Lactic Acid Production by Lactiplantibacillus plantarum AC 11S—Kinetics and Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Growth Media, and Culture Conditions
2.2. The LAB Species Identification
2.3. In Vitro Assessment of Acid Tolerance of L. plantarum AC 11S
2.4. Analytical Procedures
2.5. Fermentation
2.6. Modeling
3. Results and Discussion
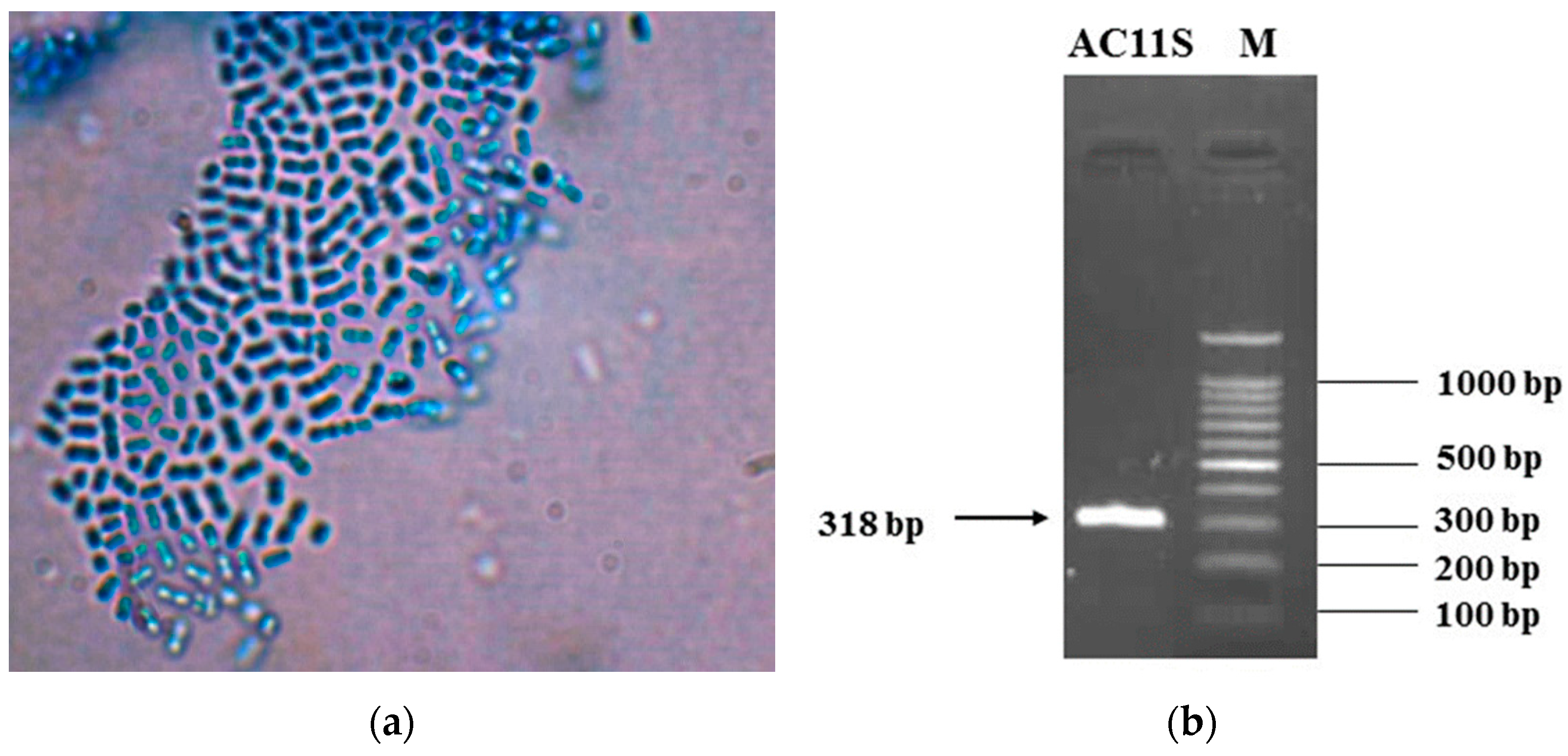
3.1. Isolation and Identification of Lactiplantibacillus plantarum AC 11S
3.2. Viability of L. plantarum AC 11S in an Acidic Environment
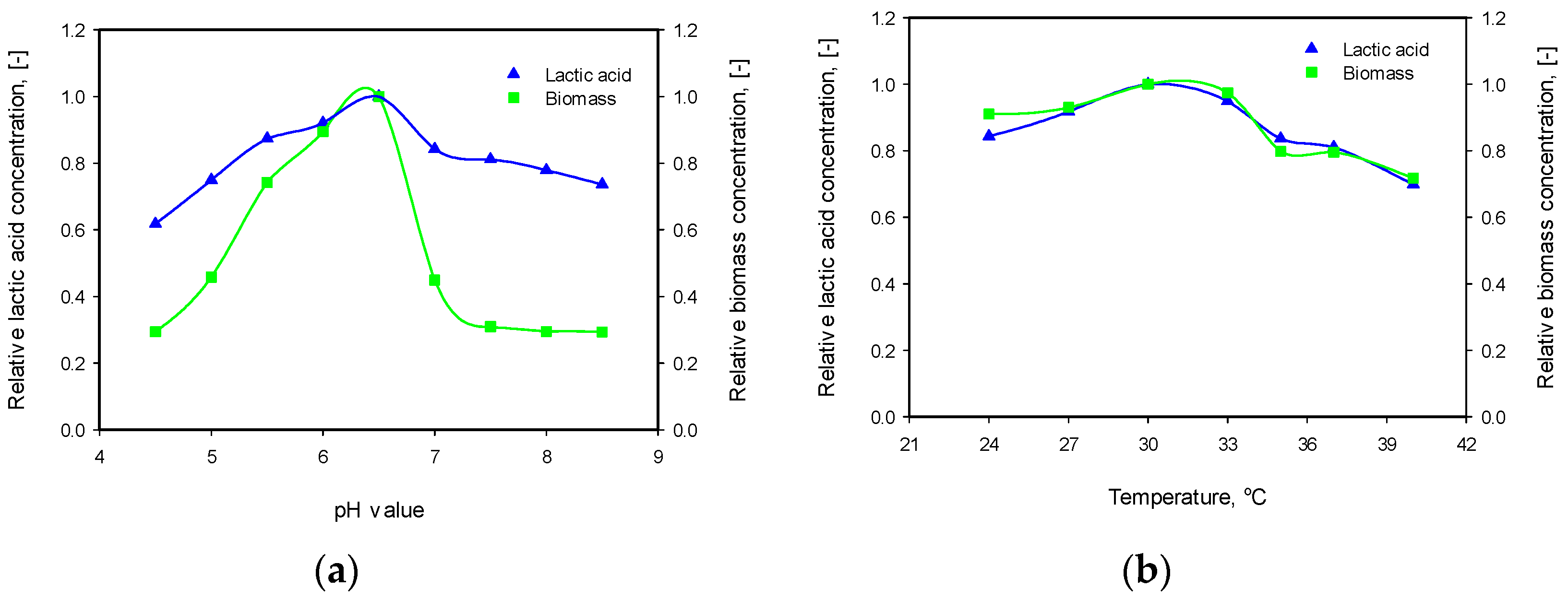
3.3. Influence of pH and Temperature
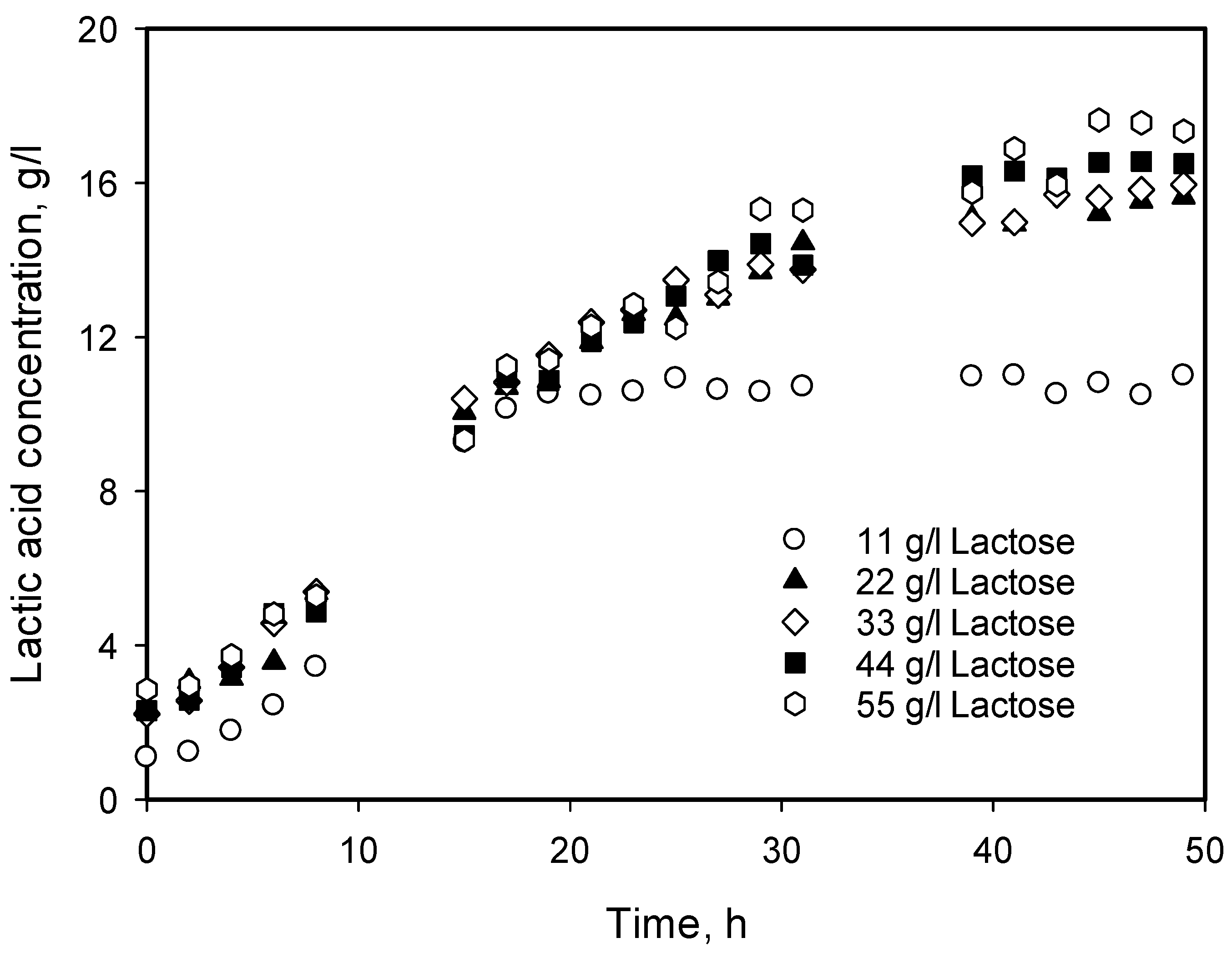
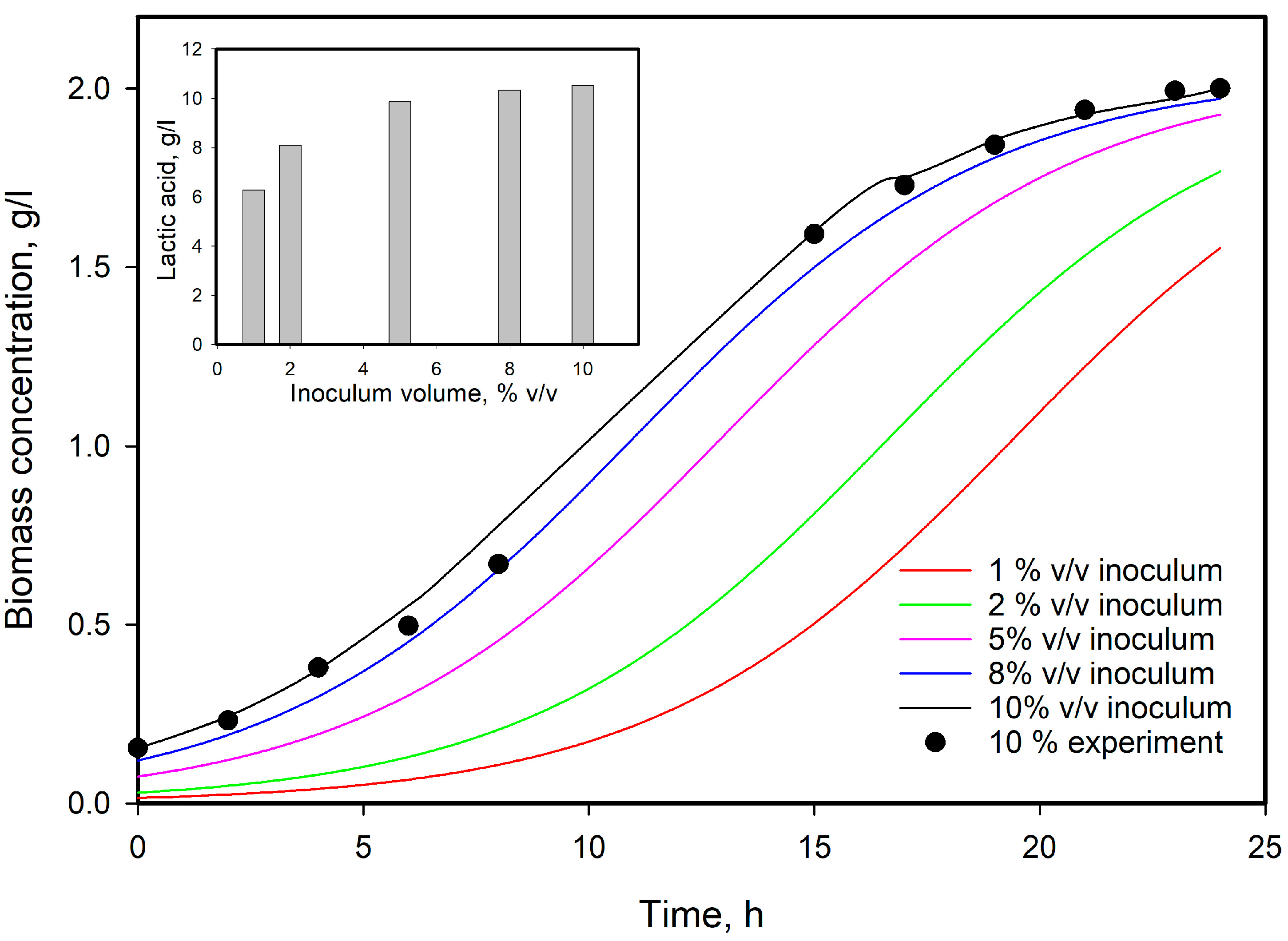
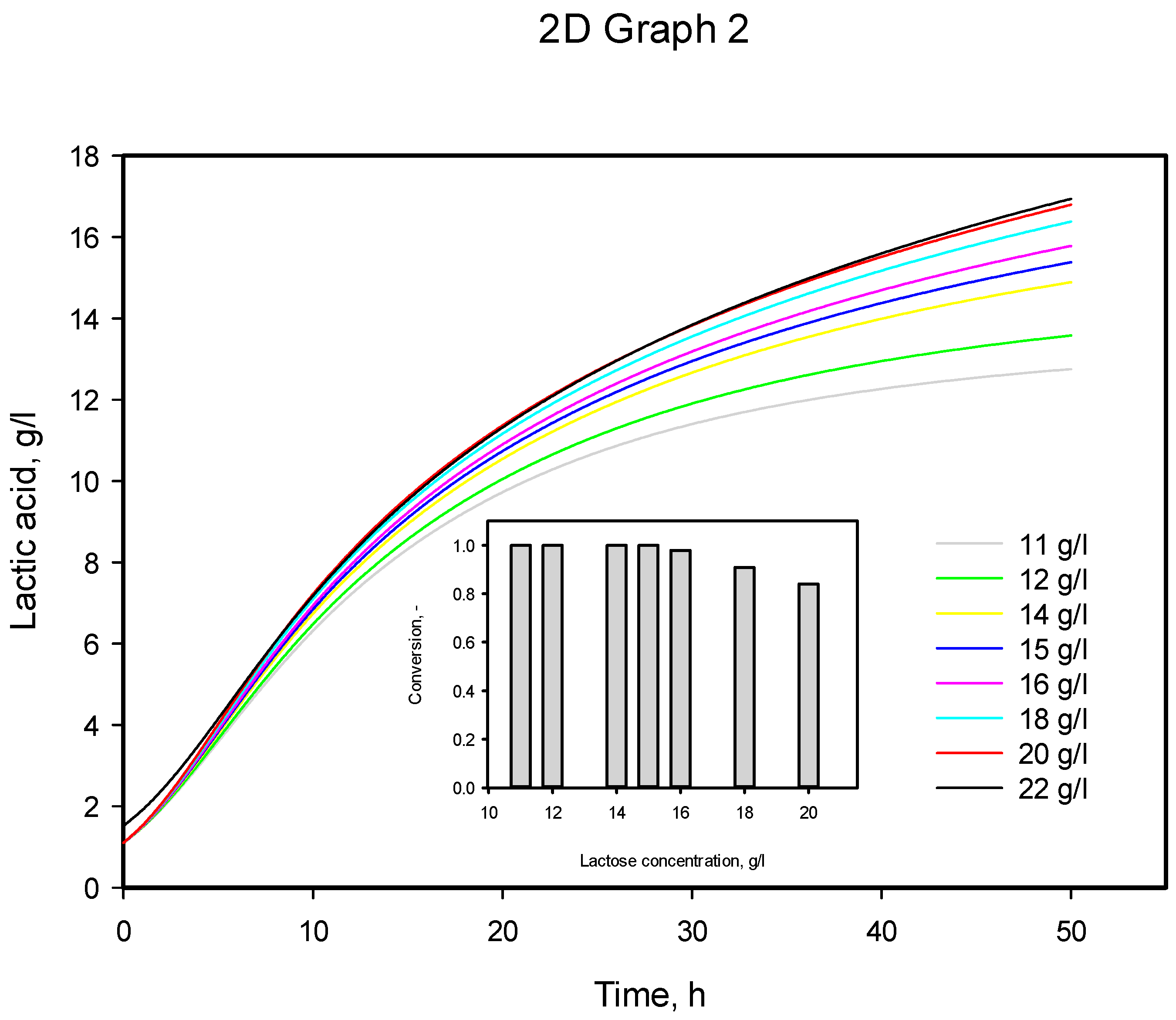
3.4. Influence of Initial Substrate Concentration
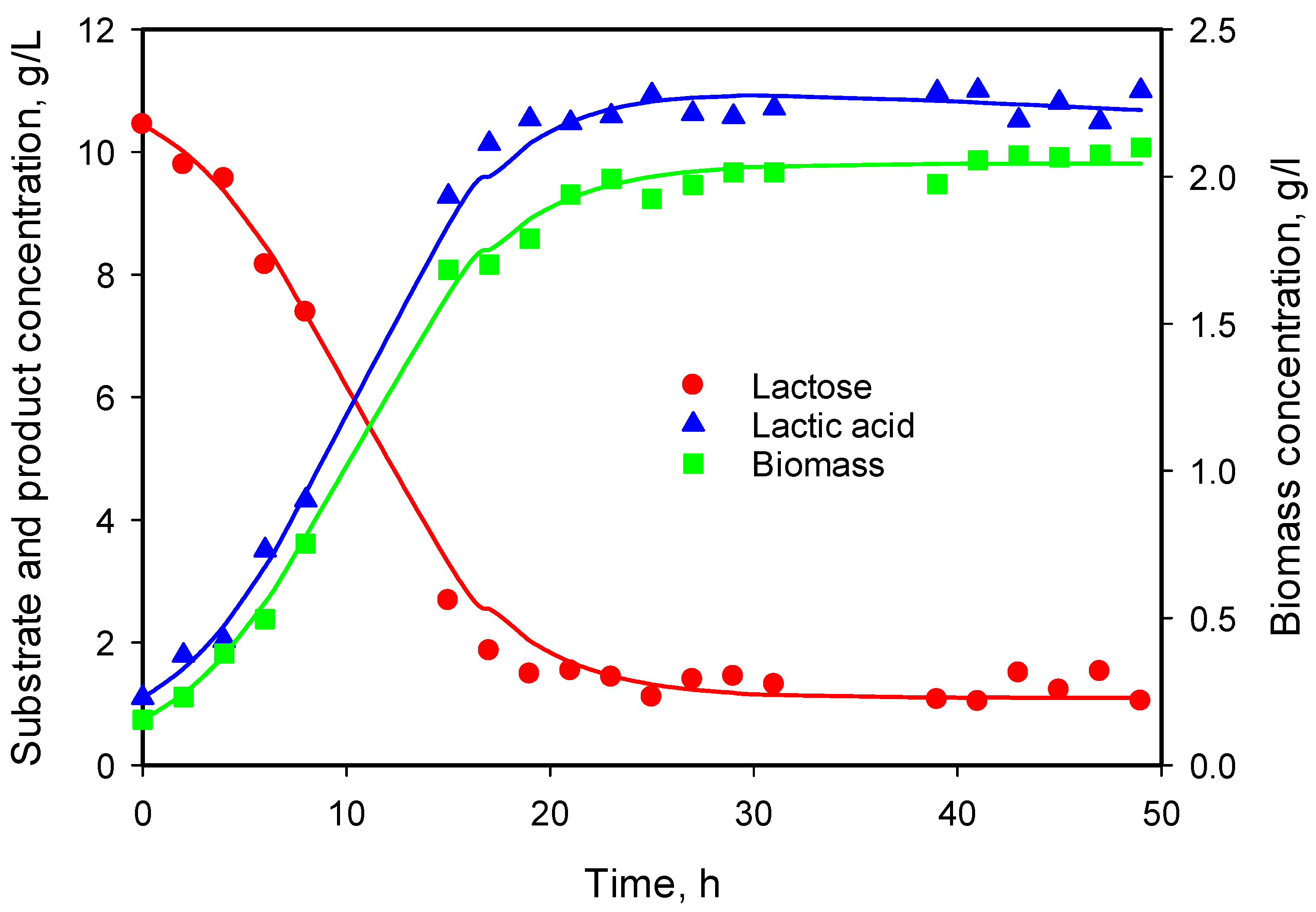
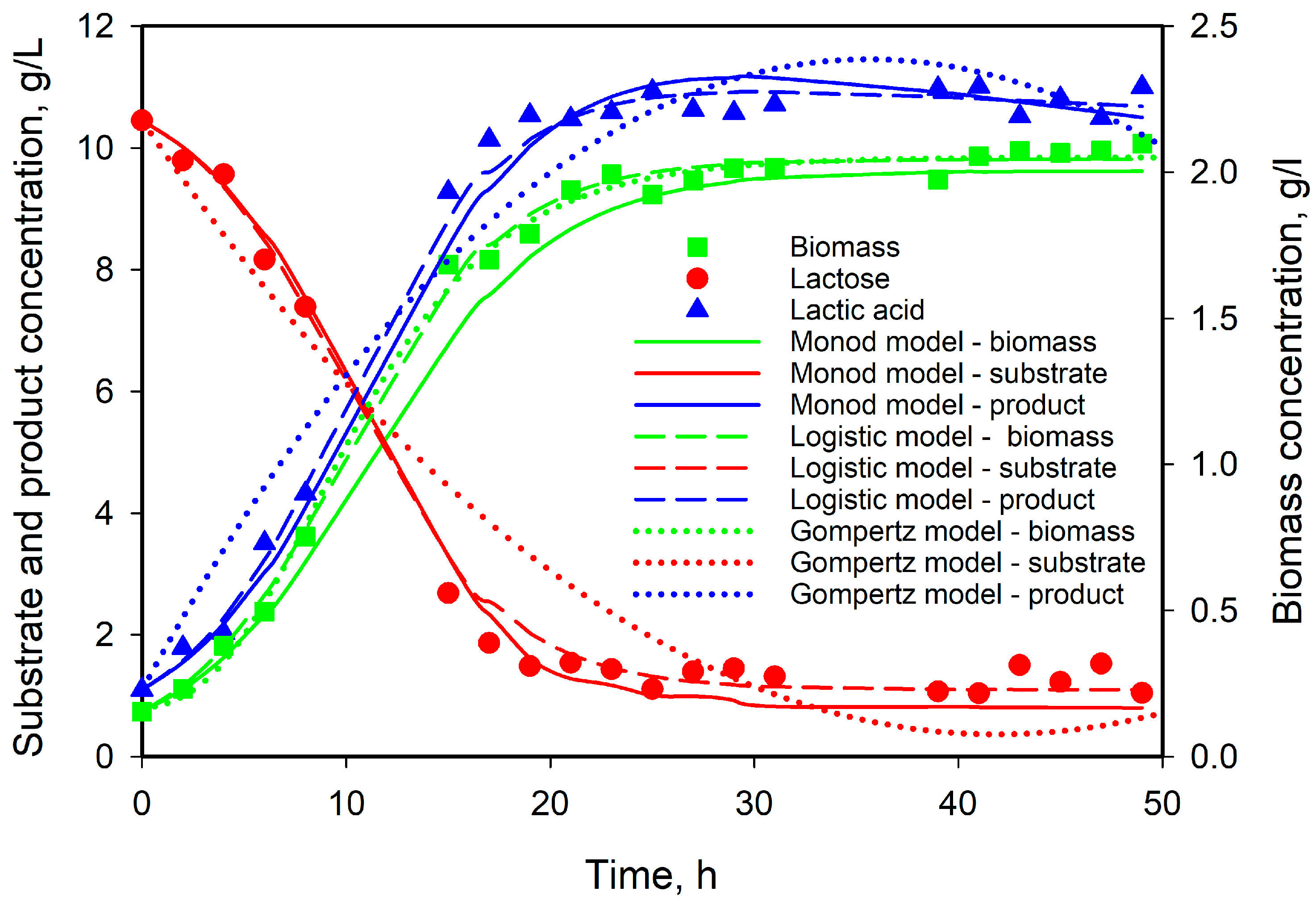
3.5. Modeling of Cell Growth, Substrate Consumption, and Product Accumulation
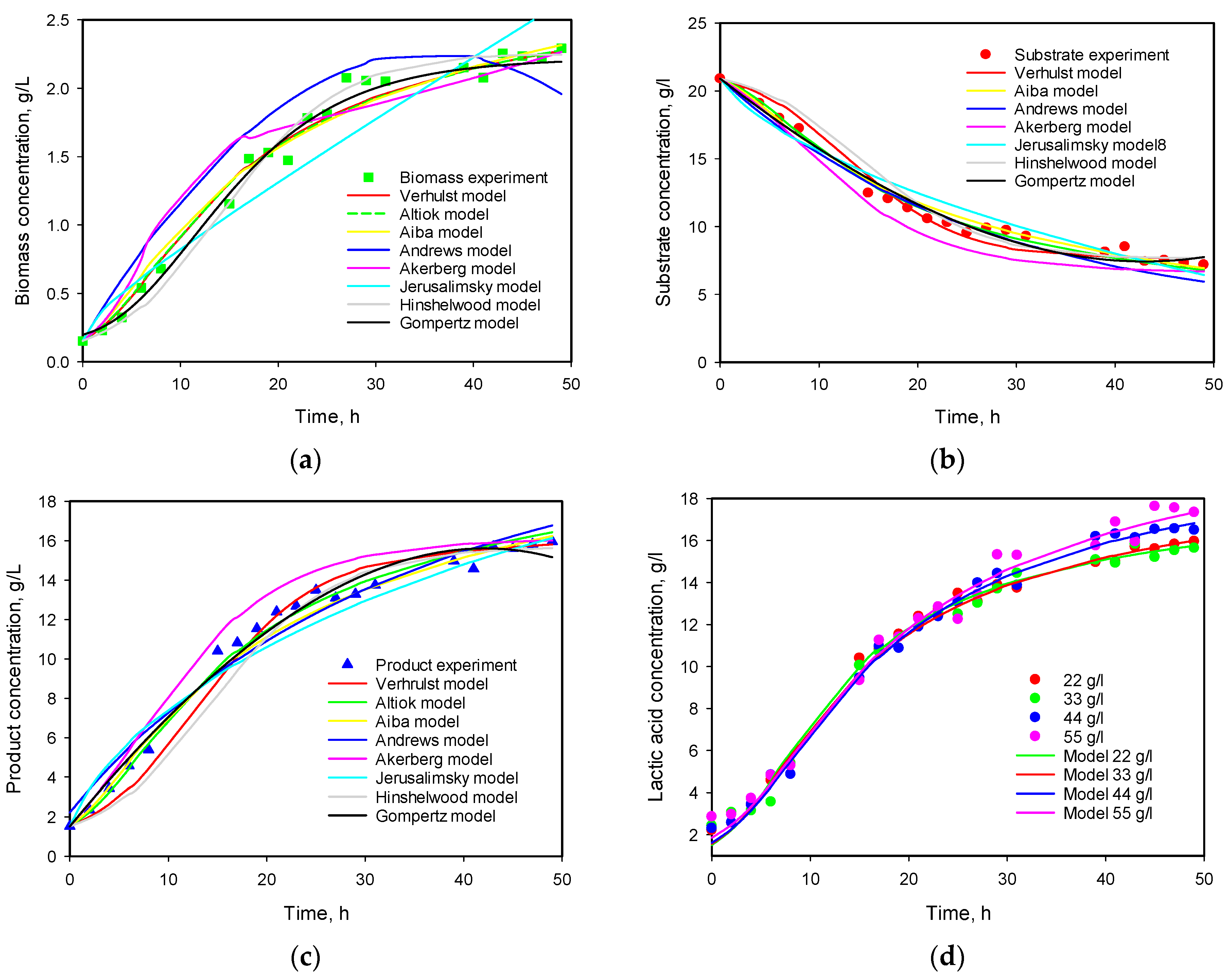
3.6. Model Predictions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatti-Kaul, R.; Chen, L.; Dishisha, T.; El Enshasy, H. Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS Microbiol. Lett. 2018, 365, fny213. [Google Scholar] [CrossRef]
- Filannin, P.; De Angelis, M.; Di Cagno, R.; Gozzi, G.; Riciputi, Y.; Gobbetti, M. How Lactobacillus plantarum shapes its transcriptome in response to contrasting habitats. Environ. Microbiol. 2018, 20, 3700–3716. [Google Scholar] [CrossRef]
- Chen, P.-T.; Hong, Z.-S.; Cheng, C.-L.; Ng, I.-S.; Lo, Y.-C.; Nagarajan, D.; Chang, J.-S. Exploring fermentation strategies for enhanced lactic acid production with polyvinyl alcohol-immobilized Lactobacillus plantarum using microalgae as feedstock. Bioresour. Technol. 2020, 308, 123266. [Google Scholar] [CrossRef]
- Yetiman, A.E.; Keskin, A.; Nur, D.B.; Kotil, S.E.; Ortakci, F.; Dogan, M. Characterization of genomic, physiological, and probiotic features Lactiplantibacillus plantarum DY46 strain isolated from traditional lactic acid fermented shalgam beverage. Food Biosci. 2022, 46, 101499. [Google Scholar] [CrossRef]
- Yadav, N.; Nain, L.; Khare, K.S. One-pot production of lactic acid from rice straw pretreated with ionic liquid. Bioresour. Technol. 2021, 323, 124563. [Google Scholar] [CrossRef]
- David, A.N.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Co-valorization of corn cobs and dairy wastewater for simultaneous sacharification and lactic acid production: Process optimization and kinetic assessment. Bioresour. Technol. 2022, 348, 126815. [Google Scholar] [CrossRef]
- Haokok, C.; Lunprom, S.; Reungsang, A.; Salakkam, A. Efficient production of lactic acid from cellulose and xylan in sugarcane bagasse by newly isolated Lactiplantibacillus plantarum and Levilactobacillus brevis through simultaneous saccharification and co-fermentation process. Heliyon 2023, 9, e17935. [Google Scholar] [CrossRef] [PubMed]
- Derabli, B.; Nancib, A.; Nancib, N.; Aníbal, J.; Raposo, S.; Rodrigues, B.; Boudrant, J. Opuntia ficus indica waste as a cost effective carbon source for lactic acid production by Lactobacillus plantarum. Food Chem. 2022, 370, 131005. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Mathews, A.P. Lactic acid production from lactose by Lactobacillus plantarum: Kinetic model and effects of pH, substrate, and oxygen. Biochem. Eng. J. 1999, 3, 163–170. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Prot. 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Ciarrocchi, A.; Prete, R. Lactic Acid Bacteria: Lactobacillus spp. In Lactobacillus plantarum. Reference Module in Food Sciences; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Zacharof, M.-P.; Lovitt, R.W. Modelling and Simulation of Cell Growth Dynamics, Substrate Consumption, and Lactic Acid Production Kinetics of Lactococcus lactis. Biotechnol. Bioprocess. Eng. 2013, 18, 52–64. [Google Scholar] [CrossRef]
- Bouguettoucha, A.; Balannec, B.; Amrane, A. Unstructured Models for Lactic Acid Fermentation—A Review. Food Technol. Biotechnol. 2011, 49, 3–12. [Google Scholar]
- Gordeev, L.S.; Koznov, A.V.; Skichko, A.S.; Gordeeva, Y.L. Unstructured Mathematical Models of Lactic Acid Biosynthesis Kinetics: A Review. Theor. Found. Chem. Eng. 2017, 51, 175–190. [Google Scholar] [CrossRef]
- Danova, S.; Georgieva, R.; Koleva, P.; Tropcheva, R.; Manasiev, J.; Nikolova, D. Biodiversity and activity of Lactic acid bacteria from traditional Bulgarian milk products. Sci. Work. UFT—Plovdiv. 2009, 56, 275–280. [Google Scholar]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef]
- Georgieva, R.N.; Iliev, I.N.; Chipeva, V.A.; Dimitonova, S.P.; Samelis, J.; Danova, S.T. Identification and in vitro characterisation of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J. Basic Microbiol. 2008, 48, 234–244. [Google Scholar] [CrossRef]
- Verhulst, P.-F. Notice sur la loi que la population suit dans son accroissement. Corr. Math. Phys. 1838, 10, 113–121. [Google Scholar]
- Altıok, D.; Tokatlı, F.; Harsa, Ş. Kinetic modelling of lactic acid production from whey by Lactobacillus casei (NRRL B-441). J. Chem. Technol. Biotechnol. 2006, 81, 1190–1197. [Google Scholar] [CrossRef]
- Aiba, S.; Shoda, M.; Nagatani, M. Kinetics of product inhibition in alcohol fermentation. Biotechnol. Bioeng. 1968, 10, 845–864. [Google Scholar] [CrossRef]
- Andrews, J.F. A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioeng. 1968, 10, 707–723. [Google Scholar] [CrossRef]
- Åkerberg, C.; Hofvendahl, K.; Zacchi, G.; Hahn-Hägerdal, B. Modelling the infuence of pH, temperature, glucose and lactic acid concentrations on the kinetics of lactic acid production by Lactococcus lactis ssp. lactis ATCC 19435 in whole-wheat flour. Appl. Microbiol. Biotechnol. 1998, 49, 682–690. [Google Scholar]
- Ierusalimskiĭ, N.D.; Neronova, N.M. Quantitative relationship between the concentration of metabolic products and the rate of growth of microorganisms. Dokl. Akad. Nauk. SSSR 1965, 161, 1437–1440. (In Russian) [Google Scholar]
- Hinshelwood, C.N. The Chemical Kinetics of the Bacterial Cell, 1st ed.; Clarendon Press: Oxford, UK, 1946. [Google Scholar]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van ‘t Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Luedeking, R.; Piret, E.L. A Kinetic Study of the Lactic Acid Fermentation. Batch Process at Controlled pH. J. Biochem. Microbiol. Technol. Eng. 1959, 1, 393–412. [Google Scholar] [CrossRef]
- Brinques, G.B.; Ayub, M.A.Z. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J. Food Eng. 2011, 103, 123–128. [Google Scholar] [CrossRef]
- van de Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. Antonie Leeuwenhoek 2002, 82, 187–216. [Google Scholar] [CrossRef]
- Wall, T.; Båth, K.; Britton, R.A.; Jonsson, H.; Versalovic, J.; Roos, S. The early response to acid shock in Lactobacillus reuteri involves the ClpL chaperone and a putative Cell Wall-altering esterase. Appl. Environ. Microbiol. 2007, 73, 3924–3935. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Albano, H.; Silva, B.; Almeida, M.H.; Nogueira, T.; Teixeira, P. Caracterization of a Lactiplantibacillus plantarum R23 Isolated from Arugula by Whole-Genome Sequencing and Its Bacteriocin Production Ability. Int. J. Environ. Res. Public Health 2021, 18, 5515. [Google Scholar] [CrossRef]
- Bomrungnok, W.; Sonomoto, K.; Pinitglang, S.; Wongwicharn, A. Single Step Lactic Acid Production from Cassava Starch by Lactobacillus plantarum SW14 in Conventional Continuous and Continuous with High Cell Density. APCBEE Procedia 2012, 2, 97–103. [Google Scholar] [CrossRef]
- Jang, H.-Y.; Kim, M.J.; Bae, M.; Hwang, I.M.; Lee, J.-H. Transcriptional analysis of the molecular mechanism underlying the response of Lactiplantibacillus plantarum to lactic acid stress conditions. Heliyon 2023, 9, e16520. [Google Scholar] [CrossRef] [PubMed]
- Baltà-Foix, R.; Serrano-Adrover, C.; López-Cano, A.; Gifre-Renom, L.; Sanchez-Chardi, A.; Arís, A.; Garcia-Fruitós, E. Lactiplantibacillus plantarum: A new example of inclusion body producing bacteria. Microb. Cell Factories 2023, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Adesokan, I.A.; Odetoyinbo, B.B.; Okanlawon, B.M. Optimization of lactic acid production by lactic acid bacteria isolated from some traditional fermented food in Nigeria. Pak. J. Nutr. 2009, 8, 611–615. [Google Scholar]
- Venkatesh, K.V.; Okos, M.R.; Wankat, P.C. Kinetic Model of Growth and Lactic Acid Production from Lactose by Lactobacillus bulgaricus. Process Biochem. 1993, 28, 231–241. [Google Scholar] [CrossRef]
- Lunelli, B.H.; Melo, D.N.C.; de Morais, E.R.; Victorino, I.R.S.; Vasco de Toledo, E.C.; Maciel, M.R.W.; Filho, R.M. Real-time optimization for lactic acid production from sucrose fermentation by Lactobacillus plantarum. Comput. Aided Chem. Eng. 2011, 29, 1396–1400. [Google Scholar]
- Peetermans, A.; Foulquié-Moreno, M.R.; Thevelein, J.M. Mechanisms underlying lactic acid tolerance and its influence on lactic acid production in Saccharomyces cerevisiae. Microb. Cell 2021, 8, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Mercier, P.; Yerushalmi, L.; Rouleau, D.; Dochain, D. Kinetics of Lactic Acid Fermentation on Glucose and Corn by Lactobacillus amylophilus. J. Chem. Technol. Biotechnol. 1992, 55, 111–121. [Google Scholar] [CrossRef]
- Kostov, G.; Angelov, M.; Denkova, Z.; Dobrev, I.; Goranov, B. Lactic acid production with Lactobacillus casei ssp. rhamnosus NBIMCC 1013: Modeling and optimization of the nutrient medium. Eng. Life Sci. 2011, 11, 517–527. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Qi, B.; Chen, X.; Su, Z.; Wan, Y. Optimizing l-(+)-lactic acid production by thermophile Lactobacillus plantarum As.1.3 using alternative nitrogen sources with response surface method. Biochem. Eng. J. 2010, 52, 212–219. [Google Scholar] [CrossRef]
- Tomás, M.S.J.; Ocaña, V.S.; Wiese, B.; Nader-Macías, M.E. Growth and lactic acid production by vaginal Lactobacillus acidophilus CRL 1259, and inhibition of uropathogenic Escherichia coli. J. Med. Microbiol. 2003, 52, 1117–1124. [Google Scholar] [CrossRef]
- Amrane, A.; Prigent, Y. Mathematical Model for Lactic Acid Production from Lactose in Batch Culture: Model Development and Simulation. J. Chem. Technol. Biotechnol. 1994, 60, 241–246. [Google Scholar] [CrossRef]
- Colucci Cante, R.; Gallo, M.; Nigro, F.; Passannanti, F.; Budelli, A.; Nigro, R. Mathematical Modeling of Lactobacillus paracasei CBA L74 Growth during Rice Flour Fermentation Performed with and without pH Control. Appl. Sci. 2021, 11, 2921. [Google Scholar] [CrossRef]
- Barragán, P.J.; Sánchez, Ó.J.; Henao-Rojas, J.C. Evaluation of the Growth Kinetics of Lactobacillus Plantarum ATCC 8014 on a Medium Based on Hydrolyzed Bovine Blood Plasma at Laboratory and Bench-Scale Levels and Its Application as a Starter Culture in a Meat Product. Fermentation 2020, 6, 45. [Google Scholar] [CrossRef]
- Palaniraj, R.; Nagarajan, P. Kinetic Studies in Production of Lactic Acid from Waste Potato Starch Using Lactobacillus casei. Int. J. Chem. Technol. Res. 2012, 4, 1601–1614. [Google Scholar]
- Vázquez, J.A.; Murado, M.A. Unstructured mathematical model for biomass, lactic acid and bacteriocin production by lactic acid bacteria in batch fermentation. J. Chem. Technol. Biotechnol. 2008, 83, 91–96. [Google Scholar] [CrossRef]
- Trontel, A.; Baršić, V.; Slavica, A.; Šantek, B.; Novak, S. Modelling the Effect of Different Substrates and Temperature on the Growth and Lactic Acid Production by Lactobacillus amylovorus DSM 20531T in Batch Process. Food Technol. Biotechnol. 2010, 48, 352–361. [Google Scholar]
- Goranov, B.; Shopska, V.; Denkova, R.; Kostov, G. Kinetics of Batch Fermentation in the Cultivation of a Probiotic Strain Lactobacillus delbrueckii ssp. Bulgaricus B1. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 61–72. [Google Scholar] [CrossRef]
- Gonçalves, L.M.D.; Xavier, A.M.R.B.; Almeida, J.S.; Carrondo, M.J.T. Concomitant substrate and product inhibition kinetics in lactic acid production. Enzym. Microb. Technol. 1991, 13, 314–319. [Google Scholar] [CrossRef]
- Anuradha, R.; Suresh, A.K.; Venkatesh, K.V. Simultaneous saccharification and fermentation of starch to lactic acid. Process Biochem. 1999, 35, 367–375. [Google Scholar] [CrossRef]
- Balannec, B.; Bouguettoucha, A.; Amrane, A. Unstructured model for batch cultures without pH control of Lactobacillus helveticus—Inhibitory effect of the undissociated lactic acid. Biochem. Eng. J. 2007, 35, 289–294. [Google Scholar] [CrossRef]
- Biazar, J.; Tango, M.; Babolian, E.; Islam, R. Solution of the kinetic modeling of lactic acid fermentation using Adomian decomposition method. Appl. Math. Comput. 2003, 144, 433–439. [Google Scholar] [CrossRef]
- Ben Youssef, C.; Goma, G.; Olmos-Dichara, A. Kinetic modelling of Lactobacillus casei ssp. rhamnosus growth and lactic acid production in batch cultures under various medium conditions. Biotechnol. Lett. 2005, 27, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G.M. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef]
- Sharma, V.; Mishra, H.N. Unstructured kinetic modeling of growth and lactic acid production by Lactobacillus plantarum NCDC 414 during fermentation of vegetable juices. LWT Food Sci. Technol. 2014, 59, 1123–1128. [Google Scholar] [CrossRef]


| No. | Model Name | Equation of Biomass Specific Growth Rate | Reference |
|---|---|---|---|
| 1 | Verhulst | if n = 1 | [19] |
| 2 | Altıok | if n1, n2 = 1 | [20] |
| 3 | Aiba | [21] | |
| 4 | Andrews | [22] | |
| 5 | Akerberg | [23] | |
| 6 | Monod–Jerusalimsky | [24] | |
| 7 | Hinshelwood | [25] |
| Model | μmax | KS | Xmax | λ | A | YX/S | YP/S | α | β | Q |
|---|---|---|---|---|---|---|---|---|---|---|
| Monod | 0.420 | 5.511 | 0.175 | 4.016 | 5.965 | 0.0197 | 1.019 | |||
| Verhulst | 0.253 | 2.045 | 0.202 | 6.394 | 5.396 | 0.0077 | 0.352 | |||
| Gompertz | 0.048 | 2.959 | 1.925 | 0.205 | 5.986 | 6.086 | 0.0042 | 3.846 | ||
| Logistic | 0.138 | 4.0705 | 1.875 | 0.229 | 2.011 | 4.591 | 0.0 | 1.949 |
| Model No | μmax | KS | KP | Ki | Xmax | Pmax | n1 | n2 | YX/S | YP/S | α | β | Q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.179 | 1.754 | 0.121 | 3.650 | 8.337 | 0.0156 | 0.991 | ||||||
| 0.322 | 3.673 | 2.060 | 0.154 | 3.346 | 7.015 | 0.0014 | 0.348 | ||||||
| 2 | 0.257 | 2.268 | 19.389 | 0.419 | 3.803 | 7.263 | 0.0069 | 0.494 | |||||
| 0.302 | 3.013 | 17.087 | 2.248 | 1.767 | 0.151 | 2.951 | 6.948 | 0.0029 | 0.305 | ||||
| 3 | 0.365 | 3.464 | 0.247 | 0.155 | 1.855 | 6.784 | 0.005 | 0.463 | |||||
| 4 | 0.168 | 0.550 | 0.077 | 0.144 | 5.269 | 6.232 | 0.0549 | 4.099 | |||||
| 5 | 0.113 | 17.306 | 2.506 | 6.406 | 0.011 | 58.27 | 29.07 | 1.2367 | 4.71 | ||||
| 6 | 0.185 | 4.293 | 1.052 | 0.114 | 1.231 | 7.287 | 0.0231 | 3.287 | |||||
| 7 | 0.233 | 4.847 | 0.064 | 0.158 | 3.262 | 6.524 | 0.0028 | 1.348 |
| Microorganism | Substrate | Growth Model | μmax (h−1) | Reference |
|---|---|---|---|---|
| L. amylophilus | Glucose 20 g/L | 0.32 (pH 6.5) | [39] | |
| L. casei | Lactose 20 g/L | 0.511 | [40] | |
| L. plantarum | Glucose 100 g/L | 0.64 | [41] | |
| L. lactis | Glucose 20 g/L | 0.66 (pH 6.5) | [13] | |
| L. acidophilus | Glucose 10 g/L | Four-parameter Gompertz model | 0.35 (pH 6.5, 30 °C) | [42] |
| 0.43 (pH 6.5, 37 °C) | ||||
| L. helveticus | Whey ultrafiltrate powder | 0.56 | [43] | |
| L. paracasei | Rice flour | 0.993 | [44] | |
| 0.619 | ||||
| 0.811 | ||||
| L. plantarum | Sucrose 20 g/L | . | 0.0545 | [45] |
| L. plantarum | Hydrolyzed wheat flour | 0.403 | [23] | |
| L. plantarum | Lactose 40 g/L | 0.364 (pH 6.0) | [9] | |
| L. plantarum | Dairy waste water | 0.35 | [6] | |
| L. casei | Waste potato starch | 0.115 | [46] | |
| L. lactis | Glucose | 0.687 | [47] | |
| L. amylovorus | Glucose | 0.58 | [48] | |
| Sucrose | 0.32 | |||
| Starch | 0.61 | |||
| L. delbrueckii | Glucose 10 g/L | Logistic equation | 0.031 | [49] |
| L. delbrueckii | Glucose | 0.55 | [50] | |
| 0.59 | ||||
| 0.58 | ||||
| L. casei | Whey lactose | 0.265 | [20] | |
| L. delbrueckii | Potato starch | 0.372 (pH 5.5) | [51] | |
| L. helveticus | Whey lactose | 0.64 | [52] | |
| L. helveticus | Lactose 50 g/L | 0.25 | [53] | |
| L. casei | Glucose 50 g/L | 0.45 | [54] | |
| L. plantarum | Irish brown seaweeds | Modified Gompertz equation | [55] | |
| L. digitata | 0.4 | |||
| L. saccharina | 0.29 | |||
| L. plantarum | Glucose—20 g/L Vegetable juice—3 g/L RS | Modified Gompertz equation | 0.6 | [56] |
| 0.45 | ||||
| L. plantarum | Glucose—20 g/L Vegetable juice—3 g/L RS | Logistic equation | 0.62 | [56] |
| 0.53 |
| Initial Substrate Concentration, g/L | α exp | α calc | µmax, calc |
|---|---|---|---|
| 11 | 5.091 | 6.850 | 0.249 |
| 22 | 6.748 | 6.782 | 0.334 |
| 33 | 6.959 | 6.768 | 0.343 |
| 44 | 6.947 | 7.197 | 0.311 |
| 55 | 7.651 | 7.195 | 0.309 |
| Mean value | 6.679 | 6.958 | 0.309 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova-Krumova, P.; Danova, S.; Atanasova, N.; Yankov, D. Lactic Acid Production by Lactiplantibacillus plantarum AC 11S—Kinetics and Modeling. Microorganisms 2024, 12, 739. https://doi.org/10.3390/microorganisms12040739
Popova-Krumova P, Danova S, Atanasova N, Yankov D. Lactic Acid Production by Lactiplantibacillus plantarum AC 11S—Kinetics and Modeling. Microorganisms. 2024; 12(4):739. https://doi.org/10.3390/microorganisms12040739
Chicago/Turabian StylePopova-Krumova, Petya, Svetla Danova, Nikoleta Atanasova, and Dragomir Yankov. 2024. "Lactic Acid Production by Lactiplantibacillus plantarum AC 11S—Kinetics and Modeling" Microorganisms 12, no. 4: 739. https://doi.org/10.3390/microorganisms12040739
APA StylePopova-Krumova, P., Danova, S., Atanasova, N., & Yankov, D. (2024). Lactic Acid Production by Lactiplantibacillus plantarum AC 11S—Kinetics and Modeling. Microorganisms, 12(4), 739. https://doi.org/10.3390/microorganisms12040739






