Immunogenicity Analysis and Identification of Potential T-Cell Epitopes in C129R Protein of African Swine Fever Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Protein
2.2. Animals
2.3. Expression and Purification of Recombinant C129R Protein
2.4. Western Blot Analysis
2.5. Screen of the T-Cell Epitopes on the C129R Protein
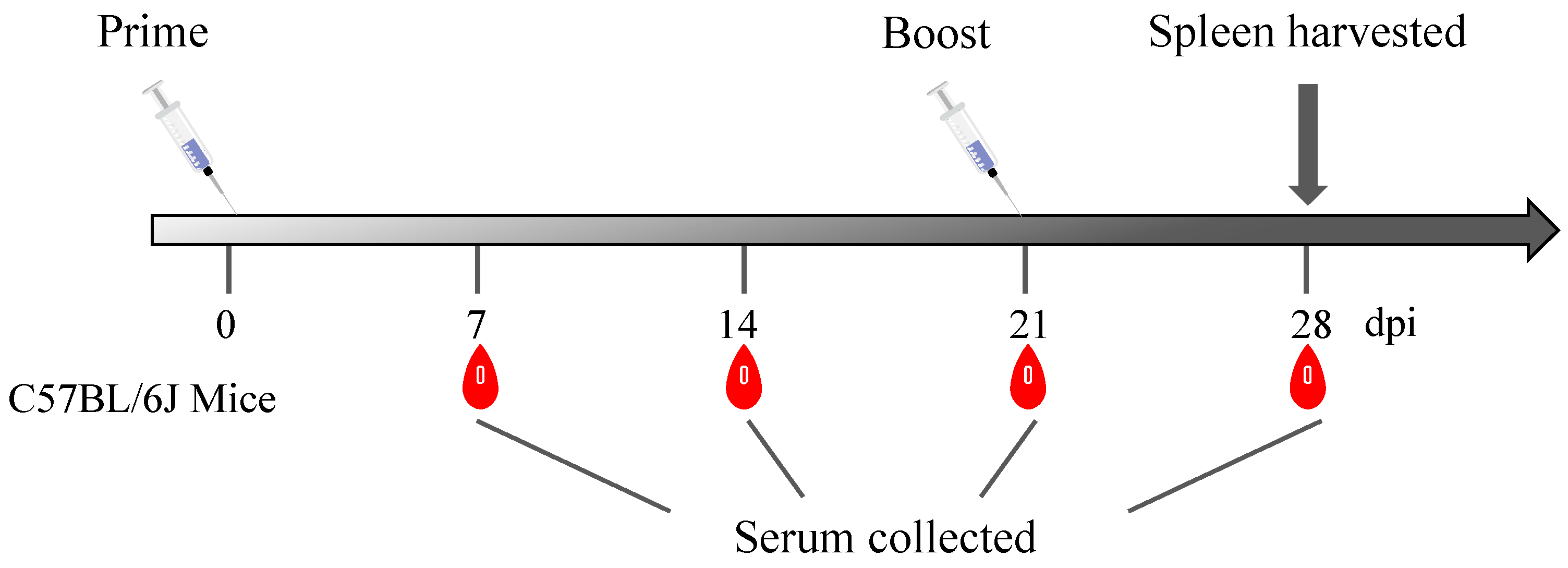
2.6. Animal Immunization
2.7. Isolation Mice Spleen Lymphocytes
2.8. IFN-γ ELISpot Assays
2.9. Indirect ELISA
2.10. CD4+/CD8+ T-Cell
2.11. Detection of IFN-γ, IL-2, IL-4, and IL-10
2.12. Conservative Analysis of the T-Cell Epitopes
2.13. Statistical Analysis
3. Results
3.1. C129R Protein Was Expressed in 293F
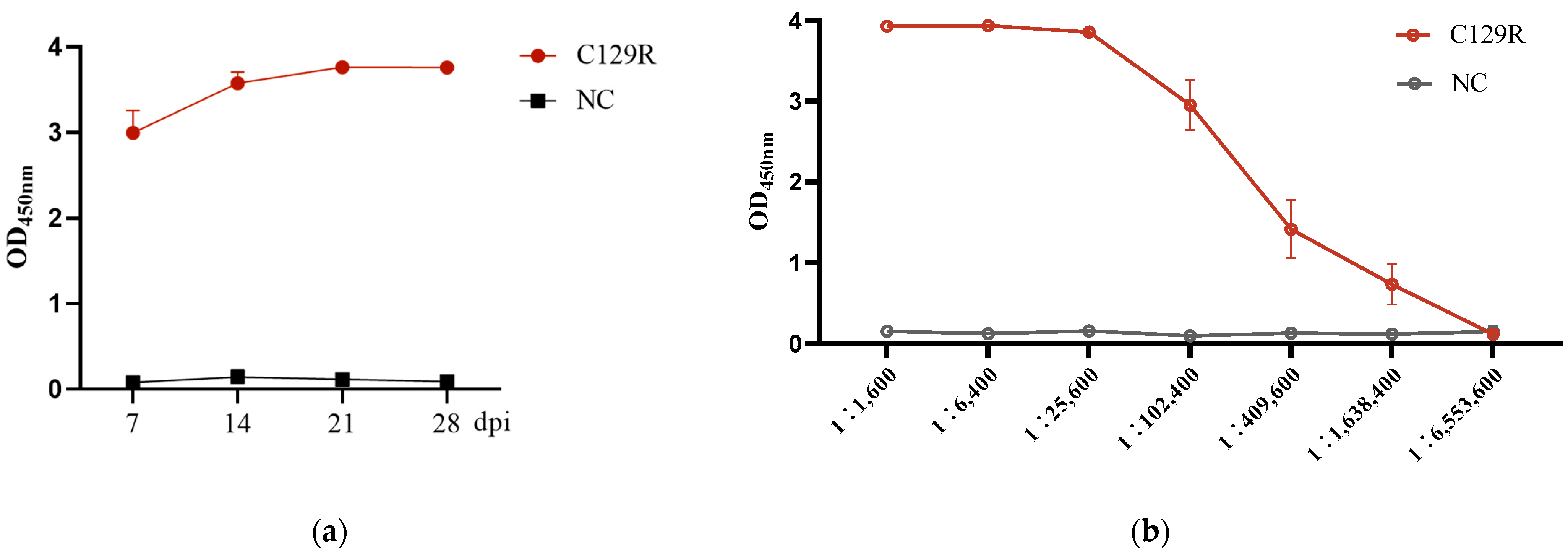
3.2. C129R Protein Induced the Humoral Immune Response
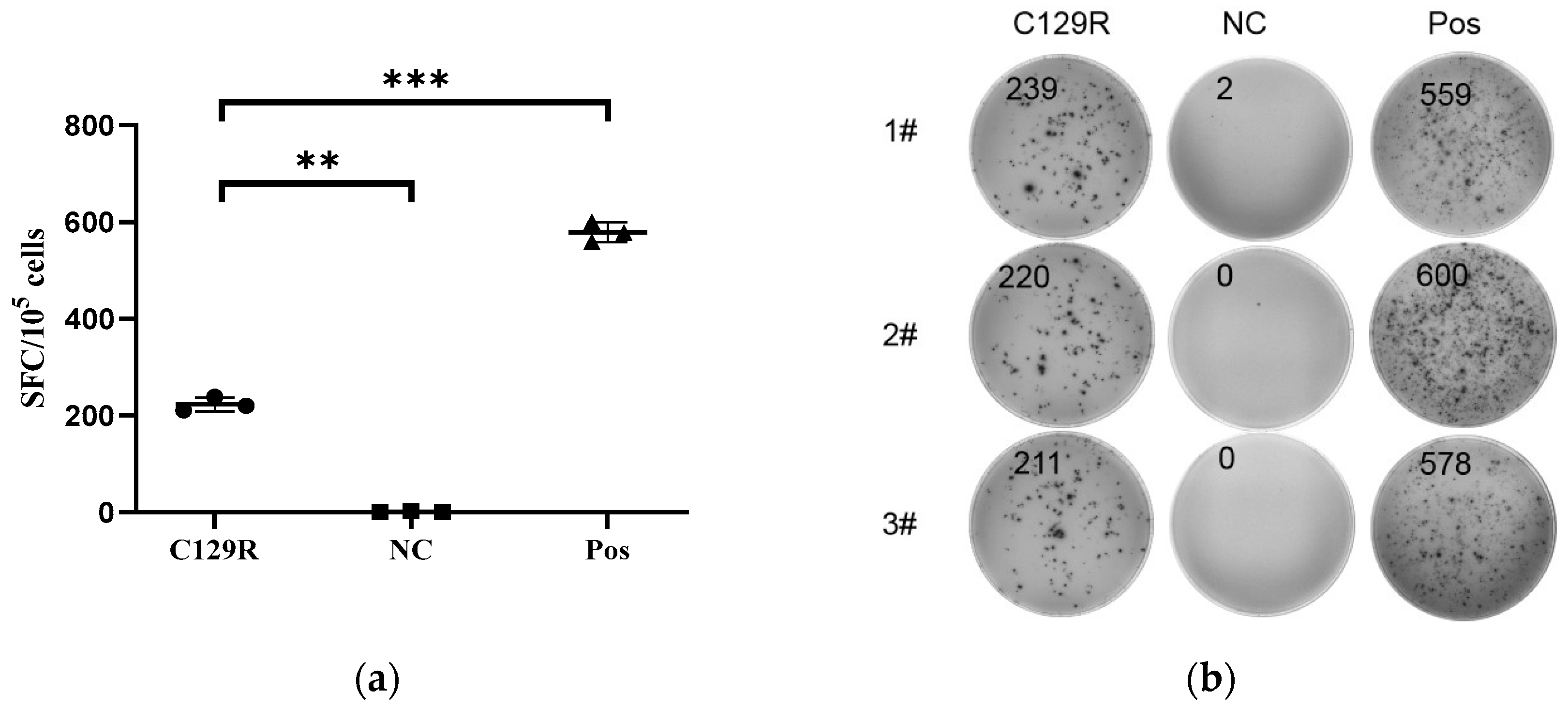
3.3. C129R Protein Activated the Cellular Immunity and Induced IFN-γ Production
3.4. C129R Protein Increased CD4+/CD8+ T-Cell Ratios of Splenic Lymphocytes
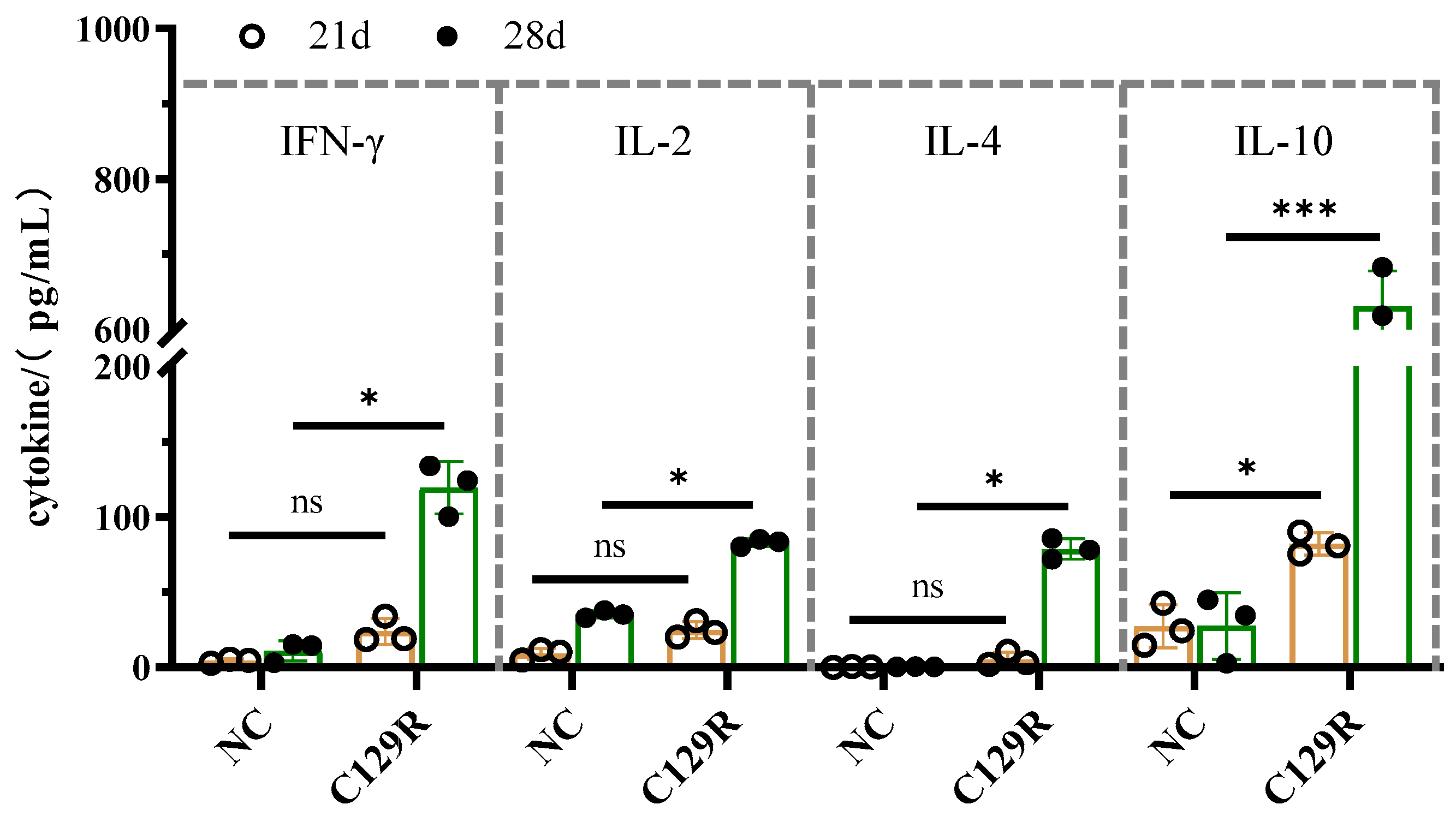
3.5. Effect of C129R Protein on Th1 and Th2 Immune Responses
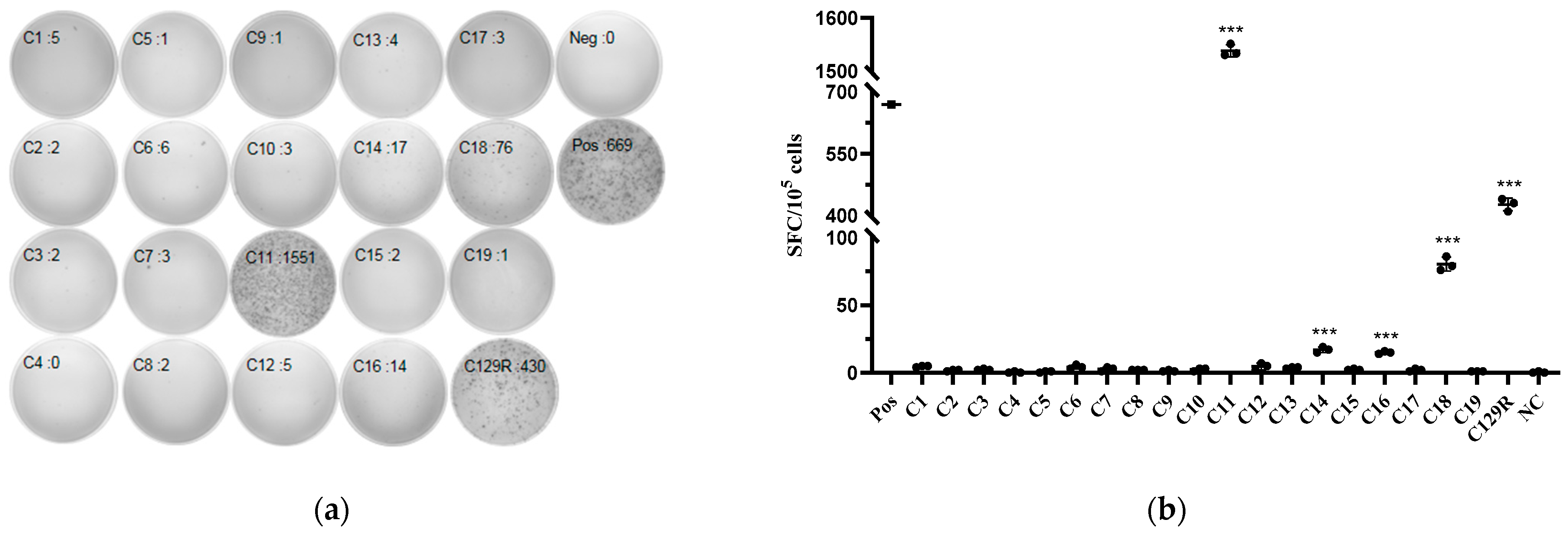
3.6. C11(53−61aa), C14(81−89aa), C16(97−105aa), and C18(116−124aa) Were the Potential T-Cell Epitopes in C129R Protein
3.7. Epitope Conservation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penrith, M.L.; Bastos, A.D.; Etter, E.M.; Beltrán-Alcrudo, D. Epidemiology of African swine fever in Africa today: Sylvatic cycle versus socio-economic imperatives. Transbound. Emerg. Dis. 2019, 66, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef]
- Wang, Z.; Ai, Q.; Huang, S.; Ou, Y.; Gao, Y.; Tong, T.; Fan, H. Immune escape mechanism and vaccine research progress of African swine fever virus. Vaccines 2022, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Qiu, H.-J. African swine fever: An unprecedented disaster and challenge to China. Infect. Dis. Poverty 2018, 7, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Ru, Y.; Yang, W.; Ren, J.; Hao, R.; Qin, X.; Li, D.; Zheng, H. Research progress on the proteins involved in African swine fever virus infection and replication. Front. Immunol. 2022, 13, 947180. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African swine fever virus–persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef]
- Arias, M.; Jurado, C.; Gallardo, C.; Fernández-Pinero, J.; Sánchez-Vizcaíno, J.M. Gaps in African swine fever: Analysis and priorities. Transbound. Emerg. Dis. 2018, 65, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Rock, D.L. Challenges for African swine fever vaccine development—“… perhaps the end of the beginning.”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef]
- Takamatsu, H.-H.; Denyer, M.S.; Lacasta, A.; Stirling, C.M.; Argilaguet, J.M.; Netherton, C.L.; Oura, C.A.; Martins, C.; Rodríguez, F. Cellular immunity in ASFV responses. Virus Res. 2013, 173, 110–121. [Google Scholar] [CrossRef]
- Netherton, C.L.; Goatley, L.C.; Reis, A.L.; Portugal, R.; Nash, R.H.; Morgan, S.B.; Gault, L.; Nieto, R.; Norlin, V.; Gallardo, C. Identification and immunogenicity of African swine fever virus antigens. Front. Immunol. 2019, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, Y.; Li, J.; Wang, A.; Meng, X.; Chen, L.; Wei, J.; Tong, W.; Kong, N.; Yu, L. Screening and identification of the dominant antigens of the African swine fever virus. Front. Vet. Sci. 2023, 10, 1175701. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; McCluskie, M.J. The quantification of antigen-specific T cells by IFN-γ ELISpot. Methods Mol. Biol. 2021, 2183, 525–536. [Google Scholar] [PubMed]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J. Virol. 2017, 91, e01760-16. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S. African swine fever virus: A review. Life 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, X.; Wang, Q.; Wang, X.; Yang, X.; Li, S.; Zhao, X.-T.; Yuan, M.; Liu, X.; Qiu, H.-J.; et al. Identification of the p34 Protein of African Swine Fever Virus as a Novel Viral Antigen with Protection Potential. Viruses 2023, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puertas, P.; Rodriguez, F.; Oviedo, J.M.; Ramiro-Ibanez, F.; Ruiz-Gonzalvo, F.; Alonso, C.; Escribano, J.M. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 1996, 70, 5689–5694. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Richt, J.A. Subunit vaccine approaches for African swine fever virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef]
- Hühr, J.; Schäfer, A.; Schwaiger, T.; Zani, L.; Sehl, J.; Mettenleiter, T.C.; Blome, S.; Blohm, U. Impaired T-cell responses in domestic pigs and wild boar upon infection with a highly virulent African swine fever virus strain. Transbound. Emerg. Dis. 2020, 67, 3016–3032. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Pérez-Martín, E.; Nofrarías, M.; Gallardo, C.; Accensi, F.; Lacasta, A.; Mora, M.; Ballester, M.; Galindo-Cardiel, I.; López-Soria, S.; et al. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS ONE 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Schmittel, A.; Keilholz, U.; Bauer, S.; Kuhne, U.; Stevanovic, S.; Thiel, E.; Scheibenbogen, C. Application of the IFN-γ ELISPOT assay to quantify T cell responses against proteins. J. Immunol. Methods 2001, 247, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Portugal, R. ELISpot Assay for the Detection of ASFV-Specific Interferon-Gamma (IFN-γ)-Producing Cells. In African Swine Fever Virus: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 169–178. [Google Scholar]
- Schumacher, T.N.; Heemels, M.-T.; Neefjes, J.J.; Kast, W.M.; Melief, C.J.; Ploegh, H.L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell 1990, 62, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Camós, L.; López, E.; Navas, M.J.; Pina-Pedrero, S.; Accensi, F.; Correa-Fiz, F.; Park, C.; Carrascal, M.; Domínguez, J.; Salas, M.L. Identification of promiscuous African swine fever virus T-cell determinants using a multiple technical approach. Vaccines 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Buan, A.K.G.; Reyes, N.A.L.; Pineda, R.N.B.; Medina, P.M.B. In silico design and evaluation of a multi-epitope and multi-antigenic African swine fever vaccine. ImmunoInformatics 2022, 8, 100019. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, W.; Gao, Z.; Yang, S.; Zhou, G.; Chang, Y.; Ma, Y.; Liang, X.; Shao, J.; Chang, H. Antigenicity and immunogenicity of recombinant proteins comprising African swine fever virus proteins p30 and p54 fused to a cell-penetrating peptide. Int. Immunopharmacol. 2021, 101, 108251. [Google Scholar] [CrossRef]
- Dodantenna, N.; Ranathunga, L.; Chathuranga, W.G.; Weerawardhana, A.; Cha, J.-W.; Subasinghe, A.; Gamage, N.; Haluwana, D.; Kim, Y.; Jheong, W.; et al. African swine fever virus EP364R and C129R target cyclic GMP-AMP to inhibit the cGAS-STING signaling pathway. J. Virol. 2022, 96, e01022. [Google Scholar] [CrossRef]



| Number | Start–End | Peptide | Solvent | Number | Start–End | Peptide | Solvent |
|---|---|---|---|---|---|---|---|
| C1 | 1–9 | MEHPSTNYT | ddH2O | C2 | 6–14 | TNYTPEQQH | ddH2O |
| C3 | 12–20 | QQHEKLKHY | ddH2O | C4 | 18–26 | KHYVLIPKH | ddH2O |
| C5 | 20–28 | YVLIPKHLW | DMSO | C6 | 25–33 | KHLWSYIKY | DMSO |
| C7 | 31–39 | IKYGTHVRY | ddH2O | C8 | 38–46 | RYYTTQNVF | DMSO |
| C9 | 43–51 | QNVFRVGGF | ddH2O | C10 | 48–56 | VGGFVLQNP | ddH2O |
| C11 | 53–61 | LQNPYEAVI | ddH2O | C12 | 58–63 | EAVIKNEVK | CH2O2 |
| C13 | 77–85 | TKAKGHVTW | ddH2O | C14 | 81–89 | GHVTWAVPY | ddH2O |
| C15 | 88–96 | PYDNISKLY | DMSO | C16 | 97–105 | AKPDAIMLT | ddH2O |
| C17 | 109–117 | NVEKALHAL | ddH2O | C18 | 116–124 | ALNQNVLTL | DMSO |
| C19 | 121–129 | VLTLASKIR | DMSO |
| Genotype | Strain | GenBank Accession No. | Source |
|---|---|---|---|
| I | Portugal/L60 | KM262844 | Portugal |
| SD SY-1 | MZ945537 | China | |
| HeN ZZ-P1 | MZ945536.1 | China | |
| Italy/47/Ss/2008 | KX354450.1 | Italy | |
| OUR T88/3 | AM712240 | Portugal | |
| Spain/E75 | NC_044958.1 | Spain | |
| Spain/BA71V | AM712239 | Spain | |
| Benin-97/1 | NC_044956.1 | Benin | |
| Portugal/NHV/1968 | KM262845.1 | Portugal | |
| II | Georgia_2007/1 | FR682468.2 | Georgia |
| Georgia_2008/2 | MH910495 | Georgia | |
| AES01 | MK940252.1 | China | |
| HuB20 | MW521382.1 | China | |
| SY18 | MH766894.3 | China | |
| Wuhan 2019-1 | MN393476.1 | China | |
| AnhuiXCGQ | MK128995 | China | |
| HLJ/18 | MK333180.1 | China | |
| DB/LN/2018 | MK333181.1 | China | |
| IX | R7 | MH025917 | Uganda |
| R8 | MH025916 | Uganda | |
| Kenya/Ken06.Bus | KM111295.1 | Kenya | |
| Uganda/R35/2015 | MH025920.1 | Uganda | |
| X | Uvriva B53 | MT956648 | Democratic Republic of the Congo |
| BUR/18/Rutana | MW856067 | Burundi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, W.; Huang, Y.; He, Y.; Chu, Y.; Tao, C.; Pang, Z.; Wang, Z.; Zhu, H.; Jia, H. Immunogenicity Analysis and Identification of Potential T-Cell Epitopes in C129R Protein of African Swine Fever Virus. Microorganisms 2024, 12, 1056. https://doi.org/10.3390/microorganisms12061056
Zhai W, Huang Y, He Y, Chu Y, Tao C, Pang Z, Wang Z, Zhu H, Jia H. Immunogenicity Analysis and Identification of Potential T-Cell Epitopes in C129R Protein of African Swine Fever Virus. Microorganisms. 2024; 12(6):1056. https://doi.org/10.3390/microorganisms12061056
Chicago/Turabian StyleZhai, Wenzhu, Ying Huang, Yuheng He, Yuanyuan Chu, Chunhao Tao, Zhongbao Pang, Zhen Wang, Hongfei Zhu, and Hong Jia. 2024. "Immunogenicity Analysis and Identification of Potential T-Cell Epitopes in C129R Protein of African Swine Fever Virus" Microorganisms 12, no. 6: 1056. https://doi.org/10.3390/microorganisms12061056
APA StyleZhai, W., Huang, Y., He, Y., Chu, Y., Tao, C., Pang, Z., Wang, Z., Zhu, H., & Jia, H. (2024). Immunogenicity Analysis and Identification of Potential T-Cell Epitopes in C129R Protein of African Swine Fever Virus. Microorganisms, 12(6), 1056. https://doi.org/10.3390/microorganisms12061056






