food Prot. 2019, 82, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-Containing Essential Oils Loaded onto Chitosan/Polyvinyl Alcohol Blended Films and Their Ability to Eradicate Staphylococcus aureus or Pseudomonas aeruginosa from Infected Microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Sofer, D.; Gilboa-Garber, N.; Belz, A.; Garber, N.C. “Subinhibitory” Erythromycin Represses Production of Pseudomonas aeruginosa Lectins, Autoinducer and Virulence Factors. Chemotherapy 1999, 45, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Tateda, K.; Comte, R.; Pechere, J.-C.; Köhler, T.; Yamaguchi, K.; Van Delden, C. Azithromycin Inhibits Quorum Sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2001, 45, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Henkel, M.; Schmidberger, A.; Kühnert, C.; Beuker, J.; Bernard, T.; Schwartz, T.; Syldatk, C.; Hausmann, R. Kinetic Modeling of the Time Course of N-Butyryl-Homoserine Lactone Concentration during Batch Cultivations of Pseudomonas aeruginosa PAO1. Appl. Microbiol. Biotechnol. 2013, 97, 7607–7616. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, N.; Huang, B.; Cai, R.; Wu, B.; Shunmei, E.; Fang, C.; Chen, C. Mechanism of Azithromycin Inhibition of HSL Synthesis in Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 24299. [Google Scholar] [CrossRef]
- El-Mowafy, S.A.; Abd El Galil, K.H.; Habib, E.-S.E.; Shaaban, M.I. Quorum Sensing Inhibitory Activity of Sub-Inhibitory Concentrations of β-Lactams. Afr. Health Sci. 2017, 17, 199–207. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Chai, M.; Li, X.; Deng, Y.; Jin, Q.; Ji, J. Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management. ACS Nano 2020, 14, 5686–5699. [Google Scholar] [CrossRef]
- Kłodzińska, S.N.; Wan, F.; Jumaa, H.; Sternberg, C.; Rades, T.; Nielsen, H.M. Utilizing Nanoparticles for Improving Anti-Biofilm Effects of Azithromycin: A Head-to-Head Comparison of Modified Hyaluronic Acid Nanogels and Coated Poly (Lactic-Co-Glycolic Acid) Nanoparticles. J. Colloid Interface Sci. 2019, 555, 595–606. [Google Scholar] [CrossRef]
- Jiang, K.; Yan, X.; Yu, J.; Xiao, Z.; Wu, H.; Zhao, M.; Yue, Y.; Zhou, X.; Xiao, J.; Lin, F. Design, Synthesis, and Biological Evaluation of 3-Amino-2-Oxazolidinone Derivatives as Potent Quorum-Sensing Inhibitors of Pseudomonas aeruginosa PAO1. Eur. J. Med. Chem. 2020, 194, 112252. [Google Scholar] [CrossRef] [PubMed]
- Miller, K. Bacterial Communication and Its Role as a Target for Nanoparticle-Based Antimicrobial Therapy. Ph.D. Thesis, University of South Carolina, Columbia, SC, USA, 2015. [Google Scholar]
- Franco, D.; Zagami, R.; De Plano, L.M.; Burduja, N.; Guglielmino, S.P.P.; Scolaro, L.M.; Mazzaglia, A. Antimicrobial and Antibiofilm Photodynamic Action of Photosensitizing Nanoassemblies Based on Sulfobutylether-β-Cyclodextrin. Molecules 2023, 28, 2493. [Google Scholar] [CrossRef] [PubMed]
- Thanh Nguyen, H.; Goycoolea, F.M. Chitosan/Cyclodextrin/TPP Nanoparticles Loaded with Quercetin as Novel Bacterial Quorum Sensing Inhibitors. Molecules 2017, 22, 1975. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Gómez-Gómez, B.; Arregui, L.; Serrano, S.; Santos, A.; Pérez-Corona, T.; Madrid, Y. Unravelling Mechanisms of Bacterial Quorum Sensing Disruption by Metal-Based Nanoparticles. Sci. Total Environ. 2019, 696, 133869. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Vigneshwari, L.; Rajavel, T.; Durgadevi, R.; Kannappan, A.; Balamurugan, K.; Pandima Devi, K.; Veera Ravi, A. Biogenic Synthesis of Silver Nanoparticles Using Piper Betle Aqueous Extract and Evaluation of Its Anti-Quorum Sensing and Antibiofilm Potential against Uropathogens with Cytotoxic Effects: An in Vitro and in Vivo Approach. Env. Sci. Pollut. Res. 2018, 25, 10538–10554. [Google Scholar] [CrossRef]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm Inhibition and Anti-Quorum Sensing Activity of Phytosynthesized Silver Nanoparticles against the Nosocomial Pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef]
- Kumar, S.; Paliya, B.S.; Singh, B.N. Superior Inhibition of Virulence and Biofilm Formation of Pseudomonas aeruginosa PAO1 by Phyto-Synthesized Silver Nanoparticles through Anti-Quorum Sensing Activity. Microb. Pathog. 2022, 170, 105678. [Google Scholar] [CrossRef]
- Saeki, E.K.; Martins, H.M.; de Camargo, L.C.; Anversa, L.; Tavares, E.R.; Yamada-Ogatta, S.F.; Lioni, L.M.Y.; Kobayashi, R.K.T.; Nakazato, G. Effect of Biogenic Silver Nanoparticles on the Quorum-Sensing System of Pseudomonas aeruginosa PAO1 and PA14. Microorganisms 2022, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, S.L.; Shaaban, M.I. Inhibition of Quorum Sensing and Virulence Factors of Pseudomonas aeruginosa by Biologically Synthesized Gold and Selenium Nanoparticles. Antibiotics 2021, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Alotaibi, S.H. Biofabrication of Gold Nanoparticles Using Capsicum Annuum Extract and Its Antiquorum Sensing and Antibiofilm Activity against Bacterial Pathogens. ACS Omega 2021, 6, 16670–16682. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, B.; Arregui, L.; Serrano, S.; Santos, A.; Pérez-Corona, T.; Madrid, Y. Selenium and Tellurium-Based Nanoparticles as Interfering Factors in Quorum Sensing-Regulated Processes: Violacein Production and Bacterial Biofilm Formation. Metallomics 2019, 11, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Rajivgandhi, G.N.; Quero, F.; Li, W.-J. Anti-Quorum Sensing and Anti-Biofilm Activity of Nickel Oxide Nanoparticles against Pseudomonas aeruginosa. J. Environ. Chem. Eng. 2020, 8, 104533. [Google Scholar] [CrossRef]
- Calabrese, G.; Franco, D.; Petralia, S.; Monforte, F.; Condorelli, G.G.; Squarzoni, S.; Traina, F.; Conoci, S. Dual-Functional Nano-Functionalized Titanium Scaffolds to Inhibit Bacterial Growth and Enhance Osteointegration. Nanomaterials 2021, 11, 2634. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; De Luca, G.; Franco, D.; Morganti, D.; Rizzo, M.G.; Bonavita, A.; Neri, G.; Fazio, E.; Neri, F.; Fazio, B.; et al. Structural and Antibacterial Studies of Novel ZnO and ZnxMn(1-x)O Nanostructured Titanium Scaffolds for Biomedical Applications. Biomater. Adv. 2023, 145, 213193. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Husain, F.M.; Zia, Q.; Ahmad, E.; Jamal, A.; Alaidarous, M.; Banawas, S.; Alam, M.D.M.; Alshehri, B.A.; Jameel, M.; et al. Anti-Quorum Sensing and Anti-Biofilm Activity of Zinc Oxide Nanospikes. ACS Omega 2020, 5, 32203–32215. [Google Scholar] [CrossRef]
- Naik, K.; Kowshik, M. Anti-Quorum Sensing Activity of AgCl-TiO2 Nanoparticles with Potential Use as Active Food Packaging Material. J. Appl. Microbiol. 2014, 117, 972–983. [Google Scholar] [CrossRef]
- Barnaby, R.; Koeppen, K.; Stanton, B.A. Cyclodextrins Reduce the Ability of Pseudomonas aeruginosa Outer-Membrane Vesicles to Reduce CFTR Cl−Secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L206–L215. [Google Scholar] [CrossRef]
- Ziegler, E.W.; Brown, A.B.; Nesnas, N.; Chouinard, C.D.; Mehta, A.K.; Palmer, A.G. β-Cyclodextrin Encapsulation of Synthetic AHLs: Drug Delivery Implications and Quorum-Quenching Exploits. ChemBioChem 2021, 22, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Molnár, M.; Fenyvesi, É.; Berkl, Z.; Németh, I.; Fekete-Kertész, I.; Márton, R.; Vaszita, E.; Varga, E.; Ujj, D.; Szente, L. Cyclodextrin-Mediated Quorum Quenching in the Aliivibrio fischeri Bioluminescence Model System—Modulation of Bacterial Communication. Int. J. Pharm. 2021, 594, 120150. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, M.; Yi, G.; Liao, L.; Cheng, Q.; Zhu, J.; Zhang, B.; Wang, Y.; Chen, Y.; Zeng, M. Screening Strategies for Quorum Sensing Inhibitors in Combating Bacterial Infections. J. Pharm. Anal. 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Weng, L.-X.; Dong, Y.-H.; Zhang, L.-H. Specificity and Enzyme Kinetics of the Quorum-Quenching N-Acyl Homoserine Lactone Lactonase (AHL-Lactonase). J. Biol. Chem. 2004, 279, 13645–13651. [Google Scholar] [CrossRef] [PubMed]
- Kusada, H.; Zhang, Y.; Tamaki, H.; Kimura, N.; Kamagata, Y. Novel N-Acyl Homoserine Lactone-Degrading Bacteria Isolated from Penicillin-Contaminated Environments and Their Quorum-Quenching Activities. Front. Microbiol. 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Torabi Delshad, S.; Soltanian, S.; Sharifiyazdi, H.; Haghkhah, M.; Bossier, P. Identification of N-Acyl Homoserine Lactone-Degrading Bacteria Isolated from Rainbow Trout (Oncorhynchus mykiss). J. Appl. Microbiol. 2018, 125, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Saha, D.; Bhuyan, S.; Jha, A.N.; Mandal, M. Quorum Quenching: A Drug Discovery Approach Against Pseudomonas aeruginosa. Microbiol. Res. 2022, 264, 127173. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Kannappan, A.; Thiyagarajan, S.; Srinivasan, R.; Jeyapragash, D.; Paul, J.B.J.; Velmurugan, P.; Ravi, A.V. AHL-Lactonase Producing Psychrobacter SP. From Palk Bay Sediment Mitigates Quorum Sensing-Mediated Virulence Production in Gram Negative Bacterial Pathogens. Front. Microbiol. 2021, 12, 634593. [Google Scholar] [CrossRef]
- Rémy, B.; Plener, L.; Decloquement, P.; Armstrong, N.; Elias, M.; Daudé, D.; Chabrière, É. Lactonase Specificity Is Key to Quorum Quenching in Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 762. [Google Scholar] [CrossRef]
- Khalid, S.J.; Ain, Q.; Khan, S.J.; Jalil, A.; Siddiqui, M.F.; Ahmad, T.; Badshah, M.; Adnan, F. Targeting Acyl Homoserine Lactones (AHLs) by the Quorum Quenching Bacterial Strains to Control Biofilm Formation in Pseudomonas aeruginosa. Saudi J. Biol. Sci. 2022, 29, 1673–1682. [Google Scholar] [CrossRef]
- Gupta, K.; Chhibber, S. Biofunctionalization of Silver Nanoparticles with Lactonase Leads to Altered Antimicrobial and Cytotoxic Properties. Front. Mol. Biosci. 2019, 6, 450649. [Google Scholar] [CrossRef] [PubMed]
- Vinoj, G.; Pati, R.; Sonawane, A.; Vaseeharan, B. In Vitro Cytotoxic Effects of Gold Nanoparticles Coated with Functional Acyl Homoserine Lactone Lactonase Protein from Bacillus licheniformis and Their Antibiofilm Activity against Proteus Species. Antimicrob. Agents Chemother. 2015, 59, 763–771. [Google Scholar] [CrossRef]
- Vogel, J.; Jansen, L.; Setroikromo, R.; Cavallo, F.M.; van Dijl, J.M.; Quax, W.J. Fighting Acinetobacter baumannii Infections with the Acylase PvdQ. Microbes Infect. 2022, 24, 104951. [Google Scholar] [CrossRef]
- De Celis, M.; Serrano-Aguirre, L.; Belda, I.; Liébana-García, R.; Arroyo, M.; Marquina, D.; de la Mata, I.; Santos, A. Acylase Enzymes Disrupting Quorum Sensing Alter the Transcriptome and Phenotype of Pseudomonas aeruginosa, and the Composition of Bacterial Biofilms from Wastewater Treatment Plants. Sci. Total Environ. 2021, 799, 149401. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, Q.; Dong, L.; Wu, W.; Liang, Z.; Dong, Z.; Ye, H.; Liao, L.; Zhang, L.-H. A Bacterial Isolate Capable of Quenching Both Diffusible Signal Factor- and N-Acylhomoserine Lactone-Family Quorum Sensing Signals Shows Much Enhanced Biocontrol Potencies. J. Agric. Food Chem. 2022, 70, 7716–7726. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Ivanova, K.; Tied, A.; Heinze, T.; Tzanov, T. Layer-By-Layer Coating of Aminocellulose and Quorum Quenching Acylase on Silver Nanoparticles Synergistically Eradicate Bacteria and Their Biofilms. Adv. Funct. Mater. 2020, 30, 2001284. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, L.; Li, Q. A Bioactive Pol (Vinylidene Fluoride)/Graphene Oxide@acylase Nanohybrid Membrane: Enhanced Anti-Biofouling Based on Quorum Quenching. J. Membr. Sci. 2018, 547, 110–122. [Google Scholar] [CrossRef]
- Grover, N.; Plaks, J.G.; Summers, S.R.; Chado, G.R.; Schurr, M.J.; Kaar, J.L. Acylase-Containing Polyurethane Coatings with Anti-Biofilm Activity. Biotechnol. Bioeng. 2016, 113, 2535–2543. [Google Scholar] [CrossRef]
- De Lamo Marin, S.; Xu, Y.; Meijler, M.M.; Janda, K.D. Antibody Catalyzed Hydrolysis of a Quorum Sensing Signal Found in Gram-Negative Bacteria. Bioorganic Med. Chem. Lett. 2007, 17, 1549–1552. [Google Scholar] [CrossRef]
- Kaufmann, G.F.; Park, J.; Mee, J.M.; Ulevitch, R.J.; Janda, K.D. The Quorum Quenching Antibody RS2-1G9 Protects Macrophages from the Cytotoxic Effects of the Pseudomonas aeruginosa Quorum Sensing Signalling Molecule N-3-Oxo-Dodecanoyl-Homoserine Lactone. Mol. Immunol. 2008, 45, 2710–2714. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Wolter, D.J.; Mishra, M.; Hayden, H.S.; Radey, M.C.; Merrihew, G.; MacCoss, M.J.; Burns, J.; Wozniak, D.J.; Parsek, M.R.; et al. Staphylococcus aureus Protein a Mediates Interspecies Interactions at the Cell Surface of Pseudomonas aeruginosa. mBio 2016, 7, e00538-16. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Velliyagounder, K.; Ragunath, C.; Rohde, H.; Mack, D.; Knobloch, J.K.-M.; Ramasubbu, N. Genes Involved in the Synthesis and Degradation of Matrix Polysaccharide in Actinobacillus Actinomycetemcomitans and Actinobacillus Pleuropneumoniae Biofilms. J. Bacteriol. 2004, 186, 8213–8220. [Google Scholar] [CrossRef] [PubMed]
- Chaignon, P.; Sadovskaya, I.; Ragunah, C.; Ramasubbu, N.; Kaplan, J.B.; Jabbouri, S. Susceptibility of Staphylococcal Biofilms to Enzymatic Treatments Depends on Their Chemical Composition. Appl. Microbiol. Biotechnol. 2007, 75, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Dobrynina, O.Y.; Bolshakova, T.N.; Umyarov, A.M.; Boksha, I.S.; Lavrova, N.V.; Grishin, A.V.; Lyashchuk, A.M.; Galushkina, Z.M.; Avetisian, L.R.; Chernukha, M.Y.; et al. Disruption of Bacterial Biofilms Using Recombinant Dispersin B. Microbiology 2015, 84, 498–501. [Google Scholar] [CrossRef]
- Chen, K.-J.; Lee, C.-K. Twofold Enhanced Dispersin B Activity by N-Terminal Fusion to Silver-Binding Peptide for Biofilm Eradication. Int. J. Biol. Macromol. 2018, 118, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, E.; Vighetto, C.; Di Martino, P.; Larreta Garde, V.; Seyer, D. Synergistic Antibiofilm Efficacy of Various Commercial Antiseptics, Enzymes and EDTA: A Study of Pseudomonas aeruginosa and Staphylococcus aureus Biofilms. Int. J. Antimicrob. Agents 2016, 48, 181–188. [Google Scholar] [CrossRef]
- Torelli, R.; Cacaci, M.; Papi, M.; Paroni Sterbini, F.; Martini, C.; Posteraro, B.; Palmieri, V.; De Spirito, M.; Sanguinetti, M.; Bugli, F. Different Effects of Matrix Degrading Enzymes towards Biofilms Formed by E. faecalis and E. faecium Clinical Isolates. Colloids Surf. B Biointerfaces 2017, 158, 349–355. [Google Scholar] [CrossRef]
- Sugimoto, S.; Sato, F.; Miyakawa, R.; Chiba, A.; Onodera, S.; Hori, S.; Mizunoe, Y. Broad Impact of Extracellular DNA on Biofilm Formation by Clinically Isolated Methicillin-Resistant and -Sensitive Strains of Staphylococcus aureus. Sci. Rep. 2018, 8, 2254. [Google Scholar] [CrossRef]
- Sharma, P.; Garg, N.; Sharma, A.; Capalash, N.; Singh, R. Nucleases of Bacterial Pathogens as Virulence Factors, Therapeutic Targets and Diagnostic Markers. Int. J. Med. Microbiol. 2019, 309, 151354. [Google Scholar] [CrossRef]
- Rubini, D.; Banu, S.F.; Nisha, P.; Murugan, R.; Thamotharan, S.; Percino, M.J.; Subramani, P.; Nithyanand, P. Essential Oils from Unexplored Aromatic Plants Quench Biofilm Formation and Virulence of Methicillin Resistant Staphylococcus aureus. Microb. Pathog. 2018, 122, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.-M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted Disruption of the Extracellular Polymeric Network of Pseudomonas aeruginosa Biofilms by Alginate oligosaccharides. NPJ Biofilms Microbiomes 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Antibacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing Antibiofilm Activity with Functional Chitosan Nanoparticles Targeting Biofilm Cells and Biofilm Matrix. Carbohydr. Polym. 2018, 200, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Giglio, K.M.; Nelson, J.L.; Sondermann, H.; Travis, A.J. Ferromagnetic Nanoparticles with Peroxidase-like Activity Enhance the Cleavage of Biological Macromolecules for Biofilm Elimination. Nanoscale 2014, 6, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Ludwig, R.; Schneider-Stickler, B. Co-Immobilization of Cellobiose Dehydrogenase and Deoxyribonuclease I on Chitosan Nanoparticles against Fungal/Bacterial Polymicrobial Biofilms Targeting Both Biofilm Matrix and Microorganisms. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110499. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Ren, J.; Qu, X. A Series of MOF/Ce-Based Nanozymes with Dual Enzyme-like Activity Disrupting Biofilms and Hindering Recolonization of Bacteria. Biomaterials 2019, 208, 21–31. [Google Scholar] [CrossRef]
- Van der Borden, A.J.; van der Werf, H.; van der Mei, H.C.; Busscher, H.J. Electric Current-Induced Detachment of Staphylococcus Epidermidis Biofilms from Surgical Stainless Steel. Appl. Environ. Microbiol. 2004, 70, 6871–6874. [Google Scholar] [CrossRef]
- Davis, C.P.; Shirtliff, M.E.; Trieff, N.M.; Hoskins, S.L.; Warren, M.M. Quantification, Qualification, and Microbial Killing Efficiencies of Antimicrobial Chlorine-Based Substances Produced by Iontophoresis. Antimicrob. Agents Chemother. 1994, 38, 2768–2774. [Google Scholar] [CrossRef]
- Borriello, G.; Werner, E.; Roe, F.; Kim, A.M.; Ehrlich, G.D.; Stewart, P.S. Oxygen Limitation Contributes to Antibiotic Tolerance of Pseudomonas Aeruginosa in Biofilms. Antimicrob. Agents Chemother. 2004, 48, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Rouse, M.S.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. The Electricidal Effect: Reduction of Staphylococcus and Pseudomonas Biofilms by Prolonged Exposure to Low-Intensity Electrical Current. Antimicrob. Agents Chemother. 2009, 53, 41–45. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. New Perspectives in Biofilm Eradication. ACS Infect. Dis. 2018, 4, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Grodzinsky, A.J.; Wang, D.I. Augmentation of Mass Transfer through Electrical Means for Hydrogel-Entrapped Escherichia Coli Cultivation. Biotechnol. Bioeng. 1995, 48, 149–157. [Google Scholar] [CrossRef]
- Stewart, P.S.; Wattanakaroon, W.; Goodrum, L.; Fortun, S.M.; McLeod, B.R. Electrolytic Generation of Oxygen Partially Explains Electrical Enhancement of Tobramycin Efficacy against Pseudomonas Aeruginosa Biofilm. Antimicrob. Agents Chemother. 1999, 43, 292–296. [Google Scholar] [CrossRef]
- Blenkinsopp, S.A.; Khoury, A.E.; Costerton, J.W. Electrical Enhancement of Biocide Efficacy against Pseudomonas Aeruginosa Biofilms. Appl. Environ. Microbiol. 1992, 58, 3770–3773. [Google Scholar] [CrossRef] [PubMed]
- Sabelnikov, A.G.; Cymbalyuk, E.S.; Gongadze, G.; Borovyagin, V.L. Escherichia Coli Membranes during Electrotransformation: An Electron Microscopy Study. Biochim. Biophys. Acta 1991, 1066, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Rozek, A. Role of Membranes in the Activities of Antimicrobial Cationic Peptides. FEMS Microbiol. Lett. 2002, 206, 143–149. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Rouse, M.S.; Mandrekar, J.N.; Sampedro, M.F.; Steckelberg, J.M.; Patel, R. Effect of Electrical Current on the Activities of Antimicrobial Agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis Biofilms. Antimicrob. Agents Chemother. 2009, 53, 35–40. [Google Scholar] [CrossRef]
- Kim, Y.W.; Subramanian, S.; Gerasopoulos, K.; Ben-Yoav, H.; Wu, H.-C.; Quan, D.; Carter, K.; Meyer, M.T.; Bentley, W.E.; Ghodssi, R. Effect of Electrical Energy on the Efficacy of Biofilm Treatment Using the Bioelectric Effect. Npj Biofilms Microbiomes 2015, 1, 15016. [Google Scholar] [CrossRef]
- Kovalova, Z.; Leroy, M.; Kirkpatrick, M.J.; Odic, E.; Machala, Z. Corona Discharges with Water Electrospray for Escherichia Coli Biofilm Eradication on a Surface. Bioelectrochemistry 2016, 112, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Górski, A. Bacteriophages and Lysins in Biofilm Control. Virol. Sin. 2020, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Dams, D.; Brøndsted, L.; Drulis-Kawa, Z.; Briers, Y. Engineering of Receptor-Binding Proteins in Bacteriophages and Phage Tail-like Bacteriocins. Biochem. Soc. Trans. 2019, 47, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage Endolysins as Novel Antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Mao, J.; Xie, J. Bacteriophage Polysaccharide Depolymerases and Biomedical Applications. BioDrugs 2014, 28, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Fokine, A.; Mahalingam, M.; Zhang, Z.; Garcia-Doval, C.; van Raaij, M.J.; Rossmann, M.G.; Rao, V.B. Molecular Anatomy of the Receptor Binding Module of a Bacteriophage Long Tail Fiber. PLoS Pathog. 2019, 15, e1008193. [Google Scholar] [CrossRef]
- Cisek, A.A.; Dąbrowska, I.; Gregorczyk, K.P.; Wyżewski, Z. Phage Therapy in Bacterial Infections Treatment: One Hundred Years After the Discovery of Bacteriophages. Curr. Microbiol. 2017, 74, 277–283. [Google Scholar] [CrossRef]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Morris, J.; Kelly, N.; Elliott, L.; Grant, A.; Wilkinson, M.; Hazratwala, K.; McEwen, P. Evaluation of Bacteriophage Anti-Biofilm Activity for Potential Control of Orthopedic Implant-Related Infections Caused by Staphylococcus aureus. Surg. Infect. 2019, 20, 16–24. [Google Scholar] [CrossRef]
- Maszewska, A.; Zygmunt, M.; Grzejdziak, I.; Różalski, A. Use of Polyvalent Bacteriophages to Combat Biofilm of Proteus Mirabilis Causing Catheter-associated Urinary Tract Infections. J. Appl. Microbiol. 2018, 125, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Rakov, C.; Ben Porat, S.; Alkalay-Oren, S.; Yerushalmy, O.; Abdalrhman, M.; Gronovich, N.; Huang, L.; Pride, D.; Coppenhagen-Glazer, S.; Nir-Paz, R.; et al. Targeting Biofilm of MDR Providencia Stuartii by Phages Using a Catheter Model. Antibiotics 2021, 10, 375. [Google Scholar] [CrossRef]
- Ben-Zaken, H.; Kraitman, R.; Coppenhagen-Glazer, S.; Khalifa, L.; Alkalay-Oren, S.; Gelman, D.; Ben-Gal, G.; Beyth, N.; Hazan, R. Isolation and Characterization of Streptococcus Mutans Phage as a Possible Treatment Agent for Caries. Viruses 2021, 13, 825. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, Z.; Kermanshahi, R.; Dallal, M.M.S.; Erfani, Y.; Ranjbar, R. Isolation of the Bacteriophages Inhibiting the Expression of the Genes Involved in Biofilm Formation by Streptococcus mutans. Jundishapur. J. Microbiol. 2021, 14, e113206. [Google Scholar] [CrossRef]
- Manoharadas, S.; Altaf, M.; Alrefaei, A.F.; Hussain, S.A.; Devasia, R.M.; Badjah Hadj, A.Y.M.; Abuhasil, M.S.A. Microscopic Analysis of the Inhibition of Staphylococcal Biofilm Formation by Escherichia coli and the Disruption of Preformed Staphylococcal Biofilm by Bacteriophage. Microsc. Res. Technol. 2021, 84, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Abou Zeid, A.A.; Swelim, M.A.; Reda, F.M.; Abd El Haveez, A.M.; Nasr-Eldin, M.A. Effectiveness of Four Lytic Phages against Biofilm-Producing and Multidrug-Resistant Escherichia coli. Benha J. Appl. Sci. 2021, 6, 53–65. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review on The Recent Use of Phage-Based Strategies with Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, B.; Olszak, T.; Drulis-Kawa, Z. Applications of Bacteriophages versus Phage Enzymes to Combat and Cure Bacterial Infections: An Ambitious and Also a Realistic Application? Appl. Microbiol. Biotechnol. 2018, 102, 2563–2581. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, J.; Yan, G.; Lei, L.; Wang, S.; Yu, L.; Zhou, L.; Gao, A.; Feng, X.; Han, W.; et al. Identification and Characterization of Dpo42, a Novel Depolymerase Derived from the Escherichia coli Phage vB_EcoM_ECOO78. Front. Microbiol. 2017, 8, 273039. [Google Scholar]
- Gutiérrez, D.; Briers, Y.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Role of the Pre-Neck Appendage Protein (Dpo7) from Phage vB_SepiS-phiIPLA7 as an Anti-Biofilm Agent in Staphylococcal Species. Front. Microbiol. 2015, 6, 167086. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, R.; Xu, M.; Liu, Y.; Zhu, X.; Qiu, J.; Liu, Q.; He, P.; Li, Q. A Novel Polysaccharide Depolymerase Encoded by the Phage SH-KP152226 Confers Specific Activity Against Multidrug-Resistant Klebsiella Pneumoniae via Biofilm Degradation. Front. Microbiol. 2019, 10, 2768. [Google Scholar] [CrossRef] [PubMed]
- Shahed-Al-Mahmud, M.; Roy, R.; Sugiokto, F.G.; Islam, M.N.; Lin, M.-D.; Lin, L.-C.; Lin, N.-T. Phage φAB6-Borne Depolymerase Combats Acinetobacter Baumannii Biofilm Formation and Infection. Antibiotics 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Schuch, R.; Khan, B.K.; Raz, A.; Rotolo, J.A.; Wittekind, M. Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent. Antimicrob. Agents Chemother. 2017, 61, e02666-16. [Google Scholar] [CrossRef]
- Cha, Y.; Son, B.; Ryu, S. Effective Removal of Staphylococcal Biofilms on Various Food Contact Surfaces by Staphylococcus aureus Phage Endolysin LysCSA13. Food Microbiol. 2019, 84, 103245. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, X.; Wang, L.; Li, G.; Cong, C.; Li, R.; Cui, H.; Murtaza, B.; Xu, Y. The Endolysin of the Acinetobacter Baumannii Phage vB_AbaP_D2 Shows Broad Antibacterial Activity. Microb. Biotechnol. 2021, 14, 403–418. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Wang, J.; Yu, J.; Wei, H. A Novel Chimeric Lysin with Robust Antibacterial Activity against Planktonic and Biofilm Methicillin-Resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 40182. [Google Scholar] [CrossRef]
- Vasina, D.V.; Antonova, N.P.; Grigoriev, I.V.; Yakimakha, V.S.; Lendel, A.M.; Nikiforova, M.A.; Pochtovyi, A.A.; Remizov, T.A.; Usachev, E.V.; Shevlyagina, N.V.; et al. Discovering the Potentials of Four Phage Endolysins to Combat Gram-Negative Infections. Front. Microbiol. 2021, 12, 748718. [Google Scholar] [CrossRef]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.-A. Synergistic Interaction Between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, D.; Taha, M.; Yi, Q.; Ramirez-Arcos, S.; Diallo, J.-S.; Carli, A.; Abdelbary, H. Does Treatment Order Matter? Investigating the Ability of Bacteriophage to Augment Antibiotic Activity against Staphylococcus aureus Biofilms. Front. Microbiol. 2018, 9, 303285. [Google Scholar] [CrossRef] [PubMed]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-Threatening Prosthetic Knee Klebsiella Pneumoniae Infection: Case Report and In Vitro Characterization of Anti-Biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef]
- Stachler, E.; Kull, A.; Julian, T.R. Bacteriophage Treatment before Chemical Disinfection Can Enhance Removal of Plastic-Surface-Associated Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2021, 87, e00980-21. [Google Scholar] [CrossRef] [PubMed]
- Seethalakshmi, P.S.; Rajeev, R.; Kiran, G.S.; Selvin, J. Shrimp Disease Management for Sustainable Aquaculture: Innovations from Nanotechnology and Biotechnology. Aquacult. Int. 2021, 29, 1591–1620. [Google Scholar] [CrossRef]
- Yu, P.; Wang, Z.; Marcos-Hernandez, M.; Zuo, P.; Zhang, D.; Powell, C.; Pan, A.Y.; Villagrán, D.; Wong, M.S.; Alvarez, P.J.J. Bottom-up Biofilm Eradication Using Bacteriophage-Loaded Magnetic Nanocomposites: A Computational and Experimental Study. Environ. Sci. Nano 2019, 6, 3539–3550. [Google Scholar] [CrossRef]
- Koskella, B.; Meaden, S. Understanding Bacteriophage Specificity in Natural Microbial Communities. Viruses 2013, 5, 806–823. [Google Scholar] [CrossRef] [PubMed]
- Huss, P.; Raman, S. Engineered Bacteriophages as Programmable Biocontrol Agents. Curr. Opin. Biotechnol. 2020, 61, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.K.; Collins, J.J. Dispersing Biofilms with Engineered Enzymatic Bacteriophage. Proc. Natl. Acad. Sci. USA 2007, 104, 11197–11202. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Lamas-Samanamud, G.R. Inhibition of Biofilm Formation by T7 Bacteriophages Producing Quorum-Quenching Enzymes. Appl. Environ. Microbiol. 2014, 80, 5340. [Google Scholar] [CrossRef] [PubMed]
- Born, Y.; Fieseler, L.; Thöny, V.; Leimer, N.; Duffy, B.; Loessner, M.J. Engineering of Bacteriophages Y2::dpoL1-C and Y2::luxAB for Efficient Control and Rapid Detection of the Fire Blight Pathogen, Erwinia Amylovora. Appl. Environ. Microbiol. 2017, 83, e00341-17. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered Bacteriophages for Treatment of a Patient with a Disseminated Drug-Resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Li, M.; Shi, D.; Li, Y.; Xiao, Y.; Chen, M.; Chen, L.; Du, H.; Zhang, W. Recombination of T4-like Phages and Its Activity against Pathogenic Escherichia coli in Planktonic and Biofilm Forms. Virol. Sin. 2020, 35, 651–661. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous Fusion Phage: Novel Expression Vectors That Display Cloned Antigens on the Virion Surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Ali, S.Z.; Al-Shahrani, A.M.; Kamal, M.A.; Ashraf, G.M. Phage Display Technique: A Novel Medicinal Approach to Overcome an Tibiotic Resistance by Using Peptide-Based Inhibitors Against β-Lactamases. Curr. Drug Metab. 2017, 18, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Flachbartova, Z.; Pulzova, L.; Bencurova, E.; Potocnakova, L.; Comor, L.; Bednarikova, Z.; Bhide, M. Inhibition of Multidrug Resistant Listeria Monocytogenes by Peptides Isolated from Combinatorial Phage Display Libraries. Microbiol. Res. 2016, 188–189, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.W.; Yang, L.; De Plano, L.M.; Stackhouse, M.A.; Petrenko, V.A. Evolution of a Landscape Phage Library in a Mouse Xenograft Model of Human Breast Cancer. Viruses 2019, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Gillespie, J.W.; Xu, H.; O’Dell, T.; De Plano, L.M. Combinatorial Avidity Selection of Mosaic Landscape Phages Targeted at Breast Cancer Cells—An Alternative Mechanism of Directed Molecular Evolution. Viruses 2019, 11, 785. [Google Scholar] [CrossRef]
- Plano, L.M.D.; Franco, D.; Rizzo, M.G.; Zammuto, V.; Gugliandolo, C.; Silipigni, L.; Torrisi, L.; Guglielmino, S.P.P. Role of Phage Capsid in the Resistance to UV-C Radiations. Int. J. Mol. Sci. 2021, 22, 3408. [Google Scholar] [CrossRef]
- De Plano, L.M.; Silipigni, L.; Torrisi, L.; Torrisi, A.; Cutroneo, M.; Havranek, V.; Mackova, A.; Zammuto, V.; Gugliandolo, C.; Rizzo, M.G.; et al. Incidence of Phage Capsid Organization on the Resistance to High Energy Proton Beams. Appl. Sci. 2022, 12, 988. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iaconis, A.; De Plano, L.M.; Caccamo, A.; Franco, D.; Conoci, S. Anti-Biofilm Strategies: A Focused Review on Innovative Approaches. Microorganisms 2024, 12, 639. https://doi.org/10.3390/microorganisms12040639
Iaconis A, De Plano LM, Caccamo A, Franco D, Conoci S. Anti-Biofilm Strategies: A Focused Review on Innovative Approaches. Microorganisms. 2024; 12(4):639. https://doi.org/10.3390/microorganisms12040639
Chicago/Turabian StyleIaconis, Antonella, Laura Maria De Plano, Antonella Caccamo, Domenico Franco, and Sabrina Conoci. 2024. "Anti-Biofilm Strategies: A Focused Review on Innovative Approaches" Microorganisms 12, no. 4: 639. https://doi.org/10.3390/microorganisms12040639
APA StyleIaconis, A., De Plano, L. M., Caccamo, A., Franco, D., & Conoci, S. (2024). Anti-Biofilm Strategies: A Focused Review on Innovative Approaches. Microorganisms, 12(4), 639. https://doi.org/10.3390/microorganisms12040639








