Purpureocillium jiangxiense sp. nov.: Entomopathogenic Effects on Ostrinia furnacalis and Galleria mellonella
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Fungal Isolation and Culture
2.3. Morphological Observations
2.4. DNA Extraction, PCR, and Sequencing
2.5. Phylogenetic Analyses
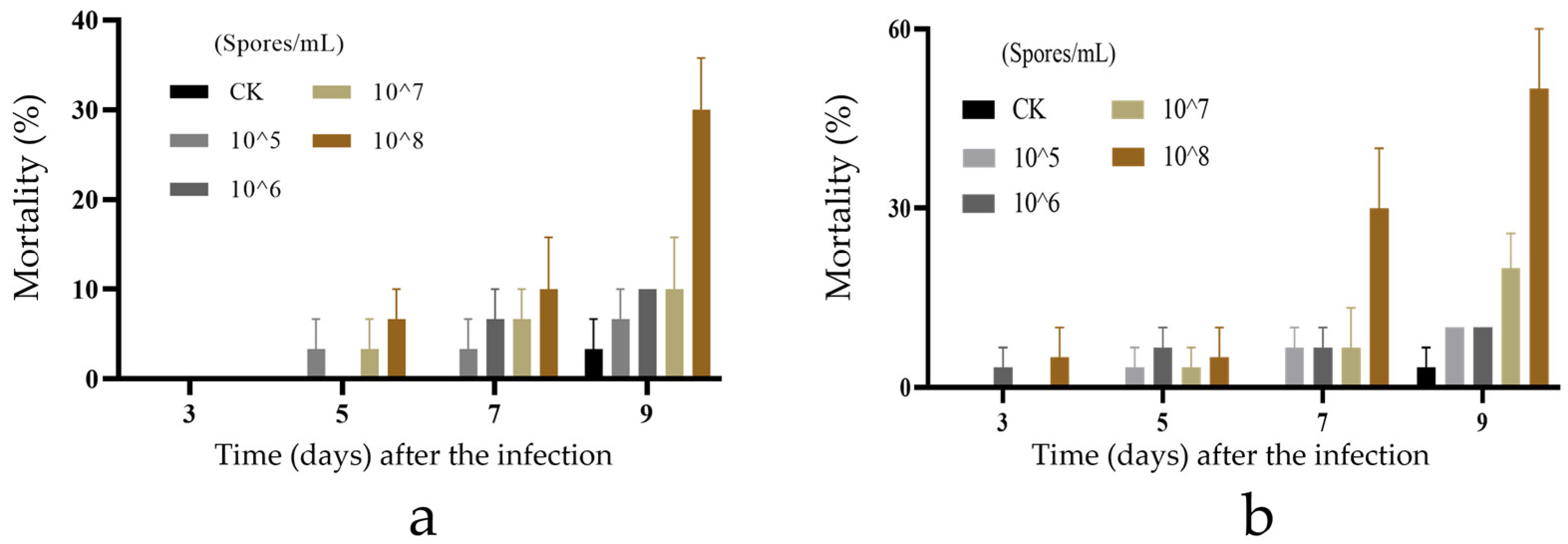
2.6. Virulence Assay of Purpureocillium Isolates
3. Results
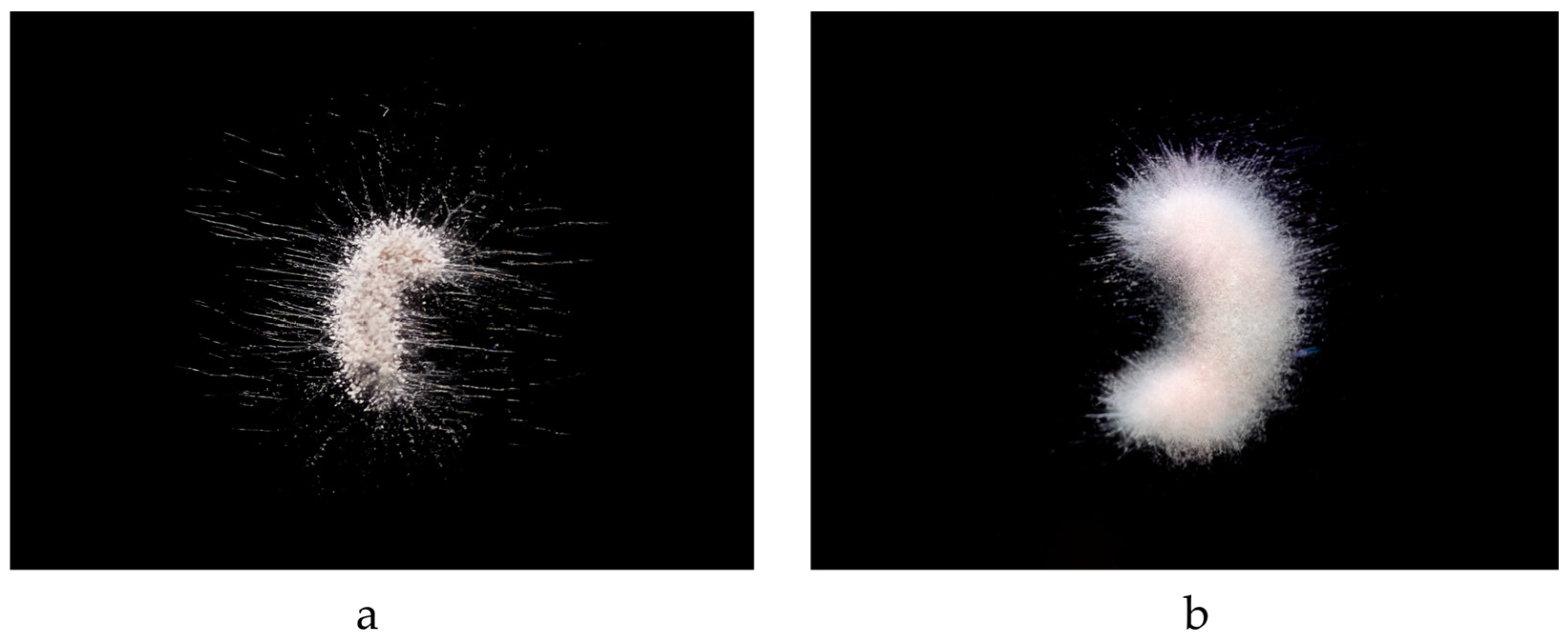
3.1. Morphological Features
3.2. Phylogenetic Analyses
3.3. Biological Activity of New Species of Purpureocillium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karunarathna, S.C.; Ashwath, N.; Jeewon, R. Editorial: The Potential of Fungi for Enhancing Crops and Forestry Systems. Front. Microbiol. 2021, 12, 813051. [Google Scholar] [CrossRef]
- Horianopoulos, L.C.; Gluck-Thaler, E.; Gelber, I.B.; Cowen, L.E.; Geddes-McAlister, J.; Landry, C.R.; Schwartz, I.S.; Scott, J.A.; Sellam, A.; Sheppard, D.C.; et al. The Canadian Fungal Research Network: Current challenges and future opportunities. Can. J. Microbiol. 2021, 67, 13–22. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Cardona, N.L.; Franco-Sierra, N.D.; Alvarez, J.C. Complete mitogenome of the biocontroller fungus Purpureocillium sp (Ascomycota, Ophiocordycipitaceae, Hypocreales). Mitochondrial DNA B 2018, 3, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Yeh, L.K.; Ma, D.; Lin, H.C.; Sun, C.C.; Tan, H.Y.; Chen, H.C.; Chen, S.Y.; Sun, P.L.; Hsiao, C.H. Paecilomyces/Purpureocillium keratitis: A consecutive study with a case series and literature review. Med. Mycol. 2020, 58, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef] [PubMed]
- Baron, N.C.; Pollo, A.D.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. Peerj 2020, 8, e9005. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, P.; Eder, R.; Consoli, E.; Krauss, J.; Kiewnick, S. Integrated control of Meloidogyne incognita in tomatoes using fluopyram and Purpureocillium lilacinum strain 251. Crop Prot. 2019, 124, 104874. [Google Scholar] [CrossRef]
- Kumar, K.K.; Arthurs, S. Recent advances in the biological control of citrus nematodes: A review. Biol. Control 2021, 157, 104593. [Google Scholar] [CrossRef]
- Bao, Z.X.; Liu, R.; Li, C.Q.; Pan, X.R.; Zhao, P.J. Pathogenicity and Metabolites of Purpureocillium lavendulum YMF1.00683 against Meloidogyne incognita. Pathogens 2022, 11, 795. [Google Scholar] [CrossRef]
- Xu, W.F.; Yang, J.L.; Meng, X.K.; Gu, Z.G.; Zhang, Q.L.; Lin, L.B. Understanding the Transcriptional Changes during Infection of Meloidogyne incognita Eggs by the Egg-Parasitic Fungus Purpureocillium lilacinum. Front. Microbiol. 2021, 12, 617710. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, F.F.; Li, H.; Zhang, W.T.; Wang, Q.; Zhang, B.X.; Sun, Y.X.; Rao, X.J. Virulence of the Bio-Control Fungus Purpureocillium lilacinum Against Myzus persicae (Hemiptera: Aphididae) and Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.; Pacheco-Esquivel, J.; Carrasco-Rueda, F.; Christopher, Y.; Gonzalez, C.; Ramos, D.; Urbina, H.; Blackwell, M. Zombie bugs? The fungus Purpureocillium cf. lilacinum may manipulate the behavior of its host bug Edessa rufomarginata. Mycologia 2014, 106, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Hernández, R.A.; Toledo, J.; Valle-Mora, J.; Holguín-Meléndez, F.; Liedo, P.; Huerta-Palacios, G. Pathogenicity and virulence of Purpureocillium lilacinum (Hypocreales: Ophiocordycipitaceae) on Mexican fruit fly adults. Fla. Entomol. 2019, 102, 309–314. [Google Scholar] [CrossRef]
- Mustu, M.; Demirci, F.; Köksal, M.; Serbes, C.; Armagan, B. Mortality effects of Isaria farinosa and Purpureocillium lilacinum (Sordariomycetes: Hypocreales) on the two spotted spider mite Tetranychus urticae (Acari: Tetranychidae) and its predator Neoseiulus californicus (Acari: Phytoseiidae) under controlled conditions. Entomol. Gen. 2016, 35, 243–252. [Google Scholar] [CrossRef]
- Chen, W.; Xie, W.W.; Cai, W.; Thaochan, N.; Hu, Q.B. Entomopathogenic Fungi Biodiversity in the Soil of Three Provinces Located in Southwest China and First Approach to Evaluate Their Biocontrol Potential. J. Fungi 2021, 7, 984. [Google Scholar] [CrossRef]
- Chatterton, N.J.; Hsiao, C.; Asay, K.H.; Jensen, K.B.; Wang, R.R. Nucleotide sequence of the internal transcribed spacer region of rDNA in barley, Hordeum vulgare L. (Gramineae). Plant Mol. Biol. 1992, 20, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.H.; Hyten, A.S.; Spatafora, J.W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.L.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- NY/T 1154.6-2006; Pesticides guidelines for laboratory bioactivity tests. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2006.
- Li, J.P.; Zhang, G.D.; Yu, H.; Huang, L.D.; Zeng, W.B.; Wang, Y.B. Complete mitochondrial genome of the important bio-control fungus Purpureocillium lilacinum (Ophiocordycipitaceae, Hypocreales) and its phylogenetic analysis. Mitochondrial DNA B 2020, 5, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Medina, R.P.; Ponce-Angulo, D.G.; Raymundo, T.; Müller-Morales, C.A.; Escudero-Leyva, E.; Guillén, J.C.; Bautista-de Lucio, V.M. Purpureocillium roseum sp. nov. A new ocular pathogen for humans and mice resistant to antifungals. Mycoses 2021, 64, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Herrero, N. A novel monopartite dsRNA virus isolated from the entomopathogenic and nematophagous fungus Purpureocillium lilacinum. Arch. Virol. 2016, 161, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Isaac, G.S.; El-Deriny, M.M.; Taha, R.G. Efficacy of Purpureocillium lilacinum AUMC 10149 as biocontrol agent against root-knot nematode Meloidogyne incognita infecting tomato plant. Braz. J. Biol. 2021, 84, 253–261. [Google Scholar] [CrossRef]
- Perdomo, H.; Cano, J.; Gené, J.; García, D.; Hernández, M.; Guarro, J. Polyphasic analysis of Purpureocillium lilacinum isolates from different origins and proposal of the new species Purpureocillium lavendulum. Mycologia 2013, 105, 151–161. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Hurdeal, V.G.; Pem, D.; Dissanayake, L.S.; Wijesinghe, S.N.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Francisco, C.S.; Ma, X.; Zwyssig, M.M.; McDonald, B.A.; Palma-Guerrero, J. Morphological changes in response to environmental stresses in the fungal plant pathogen Zymoseptoria tritici. Sci. Rep. 2019, 9, 9642. [Google Scholar] [CrossRef]
- Rudolph, S.; Maciá-Vicente, J.G.; Lotz-Winter, H.; Schleuning, M.; Piepenbring, M. Temporal variation of fungal diversity in a mosaic landscape in Germany. Stud. Mycol. 2018, 89, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Carter, D.A. Cellular plasticity of pathogenic fungi during infection. PLoS Pathog. 2020, 16, e1008571. [Google Scholar] [CrossRef]
- Hebert, P.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Jorna, J.; Linde, J.B.; Searle, P.C.; Jackson, A.C.; Nielsen, M.E.; Nate, M.S.; Saxton, N.A.; Grewe, F.; Herrera-Campos, M.D.; Spjut, R.W.; et al. Species boundaries in the messy middle-A genome-scale validation of species delimitation in a recently diverged lineage of coastal fog desert lichen fungi. Ecol. Evol. 2021, 11, 18615–18632. [Google Scholar] [CrossRef]
- Dunn, C.W.; Zapata, F.; Munro, C.; Siebert, S.; Hejnol, A. Pairwise comparisons across species are problematic when analyzing functional genomic data. Proc. Natl. Acad. Sci. USA 2018, 115, E409–E417. [Google Scholar] [CrossRef] [PubMed]
- Stott, C.M.; Bobay, L.M. Impact of homologous recombination on core genome phylogenies. BMC Genom. 2020, 21, 829. [Google Scholar] [CrossRef] [PubMed]
- Heled, J.; Drummond, A.J. Bayesian Inference of Species Trees from Multilocus Data. Mol. Biol. Evol. 2010, 27, 570–580. [Google Scholar] [CrossRef]
- Roberts, E.; Eargle, J.; Wright, D.; Luthey-Schulten, Z. MultiSeq: Unifying sequence and structure data for evolutionary analysis. BMC Bioinform. 2006, 7, 382. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Carlsen, T.; Kjoller, R.; Koljalg, U.; Pennanen, T.; Rosendahl, S.; Stenlid, J.; et al. Fungal community analysis by high-throughput sequencing of amplified markers—A user’s guide. New Phytol. 2013, 199, 288–299. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef] [PubMed]
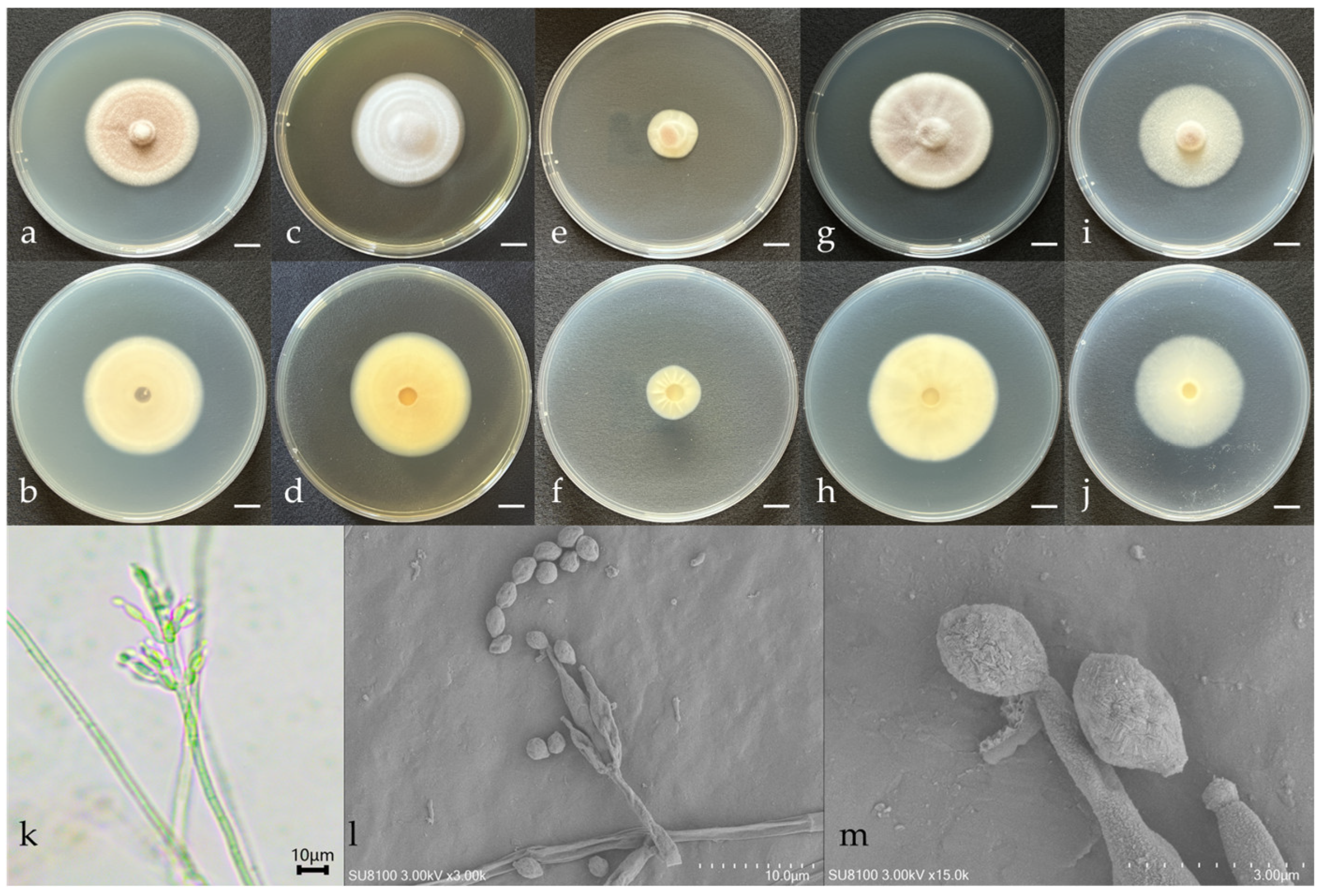

| Sample Number | Strain | Collection Site | Vegetation | Longitude and Latitude |
|---|---|---|---|---|
| JX13B | JX13B01 | Nanchang City, Jiangxi Province, China | forest | 115°45′18″ E, 28°47′28″ N |
| JX17D | JX17D04 | Fuzhou City, Jiangxi Province, China | orchard | 116°59′11″ E, 27°21′32″ N |
| Species | Voucher Information | GenBank Accession Number | ||
|---|---|---|---|---|
| ITS | nrLSU | tef1 | ||
| P. atypicola | NBRC 106945 | LC008212 | LC008347 | |
| P. lavendulum | CBS 128677 | MH864976 | NG067468 | |
| P. lavendulum | FMR 10376 | FR734106 | FR775489 | FR775516 |
| P. lilacinum | CBS 284.36 | AY624189 | MH876593 | EF468792 |
| CBS 432.87 | HQ842819 | MH873778 | AY624228 | |
| P. roseum | IOM 325363 | MT560195 | MT560197 | |
| P. sodanum | IBRC-M 30175 | KX668542 | ||
| P. jiangxiense sp.nov. | JX17D04 | PP555636 | PP555645 | PP658209 |
| P. jiangxiense sp.nov. | JX13B01T | PP555637 | PP555646 | PP658210 |
| C. gunnii | ARSEF 6828 | HM140630 | HM140633 | HM140636 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Tang, Y.; Liu, T.; Hu, H.; Ou, C.; Hu, Q.; Weng, Q. Purpureocillium jiangxiense sp. nov.: Entomopathogenic Effects on Ostrinia furnacalis and Galleria mellonella. Microorganisms 2024, 12, 1041. https://doi.org/10.3390/microorganisms12061041
Chen W, Tang Y, Liu T, Hu H, Ou C, Hu Q, Weng Q. Purpureocillium jiangxiense sp. nov.: Entomopathogenic Effects on Ostrinia furnacalis and Galleria mellonella. Microorganisms. 2024; 12(6):1041. https://doi.org/10.3390/microorganisms12061041
Chicago/Turabian StyleChen, Wei, Yanhong Tang, Tongyi Liu, Hongwang Hu, Cuiyi Ou, Qiongbo Hu, and Qunfang Weng. 2024. "Purpureocillium jiangxiense sp. nov.: Entomopathogenic Effects on Ostrinia furnacalis and Galleria mellonella" Microorganisms 12, no. 6: 1041. https://doi.org/10.3390/microorganisms12061041





