The Antimicrobial and Antibiofilm Abilities of Fish Oil Derived Polyunsaturated Fatty Acids and Manuka Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the Methylglyoxal (MGO) Concentration of Manuka Honey Samples
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4. Minimum Biofilm Eradication Concentration (MBEC)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guy, R.A. History of cod liver oil as a remedy. Am. J. Dis. Child. 1923, 26, 112–116. [Google Scholar] [CrossRef]
- Jones, R. Honey and healing through the ages. J. ApiProduct ApiMedical Sci. 2009, 1, 2–5. [Google Scholar] [CrossRef]
- Banoub, D. Buying vitamins: Newfoundland cod liver oil and the real subsumption of nature, 1919–1939. Geoforum 2018, 92, 1–8. [Google Scholar] [CrossRef]
- Chandola, H.M.; Tanna, I. Role of Omega-3 Fatty Acids in Brain and Neurological Health with Special Reference to Clinical Depression. In Omega-3 Fatty Acids in Brain and Neurological Health; Elsevier: Amsterdam, The Netherlands, 2014; pp. 163–179. [Google Scholar]
- Uauy, R.; Hoffman, D.R.; Periano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Demarquoy, J.; Le Borgne, F. Biosynthesis, metabolism and function of protectins and resolvins. Clin. Lipidol. 2017, 9, 683–693. [Google Scholar] [CrossRef]
- Colby, J.K.; Abdulnour, R.-E.E.; Sham, H.P.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Hellmann, J.; Wong, B.; Cui, Y.; El-Chemaly, S.; et al. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Protective for Injured Epithelia. Am. J. Pathol. 2016, 186, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, Z.; Dong, J.; Zhang, J.; Xia, Y.; Shu, R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb. Pathog. 2016, 99, 196–203. [Google Scholar] [CrossRef]
- Sun, M.; Dong, J.; Xia, Y.; Shu, R. Antibacterial activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against planktonic and biofilm growing Streptococcus mutans. Microb. Pathog. 2017, 107, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Simplice, M.R.; Macaire, W.H.; Hervé, N.N.F.; Fabrice, T.D.; Justin, D.D.; François, T.; Jules-Roger, K. Chemical composition and antibacterial activity of oils from Chrysicthys nigrodigitatus and Hepsetus odoe, two freshwater fishes from Yabassi, Cameroon. Lipids Health Dis. 2018, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Le, P.N.T.; Desbois, A.P. Antibacterial Effect of Eicosapentaenoic Acid against Bacillus cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target. Mar. Drugs 2017, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Gutierrez-Gines, M.J.; Maxfield, A.; Gaw, S.; Dickinson, N.; Horswell, J.; Robinson, B. Chemical elements and the quality of mānuka (Leptospermum scoparium) honey. Foods 2021, 10, 1670. [Google Scholar] [CrossRef]
- Green, K.J.; Lawag, I.L.; Locher, C.; Hammer, K.A. Correlation of the antibacterial activity of commercial manuka and Leptospermum honeys from Australia and New Zealand with methylglyoxal content and other physicochemical characteristics. PLoS ONE 2022, 17, e0272376. [Google Scholar] [CrossRef] [PubMed]
- Kilty, S.J.; Duval, M.; Chan, F.T.; Ferris, W.; Slinger, R. Methylglyoxal: (Active agent of manuka honey) in vitro activity against bacterial biofilms. Int. Forum Allergy Rhinol. 2011, 1, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka honey is bactericidal against Pseudomonas aeruginosa and results in differential expression of oprF and algD. Microbiology 2012, 158, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Wootton, M.; Howe, R.; Cooper, R. A demonstration of the susceptibility of clinical isolates obtained from cystic fibrosis patients to manuka honey. Arch. Microbiol. 2015, 3, 597–601. [Google Scholar] [CrossRef][Green Version]
- Shukrimi, A.; Sulaiman, A.R.; Halim, A.Y.; Azril, A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Med. J. Malaysia 2008, 63, 44–46. Available online: http://www.e-mjm.org/2008/v63n1/Wagner_Type_II_Diabetic_Foot_Ulcers.pdf (accessed on 19 October 2018).
- Cooper, R.; Molan, P.; Krishnamoorthy, L.; Harding, K. Manuka Honey Used to Heal a Recalcitrant Surgical Wound. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 758–759. [Google Scholar] [CrossRef]
- Lee Ventola, C. The Antibiotic Resistance Crisis Part 1: Causes and Threats. P&T 2015, 40, 277–283. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521/pdf/ptj4004277.pdf (accessed on 30 October 2018).
- Barber, S.; Sutherland, N. O’Neill Review into Antibiotic Resistance. 2017. Available online: https://researchbriefings.files.parliament.uk/documents/CDP-2017-0074/CDP-2017-0074.pdf (accessed on 6 April 2020).
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation–Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Kazek-Kęsik, A.; Brzychczy-Włoch, M.; Łos, M.J.; Ateba, C.N.; Mehrbod, P.; Ghavami, S.; Shyntum, D.Y. Recent Advances in the Control of Clinically Important Biofilms. Int. J. Mol. Sci. 2022, 23, 9526. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Moreno-Ruiz, E.; Goyard, S.; d’Enfert, C.; Janbon, G. Biofilm Formation Studies in Microtiter Plate Format; Humana Press: Totowa, NJ, USA, 2012; pp. 369–377. [Google Scholar]
- EUCAST. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, PIX-XV. [Google Scholar]
- Hobby, C.R.; Herndon, J.L.; Morrow, C.A.; Peters, R.E.; Symes, S.J.K.; Giles, D.K. Exogenous fatty acids alter phospholipid composition, membrane permeability, capacity for biofilm formation, and antimicrobial peptide susceptibility in Klebsiella pneumoniae. Microbiologyopen 2019, 8, e00635. [Google Scholar] [CrossRef]
- Norris, P.C.; Arnardottir, H.; Sanger, J.M.; Fichtner, D.; Keyes, G.S.; Serhan, C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Perween, N.; Prakash, S.K.; Siddiqui, O. Multi Drug Resistant Klebsiella Isolates in Burn Patients: A Comparative Study. J. Clin. Diagn. Res. 2015, 9, DC14-6. [Google Scholar] [PubMed]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer-A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199. [Google Scholar] [CrossRef]
- Halim, M.M.A.; Eyada, I.K.; Tongun, R.M. Prevalence of multidrug drug resistant organisms and hand hygiene compliance in surgical NICU in Cairo University Specialized Pediatric Hospital. Egypt. Pediatr. Assoc. Gaz. 2018, 66, 103–111. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int. J. Food Microbiol. 2007, 113, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Burton, N.; Cooper, R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2536–2542. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; Mcdougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT-Food Sci. Technol. 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Kateel, R.; Bhat, G.; Baliga, S.; Augustine, A.J.; Ullal, S.; Adhikari, P. Antibacterial action of Tropical honey on various bacteria obtained from diabetic foot ulcer. Complement. Ther. Clin. Pract. 2018, 30, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Islam, M.A.; Gan, S.H.; Khalil, M.I. Honey: A potential therapeutic agent for managing diabetic wounds. Evid.-Based Complement. Altern. Med. 2014, 2014, 169130. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Riaz, S.; Mazhar, S.; Essa, R.; Maryam, M.; Saleem, Y.; Syed, Q.; Perveen, I.; Bukhari, B.; Ashfaq, S.; et al. Microbial production of docosahexaenoic acid (DHA): Biosynthetic pathways, physical parameter optimization, and health benefits. Arch. Microbiol. 2023, 205, 321. [Google Scholar] [CrossRef] [PubMed]

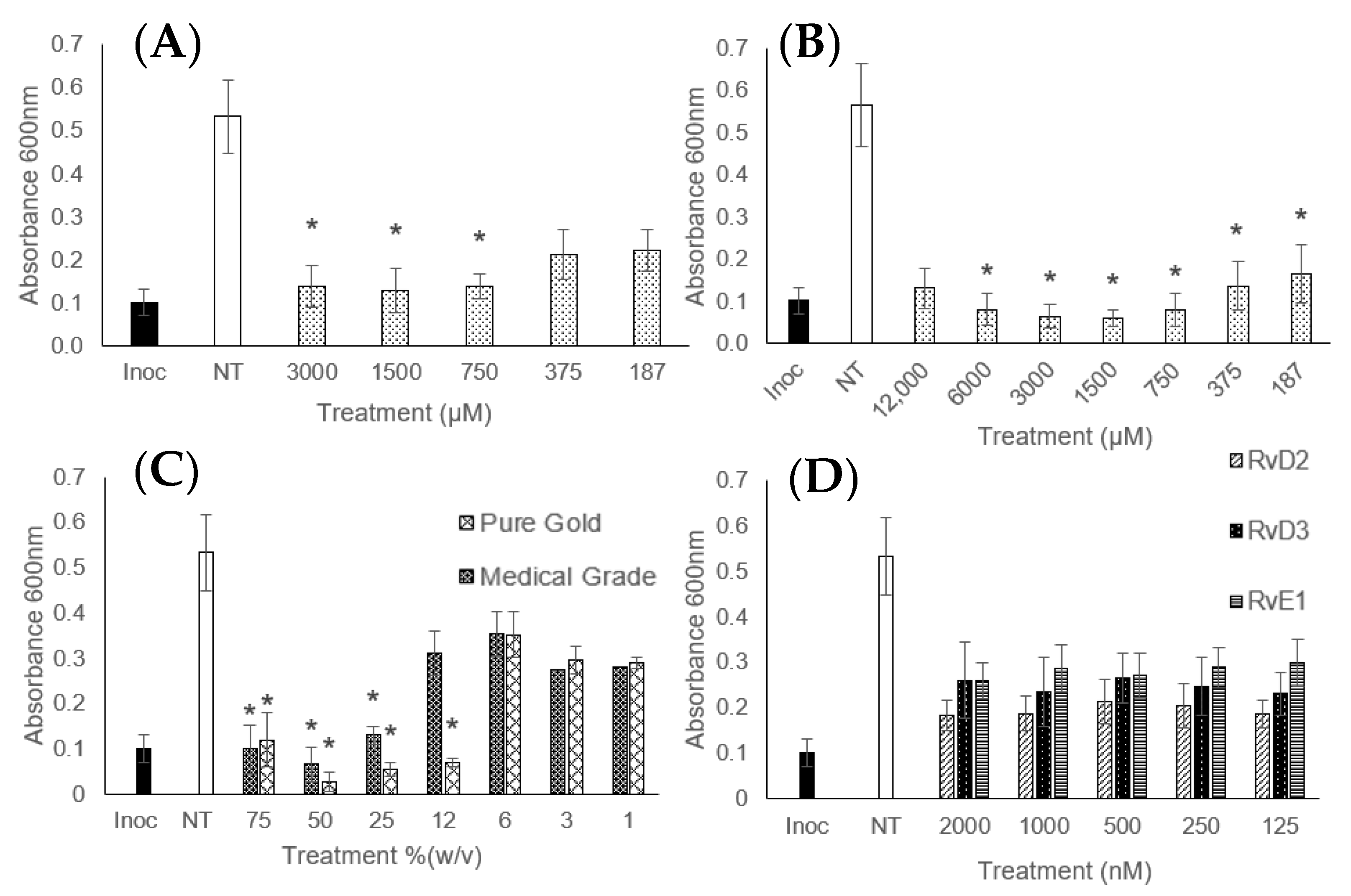
| Treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHA (µM) | EPA (µM) | RvD2 (nM) | RvD3 (nM) | RvE1 (nM) | ||||||||
| MIC | MBC | MBEC | MIC | MBC | MBEC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. epidermidis | 1500 | 6000 | – | 375 | 3000 | – | PI 31 | – | PI 125 | – | PI 125 | – |
| S. aureus | 375 | 3000 | – | 375 | 1500 | – | PI 31 | – | PI 125 | – | PI 125 | – |
| MRSA | 750 | 6000 | 24,000 | 187 | 1500 | 6000 | 31 | NBA | PI 125 | – | PI 125 | – |
| E. coli | 0.25 | 12,000 | 24,000 | 375 | 3000 | 6000 | 125 | NBA | 125 | NBA | 125 | NBA |
| CRE | PI 187 | – | – | PI 187 | – | – | PI 125 | – | PI 125 | – | PI 125 | – |
| K. pneumoniae | NIA | – | NBA | PI 375 | – | NBA | NIA | – | PI 2000 | – | PI 2000 | – |
| P. aeruginosa | PI 187 | – | – | PI 187 | – | – | NIA | – | PI 125 | – | NIA | – |
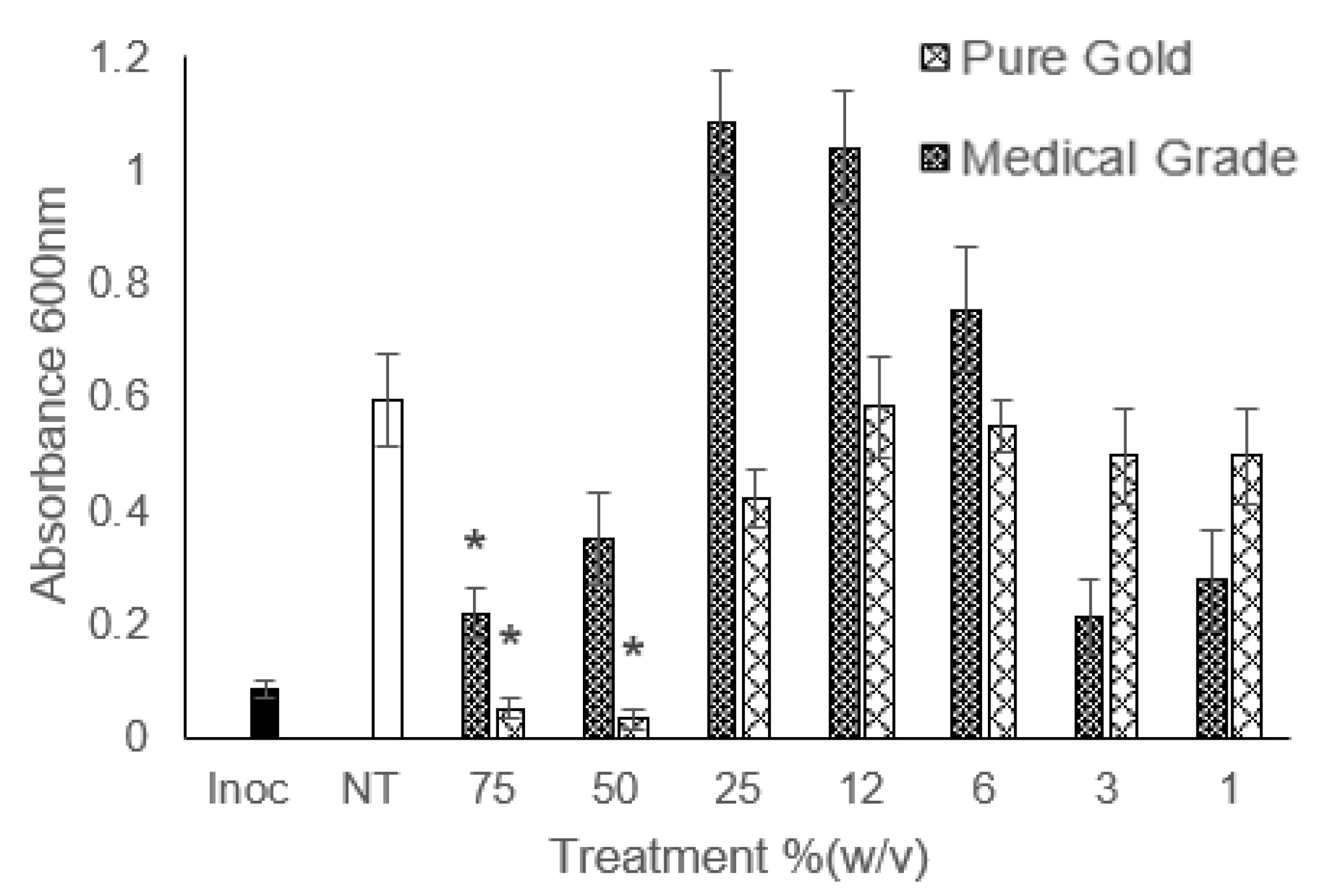
| Pure Gold Honey (%w/v) | Medical-Grade Honey (%w/v) | Pure Gold MGO Equivalent (%w/v) | Medical-Grade MGO Equivalent (%w/v) | |||||||||
| Organism | MIC | MBC | MBEC | MIC | MBC | MIC | MBC | MIC | MBC | |||
| S. epidermidis | 12.5 | 25 | – | 25 | 75 | 12.5 | – | 25 | – | |||
| MRSA | 12.5 | 25 | 75 | 25 | NBA | 12.5 | 12.5 | 25 | 50 | |||
| K. pneumoniae | 50 | 50 | 50 | 50 | NBA | 50 | – | 50 | – | |||
| S. aureus | 12.5 | 25 | – | 25 | NBA | 12.5 | – | 25 | – | |||
| E. coli | 50 | 75 | 50 | 50 | NBA | 50 | – | 50 | – | |||
| CRE | 50 | 50 | – | 50 | NBA | 50 | 50 | 50 | 75 | |||
| P. aeruginosa | 50 | 75 | – | 75 | NBA | 50 | – | 75 | – | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clare, J.; Lindley, M.R.; Ratcliffe, E. The Antimicrobial and Antibiofilm Abilities of Fish Oil Derived Polyunsaturated Fatty Acids and Manuka Honey. Microorganisms 2024, 12, 778. https://doi.org/10.3390/microorganisms12040778
Clare J, Lindley MR, Ratcliffe E. The Antimicrobial and Antibiofilm Abilities of Fish Oil Derived Polyunsaturated Fatty Acids and Manuka Honey. Microorganisms. 2024; 12(4):778. https://doi.org/10.3390/microorganisms12040778
Chicago/Turabian StyleClare, Jenna, Martin R. Lindley, and Elizabeth Ratcliffe. 2024. "The Antimicrobial and Antibiofilm Abilities of Fish Oil Derived Polyunsaturated Fatty Acids and Manuka Honey" Microorganisms 12, no. 4: 778. https://doi.org/10.3390/microorganisms12040778
APA StyleClare, J., Lindley, M. R., & Ratcliffe, E. (2024). The Antimicrobial and Antibiofilm Abilities of Fish Oil Derived Polyunsaturated Fatty Acids and Manuka Honey. Microorganisms, 12(4), 778. https://doi.org/10.3390/microorganisms12040778







