Abstract
A Gram-positive, rod-shaped, aerobic, motile, and spore-forming bacterium, designated SCL10, was isolated from Acaudina molpadioides exposure to Co-60 radiation. In this study, whole-genome sequencing was performed to identify the strain as Bacillus cereus and functional characterization, with a focus on stress resistance. The genome of the B. cereus SCL10 strain was sequenced and assembled, revealing a size of 4,979,182 bp and 5167 coding genes. The genes involved in biological functions were annotated by using the GO, COG, KEGG, NR, and Swiss-Prot databases. The results showed that genes related to alkyl hydroperoxide reductase (ahpC, ahpF), DNA-binding proteins from starved cells (dps), spore and biofilm formation (spoVG, spo0A, gerP), cold shock-like protein (cspC, cspE), ATP-dependent chaperone (clpB), and photolyase, small, acid-soluble spore protein (SASP) and DNA repair protein (recA, radD) could explain the stress resistance. These findings suggest that antioxidant activity, sporulation, biofilm formation, and DNA protection may be considered as the main resistance mechanisms under exposure to radiation in the B. cereus SCL10 strain.
1. Introduction
Bacillus cereus is a well-known foodborne pathogenic bacterium responsible for two types of food-associated gastrointestinal diseases, diarrheal and emetic, which are induced by toxins [1]. The diarrheal syndrome is caused by hemolysin BL (HBL), nonhemolytic enterotoxin (NHE), and cytotoxin K (CytK, also known as hemolysin IV); and the emetic syndrome is caused by cereulide [2]. Diarrhea and emesis caused by B. cereus are closely related to food infections, especially in rice, pasta, pastries, dairy, vegetables, and meat products. The diseases are generally mild and self-limiting, making it difficult for health authorities to determine the actual incidence [3]. The proportion of B. cereus infections is underestimated, and severe and fatal outbreaks have also been reported [4]. Despite the optimized combination of sterilization technologies such as heat, irradiation, and chemical reagents, B. cereus can still survive and pose a formidable challenge for food industries worldwide [5]. In addition to toxins and virulence factors, adaptability and resistance to the environment are also reasons for its pathogenicity and survivability [6].
In hostile environments, B. cereus has a self-protection system and develops resistance accordingly. As in most bacteria, once the level of reactive oxygen species (ROS) exceeds the capacity of endogenous antioxidant defense, the redox balance is disrupted and the oxidative stress system is activated [7]. Antioxidant defenses such as catalases, peroxiredoxins, and superoxide dismutases will promote anti-stress effects as one of the resistance strategies [8]. In addition, spores and biofilms have proven to be two forms of resistance mechanisms, leading to a high adhesion capacity on various substrates [9]. Meanwhile, resistance is affected by sporulation and biofilm-forming conditions. Spores and biofilms can both withstand extreme conditions, such as chemical agents, wetness, dry heat, low pH values, and radiation, an expanding contamination of food spoilage, and human diseases [10]. The resistance factors of spores include the outer layer, inner membrane, low water content, high levels of dipicolinic acid in the spore core, and DNA protection mechanism [11]. The appropriate conditions can prompt spores to return to vegetative cells through germination, which allows the application of milder inactivation procedures [12]. Biofilms are clusters of arranged bacteria that are attached to a surface and embedded in the self-produced matrix of the extracellular polymeric substance (EPS), where antibiotic resistance genes (ARGs) are easily transferred [13]. ARGs and genes associated with antioxidants, spores, and biofilms regulate the corresponding resistance.
Phenotypic resistance is known to have a genetic basis, which provides a reliable perspective on resistance mechanisms [14]. Any bacterium that survives adverse conditions of extreme temperature, radiation, and antibiotics would carry genes with different resistance levels and evolve multiple-stress resistance. Understanding the mechanisms underlying resistance contributes to cleaning procedures. Genomics presents a pathway for focusing on resistance genes and defense mechanisms. At present, whole-genome sequencing (WGS) of B. cereus is mostly employed in studies on routine surveillance, virulence, drug resistance mechanisms, and immune-related genes [15,16]. WGS can be used to analyze resistance in B. cereus at the molecular level. It is of interest to identify stress-related genes so that resistant bacteria that survive food sterilization would be better understood and more targeted sterilization methods could be applied.
The strain was isolated from the Co-60-induced watery sea cucumber (Acaudina molpadioides), designated the B. cereus SCL10 strain, which remained active and exposed to a high dose of Co-60 radiation. In this study, by combining pure culture and comparative genomics approaches, the WGS analysis was conducted to study stress-resistance genes and gene functions including metabolic pathways, protein functions, and virulence. Based on the predicted results, the sterilization and survival of B. cereus during food processing and preservation will be better understood. This study is expected to provide a genomic reference for resistance mechanism studies of B. cereus, contributing to food safety and public health.
2. Materials and Methods
2.1. Isolation and Culture of the Strain
The B. cereus SCL10 strain was isolated from the Co-60-induced watery sea cucumber (A. molpadioides), and preserved at Microbiology Laboratory, Ningbo University, China. The B. cereus SCL10 strain was cultured in a nutrient broth (NB) medium comprising peptone at 10.0 g/L, beef extract at 3.0 g/L, NaCl at 5.0 g/L, and additional NaCl at 15.0 g/L (simulated the growth environment of A. molpadioides), and an NB agar medium comprising peptone at 10.0 g/L, beef extract at 3.0 g/L, NaCl at 5.0 g/L, agar at 20.0 g/L, and additional NaCl at 15.0 g/L. The preserved strain was applied to the NB agar plate after gradient dilution, and incubated aerobically at 37. A single colony was picked and incubated into the NB at 37 °C and 150 revolutions per minute (rpm) for 12 h. The B. cereus pellet was obtained by centrifugation at 6000 rpm for 5 min and then washed twice with 0.10 mol/L phosphate buffer saline (PBS, pH 7.4). It was subsequently resuspended in the 0.10 mol/L PBS and prepared for the extraction of genomic DNA.
2.2. Genomic DNA Extraction and Whole-Genome Sequencing
According to the previous literature, genomic DNA was extracted with the SDS method [17,18]. The DNA of the B. cereus SCL10 strain was quantified using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), and PCR products were purified. The sequencing library was generated with NEBNext Ultra II DNA Library Prep Kit for Illumina (NEB, Ipswich, MA, USA), and then was analyzed for size distribution by Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and quantified by real-time PCR. The draft genome of the B. cereus SCL10 strain was sequenced with Illumina NovaSeq PE150 at LC-Bio Technology Co., Ltd. (Hangzhou, Zhejiang, China). The raw data were assembled with SOAP Denovo, SPAdes, and Abyss, filtering the low-quality reads out.
2.3. Genome Component Prediction
Genome component prediction included the prediction of the coding genes, non-coding RNAs, repetitive sequences, transposons, prophages, genomic islands, and clustered regularly interspaced short palindromic repeat (CRISPR) sequences. The GeneMarks program was used to retrieve the related coding genes and the RepeatMasker was used to search for repetitive sequences, the Tandem Repeats Finder (TRF) [19] to tandem repeats, the tRNAscan-SE to transfer RNA (tRNA) genes, the rRNAmmer to ribosome RNA (rRNA) genes, Basic Local Alignment Search Tool (blast) against the RNA families (Rfam) database [20] to small nuclear RNAs (snRNAs), the IslandPath-DIOMB program to the Genomics Islands, transposonPSI to the transposons, the PHAST to the prophages, and the CRISPR Finder to CRISPRs.
2.4. Genome Function Annotation
Eight databases were used to predict gene functions. It contained Non-Redundant Protein Sequence Database (NR), Kyoto Encyclopedia of Genes and Genomes (KEGG), Clusters of Orthologous Groups (COGs), Gene Ontology (GO), carbohydrate-active enzymes (CAZy), Transporter Classification Database (TCDB), protein families (Pfam), and Swiss-Prot Protein Sequence Database (Swiss-Prot), respectively [21]. A blast search of the whole genome of the B. cereus SCL10 strain was performed against the above eight databases. Meanwhile, we analyzed secondary metabolism gene clusters by the Antibiotics and Secondary Metabolite Analysis Shell (antiSMASH) [22]. Also, we used the Virulence Factors of Pathogenic Bacteria Database (VFDB) to study pathogenicity and used Comprehensive Antibiotic Research Database (CARD) to study antibiotic resistance genes [23].
2.5. Comparative Genomic Analysis
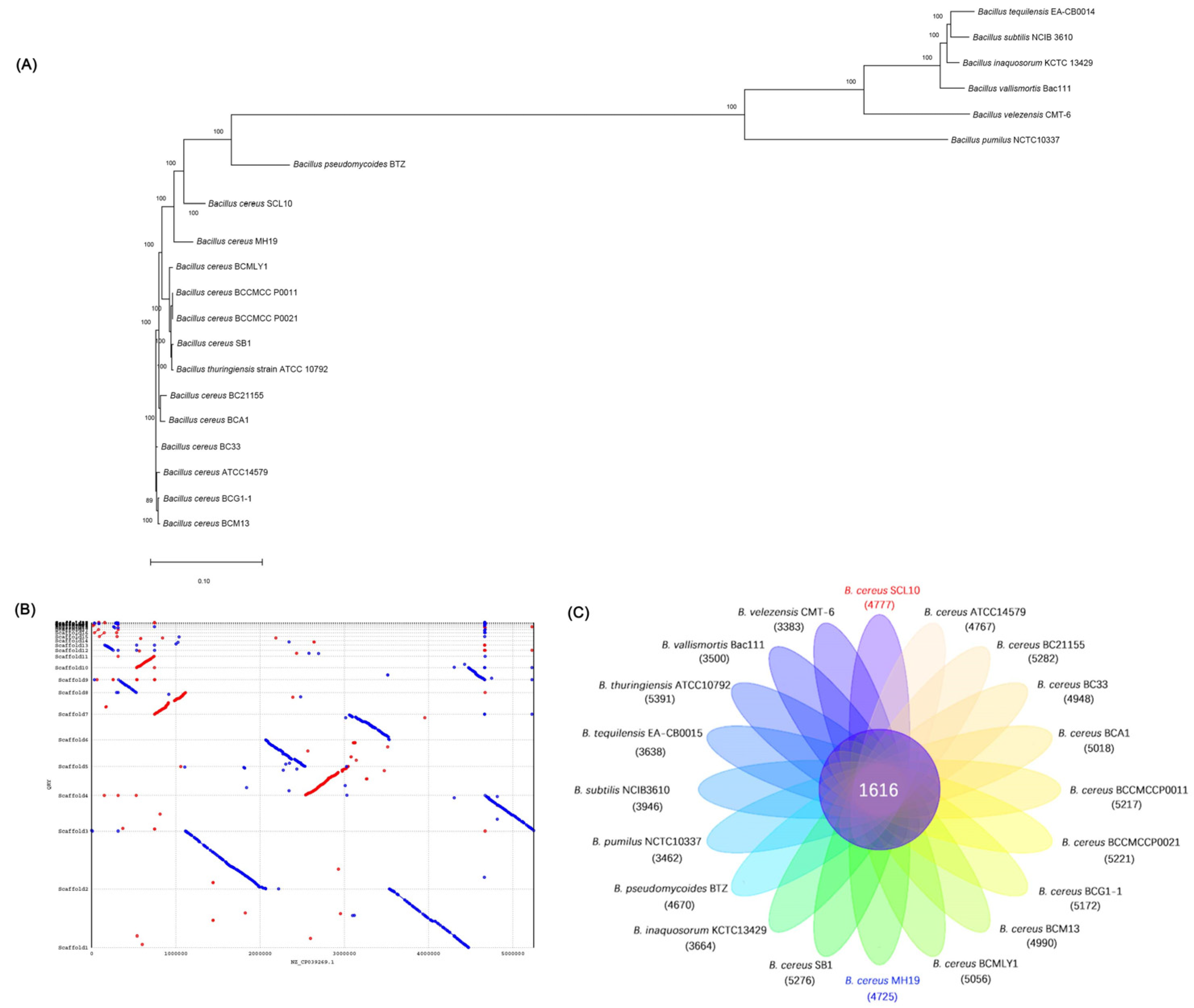
The comparative genomic analysis is applied among the B. cereus SCL10 strain and the other 19 strains (detailed strain information in Table S1), including core genes, specific genes, a synteny analysis, and a gene family phylogenetic tree. Genomic alignment between the sample genome and reference genomes was performed with the MUMmer and LASTZ. Based on the alignment results, the synteny analysis was among the B. cereus SCL10 strain and the most similar strain MH19. The gene family was constructed using blast and Hcluster_sg.
3. Results
3.1. Identification of B. cereus by WGS
When cultured on the NB agar medium, the colonies of B. cereus were white, round, flat, and soft, with irregular margins, a slightly dry and rough surface, and a special feature of surface elevation (Figure S1). The optimal matching result for the genome showed that 4955 unigenes (refers to non-redundant gene sequences obtained from whole-genome sequencing) of the strain were annotated, accounting for 95.90% of the total coding genes in the NR database (Figure 1). A total of 3478 species-related unigenes were identified for the strain B. cereus.
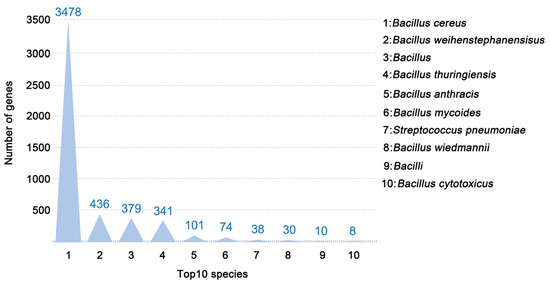
Figure 1.
The species identification of genes annotated. The top 10 species with the highest matches were selected in the Non-Redundant Protein Sequence (NR) Database. The height of the bar represents the number of genes.
3.2. Composition of the Overall B. cereus SCL10 Strain Genome
The B. cereus SCL10 strain genome consisted of 34 contigs (>500 bp) of 4,979,182 bp, with a GC% = 35.42. The Q20 and Q30 values were 97.51% and 93.25%, indicating good quality of the genome. The overall annotation information of the genome is listed in Table 1. Data validity information is provided in the Supplementary Materials (Figure S2). The assembled whole genome contained 5167 coding sequences (4955 protein-coding genes with functional assignment), and the total length was 4,167,474 bp, accounting for 83.70% of the genome. The main genome features of the B. cereus SCL10 strain included 33 transposons, 115 long terminal repeats, 25 long scattered repeats, 10 short scattered repeats, 12 prophages, and six CRISPRs. In addition, regarding non-coding RNAs, the genome encodes 89 tRNAs, 14 rRNAs, and six sRNAs.

Table 1.
Genome prediction statistics of B. cereus SCL10 strain.
3.3. General Functions of B. cereus SCL10 Strain Annotation
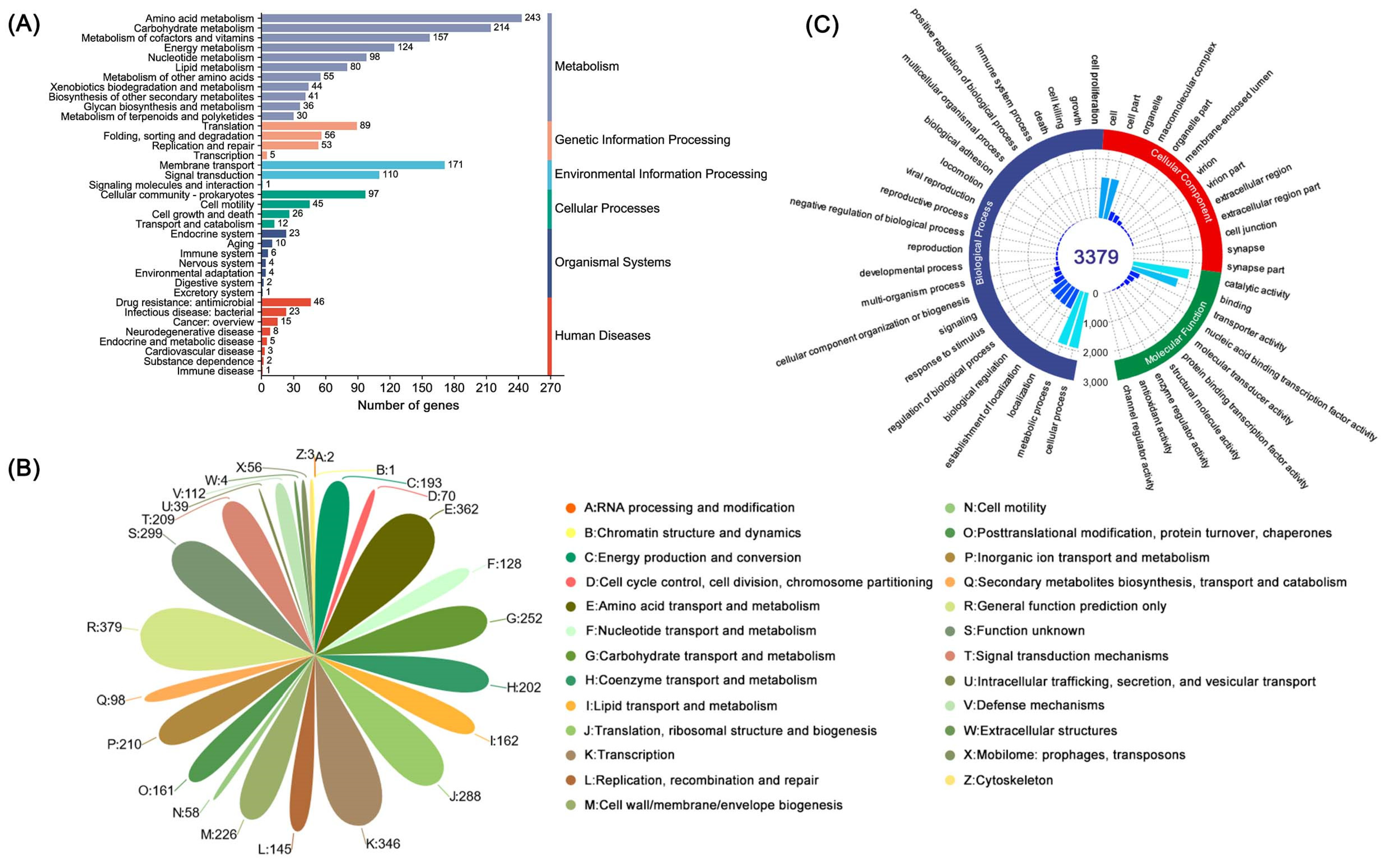
Using the GO, COG, KEGG, Pfam, Swiss-Prot, and TCDB databases as a reference, various biological functions of genes and gene clusters were annotated in the B. cereus SCL10 strain genome. The number of genes found within each category and their sources are shown below (Figure 2). KEGG pathways were associated with 2407 unigenes, which were gathered into 199 metabolic pathways, including 243 unigenes in amino acid metabolic pathways, 214 in carbohydrate metabolic pathways, and 171 in membrane transport pathways of the top three pathways (Figure 3A). In addition, 53 unigenes in metabolic pathways for replication and repair were annotated. The detailed KEGG map showed 140 unigenes involved in ABC transporters (map02010), 88 in quorum sensing (map02024), and 30 in flagellar assembly (map02040) (Table S2).
Figure 2.
The general function annotation of the B. cereus SCL10 strain involving eight databases. The number of genes annotated varies across different databases, which can be compared with a total number of 5167 coding sequence genes. The eight databases are labeled in different colors and the numbers are gene counts. COG: Cluster of Orthologous Groups of Proteins, GO: Gene Ontology, Pfam: Protein Family, Swiss-Prot: Non-redundant High-quality Proteins, KEGG: Kyoto Encyclopedia of Genes and Genomes, CAZy: Carbohydrate-active Enzymes, TCDB: Transporter Classification Database, antiSMASH: Secondary Metabolism Gene Clusters.
Figure 3.
The different function classification of the B. cereus SCL10 strain. (A) KEGG annotation. The X-axis is the number of genes, and the Y-axis is the KEGG pathway. Different colors of the columns represent different categories, and the corresponding category names are on the right side. (B) COG annotation. Different letters and colors represent different classifications, and the numbers beside the petals represent the number of genes. (C) GO annotation. The three colors of the outer circle represent the three categories, which are used to distinguish biological processes, cell components, and molecular functions. The bars indicate the number of genes with different functions at all levels of the catalog.
A total of 68.05% of unigenes (3516) were represented in 26 classes by COG functional categories (Figure 3B). The protein functions of the B. cereus SCL10 strain were associated with cell wall/membrane/envelope biogenesis (M); post-translational modification, protein turnover, and chaperones (O); replication, recombination, and repair (L); and defense mechanisms (V), with 226, 161, 145, and 112 unigenes, respectively (Table S3). Defense mechanisms included organic hydroperoxide reductase (ohrA, ohrR, osmC), DNA-binding ferritin-like protein (oxidative damage protectant), alkyl hydroperoxide reductase subunit (ahpC, ahpF), and glutathione peroxidase, and the chaperone included ATP-dependent protease (clpA, clpP).
The GO database defines three major categories: the biological process, molecular function, and cellular component (Figure 3C). The biological process had the largest number and function types of annotated genes (7741), accounting for 48.68% of all annotation information, including biological regulation, response to a stimulus, signaling, and other biological processes related to adaptation. There were 371 responses to stimulus unigenes and 57 membrane-enclosed lumen unigenes. The two remaining categories were the molecular function and cellular component. The molecular function was associated with 337 unigenes involved in transporter activities, 28 in enzyme regulation, and 9 in antioxidant activities. Importantly, protein-encoding genes associated with general stress protein and ATP-dependent chaperone ClpB were found in the GO annotation results, covering 31 and 32 classes, respectively (Table S4).
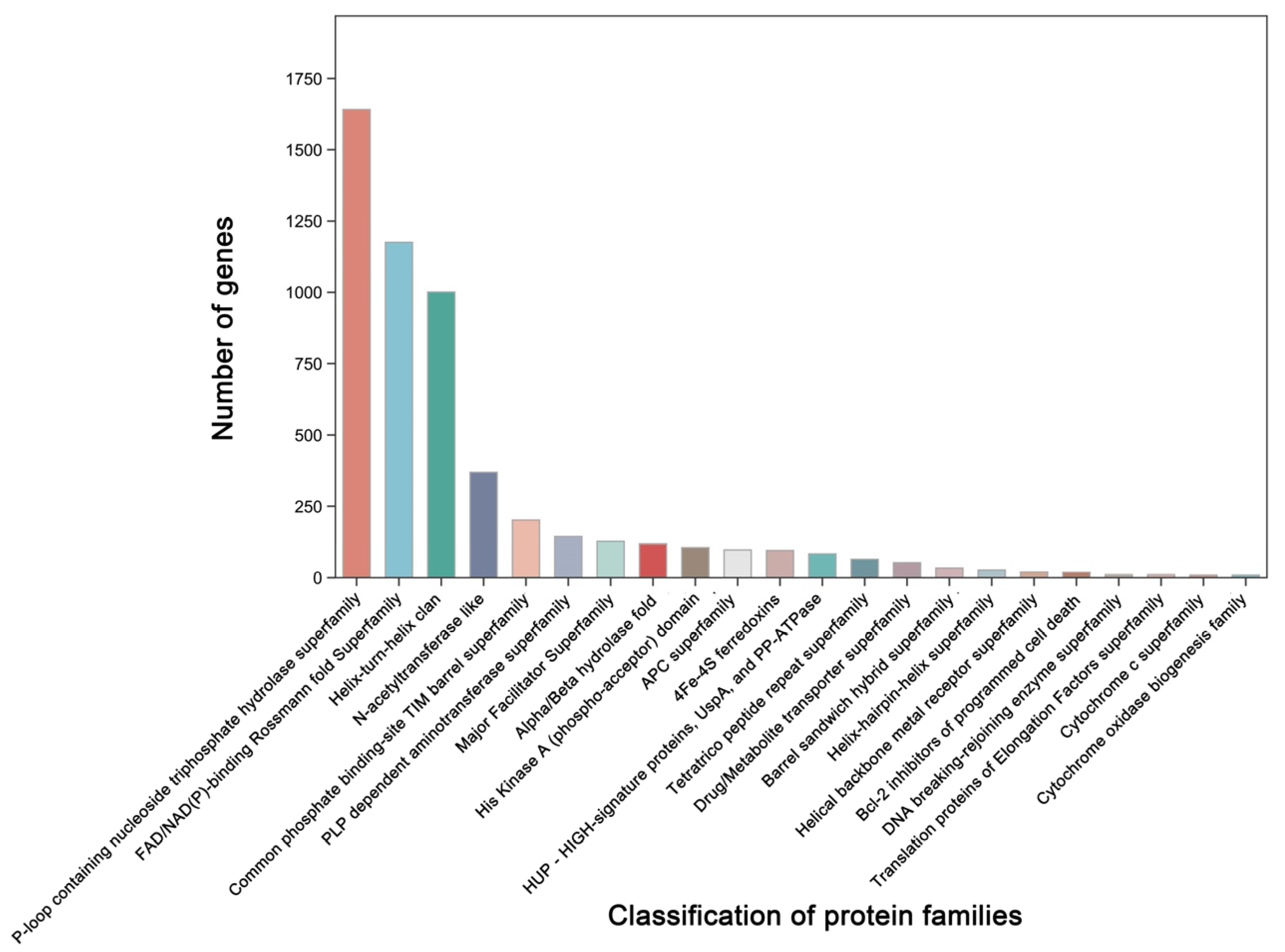
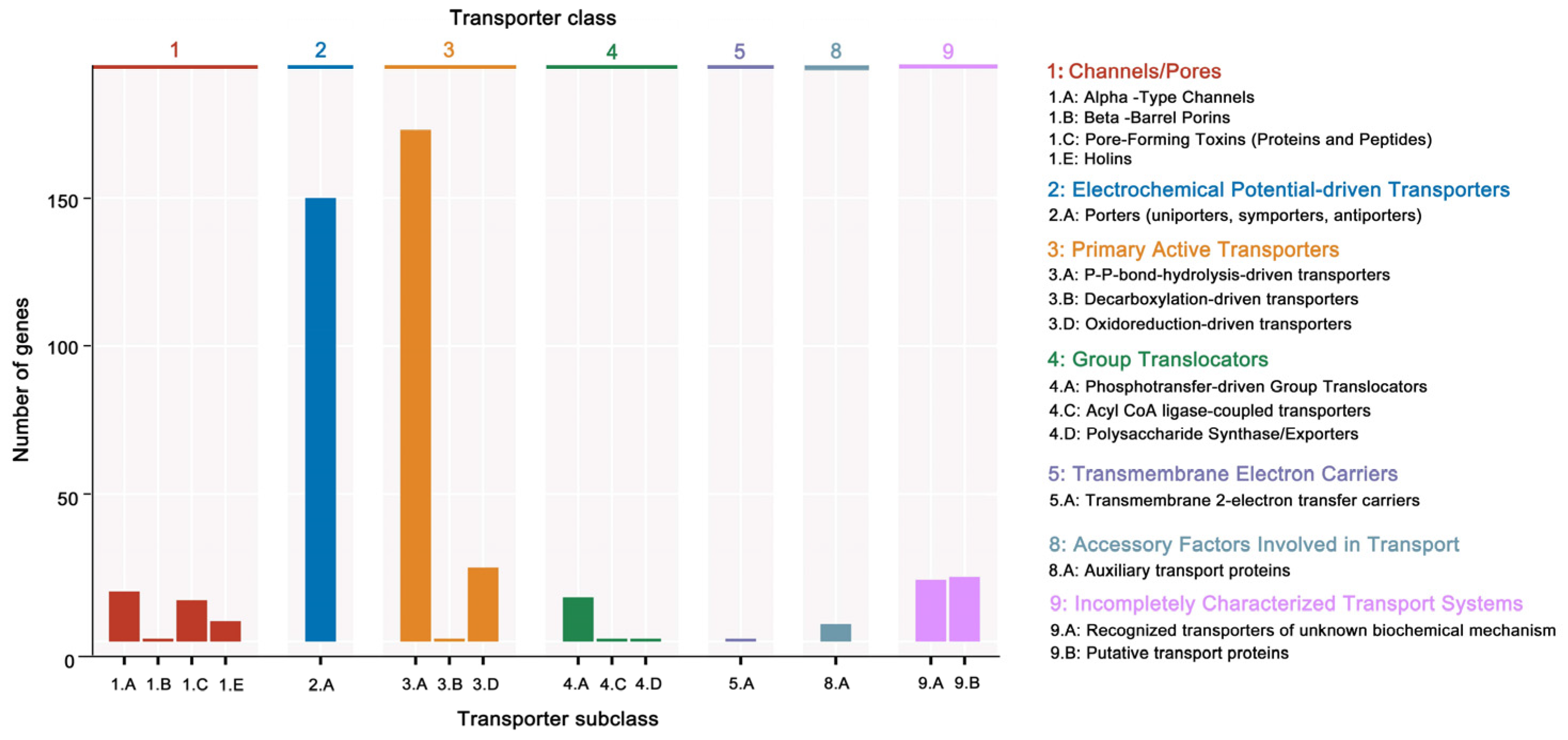
Additionally, 3379 unigenes were associated with a protein family in the Pfam database, including 18 members of bcl-2 inhibitors of programmed cell death and 10 members of the DNA breaking–rejoining enzyme superfamily (Figure 4, Table S5). A total of 2545 unigenes were annotated in the Swiss-Prot database, representing high-quality protein genes with low redundancy (Table S6). Furthermore, four types of secondary metabolism gene clusters of the B. cereus SCL10 strain were found: lassopeptide, siderophore, bacteriocin, and betalactone (Figure S3A). The classification of carbohydrate-active enzymes revealed 60 glycosyltransferases (GTs), 43 glycosidase hydrolases (GHs), 38 carbohydrate-binding modules (CBMs), and 17 carbohydrate esterases (CEs) (Figure S3B). The TCDB system, comprising 5 levels, revealed 199 major active transport proteins, 150 electrochemical potential-driven transport proteins, 39 channel/micropores, and other transport proteins (Figure 5, Table S7).
Figure 4.
The classification and gene number of protein families of the B. cereus SCL10 strain. The top 20 protein families with the highest numbers and correlations with resistance were selected in the protein families (Pfam) database.
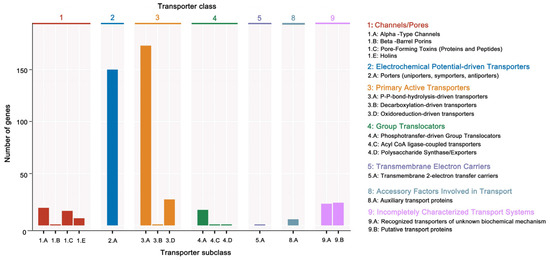
Figure 5.
The TCDB annotation of the B. cereus SCL10 strain. The TC system is classified into 5 levels, each level corresponds to a letter or number in the TC number, and each letter or number represents a specific type of transport protein. Level 2 is a more specific subcategory below Level 1, and Level 3 is a transporter protein family classification. The horizontal coordinate indicates the number of Level 2.
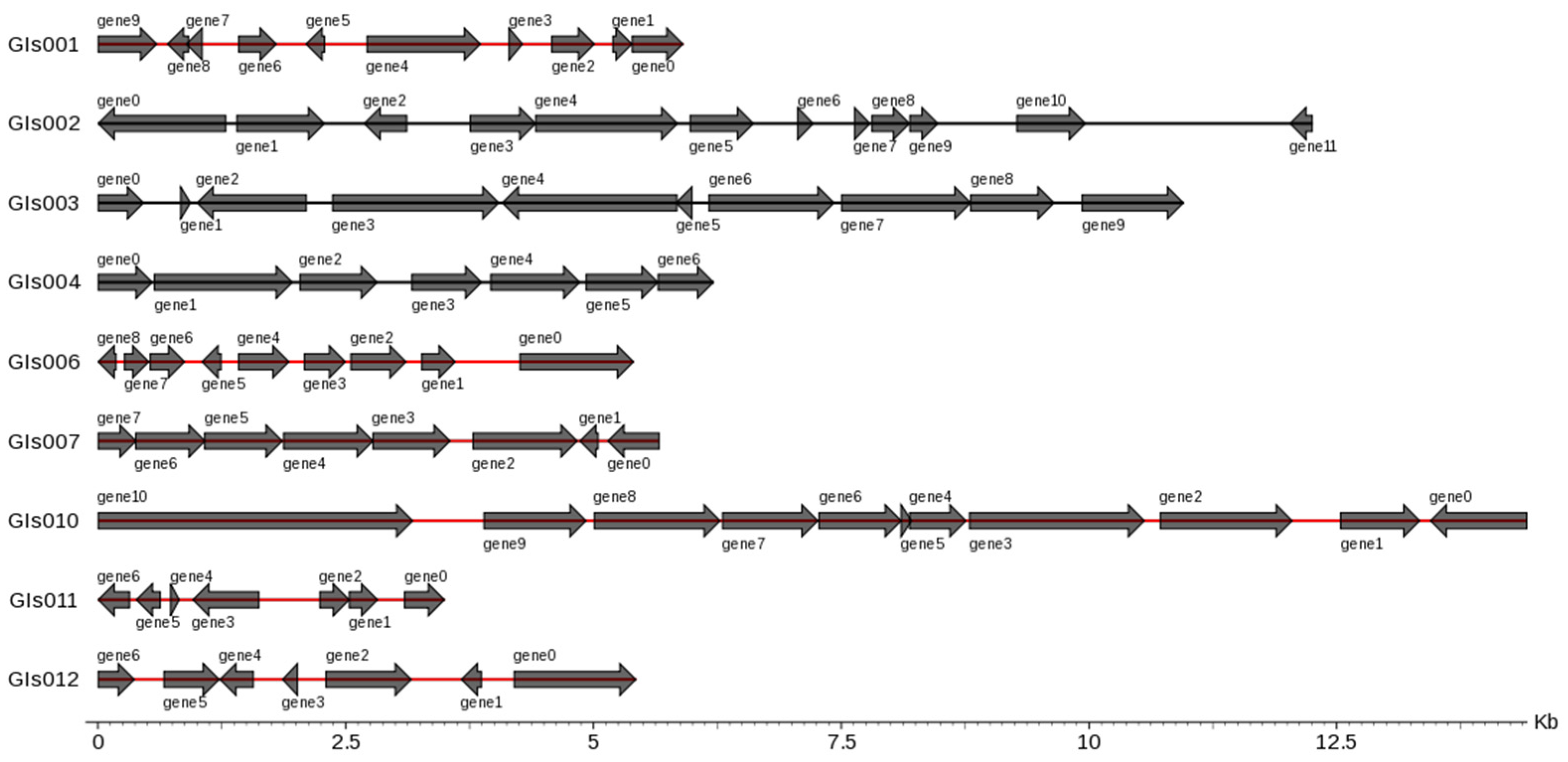
3.4. Genes Related to Flagellum and Enzymes in Genomic Island Analysis
There were 12 genomic islands (GIs) in the whole genome of the B. cereus SCL10 strain, which were related to a variety of biological functions, such as pathogenic and adaptive mechanisms (Figure 6). Genes related to the flagellum included the flagellar biosynthesis regulator flhF; flagellar biosynthesis proteins flhA, flhB, fliR, and fliP; flagellar export apparatus protein fliQ; and flagellar motor switch protein fliM. Additionally, other metabolic regulatory factors were concluded in GIs004, such as the GTP-sensing transcriptional pleiotropic repressor codY, and were included in GIs009, such as phage integrase family protein, FAD-dependent oxidoreductase, alkyl hydro peroxidase AhpD family core domain-containing protein, spore photoproduct lyase, and deoxyribodipyrimidine photolyase (Table S8).
Figure 6.
Gene distribution in gene islands of B. cereus SCL10 strain. On the left is the gene island ID, and on the right is the number and size of genes contained in the gene island. Horizontal coordinates are length scales. GIs shown in figure are less than 15 kb.
3.5. B. cereus SCL10 Strain Multiple Pathogenic Factors and Antibiotic Resistance
Genes associated with virulence and stress response were annotated in the B. cereus SCL10 strain genome. The genome harbored 260 virulence genes aligned to the VFDB database. All virulence factors were categorized into 13 types (Figure 7A). The top four types, which included immune modulation, nutritional/metabolic factor, exotoxin, and motility, had different numbers of unigenes (Figure S4). In the pathogen–host interactions, the number of PHI-related genes of the B. cereus SCL10 strain was 273, mainly distributed in the categories of reduced virulence (66.7%) and unaffected pathogenicity (13.9%) (Figure 7B). In addition, the B. cereus SCL10 strain carried 192 antibiotic resistance genes of 20 major types (Figure 7C, Table S9). The top five types containing the largest numbers of genes were as follows: 32 peptide antibiotic genes, 27 glycopeptide antibiotic resistance genes, 24 fluoroquinolone genes, 24 macrolide resistance genes, and 23 aminoglycoside resistance genes. A small number of genes that mediated resistance to antibiotics, such as diaminopyrimidines, sulfa, and streptomycin, were also found in the genome.
Figure 7.
Pathogenic factors and antibiotic resistance analysis of B. cereus SCL10 strain. (A) Distribution of virulence factors. (B) Distribution of pathogen–host interaction genes. (C) Distribution of main top 20 antibiotic resistance genes.
3.6. Comparative Genomics of B. cereus SCL10 Strain
In addition to the general functional annotation of the B. cereus SCL10 strain, comparative genomics was also carried out to analyze its specific functions in comparison with those of other Bacillus genera. The phylogenetic tree was constructed based on the alignment of 1355 single copies of homologous genes from 20 strains (Figure 8A, Table S1). The B. cereus SCL10 strain, B. cereus MH19 strain, and other Bacillus sp. strains were located on the same large branch. In addition, the B. cereus SCL10 strain and B. cereus MH19 strain were located on adjacent branches and clustered closely to each other, with a confidence level of 100. Based on the phylogenetic tree, the synteny analysis of the B. cereus SCL10 strain and B. cereus MH19 strain was performed to explore their evolutionary relationship (Figure 8B). The genome sequences of the two strains had high similarity and a large number of homologous regions. At the same time, there was also a small amount of translocation and gene deletion. The blank area showed the area not aligned, indicating that the strain had unique genomic regions. Additionally, the core genome analysis was conducted by comparing the B. cereus SCL10 strain genome and the genomes of 19 other strains. The results revealed that the number of shared orthologous genes was 1616 among the 20 strains, and the B. cereus SCL10 strain and B. cereus MH19 strain showed 4777 and 4725 genes, respectively, with the smallest difference between them (Figure 8C). A further analysis revealed that 3853 unigenes were homologous, 770 unigenes were unique to the B. cereus MH19 strain, and 920 unigenes were unique to the B. cereus SCL10 strain.
Figure 8.
Comparative genomic and evolutionary analysis of B. cereus SCL10 strain and other 19 strains. (A) Phylogenetic analysis based on single-copy genes of 20 strains. (B) Synteny analysis of B. cereus SCL10 strain and B. cereus MH19 strain. Red represents forward alignment, blue represents reverse alignment, and gaps represent possible rearrangements between two genomes. (C) Venn diagram of gene family analysis showing shared and unique genes of 20 strains.
3.7. Stress-Resistance Genes Related to Spores and Biofilms
In the B. cereus SCL10 strain genome, annotated stress-resistance genes were relevant to the general stress response and the main two aspects, spores and biofilms. From an overall perspective, five general stress response protein genes, forty-four spore-related genes, and five biofilm-related genes were identified (Table 2). These functional genes were annotated by comparison with the Swiss-Prot database. Comparison with the NR database also revealed a large number of spore-associated genes, whose identity was mostly 100. In addition to the stress response protein genes yhaX, yugI, yceD, yceC, and SH1215 annotated against the Swiss-Prot database, the universal stress protein-encoding gene uspA was also annotated against the NR database. Genes related to spores included small, acid-soluble spore protein (SASP), spore germination protein, different stage sporulation protein, spore maturation protein, septation protein, and sporulation transcription regulator, including spo0/II/III/IV/V, gerE/I/L/P, sspF/H1/H2/I/K/O/N, and so on. The sporulation membrane protein ytaF, membrane protein insertion efficiency factor yidD, and mutator protein mutT are worth noting (Table S10).

Table 2.
Genes related to general stress resistance of B. cereus SCL10 strain.
3.8. Stress-Resistance Genes Related to Other Biological Processes
In addition to the genes mentioned above, genes related to several other aspects of stress resistance, such as a cellular process, transporter, cold/salt resistance, oxidative stress resistance, transcriptional regulation, and DNA protection, were identified (Table 3). These genes, gene families, and superfamilies were derived from different databases, namely, Swiss-Prot, TCDB, Pfam, and GIs. Additional descriptions of these genes are provided in the Supplementary Materials (Table S11). Additionally, the NR database annotated (redox-sensitive) transcriptional regulation, cold tolerance, and DNA protection-related genes (Table S10). The cold/salt resistance genes were associated with cold shock proteins (cspB, cspC, cspE) and the (salt or low-temperature) stress-induced hydrophobic peptide (SHP) family. About transcriptional regulation, the genes transcriptional repressor nrdR, transcription antitermination protein nusB, redox-sensitive transcriptional repressor rex, and nutrient-sensitive regulator codY were annotated. For the DNA protection-related functions, a large number of genes were found, including DNA repair protein recFOR and radA, DNA protection during starvation proteins dps1 and dps2, UV DNA damage endonuclease uvsE, DNA breaking–rejoining enzyme superfamily, spore photoproduct lyase, and deoxyribodipyrimidine photolyase. Moreover, there were special genes associated with inhibitors of programmed cell death bcl-2 and the chloroplast envelope protein translocase (CEPT or Tic-Toc) family in the genome.

Table 3.
Other genes related to stress resistance of B. cereus SCL10 strain.
4. Discussion
Foodborne illnesses caused by pathogenic B. cereus occur worldwide, and this species poses a great challenge to the food and public health industries as a result of its extraordinary resistance, making it difficult to eradicate [24]. Present-day sterilization and detection techniques have improved the control of infection by this bacterium. Genomic studies of new isolates can uncover gene functions, explain biological mechanisms, and identify stress-resistance genes for better sterilization [25]. The B. cereus SCL10 strain, which remained active, was isolated from Co-60-inactivated watery sea cucumber (A. molpadioides) cryopreserved for more than 10 years. The survival of the SCL10 strain under radiation sterilization could be associated with predicted stress-resistance genes. Traditional experimental and identification methods have difficulty in fully analyzing the resistance of B. cereus and cannot uncover all of the stress-resistance genes. In this study, the whole genome of the B. cereus SCL10 strain was sequenced to investigate genes involved in defense response and resistance to adverse environments by WGS and a bioinformatics analysis.
Oxidative stress is an inherent defense response of organisms. Due to genetic history, oxidative damage is unavoidable in adverse environments, and organisms have evolved their metabolic systems to protect against oxidation [26]. Alkyl hydroperoxide reductase AhpC and its corresponding reducing systems (e.g., AhpF) were the first systems used by bacteria to decompose ROS [27]. The organic hydroperoxide reductase/osmotically induced bacterial protein C (Ohr/OsmC) family can defend against the attack of fatty acid hydroperoxides and peroxynitrite [28]. DNA-binding proteins from starved cells (Dps) act as oxidative DNA damage protectants [29]. The genes dps1 and dps2 were identified in the genome in this study. Dps performs dual actions to protect DNA by physical binding and reducing oxidative damage initiated by excess iron [30].
The significant induction of general stress proteins is one of the most notable stress responses of the bacterium [31]. Orthologous genes related to general stress protein (WP_001998456.1) were annotated in 31 GO functional modes, indicating a high number of annotations. Genes linked with stress response proteins such as yhaX, yugI, yceD, yceC, and SH1215 were present in the genome. Moreover, the ATP-dependent chaperone gene clpB (WP_000365408.1) was annotated in 32 GO modes, and clpA (NP_842649) and clpP (YP_007424642) were found in COG annotation. Unfolded proteins may be a common outcome of coping with different stresses. The chaperone Clp represents a key cellular component in bacterial survival and adaptation since it maintains cellular proteostasis to prevent protein misfolding [32]. Among the Clp chaperones, ClpB performs vital functions for protection in radiation environments through changes in ROS, antibiotics, and bactericidal molecules [33].
Spores, one of the special structures of Bacillus spp., can withstand adverse environments and make later virulence release possible [34]. Biofilms increase the resistance of spores or vegetative cells, which can protect B. cereus to different degrees [35]. In total, 49 low-redundancy protein-encoding genes were obtained by Swiss-Prot database annotation to determine exactly which genes regulate spore and biofilm formation. For spore formation, different stage sporulation proteins affect the corresponding stage, which can produce functionally important amino acids to construct the spore and maintain the capacity to survive and the potential for reactivation [36]. SpoVG and Spo0A are important in regulating sporulation and biofilm formation [37]. The spore germination protein GerP in the genome plays a major role in the coat (inner coat and outer coat) [38]. Spore maturation protein genes and spoII/III/IV/V were highly abundantly expressed during sporulation. The cold shock proteins CspB, CspC, and CspE also act in growth, biofilm formation during temperature decrease, and antibiotic resistance [39]. Prior studies showed that Clp also plays an important role in biofilm formation and bacterium-hesive material interactions in addition to general stress regulation [40].
As discussed above, the outer spore layer and its low-water-content core have a barrier effect under low temperatures and radiation. Moreover, DNA protection mechanisms are also available to prevent further damage to the spore [41]. Upon radiation exposure, the inner DNA is damaged; as a result, the number of DNA protection genes exhibits a wide distribution. The two major spore DNA repair pathways of Bacillus subtilis were also found in B. cereus, namely, nucleotide excision repair and spore photoproduct lyase, which are vital to resistance and DNA protection [42]. This may explain why B. cereus can remain active in food sterilized by radiation. Ultraviolet (UV) DNA damage endonuclease uvsE and DNA damage scanning disA in the genome showed that DNA was damaged. Thus, DNA protection and repair in spores can play an important role under radiation pressure. The DNA repair proteins RecA and RecFOR participate in synthesis-dependent strand annealing (SDSA) and recombination repair, during which the RecA-dependent accessory protein RadD greatly accelerates DNA strand exchange [43,44]. However, the mechanism of RecF remains to be further studied. In addition, 53 unigenes in metabolic pathways for replication and repair were annotated among the KEGG pathways. During the protection process, small, acid-soluble spore protein (SASP), an exclusive constituent of the spore core, acts as a major protectant of spore DNA since they saturate the spore DNA to alter the structures and properties dramatically [45]. Regarding photochemistry protection, UV-induced spore photoproducts (SPs) are repaired by spore photoproduct lyase (SPL), regarded as a major DNA repair enzyme in spore-forming genera [46].
5. Conclusions
This study provides a general description of the resistance of the B. cereus SCL10 strain at the gene level by WGS. The whole genome consisted of 34 scaffolds of 4,979,182 bp and 5167 coding sequences. By comparison with the GO, COG, KEGG, NR, and Swiss-Prot databases, various functional genes were annotated. The B. cereus SCL10 strain has evolved adaptive radiation-stress genes to survive under conditions of radiation with Co-60. In the annotation of stress-resistance genes, antioxidant genes included ahpC, ahpF, and dps. Spore- and biofilm-related genes included spoVG, spo0A, gerP, cspC, cspE, and clpB. DNA protection components included photolyase, SASP, and the genes recA and radD. In conclusion, a variety of genetic characteristics, especially oxidative stress-related genes, sporulation genes, biofilm-related genes, and DNA protection regulation genes, are likely to be responsible for the radiation-adapted phenotype of the B. cereus SCL10 strain. DNA protection is the most important factor in radiation resilience and mainly depends on nucleotide excision repair and the spore photoproduct lyase. We hope that our analysis of stress resistance and the underlying mechanism will provide important insight for protection against B. cereus infection and in turn contribute to food safety and public health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12061168/s1, Figure S1: The colony morphology of the B. cereus SCL10 strain; Figure S2: Data validity analysis of whole genome of B. cereus SCL10 strain; Figure S3: Gene clusters and CAZy annotation of B. cereus SCL10 strain; Figure S4: Distribution of B. cereus SCL10 strain virulence factors; Table S1: Identification of 20 strains according to the phylogenetic tree; Table S2: Gene analysis for KEGG annotation of B. cereus SCL10 strain; Table S3: Gene analysis for COG annotation of B. cereus SCL10 strain; Table S4: Gene analysis for GO annotation of B. cereus SCL10 strain; Table S5: Gene analysis for Pfam annotation of B. cereus SCL10 strain; Table S6: Gene analysis for Swiss-Prot annotation of B. cereus SCL10 strain; Table S7: Gene analysis for TCDB annotation of B. cereus SCL10 strain; Table S8: Gene analysis for GI annotation of B. cereus SCL10 strain; Table S9: Gene analysis for the main antibiotic resistance of B. cereus SCL10 strain; Table S10: Gene analysis for various functions and stress resistance of B. cereus SCL10 in NR annotation; Table S11: Gene analysis for various stress resistance of B. cereus SCL10 in classification.
Author Contributions
Conceptualization, Y.M. and J.Z.; methodology, F.L.; software, L.Z.; validation, Y.Y. and H.C.; formal analysis, Y.M.; resources, X.S. and J.Z.; data curation, Y.Y., H.C., L.Z., and F.L.; project administration, J.Z. and J.H.; visualization, Y.M. and Y.Y.; writing—original draft preparation, Y.M.; writing—review and editing, J.H.; supervision, J.Z. and J.H.; funding acquisition, J.Z. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2023YFD2402200; Natural Science Foundation of Zhejiang Province, grant number LTGC23C190001; and K.C. Wong Magna Fund of Ningbo University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA1051368. The genome was accessed on 26 December 2023.
Acknowledgments
We would like to thank the support of Zhonghua Wang.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeng, Q.; Xie, J.; Li, Y.; Gao, T.; Xu, C.; Wang, Q. Comparative genomic and functional analyses of four sequenced Bacillus cereus genomes reveal conservation of genes relevant to plant-growth-promoting traits. Sci. Rep. 2018, 8, 17009. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Enosi Tuipulotu, D.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus cereus: Epidemiology, Virulence Factors, and Host-Pathogen Interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Butcher, M.; Puiu, D.; Romagnoli, M.; Carroll, K.C.; Salzberg, S.L.; Nauen, D.W. Rapidly fatal infection with Bacillus cereus/thuringiensis: Genome assembly of the responsible pathogen and consideration of possibly contributing toxins. Diagn. Microbiol. Infect. Dis. 2021, 101, 115534. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Zou, M.; Chantapakul, T.; Chen, W.; Muhammad, A.I.; Zhou, J.; Ding, T.; Ye, X.; Liu, D. Effect of ultrasonication and thermal and pressure treatments, individually and combined, on inactivation of Bacillus cereus spores. Appl. Microbiol. Biotechnol. 2019, 103, 2329–2338. [Google Scholar] [CrossRef]
- Jovanovic, J.; Ornelis, V.F.M.; Madder, A.; Rajkovic, A. Bacillus cereus food intoxication and toxicoinfection. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3719–3761. [Google Scholar] [CrossRef] [PubMed]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2021, 12, 821535. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Majed, R.; Faille, C.; Kallassy, M.; Gohar, M. Bacillus cereus Biofilms-Same, Only Different. Front. Microbiol. 2016, 7, 1054. [Google Scholar] [CrossRef]
- Checinska, A.; Paszczynski, A.; Burbank, M. Bacillus and Other Spore-Forming Genera: Variations in Responses and Mechanisms for Survival. Annu. Rev. Food Sci. Technol. 2015, 6, 351–369. [Google Scholar] [CrossRef]
- Setlow, P. Spore Resistance Properties. Microbiol. Spectr. 2014, 2, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Delbrück, A.I.; Zhang, Y.; Heydenreich, R.; Mathys, A. Bacillus spore germination at moderate high pressure: A review on underlying mechanisms, influencing factors, and its comparison with nutrient germination. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4159–4181. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Flint, S.; Li, Y.; Ou, K.; Yuan, L.; He, G.Q. Phenotypic and genetic heterogeneity within biofilms with particular emphasis on persistence and antimicrobial tolerance. Future Microbiol. 2017, 12, 1087–1107. [Google Scholar] [CrossRef]
- Chang, T.; Rosch, J.W.; Gu, Z.; Hakim, H.; Hewitt, C.; Gaur, A.; Wu, G.; Hayden, R.T. Whole-Genome Characterization of Bacillus cereus Associated with Specific Disease Manifestations. Infect. Immun. 2018, 86, e00574-17. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Wiedmann, M.; Kovac, J. Proposal of a Taxonomic Nomenclature for the Bacillus cereus Group Which Reconciles Genomic Definitions of Bacterial Species with Clinical and Industrial Phenotypes. mBio 2020, 11, e00034-20. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Lee, E.H.; Yoon, Y.; Chua, B.; Son, A. Portable lysis apparatus for rapid single-step DNA extraction of Bacillus subtilis. J. Appl. Microbiol. 2016, 120, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Gardner, P.P.; Daub, J.; Tate, J.G.; Nawrocki, E.P.; Kolbe, D.L.; Lindgreen, S.; Wilkinson, A.C.; Finn, R.D.; Griffiths-Jones, S.; Eddy, S.R.; et al. Rfam: Updates to the RNA families database. Nucleic Acids Res. 2009, 37, D136–D140. [Google Scholar] [CrossRef]
- Li, W.; Jaroszewski, L.; Godzik, A. Tolerating some redundancy significantly speeds up clustering of large protein databases. Bioinformatics 2002, 18, 77–82. [Google Scholar] [CrossRef]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus Spores in the Food Industry: A Review on Resistance and Response to Novel Inactivation Technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Dessai, U.; McGarry, S.; Gerner-Smidt, P. Use of Whole-Genome Sequencing for Food Safety and Public Health in the United States. Foodborne Pathog. Dis. 2019, 16, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Imlay, J.A. How Microbes Defend Themselves From Incoming Hydrogen Peroxide. Front. Immunol. 2021, 12, 667343. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, F.S.; Morgan, R.W.; Christman, M.F.; Ames, B.N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J. Biol. Chem. 1989, 264, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Meireles, D.A.; da Silva Neto, J.F.; Domingos, R.M.; Alegria, T.G.P.; Santos, L.C.M.; Netto, L.E.S. Ohr-OhrR, a neglected and highly efficient antioxidant system: Structure, catalysis, phylogeny, regulation, and physiological roles. Free Radic. Biol. Med. 2022, 185, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.C.; Soo, P.C.; Chen, J.C.; Hsu, S.H.; Chen, L.C.; Chen, C.Y.; Liang, S.H.; Buu, L.M.; Chen, C.C. Differential regulation and activity against oxidative stress of Dps proteins in Bacillus cereus. Int. J. Med. Microbiol. 2013, 303, 662–673. [Google Scholar] [CrossRef]
- Shtykova, E.V.; Petoukhov, M.V.; Mozhaev, A.A. Formation of Iron Oxide Nanoparticles in the Internal Cavity of Ferritin-Like Dps Protein: Studies by Anomalous X-ray Scattering. Biochemistry 2022, 87, 511–523. [Google Scholar] [CrossRef]
- Hecker, M.; Völker, U. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 2001, 44, 35–91. [Google Scholar] [CrossRef] [PubMed]
- Illigmann, A.; Thoma, Y.; Pan, S.; Reinhardt, L.; Brötz-Oesterhelt, H. Contribution of the Clp Protease to Bacterial Survival and Mitochondrial Homoeostasis. Microb. Physiol. 2021, 31, 260–279. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Bröms, J.E.; Kumar, R.; Sjöstedt, A. The Role of ClpB in Bacterial Stress Responses and Virulence. Front. Mol. Biosci. 2021, 8, 668910. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, A.M.; Kim, D.; Jin, Y.H.; Jeong, K.C.; Mah, J.H. Biofilm-associated heat resistance of Bacillus cereus spores in vitro and in a food model, Cheonggukjang jjigae. Int. J. Food Microbiol. 2022, 363, 109505. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Fajardo-Cavazos, P.; Rebeil, R.; Slieman, T.A.; Riesenman, P.J.; Law, J.F.; Xue, Y. Bacterial endospores and their significance in stress resistance. Antonie Van. Leeuwenhoek 2002, 81, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, Z.; Liu, Q.; Liu, F.; Liu, Y.; Zhang, J.; Wang, G. SpoVG is an important regulator of sporulation and affects biofilm formation by regulating Spo0A transcription in Bacillus cereus 0-9. BMC Microbiol. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Harper, W.F., Jr. Bacillus spore awakening: Recent discoveries and technological developments. Curr. Opin. Biotechnol. 2020, 64, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Faßhauer, P.; Busche, T.; Kalinowski, J.; Mäder, U.; Poehlein, A.; Daniel, R.; Stülke, J. Functional Redundancy and Specialization of the Conserved Cold Shock Proteins in Bacillus subtilis. Microorganisms 2021, 9, 1434. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Shang, Q.; Li, Y.; Hao, H.; Zhang, Y.; Guo, Z.; Yang, G.; Xie, Z.; Wang, R. Collagen-like proteins (ClpA, ClpB, ClpC, and ClpD) are required for biofilm formation and adhesion to plant roots by Bacillus amyloliquefaciens FZB42. PLoS ONE 2015, 10, e0117414. [Google Scholar] [CrossRef]
- Taylor, W.; Camilleri, E.; Craft, D.L.; Korza, G.; Granados, M.R.; Peterson, J.; Szczpaniak, R.; Weller, S.K.; Moeller, R.; Douki, T.; et al. DNA Damage Kills Bacterial Spores and Cells Exposed to 222-Nanometer UV Radiation. Appl. Environ. Microbiol. 2020, 86, e03039-19. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Nicholson, W.L. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl. Environ. Microbiol. 1996, 62, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Bonde, N.J.; Romero, Z.J.; Chitteni-Pattu, S.; Cox, M.M. RadD is a RecA-dependent accessory protein that accelerates DNA strand exchange. Nucleic Acids Res. 2022, 50, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Nirwal, S.; Czarnocki-Cieciura, M.; Chaudhary, A.; Zajko, W.; Skowronek, K.; Chamera, S.; Figiel, M.; Nowotny, M. Mechanism of RecF-RecO-RecR cooperation in bacterial homologous recombination. Nat. Struct. Mol. Biol. 2023, 30, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Yang, L.; Jian, Y.; Setlow, P.; Li, L. Spore photoproduct within DNA is a surprisingly poor substrate for its designated repair enzyme—The spore photoproduct lyase. DNA Repair. 2017, 53, 31–42. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).