Device-Associated Infections in COVID-19 Patients: Frequency of Resistant Bacteria, Predictors and Mortality in Medellín, Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Population
2.2. Variables
2.3. Data Collection
2.4. Statistical Analysis
3. Results
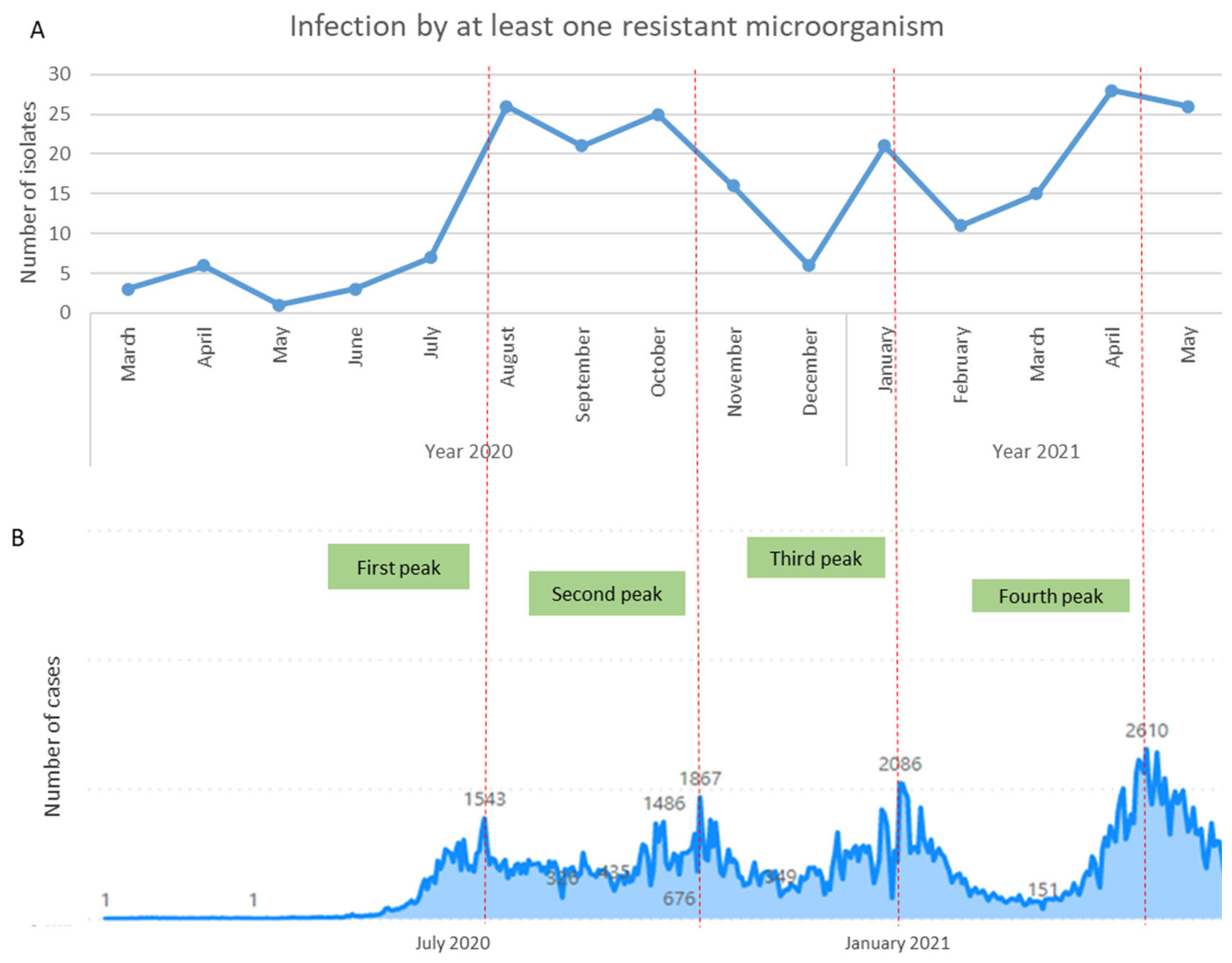
3.1. Device-Associated Infections in COVID-19 Patients
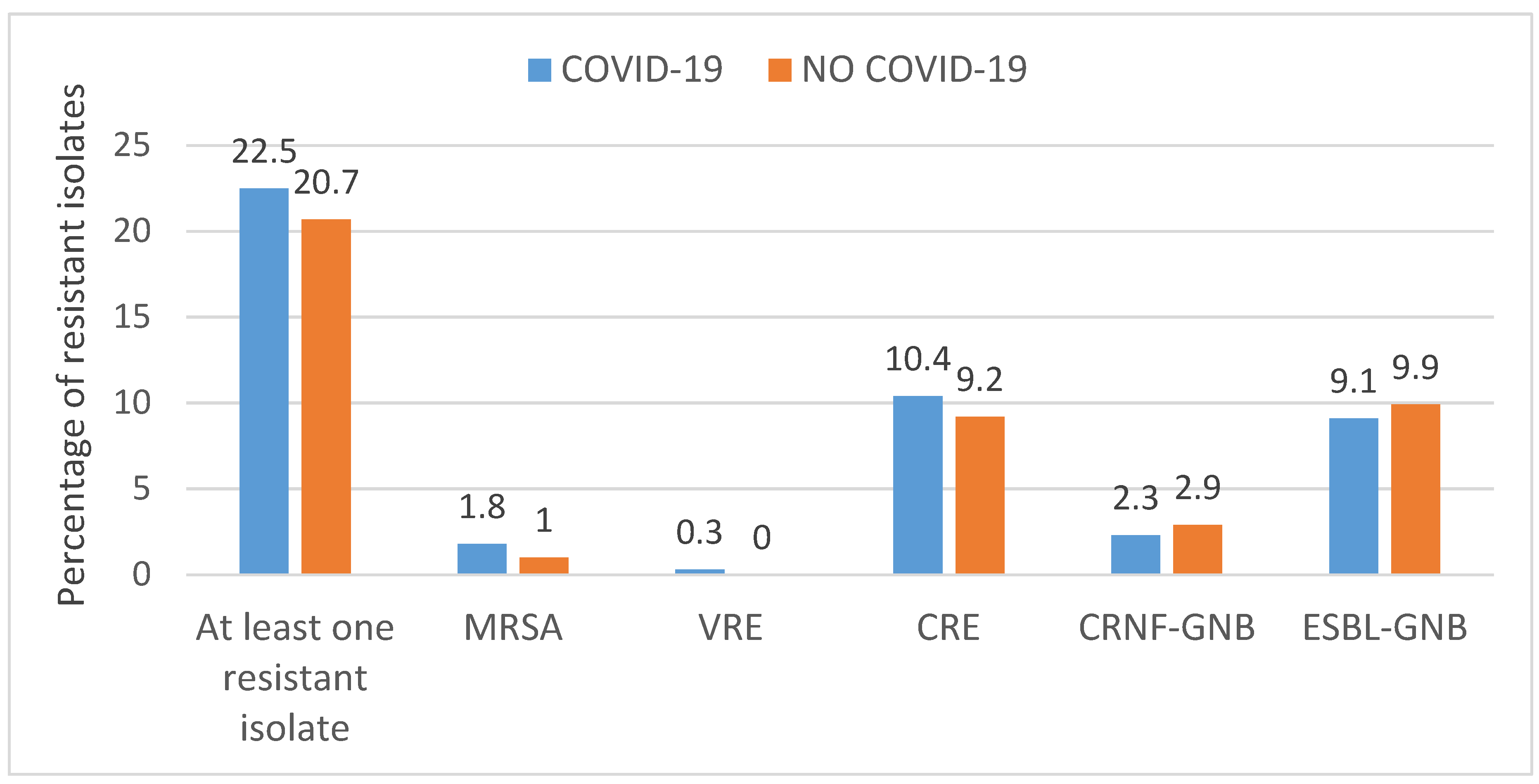
3.2. Overall Use of Antibiotics and Antimicrobial Resistance
3.3. Infections Caused by Resistant Bacteria and Predictors
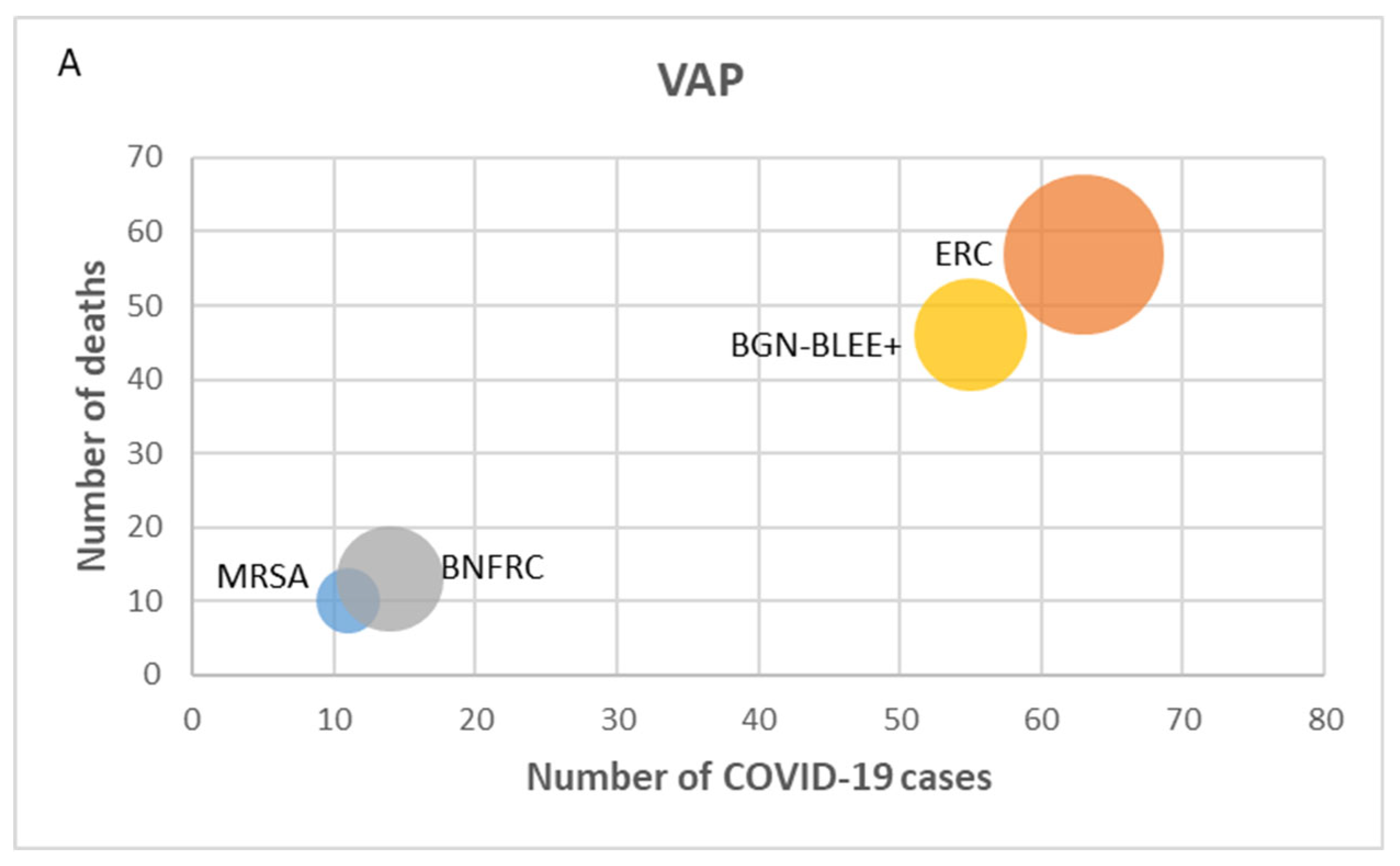
3.4. Mortality and Antimicrobial Resistance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two european point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, O.A.; González-Lara, M.F.; Villanueva-Reza, M.; De-León-Cividanes, N.; Xancal-Salvador, L.F.; Esteban-Kenel, V.; Cárdenas-Ochoa, A.; Cervantes-Sánchez, A.; Martínez-Gamboa, A.; Ochoa-Hein, E.; et al. Outbreak of NDM-1-Producing Escherichia coli in a Coronavirus Disease 2019 Intensive Care Unit in a Mexican Tertiary Care Center. Microbiol. Spectr. 2022, 10, e0201521. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garbajosa, P.; Cantón, R. COVID-19: Impact on prescribing and antimicrobial resistance. Rev. Esp. Quimioter. 2021, 34, 63–68. [Google Scholar] [CrossRef]
- Murray, A.K. The Novel Coronavirus COVID-19 Outbreak: Global Implications for Antimicrobial Resistance. Front. Microbiol. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Yadegar, A.; Hatami, B.; Asadzadeh Aghdaei, H.; Zali, M.R. Antimicrobial Resistance as a Hidden Menace Lurking Behind the COVID-19 Outbreak: The Global Impacts of Too Much Hygiene on AMR. Front. Microbiol. 2020, 11, 590683. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-H.; Liu, L.-M.; Yin, W.; Wang, W.; Guan, L.-L.; Yuan, M.-L.; Li, Y.-L.; Hu, Y.; Li, X.-Y.; Sun, B.; et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann. Am. Thorac. Soc. 2020, 17, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Gijón, D.; Ruiz-Garbajosa, P. Antimicrobial resistance in ICUs: An update in the light of the COVID-19 pandemic. Curr. Opin. Crit. Care 2020, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Beović, B.; Doušak, M.; Ferreira-Coimbra, J.; Nadrah, K.; Rubulotta, F.; Belliato, M.; Berger-Estilita, J.; Ayoade, F.; Rello, J.; Erdem, H. Antibiotic use in patients with COVID-19: A “snapshot” Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 2020, 75, 3386–3390. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud y Protección Social de Colombia. Total de Camas UCI en el País Para la Atención de COVID-19 Incrementó 91%; Ministerio de Salud y Protección Social de Colombia: Bogotá, Colombia, 2020. [Google Scholar]
- Perozo, A.; Castellano González, M.J.; Gómez Gamboa, L.P. Infecciones asociadas a la atención en salud. Enferm. Investig. 2020, 5, 48. [Google Scholar] [CrossRef]
- CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. 2017. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (accessed on 3 May 2023).
- Ospina, M.C.; Roa, M.A. Análisis de Situación en Salud: Infecciones Asociadas a la Atención en Salud—IAAS, Dimensión Vida Saludable Libre de Enfermedades Transmisibles, año 2018; Secretaría de Salud de Medellín: Medellín, Colombia, 2019. [Google Scholar]
- Porretta, A.D.; Baggiani, A.; Arzilli, G.; Casigliani, V.; Mariotti, T.; Mariottini, F.; Scardina, G.; Sironi, D.; Totaro, M.; Barnini, S.; et al. Increased risk of acquisition of new delhi metallo-beta-lactamase-producing carbapenem-resistant enterobacterales (Ndm-cre) among a cohort of COVID-19 patients in a teaching hospital in Tuscany, Italy. Pathogens 2020, 9, 635. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud (INS). Análisis del Comportamiento de las Infecciones Asociadas a la Atención en Salud (IAAS); Boletín Epidemiológico Semana Epidemiológica 44; Instituto Nacional de Salud (INS): Bogotá, Colombia, 2021. [Google Scholar]
- Lastinger, L.M.; Alvarez, C.R.; Kofman, A.; Konnor, R.Y.; Kuhar, D.T.; Nkwata, A.; Patel, P.R.; Pattabiraman, V.; Xu, S.Y.; Dudeck, M.A. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control. Hosp. Epidemiol. 2023, 44, 997–1001. [Google Scholar] [CrossRef]
- Nori, P.; Szymczak, W.; Puius, Y.; Sharma, A.; Cowman, K.; Gialanella, P.; Fleischner, Z.; Corpuz, M.; Torres-Isasiga, J.; Bartash, R.; et al. Emerging Co-Pathogens: New Delhi Metallo-beta-lactamase producing Enterobacterales Infections in New York City COVID-19 Patients. Int. J. Antimicrob. Agents 2020, 56, 106179. [Google Scholar] [CrossRef]
- Monnet, D.L.; Harbarth, S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Eurosurveillance 2020, 25, 2001886. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2020, 76, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Nebreda-Mayoral, T.; Miguel-Gómez, M.A.; March-Rosselló, G.A.; Puente-Fuertes, L.; Cantón-Benito, E.; Martínez-García, A.M.; Muñoz-Martín, A.B.; Orduña-Domingo, A. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enferm. Infecc. Microbiol. Clin. 2022, 40, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. 2021, 27, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hooper, D.C. Hospital-Acquired Infections Due to Gram-Negative Bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- Barrasa-Villar, J.I.; Aibar-Remón, C.; Prieto-Andrés, P.; Mareca-Doñate, R.; Moliner-Lahoz, J. Impact on Morbidity, Mortality, and Length of Stay of Hospital-Acquired Infections by Resistant Microorganisms. Clin. Infect. Dis. 2017, 65, 644–652. [Google Scholar] [CrossRef]
- Santoro, A.; Franceschini, E.; Meschiari, M.; Menozzi, M.; Zona, S.; Venturelli, C.; Digaetano, M.; Rogati, C.; Guaraldi, G.; Paul, M.; et al. Epidemiology and Risk Factors Associated with Mortality in Consecutive Patients with Bacterial Bloodstream Infection: Impact of MDR and XDR Bacteria. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2020; Volume 7, pp. 1–10. [Google Scholar]
- Tamma, P.D.; Goodman, K.E.; Harris, A.D.; Tekle, T.; Roberts, A.; Taiwo, A.; Simner, P.J. Comparing the Outcomes of Patients with Carbapenemase-Producing and Non-Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Bacteremia. Clin. Infect. Dis. 2016, 64, 257–264. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-acquired infections in critically-ill COVID-19 patients. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Mazzalai, E.; Alessandri, F.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 87. [Google Scholar] [CrossRef]
- Blot, S.; Ruppé, E.; Harbarth, S.; Asehnoune, K.; Poulakou, G.; Luyt, C.-E.; Rello, J.; Klompas, M.; Depuydt, P.; Eckmann, C.; et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit. Care Nurs. 2022, 70, 103227. [Google Scholar] [CrossRef] [PubMed]

| Device-Associated Infections Characteristics | Total (n = 1665) No (%) | COVID-19 (n = 1143) No (%) | NO COVID-19 (n = 522) No (%) | p Value |
|---|---|---|---|---|
| Type VAP * CLABSI * CAUTI * | 802 (48.2) 538 (32.3) 325 (19.5) | 618 (54.1) 361 (31.6) 164 (14.3) | 184 (35.2) 177 (33.9) 161 (30.8) | <0.001 0.347 <0.001 |
| Two or more DAI | 124 (8.1) | 92 (8.9) | 32 (6.6) | 0.118 |
| Time in days from hospitalization to the first DAI (Median (IQR *) | 12 (8–17) | 12 (8–16) | 13 (9–20) | <0.001 |
| Presence of devices Urinary catheter Central venous catheter Mechanical ventilation | 1100 (66.1) 1137 (68.3) 1336 (80.2) | 751 (65.7) 809 (70.8) 1007 (88.1) | 349 (66.9) 328 (62.8) 329 (63.0) | 0.645 0.001 <0.001 |
| Days with the device Median (IQR *) Urinary catheter (n = 960) Mechanic ventilation (n = 985) Central catheter (n = 986) | 13.5 (9–21) 15 (10–23) 13 (9–19) | 14 (10–21) 15 (10–22) 13 (9–19) | 12.5 (8–21) 15 (9–25) 12 (8–21) | 0.025 0.601 0.767 |
| Microorganisms (n = 989) Staphylococcus aureus Enterococcus spp. Klebsiella pneumoniae Escherichia coli Enterobacter cloacae Pseudomonas aeruginosa Acinetobacter baumannii | 60 (6.1) 88 (8.9) 285 (28.9) 159 (16.1) 60 (6.1) 108 (10.9) 3 (0.3) | 38 (6.3) 58 (9.6) 195 (32.2) 78 (12.9) 38 (6.3) 58 (9.6) 1 (0.2) | 22 (5.8) 30 (7.8) 90 (23.6) 81 (21.2) 22 (5.8) 50 (13.1) 2 (0.5) | 0.738 0.352 0.003 0.001 0.738 0.086 0.333 |
| Polymicrobial infection | 69 (6.7) | 36 (5.9) | 33 (8,6) | 0.107 |
| Outcome | Bivariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| RR | CI 95% | p Value | RR | CI 95% | p Value | |||
| Meticillin-resistant Staphylococcus aureus | ||||||||
| Age over 60 years | 1.16 | 0.26 | 5.14 | 0.840 | 1.00 | 0.27 | 3.70 | 0.997 |
| ICU stay > 14 days | 0.65 | 0.10 | 4.25 | 0.651 | 0.65 | 0.10 | 4.07 | 0.644 |
| Diabetes | 1.10 | 0.50 | 2.41 | 0.813 | 1.27 | 0.62 | 2.58 | 0.514 |
| Carbapenem-resistant Enterobacteriaceae | ||||||||
| Age over 60 years | 1.80 | 0.96 | 3.38 | 0.065 | 1.42 | 0.77 | 2.65 | 0.264 |
| ICU stay > 14 days | 2.20 | 1.51 | 3.19 | <0.001 | 2.09 | 1.39 | 3.16 | <0.001 |
| Diabetes | 1.84 | 1.30 | 2.60 | 0.001 | 1.73 | 1.21 | 2.49 | 0.003 |
| Mechanic ventilation | 1.87 | 1.00 | 3.53 | 0.054 | 2.13 | 0.99 | 4.51 | 0.049 |
| Central venous catheter | 0.68 | 0.19 | 2.36 | 0.541 | 0.53 | 0.14 | 2.02 | 0.352 |
| Carbapenem-resistant non-fermenting Gram-negative bacilli | ||||||||
| Age over 60 years | 1.22 | 0.39 | 3.77 | 0.734 | 1.19 | 0.41 | 3.45 | 0.746 |
| ICU stay > 14 days | 2.59 | 0.99 | 6.78 | 0.053 | 2.47 | 0.95 | 6.44 | 0.065 |
| Diabetes | 0.82 | 0.26 | 2.55 | 0.732 | 0.79 | 0.26 | 2.43 | 0.681 |
| Mechanic ventilation | 1.74 | 0.54 | 5.63 | 0.355 | 1.55 | 0.29 | 8.30 | 0.607 |
| Central venous catheter | 1.38 | 0.31 | 6.17 | 0.675 | 1.05 | 0.15 | 7.42 | 0.964 |
| ESBL-producing Gram-negative bacilli | ||||||||
| Age over 60 years | 0.87 | 0.56 | 1.37 | 0.555 | 0.83 | 0.50 | 1.38 | 0.476 |
| ICU stay > 14 days | 1.06 | 0.55 | 2.03 | 0.857 | 1.07 | 0.54 | 2.12 | 0.854 |
| Diabetes | 1.13 | 0.68 | 1.87 | 0.644 | 1.18 | 0.70 | 1.96 | 0.537 |
| Urinary catheter | 1.48 | 0.61 | 3.60 | 0.383 | 1.51 | 0.61 | 3.74 | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocampo, D.P.; Echeverri-Toro, L.M.; Jiménez, J.N.; Salazar, L.; Vargas, C.; Roncancio, G.; Roa, M.A.; Vanegas, J.M. Device-Associated Infections in COVID-19 Patients: Frequency of Resistant Bacteria, Predictors and Mortality in Medellín, Colombia. Microorganisms 2024, 12, 640. https://doi.org/10.3390/microorganisms12040640
Ocampo DP, Echeverri-Toro LM, Jiménez JN, Salazar L, Vargas C, Roncancio G, Roa MA, Vanegas JM. Device-Associated Infections in COVID-19 Patients: Frequency of Resistant Bacteria, Predictors and Mortality in Medellín, Colombia. Microorganisms. 2024; 12(4):640. https://doi.org/10.3390/microorganisms12040640
Chicago/Turabian StyleOcampo, Diana Patricia, Lina María Echeverri-Toro, Judy Natalia Jiménez, Lorena Salazar, Carlos Vargas, Gustavo Roncancio, Maria Alejandra Roa, and Johanna Marcela Vanegas. 2024. "Device-Associated Infections in COVID-19 Patients: Frequency of Resistant Bacteria, Predictors and Mortality in Medellín, Colombia" Microorganisms 12, no. 4: 640. https://doi.org/10.3390/microorganisms12040640





