Comparative Analysis of Laboratory-Based and Spectroscopic Methods Used to Estimate the Algal Density of Chlorella vulgaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Strain Culture Conditions
2.2. Determination of Algal Density
2.2.1. Determination of Algal Biomass by Filtration
2.2.2. Determination of Algal Biomass by Evaporation
2.2.3. Measurement of Chlorophyll a Concentration and Magnesium Content
2.2.4. Determination of Cell Count
2.2.5. Estimation of Algal Biomass Based on the Detection of Induced Chlorophyll a Fluorescence
2.2.6. Determination of Algal Optical Density by Spectrophotometry
2.3. Data Analysis
3. Results
3.1. Results of Laboratory Measurements Used for the Determination of Algal Density
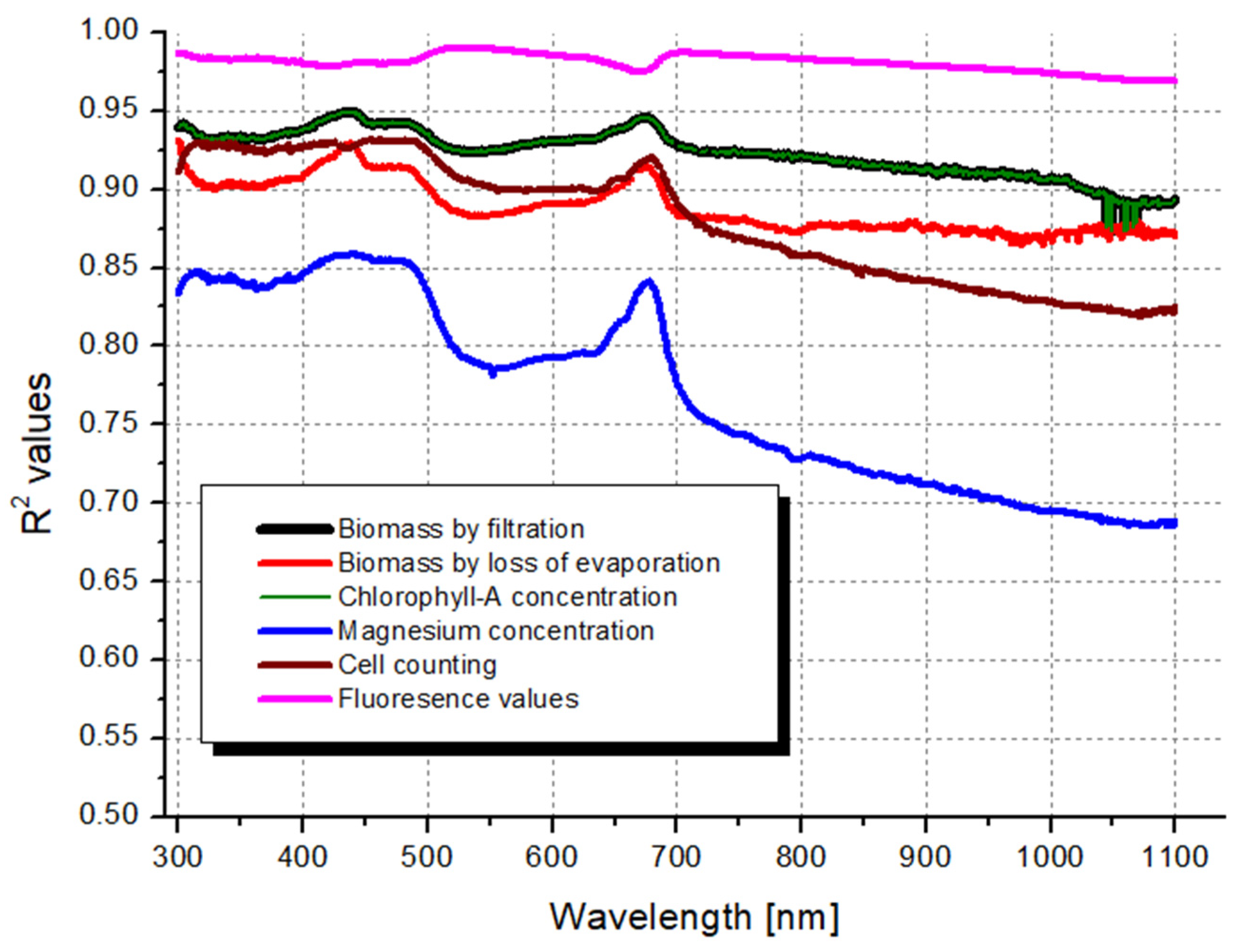
3.2. Coefficient of Determination Correlation Scanning on the Whole Spectra
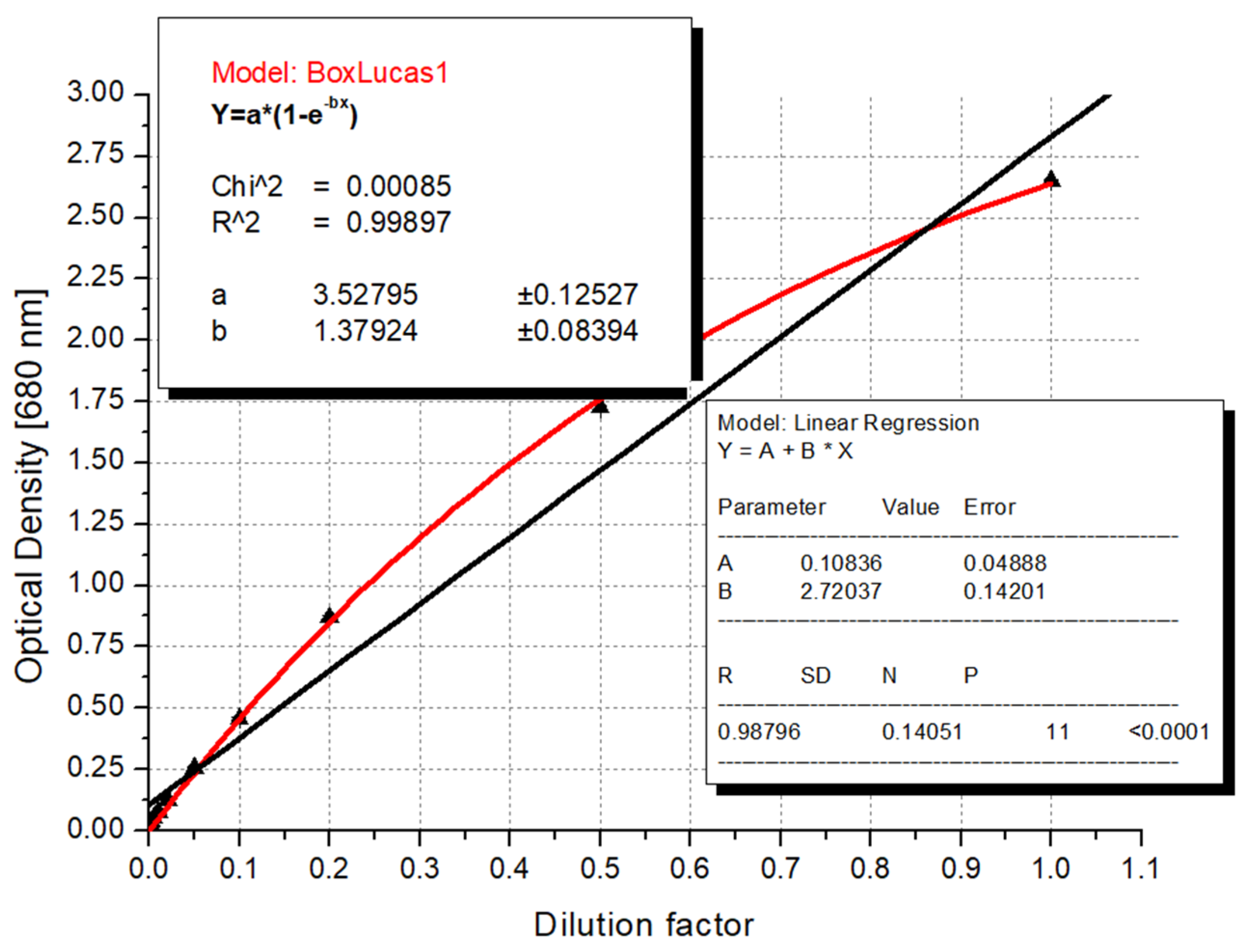
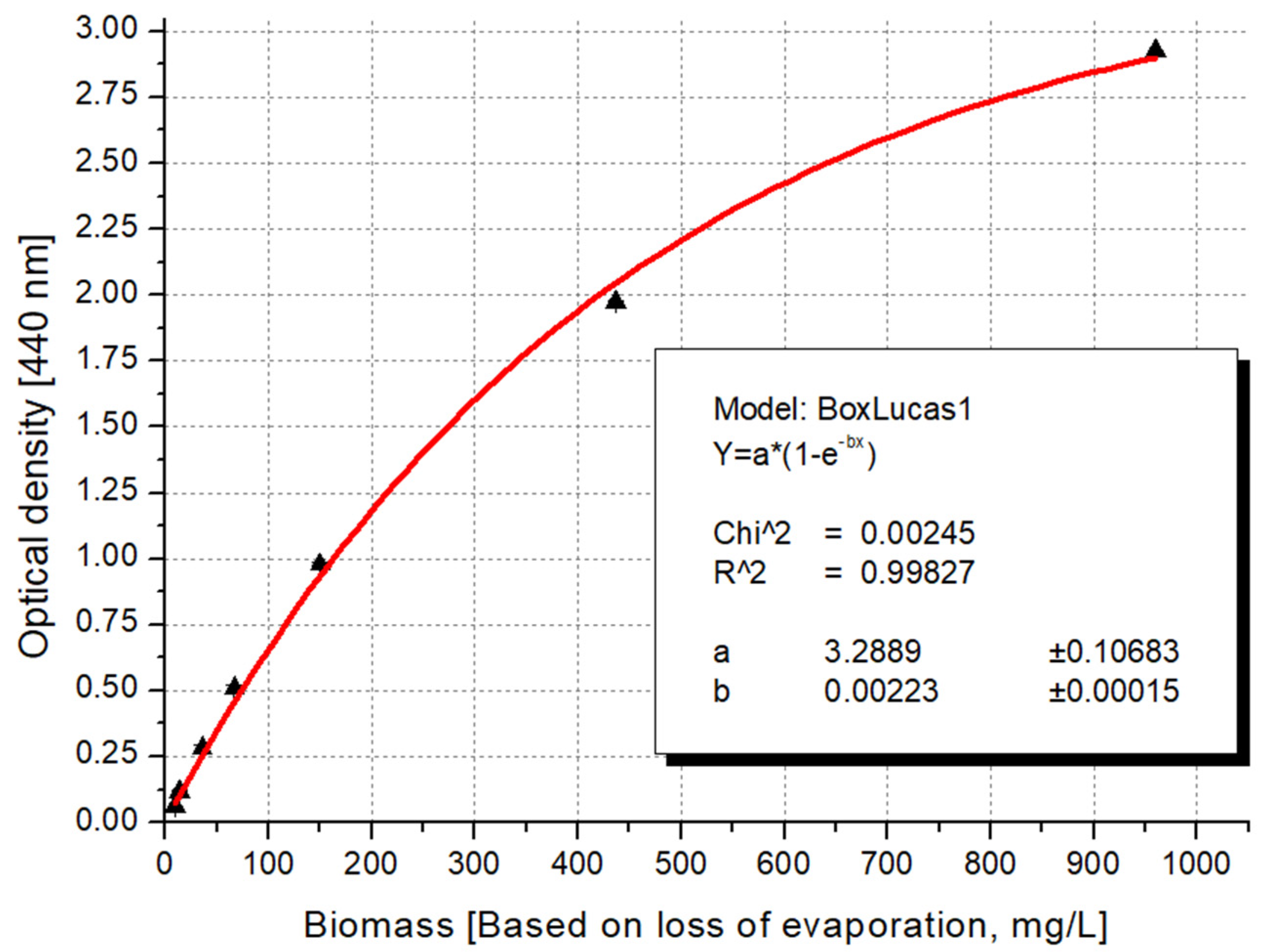
3.3. Recommended Formula for Algal Biomass Estimation
3.4. The Mathematical Basis for Using the Logistic Curve
- A: Absorbance, [ ];
- c: Concentration of algae, [mg/dm3, on dry base];
- k: A specific light absorption coefficient characteristic of the given algae, [ ];
- Amax: The maximum absorbance characteristic of the given algae, [ ].
4. Discussion
4.1. Possible Areas of Application of the Results of Our Study
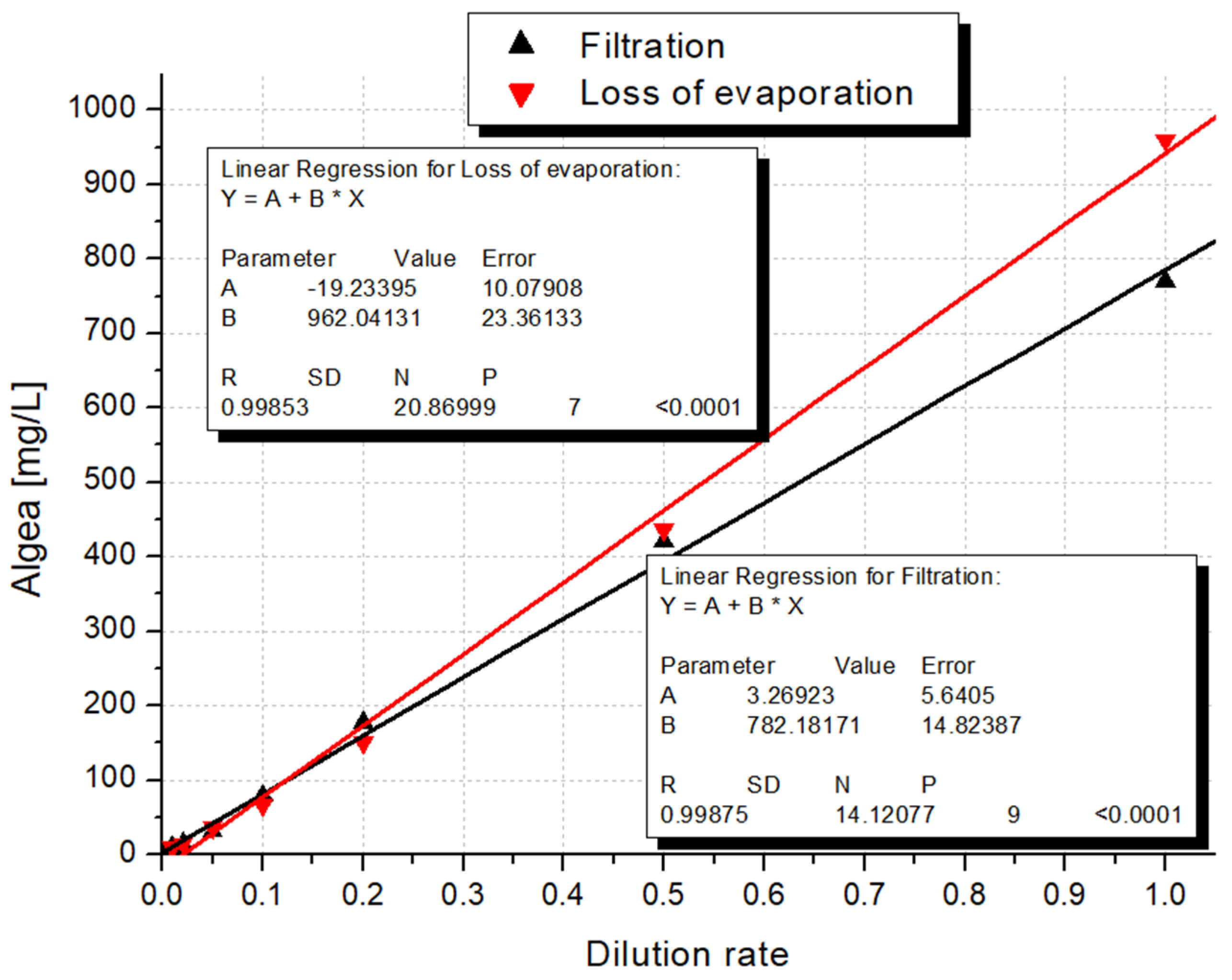
4.2. Biomass Determination: Filtration or Loss of Evaporation
4.3. Novel Automatized Method for Cell Counting Procedure and New Values for Cell Weight and Magnesium Content of Chlorella vulgaris
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutschlechner, M.; Walter, A.; Colleselli, L.; Griesbeck, C.; Schöbel, H. Enhancing carotenogenesis in terrestrial microalgae by UV-A light stress. J. Appl. Phycol. 2022, 34, 1943–1955. [Google Scholar] [CrossRef]
- De Mendonca, H.V.; Assemany, P.; Abreu, M.; Couto, E.; Maciel, A.M.; Duarte, R.L.; Reis, A. Microalgae in a global world: New solutions for old problems? Renew. Energy 2021, 165, 842–862. [Google Scholar] [CrossRef]
- Lobus, N.V. Biogeochemical role of algae in aquatic ecosystems: Basic research and applied biotechnology. J. Mar. Sci. Eng. 2022, 10, 1846. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Walcker, R.; Kardol, P.; Geisen, S.; Heger, T.; Lamentowicz, M.; Hamard, S.; Lara, E. Contribution of soil algae to the global carbon cycle. New Phytol. 2022, 234, 64–76. [Google Scholar] [CrossRef]
- Chisti, Y. Microalgae biotechnology: A brief introduction. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Manzoni Maroneze, M., Queiroz, M.I., Queiroz Zepka, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 3–23. [Google Scholar]
- Caroppo, C.; Pagliara, P. Microalgae: A Promising Future. Microorganisms 2022, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Bourgougnon, N.; Burlot, A.-S.; Jacquin, A.G. Algae for global sustainability? In Advances in Botanical Research; Jacquot, J.-P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 145–212. [Google Scholar]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- West, J.B. The strange history of atmospheric oxygen. Physiol. Rep. 2022, 10, e15214. [Google Scholar] [CrossRef]
- Grégoire, M.; Oschlies, A.; Canfield, D.; Castro, C.; Ciglenečki, I.; Croot, P.; Salin, K.; Schneider, B.; Serret, P.; Slomp, C.P.; et al. Ocean oxygen: The role of the Ocean in the oxygen we breathe and the threat of deoxygenation. In Future Science Brief No. 10 of the European Marine Board; Rodriguez Perez, A., Kellett, P., Alexander, B., Muñiz Piniella, Á., Van Elslander, J., Heymans, J.J., Eds.; Zenodo: Ostend, Belgium, 2023; pp. 4–8. [Google Scholar]
- Schaffer, J.D.; Sebetich, M.J. Effects of aquatic herbicides on primary productivity of phytoplankton in the laboratory. Bull. Environ. Contam. Toxicol. 2004, 72, 1032–1037. [Google Scholar] [CrossRef]
- Jyothi, K.; Krishna Prasad, M.; Mohan Narasimha Rao, G. Algae in fresh water ecosystem. Phykos 2016, 46, 25–31. [Google Scholar]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.; Selmeczy, G.B.; Lengyel, E.; Tapolczai, K. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia 2023, 850, 2707–2733. [Google Scholar] [CrossRef]
- Suresh, K.; Tang, T.; van Vliet, M.T.H.; Bierkens, M.F.P.; Strokal, M.; Sorger-Domenigg, F.; Wad, Y. Recent advancement in water quality indicators for eutrophication in global freshwater lakes. Environ. Res. Lett. 2023, 18, 063004. [Google Scholar] [CrossRef]
- Ballah, M.; Vishwakalyan, B.; Neetoo, H. Assessment of the physico-chemical quality and extent of algal proliferation in water from an impounding reservoir prone to eutrophication. J. Ecol. Environ. 2019, 43, 5. [Google Scholar]
- Gibbs, S.P. The evolution of algal chloroplasts. In Origins of Plastids; Lewin, R.A., Ed.; Chapman & Hall: New York, NY, USA, 1992; pp. 107–117. [Google Scholar]
- Singh, J.; Saxena, R.C. An introduction to microalgae: Diversity and significance. In Handbook of Marine Microalgae; Se-Kwon, K., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 11–24. [Google Scholar]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2023, 850, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Sadvakasova, A.K.; Kossalbayev, B.D.; Bauenova, M.O.; Balouch, H.; Leong, Y.K.; Zayadan, B.K.; Allakhverdiev, S.I. Microalgae as a key tool in achieving carbon neutrality for bioproduct production. Algal Res. 2023, 72, 103096. [Google Scholar] [CrossRef]
- Han, F.F.; Wang, W.L.; Li, Y.G.; Shen, G.M.; Wan, M.X.; Wang, J. Changes of biomass, lipid content and fatty acids composi-tion under a light-dark cyclic culture of Chlorellapyrenoidosa in response to different temperature. Bioresour. Technol. 2013, 132, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, P.; Min, M.; Ma, X.; Wang, J.; Griffith, R.; Hussain, F.; Peng, P.; Xie, Q.; Li, Y.; et al. Environment-enhancing algal biofuel production using wastewaters. Renew. Sustain. Energy Rev. 2014, 36, 256–269. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Mobin, S.; Alam, F. Some promising microalgal species for commercial applications: A review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- Nayaka, S.; Toppo, K.; Verma, S. Adaptation in algae to environmental stress and ecological conditions. In Plant Adaptation Strategies in Changing Environment; Shukla, V., Kumar, S., Kumar, N., Eds.; Springer: Singapore, 2017; pp. 103–115. [Google Scholar]
- Silva, A.; Figueiredo, S.A.; Sales, M.G.; Delerue-Matos, C. Ecotoxicity tests using the green algae Chlorella vulgaris—A useful tool in hazardous effluents management. J. Hazard. Mater. 2009, 167, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Dong, X.; Liu, Y.; Wang, C.; Baattrup-Pederson, A.; Riis, T. Using river microalgae as indicators for freshwater biomonitoring: Review of published research and future directions. Ecol. Indic. 2017, 81, 124–131. [Google Scholar] [CrossRef]
- Amin, M.; Tahir, F.; Ashfaq, H.; Akbar, I.; Razzaque, N.; Haider, M.N.; Xu, J.; Zhu, H.; Wang, N.; Shahid, A. Decontamination of industrial wastewater using microalgae integrated with biotransformation of the biomass to green products. Energy Nexus 2022, 6, 100089. [Google Scholar] [CrossRef]
- Lázár, D.; Takács, E.; Mörtl, M.; Klátyik, S.; Barócsi, A.; Kocsányi, L.; Lenk, S.; Domján, L.; Szarvas, G.; Lengyel, E.; et al. Application of a fluorescence-based instrument prototype for chlorophyll measurements and its utility in an herbicide algal ecotoxicity assay. Water 2023, 15, 1866. [Google Scholar] [CrossRef]
- Nguyen, M.-N.; Lin, C.; Nguyen, H.-L.; Le, V.-G.; Haddout, S.; Um, M.-J.; Chang, S.W.; Nguyen, D.D. Ecotoxicity of micro- and nanoplastics on aquatic algae: Facts, challenges, and future opportunities. J. Environ. Manag. 2023, 346, 118982. [Google Scholar] [CrossRef] [PubMed]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae culture quality indicators: A review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Kit, L.Y.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-mediated biosorption for effective heavy metals removal from wastewater: A review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Umamaheswari, J.; Rajamanickam, R.; Vilvanathan, S.; Shanthakumar, S.; Venkateswarlu, K.; Abinandan, S.; Megharaj, M. Removal of heavy metals and organic pollutants by marine microalgae. In Marine Organisms: A Solution to Environmental Pollution? Environmental Challenges and Solutions; Encarnação, T., Canelas Pais, A., Eds.; Springer: Singapore, 2023; pp. 29–64. [Google Scholar]
- Blanco-Vieites, M.; Suárez-Montes, D.; Delgado, F.; Álvarez-Gil, M.; Hernández Battez, A.; Rodríguez, E. Removal of heavy metals and hydrocarbons by microalgae from wastewater in the steel industry. Algal Res. 2022, 64, 102700. [Google Scholar] [CrossRef]
- Beijerinck, M.W. Culturversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. Botanische Zeitung 1890, 47, 725–739, 741–754, 757–768, 781–785. [Google Scholar]
- Gomont, M. Monographie des Oscillariées (Nostocacées Homocystées). Deuxième partie—Lyngbyées. Annales des Sciences Naturelles. Botanique 1892, 7, 91–264. [Google Scholar]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Elbaily, Z.I. A review: Importance of chlorella and different applications. Alex. J. Vet. Sci. 2020, 65, 16. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Q.; Kuča, K. Spirulina. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 959–974. [Google Scholar]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Dai, N.; Wang, Q.; Xu, B.; Chen, H. Remarkable natural biological resource of algae for medical applications. Front. Mar. Sci. 2022, 9, 2022. [Google Scholar] [CrossRef]
- Dini, I. The potential of algae in the nutricosmetic sector. Molecules 2023, 28, 4032. [Google Scholar] [CrossRef]
- Gonzalez Fernandez, M.C.; Sialve, B.; Bernet, N.; Steyer, J.-P. Impact of microalgae characteristics on their conversion to biofuel. Part II: Focus on biomethane production. Biofuels Bioprod. Biorefining 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Kuenz, A.; Grimm, D.; Rahmann, G. Versatility of algae—Exploring the potential of algae for nutrient circulation. Org. Agric. 2021, 11, 251–260. [Google Scholar] [CrossRef]
- Gors, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J. Appl. Phycol. 2009, 22, 265–276. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a dietary supplement to promote human health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factor. 2018, 17, 36. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L.; Luong-Van, T. Microalgae for use in tropical aquaculture. I. Gross chemical and fatty acid composition of twelve species of microalgae from the Northern Territory, Australia. J. Appl. Phycol. 1994, 6, 337–345. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; González-Hernández, J.C. Vitamins from microalgae. In Handbook of Food and Feed from Microalgae; Jacob-Lopes, E., Queiroz, M.I., Manzoni Maroneze, M., Queiroz Zepka, L., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 111–115. [Google Scholar]
- Sansawa, H.; Endo, H. Production of intracellular phytochemicals in Chlorella under heterotrophic conditions. J. Biosci. Bioeng. 2004, 98, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Lin, L. Chlorella: Its Ecology Structure Cultivation Bioprocess and Application; Yi Hsien Publishing Company: Taipei City, Taiwan, 2005; p. 596. [Google Scholar]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.; Mendes, M.A. Chlorella and Spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- ALIGA Microalgae. Available online: https://www.aliga.dk/products (accessed on 30 April 2024).
- Phycom. Available online: https://phycom.eu/ (accessed on 30 April 2024).
- Die Chlorella-Farm von Algomed im Herzen der Altmark. Available online: https://www.algomed.de/algenfarm/anbau/ (accessed on 30 April 2024).
- Richardson, B.; Orcutt, D.; Schwertner, H.; Martinez, C.L.; Wickline, H.E. Effects of nitrogen limitation on the growth and composition of unicellular algae in continuous culture. Appl. Microbiol. 1969, 18, 245–250. [Google Scholar] [CrossRef]
- Goldman, J.C.; Azov, Y.; Riley, C.B.; Dennett, M.R. The effect of pH in intensive microalgal cultures. I. biomass regulation. J. Exp. Mar. Biol. Ecol. 1982, 57, 1–13. [Google Scholar] [CrossRef]
- Brown, M.R.; Dunstan, G.A.; Jeffrey, S.W.; Volkman, J.K.; Barrett, S.M.; LeRoi, J.M. The influence of irradiance on the biochemical composition of the prymnesiophyte Isochrysis sp. J. Phycol. 1993, 29, 601–612. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Malcata, F.X. Effect of culture media on production of polyunsaturated fatty acids by Pavlova lutheri. Cryptogam. Algol. 2000, 21, 59–71. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Blinová, L.; Bartośová, A.; Gerulová, K. Cultivation of microalgae (Chlorella vulgaris) for biodiesel production. Res. Pap. Fac. Mater. Sci. Technol. Slovak. Univ. Technol. 2015, 23, 87–95. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Razzak, S.A.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae cultivation in photobioreactors: Sustainable solutions for a greener future. Green Chem. Eng. 2023, 2023. [Google Scholar] [CrossRef]
- Ahmad, S.; Kothari, R.; Shankarayan, R.; Tyagi, V.V. Temperature dependent morphological changes on algal growth and cell surface with dairy industry wastewater: An experimental investigation. 3 Biotech 2020, 10, 24. [Google Scholar] [CrossRef]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Zhang, Z.; Pang, Z.; Xu, S.; Wei, T.; Song, L.; Wang, G.; Zhang, J.; Yang, X. Improved carotenoid productivity and COD removal efficiency by co-culture of Rhodotorula glutinis and Chlorella vulgaris using starch wastewaters as raw material. Appl. Biochem. Biotechnol. 2019, 189, 193–205. [Google Scholar] [CrossRef]
- Geremia, E.; Ripa, M.; Catone, C.M.; Ulgiati, S. A review about microalgae wastewater treatment for bioremediation and biomass production—A new challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Morais, E.G.; Cristofoli, N.L.; Maia, I.B.; Magina, T.; Cerqueira, P.R.; Teixeira, M.R.; Gouveia, L. Microalgal systems for wastewater treatment: Technological trends and challenges towards waste recovery. Energies 2021, 14, 8112. [Google Scholar] [CrossRef]
- Rani, S.; Gunjyal, N.; Ojha, C.S.P.; Singh, R.P. Review of challenges for algae-based wastewater treatment: Strain selection, wastewater characteristics, abiotic, and biotic factors. J. Hazard. Toxic Radioact. Waste 2021, 25, 03120004. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Lau, P.S.; Wong, Y.S. Wastewater inorganic N and P removal by immobilized Chlorella vulgaris. Wat. Sci. Technol. 1994, 30, 369–374. [Google Scholar] [CrossRef]
- Shi, J.; Podola, B.; Melkonian, M. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: An experimental study. J. Appl. Phycol. 2007, 19, 417–423. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Aslan, S.; Kapdan, I.K. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol. Eng. 2006, 28, 64–70. [Google Scholar] [CrossRef]
- Mallick, N.; Rai, L.C. Removal of inorganic ions from wastewaters by immobilized microalgae. World J. Microbiol. Biotechnol. 1994, 10, 439–443. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Lemos, P.V.F.; de Souza, C.O.; Oliveira, M.B.P.P.; Chinalia, F.A. Current advances in phytoremediation and biochemical composition of Arthrospira (Spirulina) grown in aquaculture wastewater. Aquac. Res. 2022, 53, 4931–4943. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chang, C.K.; Munawaroh, H.S.H.; Kumar, P.S.; Show, P.L. Perspective of Spirulina culture with wastewater into a sustainable circular bioeconomy. Environ. Pollut. 2021, 284, 117492. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.-P.; Chew, K.W.; Ling, T.C. Application progress of bioactive compounds in microalgae on pharmaceutical and cosmetics. Chemosphere 2022, 291, 132932. [Google Scholar] [CrossRef]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Justo, G.Z.; Silva, M.R.; Queiroz, M.L. Effects of the green algae Chlorella vulgaris on the response of the host hematopoietic system to intraperitoneal Ehrlich ascites tumor transplantation in mice. Immunopharmacol. Immunotoxicol. 2001, 23, 119–132. [Google Scholar] [CrossRef]
- Morris, H.J.; Carrillo, O.V.; Almarales, Á.; Bermúdez, R.C.; Alonso, M.E.; Borges, L.; Hernández, M. Protein hydrolysates from the alga Chlorella vulgaris 87/1 with potentialities in immunonutrition. Biotecnol. Apl. 2009, 26, 162–165. [Google Scholar]
- Huesemann, M.H.; Van Wagenen, J.; Miller, T.; Chavis, A.; Hobbs, S.; Crowe, B. A screening model to predict microalgae biomass growth in photobioreactors and raceway ponds. Biotechnol. Bioeng. 2013, 110, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Rosko, J.J.; Rachlin, J.W. The effect of cadium, copper, mercury, zinc and lead on cell division, growth, and chlorophyll a content of the Chlorophyte Chlorella vulgaris. Bull. Torrey Bot. Club 1977, 104, 226–233. [Google Scholar] [CrossRef]
- Ferrando, N.S.; Benítez, H.H.; Gabellone, N.A.; Claps, M.C.; Altamirano, P.R. A quick and effective estimation of algal density by turbidimetry developed with Chlorella vulgaris cultures. Limnetica 2015, 34, 397–406. [Google Scholar]
- Flores, G.; Rodriguez-Mata, A.E.; Amabilis-Sosa, L.E.; Gonzalez-Huitron, V.A.; Hernández-González, O.; Lopéz-Peréz, P.A. A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation. Open Chem. 2020, 18, 1349–1361. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating biomass and vitality of microalgae for monitoring cultures: A roadmap for reliable measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Ribeiro Rodrigues, L.H.; Arenzon, A.; Raya-Rodriguez, M.T.; Ferreira Fontoura, N. Algal density assessed by spectrophotometry: A calibration curve for the unicellular algae Pseudokirchneriella subcapitata. J. Environ. Chem. Ecotoxicol. 2011, 3, 225–228. [Google Scholar]
- Santos-Ballardo, D.U.; Rossi, S.; Hernández, V.; Vázquez Gómez, R.; del Carmen Rendón-Unceta, M.; Caro-Corrales, J.; Valdez-Ortiz, A. A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture 2015, 448, 87–92. [Google Scholar] [CrossRef]
- Mayer, P.; Cuhel, R.; Nyholm, N. A simple in vitro fluorescence method for biomass measurements in algal growth inhibition tests. Water Res. 1997, 31, 2525–2531. [Google Scholar] [CrossRef]
- Cadondon, J.G.; Ong, P.M.B.; Vallar, E.A.; Shiina, T.; Galvez, M.C.D. Chlorophyll-a pigment measurement of Spirulina in algal growth monitoring using portable pulsed LED fluorescence lidar system. Sensors 2022, 22, 2940. [Google Scholar] [CrossRef]
- Pořízka, P.; Prochazková, P.; Prochazka, D.; Sládková, L.; Novotný, J.; Petrilak, M.; Brada, M.; Samek, O.; Pilát, Z.; Zemánek, P.; et al. Algal biomass analysis by laser-based analytical techniques—A review. Sensors 2014, 14, 17725–17752. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Ya, Q.; Du, W.C.; Wen, X.B.; Geng, Y.H.; Li, Y.G. The relationships between optical density, cell number, and biomass (dry weight) of four microalgae. Acta Ecol. Sin. 2014, 34, 6156–6163. [Google Scholar]
- Lopes, A.P.; Santos, F.M.; Silva, T.F.; Vilar, V.J.; Pires, J.C. Outdoor cultivation of the microalga Chlorella vulgaris in a new photobioreactor configuration: The effect of ultraviolet and visible radiation. Energies 2020, 13, 1962. [Google Scholar] [CrossRef]
- Fan, L.H.; Zhang, Y.T.; Zhang, L.; Chen, H.L. Evaluation of a membrane-sparged helical tubular photobioreactor for carbon dioxide biofixation by Chlorella vulgaris. J. Membr. Sci. 2008, 325, 336–345. [Google Scholar] [CrossRef]
- Fernández-Linares, L.C.; Barajas, C.G.; Páramo, E.D.; Corona, J.A.B. Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresour. Technol. 2017, 244, 400–406. [Google Scholar] [CrossRef] [PubMed]
- National Aeronautics and Space Administration, Microalgae Protocols. Available online: https://www.nasa.gov/wp-content/uploads/2018/12/microalgaeprotocols_508.pdf (accessed on 30 April 2024).
- Yatirajula, S.K.; Shrivastava, A.; Saxena, V.K.; Kodavaty, J. Flow behavior analysis of Chlorella vulgaris microalgal biomass. Heliyon 2019, 5, e01845. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F.A.; Örmeci, B. Monitoring and measurement of microalgae using the first derivative of absorbance and comparison with chlorophyll extraction method. Environ. Monit. Assess. 2018, 190, 90. [Google Scholar] [CrossRef]
- Smith, A. Non-Invasive Optical Methodologies to Evaluate and Enhance Chlorella vulgaris Biomass. Doctoral Dissertation, University of Cambridge, Cambridge, UK, 2022. [Google Scholar]
- Grósz, J.; Waltner, I.; Vekerdy, Z. First analysis results of in situ measurements for algae monitoring in lake Naplás (Hungary). Carpathian J. Earth Environ. Sci. 2019, 14, 385–398. [Google Scholar] [CrossRef]
- Lu, Y.; Tuo, Y.; Zhang, L.; Hu, X.; Huang, B.; Chen, M.; Li, Z. Vertical distribution rules and factors influencing phytoplankton in front of a drinking water reservoir outlet. Sci. Total Environ. 2023, 902, 166512. [Google Scholar] [CrossRef]
- Sakharova, E.; Krylov, A.V.; Sabitova, R.Z.; Tsvetkov, A.I.; Gambaryan, L.R.; Mamyan, A.S.; Gabrielyan, B.K.; Hayrapetyan, A.H.; Khachikyan, T.G. Horizontal and Vertical Distribution of Phytoplankton in the Alpine Lake Sevan (Armenia) during the Summer Cyanoprokaryota Bloom. Contemp. Probl. Ecol. 2020, 13, 60–70. [Google Scholar] [CrossRef]
- Kang, J.-J.; Min, J.-O.; Kim, Y.; Lee, C.-H.; Yoo, H.; Jang, H.-K.; Kim, M.-J.; Oh, H.-J.; Lee, S.-H. Vertical distribution of phytoplankton community and pigment production in the Yellow Sea and the East China Sea during the late summer season. Water 2021, 13, 3321. [Google Scholar] [CrossRef]
- Blanken, W.M.; Postma, P.R.; de Winter, L.; Wijffels, R.H.; Janssen, M.G.J. Predicting microalgae growth. Algal Res. 2016, 14, 28–38. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Sarker, N.K.; Kaparaju, P. A critical review on the status and progress of microalgae cultivation in outdoor photobioreactors conducted over 35 years (1986–2021). Energies 2023, 16, 3105. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.; Baptista, J.M.; Malcata, F. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Havlik, I.; Reardon, K.F.; Ünal, M.; Lindner, P.; Prediger, A.; Babitzky, A.; Beutel, S.; Scheper, T. Monitoring of microalgal cultivations with on-line, flow-through microscopy. Algal Res. 2013, 2, 253–257. [Google Scholar] [CrossRef]
- Bernard, O.; Gouzé, J.-L. Non-linear qualitative signal processing for biological systems: Application to the algal growth in bioreactors. Math. Biosci. 1999, 157, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Bernard, O. Hurdles and challenges for modelling and control of microalgae for CO2 mitigation and biofuel production. J. Process Control 2011, 21, 1378–1389. [Google Scholar] [CrossRef]
- Ördög, V. Contributions to the standardization of laboratory alga tests. Bot. Közlemények Bot. Publ. 1981, 68, 85–93. [Google Scholar]
- Felföldy, L. Biological Water Qualification; Water Management Institute: Budapest, Hungary, 1987; p. 190. [Google Scholar]
- ISO 10260:1992; Water Quality—Measurement of Biochemical Parameters—Spectrometric Determination of the Chlorophyll-a Concentration. International Organization for Standardization: Geneva, Switzerland, 1992; p. 6.
- Arslan, Z.; Tyson, J.F. Determination of calcium, magnesium and strontium in soils by flow injection flame atomic absorption spectrometry. Talanta 1999, 50, 929–3937. [Google Scholar] [CrossRef] [PubMed]
- Mónok, D.; Füleky, G. A talaj kadmium szennyezettségének vizsgálata angolperje (Lolium perenne L.) bioteszttel. Agrokémia és Talajt. 2017, 66, 333–347. [Google Scholar] [CrossRef]
- Rinawati, M.; Sari, L.A.; Pursetyo, K.T. Chlorophyll and carotenoids analysis spectrophotometer using method on microalgae. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012056. [Google Scholar] [CrossRef]
- Hagemann, L.T.; McCartney, M.M.; Fung, A.G.; Peirano, D.J.; Davis, C.E.; Mizaikoff, B. Portable combination of Fourier transform infrared spectroscopy and differential mobility spectrometry for advanced vapor phase analysis. Analyst 2018, 143, 5683–5691. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Ebbing, T.; Frick, K.; Schmid-Staiger, U.; Haasdonk, B.; Tovar, G.E. Improving determination of pigment contents in microalgae suspension with absorption spectroscopy: Light scattering effect and Bouguer–Lambert–Beer law. Mar. Drugs. 2023, 21, 619. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, J.F.; Kean-Meng, T.; Asencio-Alcudia, G.G.; Asyraf-Kassim, M.; Alvarez-González, C.A.; Márquez-Rocha, F.J. Sustainable microalgae and cyanobacteria biotechnology. Appl. Sci. 2022, 12, 6887. [Google Scholar] [CrossRef]
- Tekiner, M.; Kurt, M.; Ak, I.; Kurt, A. Determination of absorption coefficient of Chlorella vulgaris and Arthrospira maxima in water. AIP Conf. Proc. 2018, 1935, 120002. [Google Scholar]
- Nguy-Robertson, A.; Li, L.; Tedesco, L.P.; Wilson, J.S.; Soyeux, E. Determination of absorption coefficients for chlorophyll a, phycocyanin, mineral matter and CDOM from three central Indiana reservoirs. J. Great Lakes Res. 2013, 39, 151–160. [Google Scholar] [CrossRef]
- Clementson, L.A.; Wojtasiewicz, B. Dataset on the in vivo absorption characteristics and pigment composition of various phytoplankton species. Data Br. 2019, 25, 104020. [Google Scholar] [CrossRef]
- Bidigare, R.R.; Ondrusek, M.E.; Morrow, J.H.; Kiefer, D.A. In-vivo absorption properties of algal pigments. Ocean. Opt. X 1990, 1302, 290–302. [Google Scholar]
- ISO 7027-1:2016; Water Quality—Determination of Turbidity. International Organization for Standardization: Geneva, Switzerland, 2016; p. 9.
- Havlik, I.; Beutel, S.; Scheper, T.; Reardon, K.F. On-line monitoring of biological parameters in microalgal bioprocesses using optical methods. Energies 2022, 15, 875. [Google Scholar] [CrossRef]
- Hu, W. Dry Weight and Cell Density of Individual Algal and Cyanobacterial Cells for Algae Research and Development, Master’s Thesis. University of Missouri-Columbia, Columbia, MO, USA, 2014.
- Prabakaran, G.; Moovendhan, M.; Arumugam, A.; Matharasi, A.; Dineshkumar, R.; Sampathkumar, P. Evaluation of Chemical Composition and in vitro antiinflammatory effect of marine microalgae Chlorella vulgaris. Waste Biomass Valor 2019, 10, 3263–3270. [Google Scholar] [CrossRef]
- Tokuşoglu, Ö.; Üunal, M.K. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Maruyama, I.; Nakao, T.; Shigeno, I.; Ando, Y.; Hirayama, K. Application of unicellular algae Chlorella vulgaris for the mass-culture of marine rotifer Brachionus. Hydrobiologia 1997, 358, 133–138. [Google Scholar] [CrossRef]
- Mandalam, R.K.; Palsson, B. Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol. Bioeng. 1998, 59, 605–611. [Google Scholar] [CrossRef]
- Panahi, Y.; Pishgoo, B.; Jalalian, H.R.; Mohammadi, E.; Taghipour, H.R.; Sahebkar, A. Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepatogastroenterology 2012, 59, 2099–2103. [Google Scholar]
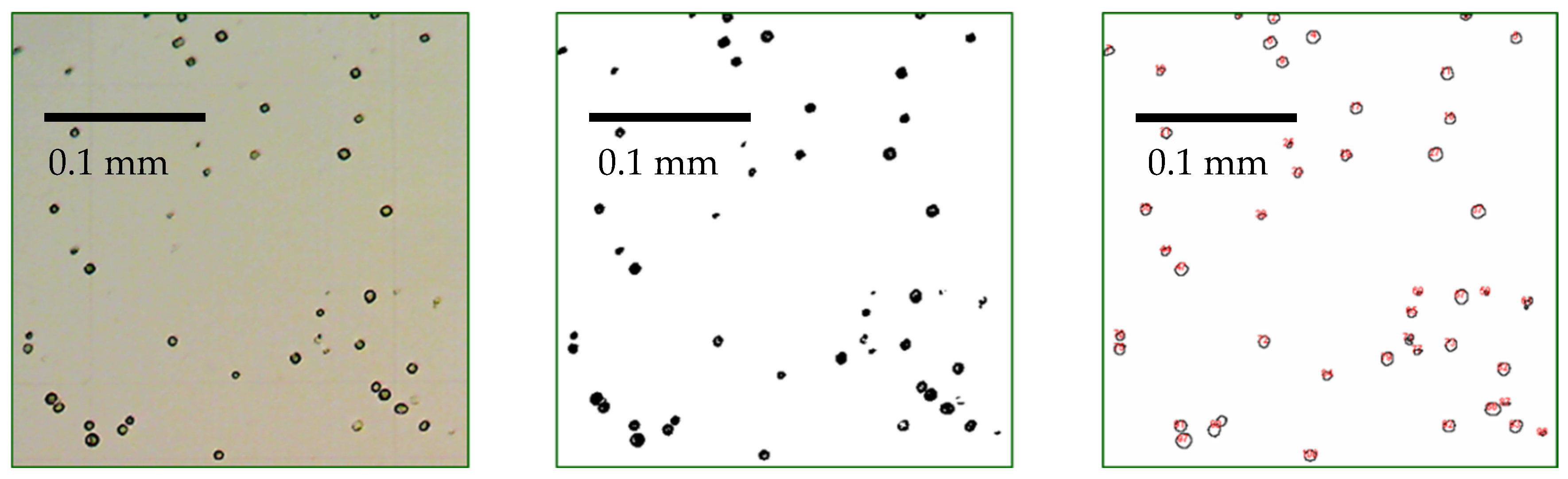

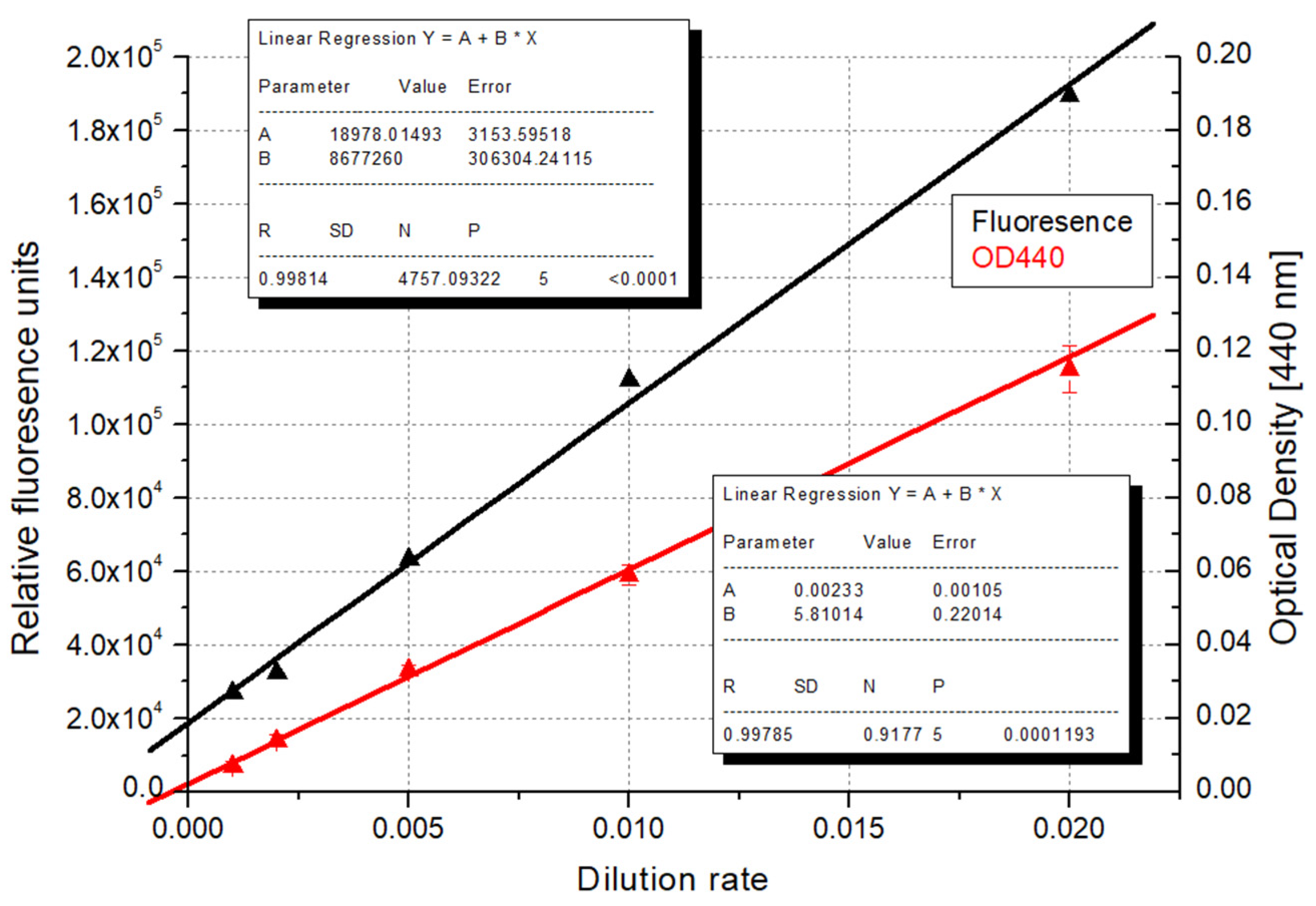
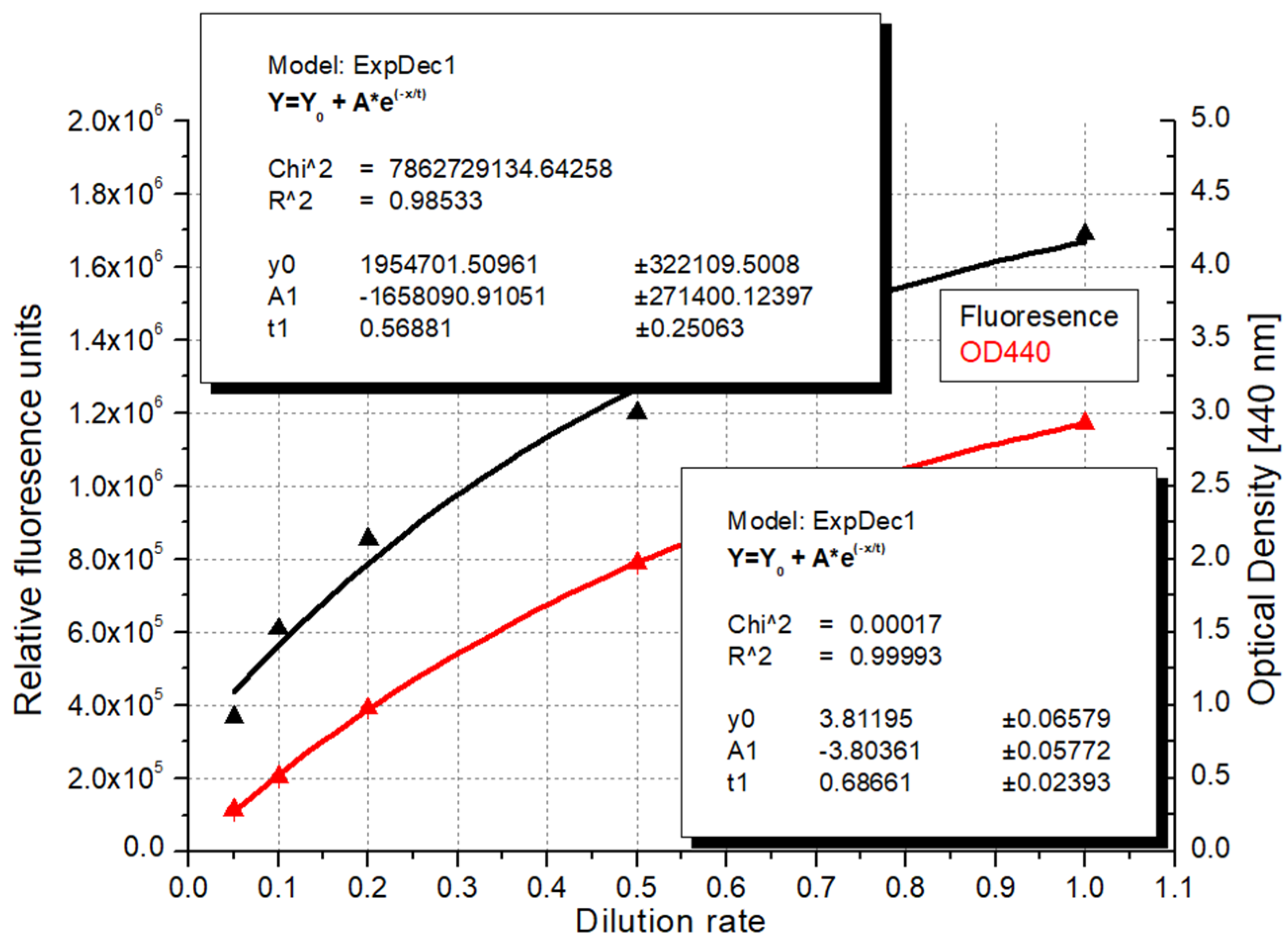

| Recommended Wavelength | R2 | References |
|---|---|---|
| 435 nm | 0.980 | [107] |
| 440 nm | 0.998 | [108] |
| 450 nm | n.a. a | [109] |
| 600 nm | n.a. | [110] |
| 670 nm | n.a. | [111] |
| 680 nm | n.a. | [112] |
| 680 and 750 nm | n.a. | [101] |
| 695 nm | 0.970 | [113] |
| Nutrient Solution Component | Concentration (g/L) |
|---|---|
| sodium nitrate | 1.000 |
| dipotassium phosphate | 0.250 |
| magnesium sulfate | 0.513 |
| ammonium chloride | 0.050 |
| calcium chloride | 0.058 |
| ferrous chloride | 0.003 |
| Dilution | Ratio a | BMe b (mg d.m. c/L) | BMf d (mg d.m./L) | Chl-a e (μg/L) | Mg f (μg/L) | CN g (106 cells/L) | RFU h |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 960.0 | 770.0 | 7515.6 | 204.5 | 108,222.7 | 1,689,562 |
| 2 | 0.5 | 436.7 | 420.0 | 4163.1 | 109.6 | 61,578.1 | 1,200,521 |
| 5 | 0.2 | 150.0 | 177.5 | 1567.0 | 27.6 | 34,113.3 | 853,994 |
| 10 | 0.1 | 66.7 | 79.3 | 892.4 | 10.6 | 13,136.7 | 610,195 |
| 20 | 0.05 | 35.8 | 31.4 | 382.1 | 28.4 | 10,664.1 | 369,087 |
| 50 | 0.02 | 14.2 | 15.0 | 182.3 | 13.8 | 3757.8 | 189,411 |
| 100 | 0.01 | 10.0 | 9.3 | 90.9 | 15.4 | 1699.2 | 112,247 |
| 200 | 0.005 | <LOD i | 2.5 | 54.6 | 17.5 | 472.7 | 63,474 |
| 500 | 0.002 | <LOD | 1.4 | 20.1 | 11.0 | 371.1 | 32,556 |
| 1000 | 0.001 | <LOD | <LOD | 12.3 | 6.9 | 105.5 | 26,938 |
| Dilution | BMf a | BMe b | Mg c | CN d | Chl-a e | RFU f | |
|---|---|---|---|---|---|---|---|
| Dilution | - | 0.998 (L) | 0.997 (L) | 0.975 (L) | 0.986 (L) | 0.998 (L) | 0.989 (Lg) |
| BMf | - | - | 0.989 (L) | 0.966 (L) | 0.993 (L) | 0.999 (L) | 0.987 (Lg) |
| BMe | - | - | - | 0.982 (L) | 0.974 (L) | 0.990 (L) | 0.974 (Ep) |
| Mg | - | - | - | - | 0.945 (L) | 0.971 (L) | 0.816 (Ep) |
| CN | - | - | - | - | - | 0.989 (L) | 0.984 (Lg) |
| Chl-a | - | - | - | - | - | - | 0.986 (Lg) |
| RFU | - | - | - | - | - | - | - |
| BMf a | BMe b | Chl-a c | Mg d | CN e | RFU f | |
|---|---|---|---|---|---|---|
| max R2 | 0.950 | 0.929 | 0.950 | 0.859 | 0.933 | 0.990 |
| R2 max wavelength | 439 | 437 | 439 | 442 | 461 | 529 |
| Dilution | BMe a (mg d.m. b/L) | Mg (μg/L) | Mg c (mg/100 g d.m.) | Chl-a d from Mg (μg/L) | Chl-a e (μg/L) | CN f (106 cells/L) | AW g (μg/106 cells) |
|---|---|---|---|---|---|---|---|
| 1× | 960 | 205 | 21.31 | 7521 | 7516 | 108,223 | 8.871 |
| 2× | 437 | 110 | 25.09 | 4029 | 4163 | 61,578 | 7.091 |
| 5× | 150 | 28 | 18.40 | 1015 | 1567 | 34,113 | 4.397 |
| 10× | 67 | 11 | 15.83 | 388 | 892 | 13,137 | 5.075 |
| Average | 20.16 ± 3.98 | 6.36 ± 2.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete, G.; Sebők, A.; Klátyik, S.; Varga, Z.I.; Grósz, J.; Czinkota, I.; Székács, A.; Aleksza, L. Comparative Analysis of Laboratory-Based and Spectroscopic Methods Used to Estimate the Algal Density of Chlorella vulgaris. Microorganisms 2024, 12, 1050. https://doi.org/10.3390/microorganisms12061050
Fekete G, Sebők A, Klátyik S, Varga ZI, Grósz J, Czinkota I, Székács A, Aleksza L. Comparative Analysis of Laboratory-Based and Spectroscopic Methods Used to Estimate the Algal Density of Chlorella vulgaris. Microorganisms. 2024; 12(6):1050. https://doi.org/10.3390/microorganisms12061050
Chicago/Turabian StyleFekete, György, András Sebők, Szandra Klátyik, Zsolt István Varga, János Grósz, Imre Czinkota, András Székács, and László Aleksza. 2024. "Comparative Analysis of Laboratory-Based and Spectroscopic Methods Used to Estimate the Algal Density of Chlorella vulgaris" Microorganisms 12, no. 6: 1050. https://doi.org/10.3390/microorganisms12061050





