Synergy of Subgroup J Avian Leukosis Virus and Chicken Infectious Anemia Virus Enhances the Pathogenicity in Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Cells, and Animals
2.2. Real-Time Quantitative PCR (qPCR) for the Detection of CIAV
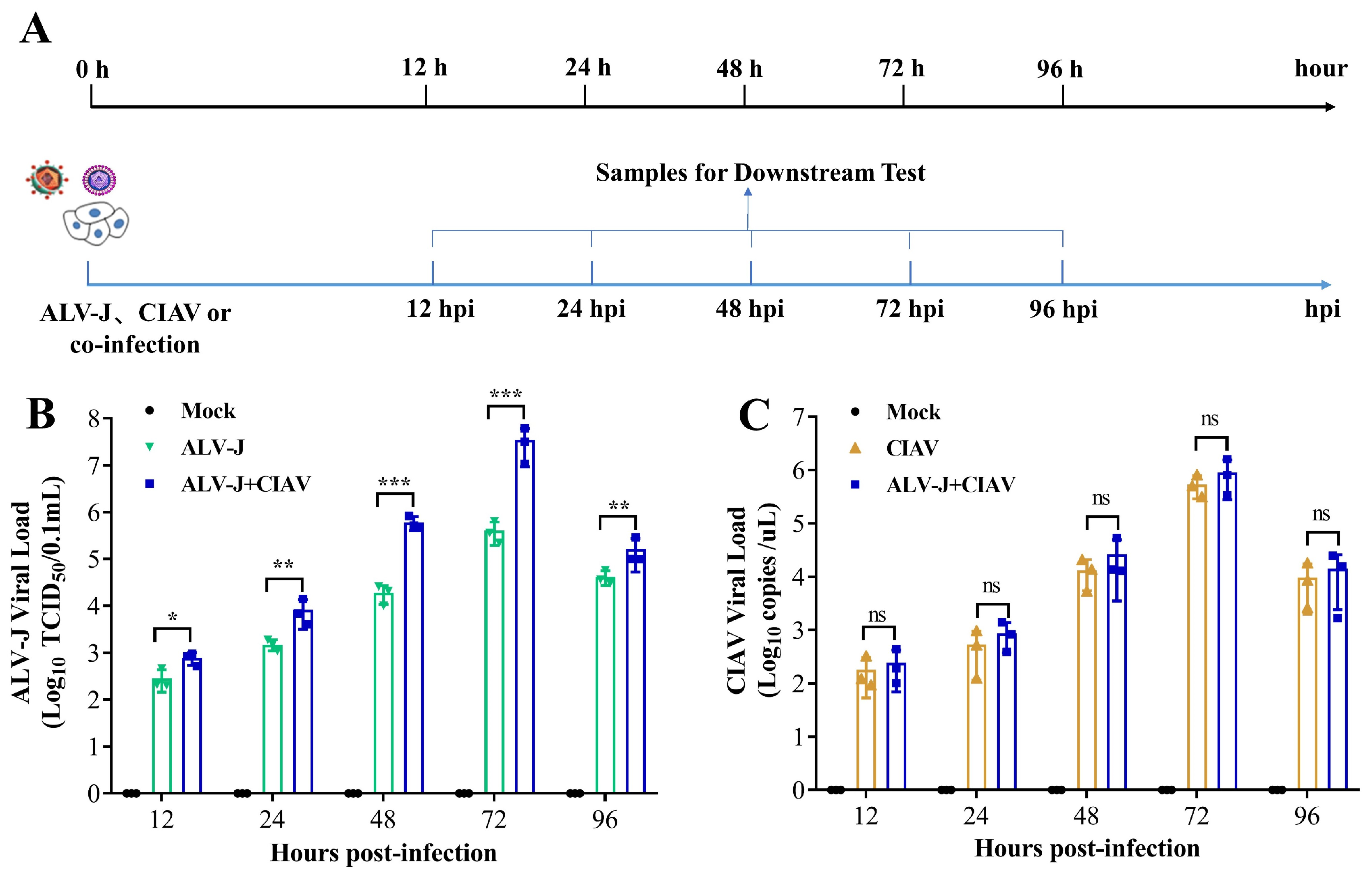
2.3. Cell Infection and Sampling Design
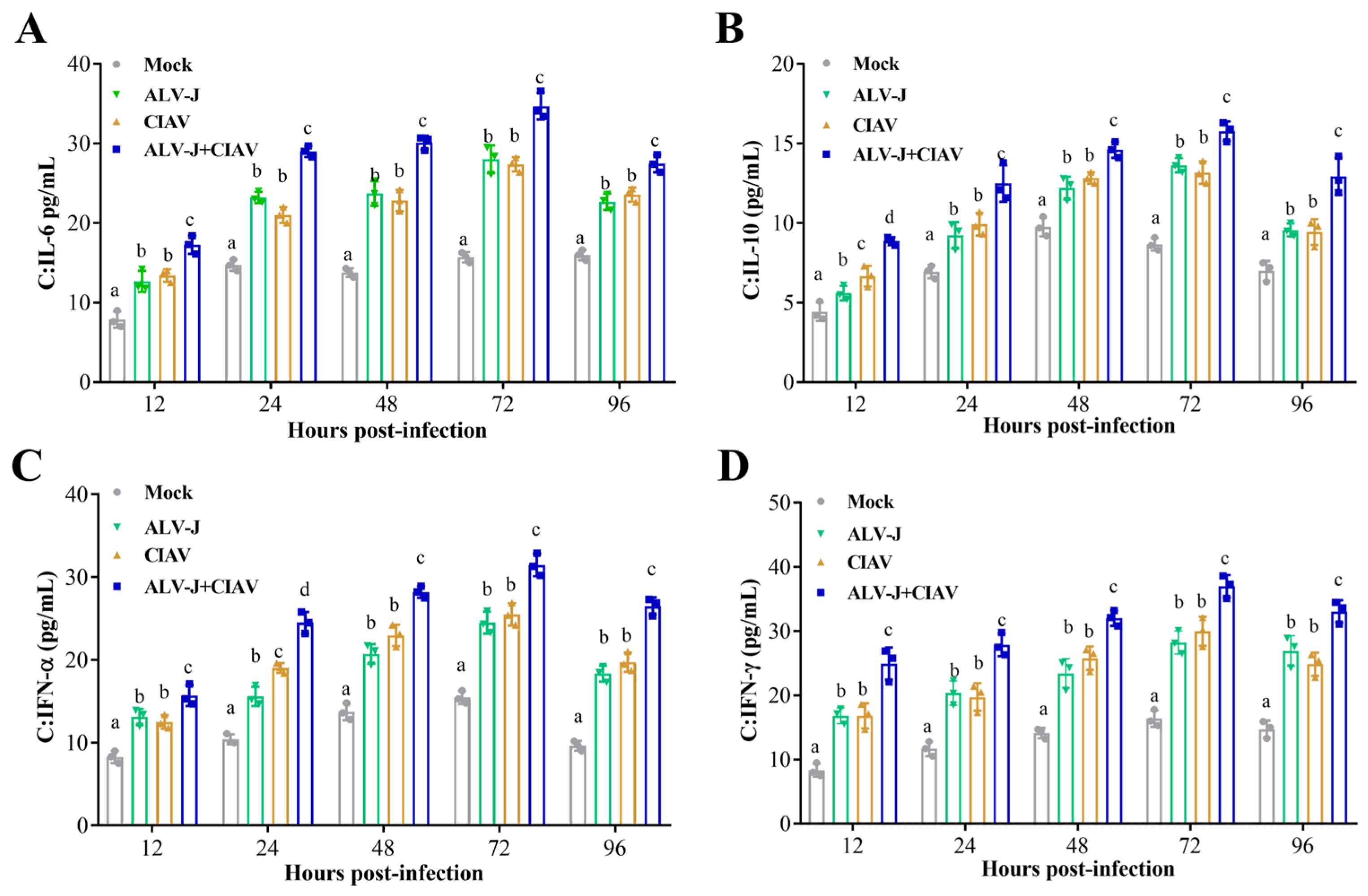
2.4. Testing Indices of Cell Infection Experiments
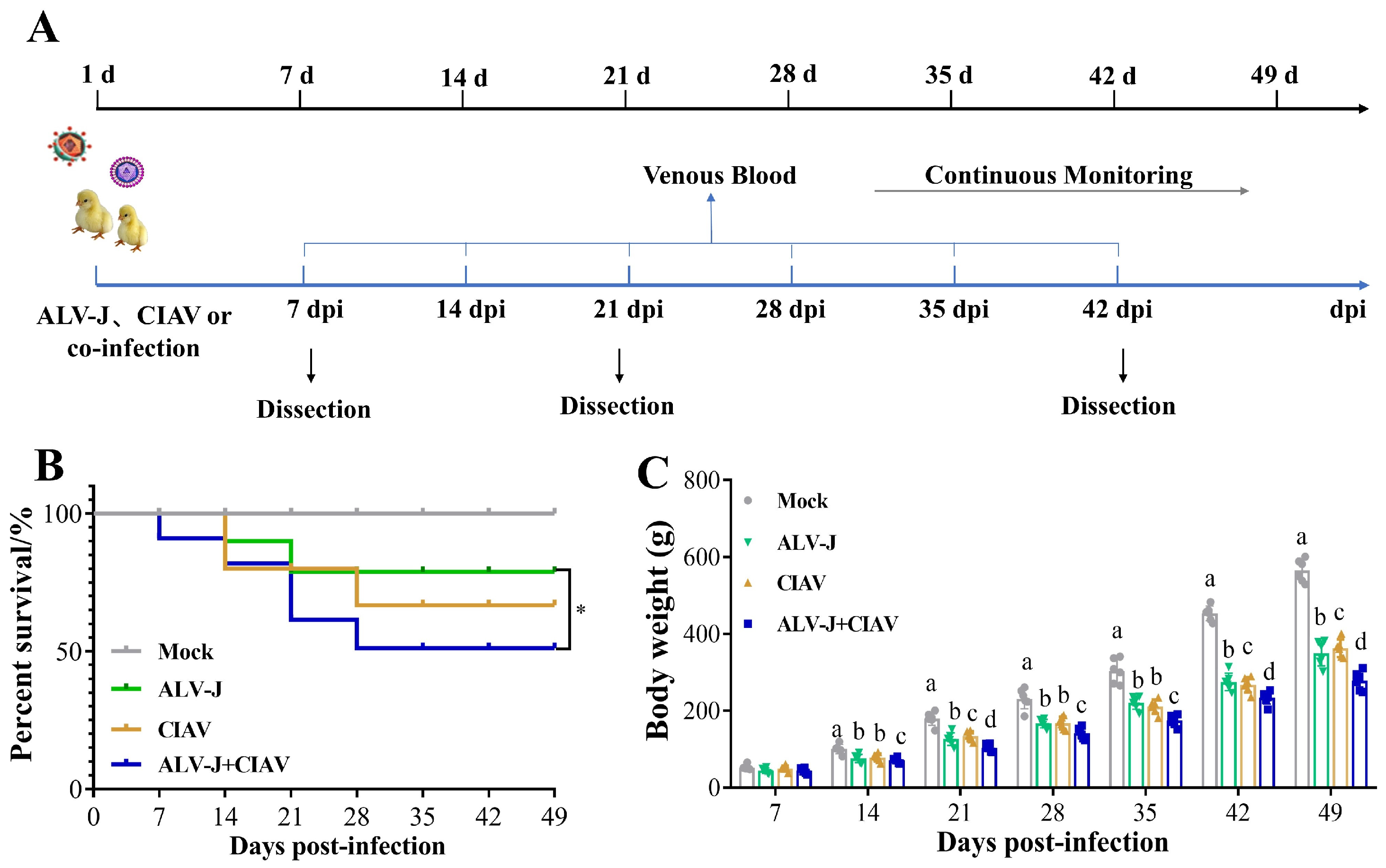
2.5. Animal Infection Experiments
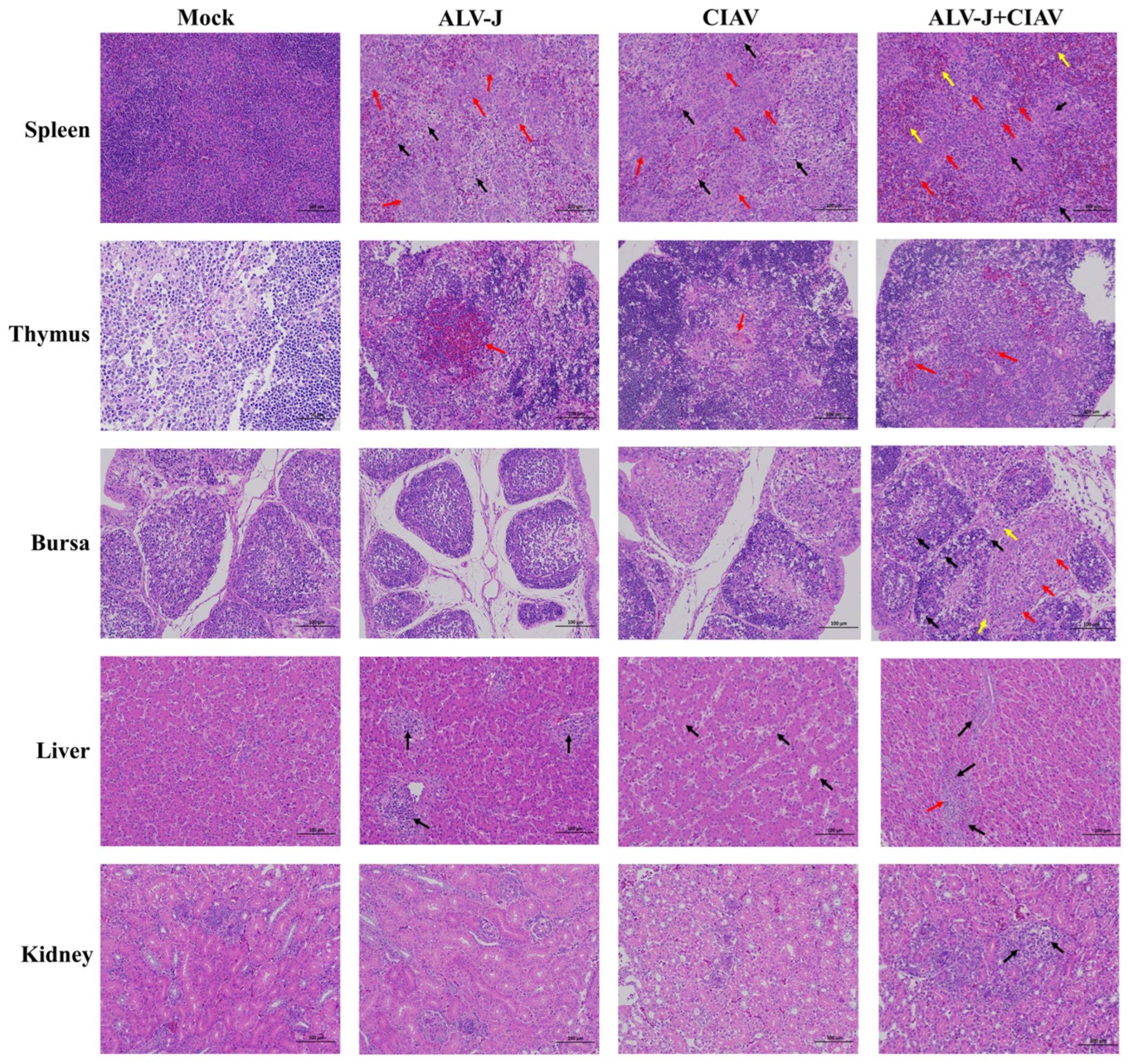
2.6. Testing Indices of Animal Experiments
2.7. Statistical Analysis
3. Results
3.1. CIAV Can Increase the Replication of ALV-J In Vitro
3.2. ALV-J and CIAV Synergistically Induce Inflammatory Mediator Secretion and Apoptosis
3.3. ALV-J and CIAV Synergistically Increase the Pathogenicity in SPF Chickens
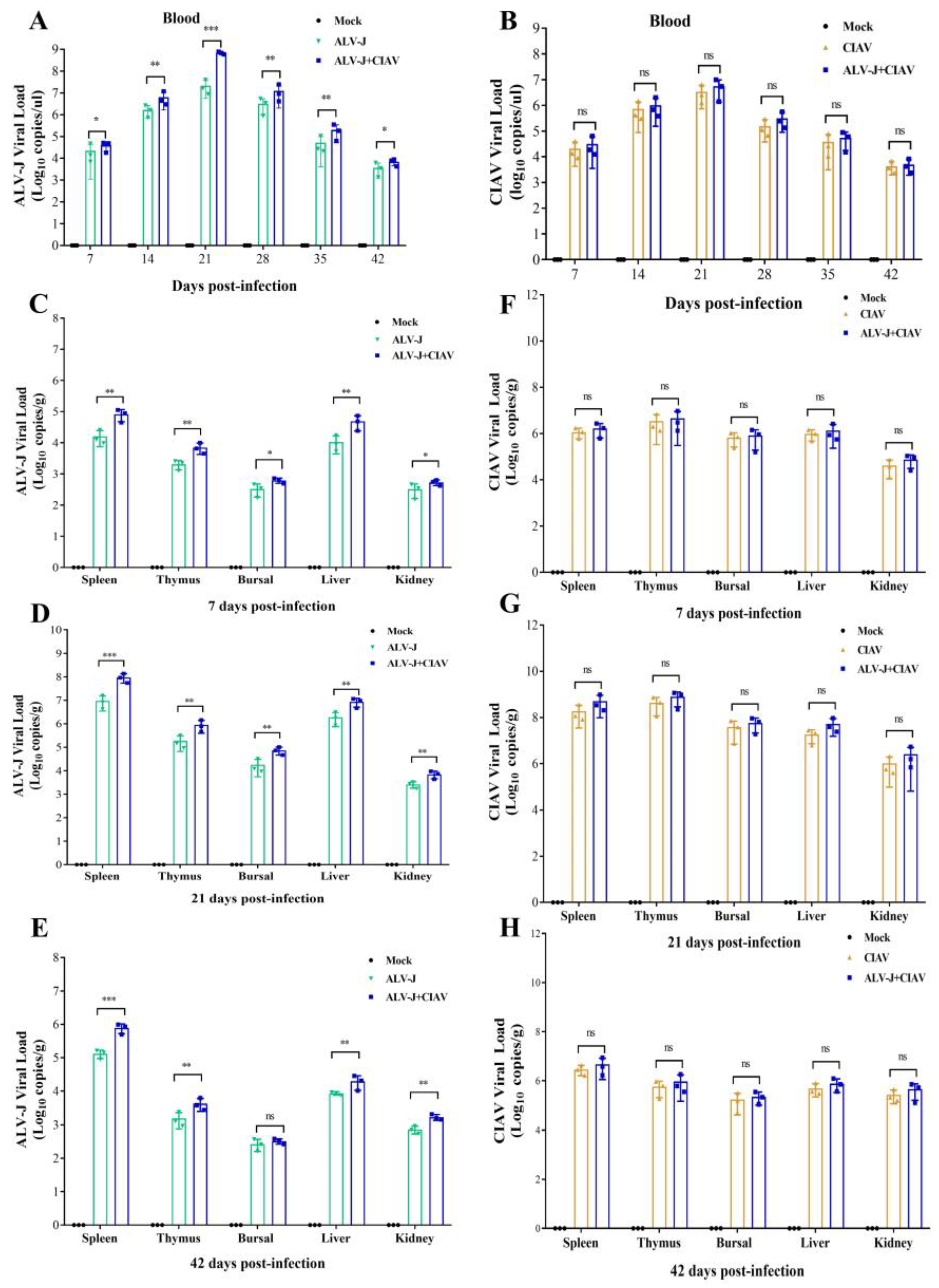
3.4. CIAV Can Promote the Replication of ALV-J In Vivo
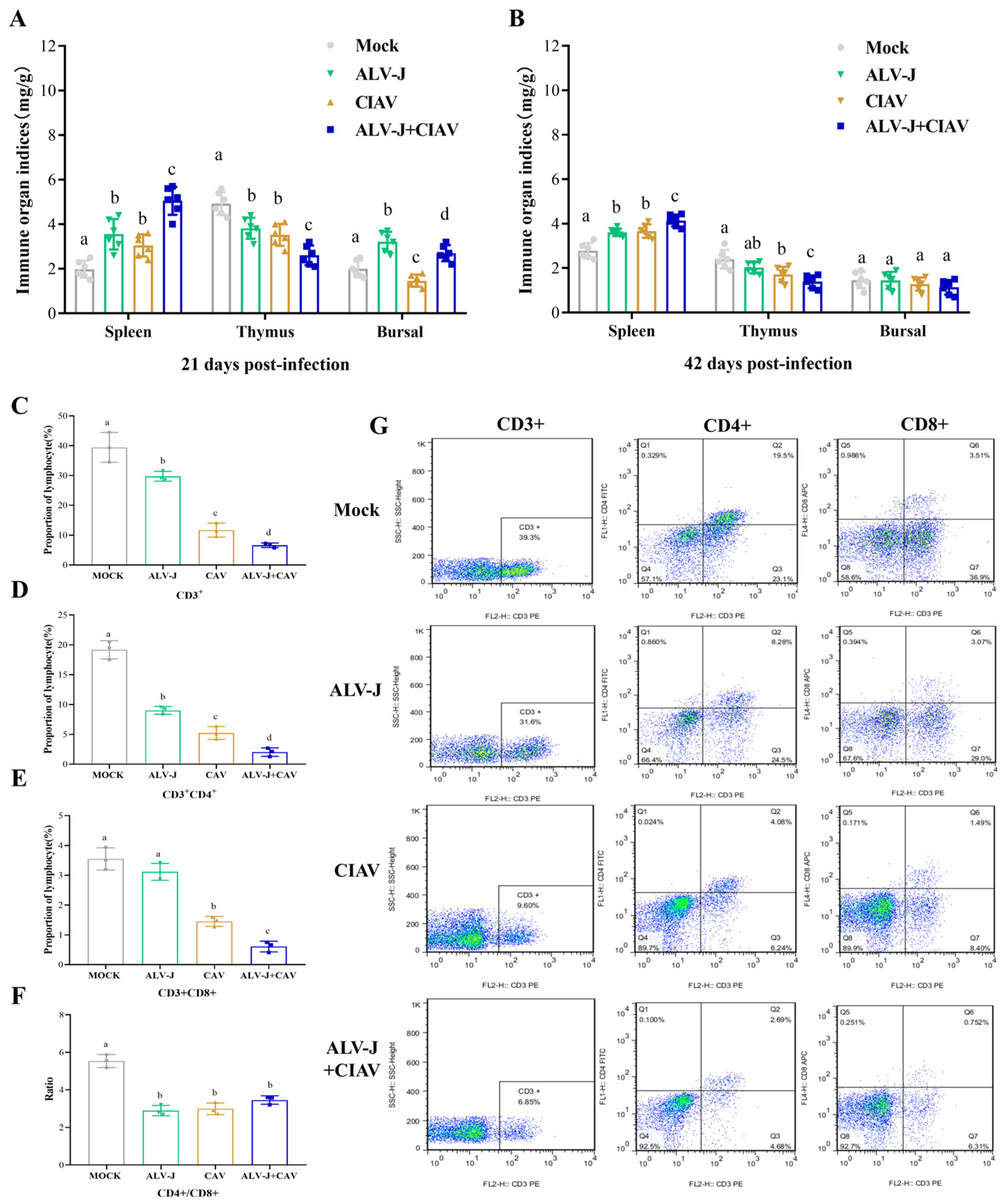
3.5. ALV-J and CIAV Synergistically Enhances the Immunosuppression in SPF Chickens
4. Discussion
4.1. Co-Infection of ALV-J and CIAV Can Significantly Aggravate the Severity of Illness In Vitro and In Vivo
4.2. CIAV Can Efficiently Promote the Replication Ability of ALV-J
4.3. Co-Infection of ALV-J and CIAV Can Aggravate Immunosuppression
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoerr, F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010, 54, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, I.M.; Schat, K.A. Virus-Induced Immunosuppression in Chickens. Avian Dis. 2018, 62, 272–285. [Google Scholar] [CrossRef]
- Song, H.; Bae, Y.; Park, S.; Kwon, H.; Lee, H.; Joh, S. Loop-mediated isothermal amplification assay for detection of four immunosuppressive viruses in chicken. J. Virol. Methods 2018, 256, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.N.; Nair, V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012, 41, 11–19. [Google Scholar] [CrossRef]
- Xu, M.; Hang, F.; Qian, K.; Shao, H.; Ye, J.; Qin, A. Chicken hepatomegaly and splenomegaly associated with novel subgroup J avian leukosis virus infection. BMC Vet. Res. 2022, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Yulong, G.; Liting, Q.; Shuai, Y.; Delong, L.; Yubao, L.; Lili, J.; Sidang, L.; Xiaomei, W. Mutations in and Expression of the Tumor Suppressor Gene p53 in Egg-Type Chickens Infected With Subgroup J Avian Leukosis Virus. Vet. Pathol. 2015, 52, 1052–1056. [Google Scholar] [CrossRef]
- Santen, V.L.V.; Joiner, K.S.; Murray, C.; Petrenko, N.; Hoerr, F.J.; Toro, H. Pathogenesis of chicken anemia virus: Comparison of the oral and the intramuscular routes of infection. Avian Dis. 2004, 48, 494–504. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhang, Y.; Chen, L.; Fang, L.; Chang, S.; Wang, Y.; Zhao, P. Construction of chicken infectious anemia virus infectious clone and study on its pathogenicity. Front. Microbiol. 2022, 13, 1016784. [Google Scholar] [CrossRef]
- Miller, M.M.; Schat, K.A. Chicken infectious anemia virus: An example of the ultimate host-parasite relationship. Avian Dis. 2004, 48, 734–745. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kwon, Y.-K.; Bae, Y.-C.; Oem, J.-K.; Lee, O.-S. Molecular characterization of chicken infectious anemia viruses detected from breeder and broiler chickens in South Korea. Poult. Sci. 2010, 89, 2426–2431. [Google Scholar] [CrossRef]
- Fatoba, A.J.; Adeleke, M.A. Chicken anemia virus: A deadly pathogen of poultry. Acta Virol. 2019, 63, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, H.; Zhang, H. Isolation, Identification, and Whole Genome Analysis of Chicken Infectious Anemia Virus in an Outbreak of Disease in Adult Layer Hens. Vet. Sci. 2023, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Wu, B.; Sun, B.; Chen, F.; Ji, J.; Ma, J.; Xie, Q. Phylogenetic and molecular characterization of chicken anemia virus in southern China from 2011 to 2012. Sci. Rep. 2013, 3, 3519. [Google Scholar] [CrossRef]
- McConnell, C.D.; Adair, B.M.; McNulty, M.S. Effects of chicken anemia virus on cell-mediated immune function in chickens exposed to the virus by a natural route. Avian Dis. 1993, 37, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-P.; Teng, M.; Li, G.-X.; Zhang, W.-K.; Wang, W.-D.; Liu, J.-L.; Li, L.-Y.; Yao, Y.; Nair, V.; Luo, J. Current Epidemiology and Co-Infections of Avian Immunosuppressive and Neoplastic Diseases in Chicken Flocks in Central China. Viruses 2022, 14, 2599. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S.; Nie, Y.; Li, W.; Li, H.; Zhang, X.; Chen, F.; Xie, Q. Synergistic Immunosuppression of Avian Leukosis Virus Subgroup J and Infectious Bursal Disease Virus Is Responsible for Enhanced Pathogenicity. Viruses 2022, 14, 2312. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ju, S.; Zhao, P.; Li, Y.; Meng, F.; Sun, P.; Cui, Z. Synergetic effects of subgroup J avian leukosis virus and reticuloendotheliosis virus co-infection on growth retardation and immunosuppression in SPF chickens. Vet. Microbiol. 2014, 172, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Wang, Q.; Shi, W.; Han, L.; Wang, J.; Ma, X.; Li, H.; Wang, F.; Su, S.; Zhao, X. Synergy of subgroup J avian leukosis virus and Eimeria tenella to increase pathogenesis in specific-pathogen-free chickens. Vet. Immunol. Immunopathol. 2016, 177, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, G.-L.; Wang, X.-M.; Du, X.-S.; Su, S.; Li, C.-G.; Nair, V.; Yao, Y.-X.; Cheng, Z.-Q. Synergistic Viral Replication of Marek’s Disease Virus and Avian Leukosis Virus Subgroup J is Responsible for the Enhanced Pathogenicity in the Superinfection of Chickens. Viruses 2018, 10, 271. [Google Scholar] [CrossRef]
- McNeilly, F.; Smyth, J.A.; Adair, B.M.; McNulty, M.S. Synergism between chicken anemia virus (CAV) and avian reovirus following dual infection of 1-day-old chicks by a natural route. Avian Dis. 1995, 39, 532–537. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Q.; Bi, X.; Shi, H.; Yang, J.; Cheng, X.; Yan, T.; Zhang, H.; Cheng, Z. The Synergy of Chicken Anemia Virus and Gyrovirus Homsa 1 in Chickens. Viruses 2023, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, K.; Pei, Y.; Xue, J.; Ruan, S.; Zhang, G. Development and Application of an MRT-qPCR Assay for Detecting Coinfection of Six Vertically Transmitted or Immunosuppressive Avian Viruses. Front. Microbiol. 2020, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Chen, W.; Zhang, X.; Zhang, H.; Li, A.; Yan, Y.; Xie, Z.; Li, H.; Lin, W.; Ma, J.; et al. Semen extracellular vesicles mediate vertical transmission of subgroup J avian leukosis virus. Virol. Sin. 2022, 37, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Krapez, U.; Barlic-Maganja, D.; Toplak, I.; Hostnik, P.; Rojs, O.Z. Biological and molecular characterization of chicken anemia virus isolates from Slovenia. Avian Dis. 2006, 50, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Farooque, M.; Li, X.; Hussain, A.; Fayyaz, A.; Bao, Y.; Xing, L.; Yu, M.; Chang, F.; Wang, S.; Liu, P.; et al. Isolation and molecular characterization of the first subgroup J avian leukosis virus from chicken in Pakistan. Infect. Genet. Evol. 2020, 85, 104425. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, L.; Jiang, Z.; Yu, Y.; Chen, X.; Xiang, Y.; Chen, J.; Li, Y.; Cao, W. Molecular characteristics of subgroup J avian leukosis virus isolated from yellow breeder chickens in Guangdong, China, during 2016–2019. Infect. Genet. Evol. 2021, 89, 104721. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.T.; Bahoussi, A.N.; Cui, X.; Shabir, S.; Wu, C.; Xing, L. Genetic diversity, distribution, and evolution of chicken anemia virus: A comparative genomic and phylogenetic analysis. Front. Microbiol. 2023, 14, 1145225. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Z.; Xu, X.; Ji, J.; Yao, L.; Kan, Y.; Xie, Q.; Bi, Y. Molecular Characteristics of Chicken Infectious Anemia Virus in Central and Eastern China from 2020 to 2022. Animals 2023, 13, 2709. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Dong, G.; Zhang, Y.; Tian, S.; Cui, Z.; Chang, S.; Zhao, P. Co-infection of fowl adenovirus with different immunosuppressive viruses in a chicken flock. Poult. Sci. 2018, 97, 1699–1705. [Google Scholar] [CrossRef]
- Wu, X.; Kong, J.; Yao, Z.; Sun, H.; Liu, Y.; Wu, Z.; Liu, J.; Zhang, H.; Huang, H.; Wang, J.; et al. A new rapid and sensitive method for detecting chicken infectious anemia virus. Front. Microbiol. 2022, 13, 994651. [Google Scholar] [CrossRef]
- Körner, C.; Krämer, B.; Schulte, D.; Coenen, M.; Mauss, S.; Fätkenheuer, G.; Oldenburg, J.; Nattermann, J.; Rockstroh, J.K.; Spengler, U. Effects of HCV co-infection on apoptosis of CD4+ T-cells in HIV-positive patients. Clin. Sci. 2009, 116, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, F.; Shu, X.; Li, Q.; Ding, Q.; Hao, C.; Yan, X.; Xu, M.; Hu, H. Co-infection of porcine epidemic diarrhoea virus and porcine deltacoronavirus enhances the disease severity in piglets. Transbound. Emerg. Dis. 2022, 69, 1715–1726. [Google Scholar] [CrossRef]
- Feng, M.; Xie, T.; Li, Y.; Zhang, N.; Lu, Q.; Zhou, Y.; Shi, M.; Sun, J.; Zhang, X. A balanced game: Chicken macrophage response to ALV-J infection. Vet. Res. 2019, 50, 20. [Google Scholar] [CrossRef]
- Heidari, M.; Zhang, H.M.; Sharif, S. Marek’s disease virus induces Th-2 activity during cytolytic infection. Viral Immunol. 2008, 21, 203–214. [Google Scholar] [CrossRef]
- Parvizi, P.; Read, L.; Abdul-Careem, M.F.; Lusty, C.; Sharif, S. Cytokine gene expression in splenic CD4(+) and CD8(+) T-cell subsets of chickens infected with Marek’s disease virus. Viral Immunol. 2009, 22, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liberto, M.C.; Zicca, E.; Pavia, G.; Quirino, A.; Marascio, N.; Torti, C.; Focà, A. Virological Mechanisms in the Coinfection between HIV and HCV. Mediat. Inflamm. 2015, 2015, 320532. [Google Scholar] [CrossRef]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Bochnakian, A.; Zhen, A.; Zisoulis, D.G.; Idica, A.; KewalRamani, V.N.; Neel, N.; Daugaard, I.; Hamdorf, M.; Kitchen, S.; Lee, K.; et al. Interferon-Inducible MicroRNA miR-128 Modulates HIV-1 Replication by Targeting TNPO3 mRNA. J. Virol. 2019, 93, e00364-19. [Google Scholar] [CrossRef] [PubMed]
- Modai, S.; Farberov, L.; Herzig, E.; Isakov, O.; Hizi, A.; Shomron, N. HIV-1 infection increases microRNAs that inhibit Dicer1, HRB and HIV-EP2, thereby reducing viral replication. PLoS ONE 2019, 14, e0211111. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, D.; Zhou, J.; Du, X.; Zhang, X.; Liu, X.; Ding, L.; Cheng, Z. miR-155 facilitates the synergistic replication between avian leukosis virus subgroup J and reticuloendotheliosis virus by targeting a dual pathway. J. Virol. 2023, 97, e0093723. [Google Scholar] [CrossRef]
- Liu, H.; Ma, K.; Liu, M.; Yang, C.; Huang, X.; Zhao, Y.; Qi, K. Histologic findings and viral antigen distribution in natural coinfection of layer hens with subgroup J avian leukosis virus, Marek’s disease virus, and reticuloendotheliosis virus. J. Vet. Diagn. Investig. 2019, 31, 761–765. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zheng, G.; Zhou, D.; Huang, L.; Dong, J.; Cheng, Z. High-frequency and activation of CD4+CD25+ T cells maintain persistent immunotolerance induced by congenital ALV-J infection. Vet. Res. 2021, 52, 119. [Google Scholar] [CrossRef] [PubMed]
- Cheraï, M.; Hamel, Y.; Baillou, C.; Touil, S.; Guillot-Delost, M.; Charlotte, F.; Kossir, L.; Simonin, G.; Maury, S.; Cohen, J.L.; et al. Generation of Human Alloantigen-Specific Regulatory T Cells Under Good Manufacturing Practice-Compliant Conditions for Cell Therapy. Cell Transplant. 2015, 24, 2527–2540. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, M.; Franchini, G. T Cell Subsets in the Germinal Center: Lessons from the Macaque Model. Front. Immunol. 2018, 9, 348. [Google Scholar] [CrossRef]
- Velu, V.; Mylvaganam, G.; Ibegbu, C.; Amara, R.R. Tfh1 Cells in Germinal Centers During Chronic HIV/SIV Infection. Front. Immunol. 2018, 9, 1272. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Li, W.; Nie, Y.; Chen, S.; Li, H.; Zhang, X.; Xie, Q.; Chen, W. Synergy of Subgroup J Avian Leukosis Virus and Chicken Infectious Anemia Virus Enhances the Pathogenicity in Chickens. Microorganisms 2024, 12, 740. https://doi.org/10.3390/microorganisms12040740
Xu H, Li W, Nie Y, Chen S, Li H, Zhang X, Xie Q, Chen W. Synergy of Subgroup J Avian Leukosis Virus and Chicken Infectious Anemia Virus Enhances the Pathogenicity in Chickens. Microorganisms. 2024; 12(4):740. https://doi.org/10.3390/microorganisms12040740
Chicago/Turabian StyleXu, Huijuan, Wenxue Li, Yu Nie, Sheng Chen, Hongxin Li, Xinheng Zhang, Qingmei Xie, and Weiguo Chen. 2024. "Synergy of Subgroup J Avian Leukosis Virus and Chicken Infectious Anemia Virus Enhances the Pathogenicity in Chickens" Microorganisms 12, no. 4: 740. https://doi.org/10.3390/microorganisms12040740





