Metagenomic Characterization and Comparative Analysis of Removable Denture-Wearing and Non-Denture-Wearing Individuals in Healthy and Diseased Periodontal Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
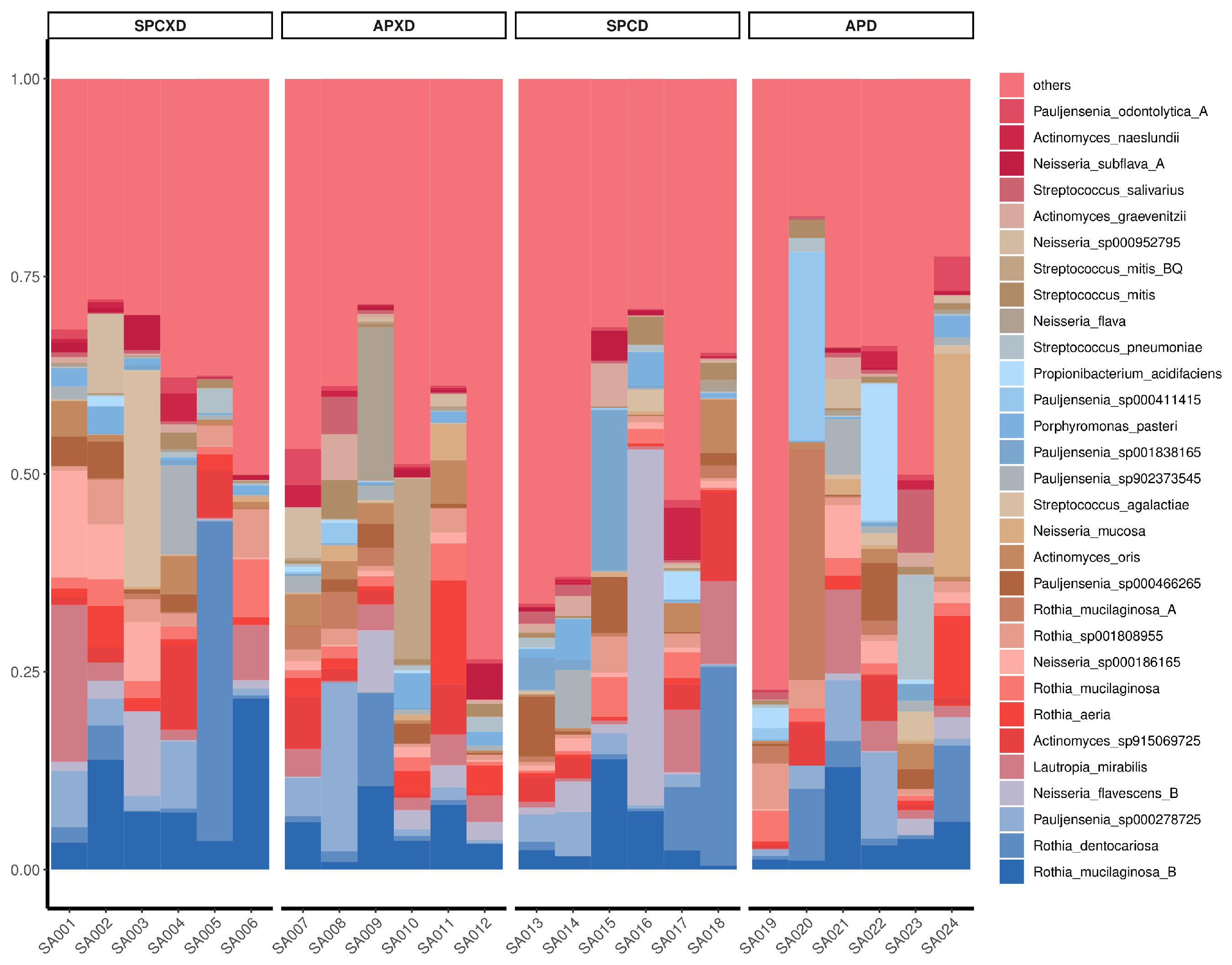
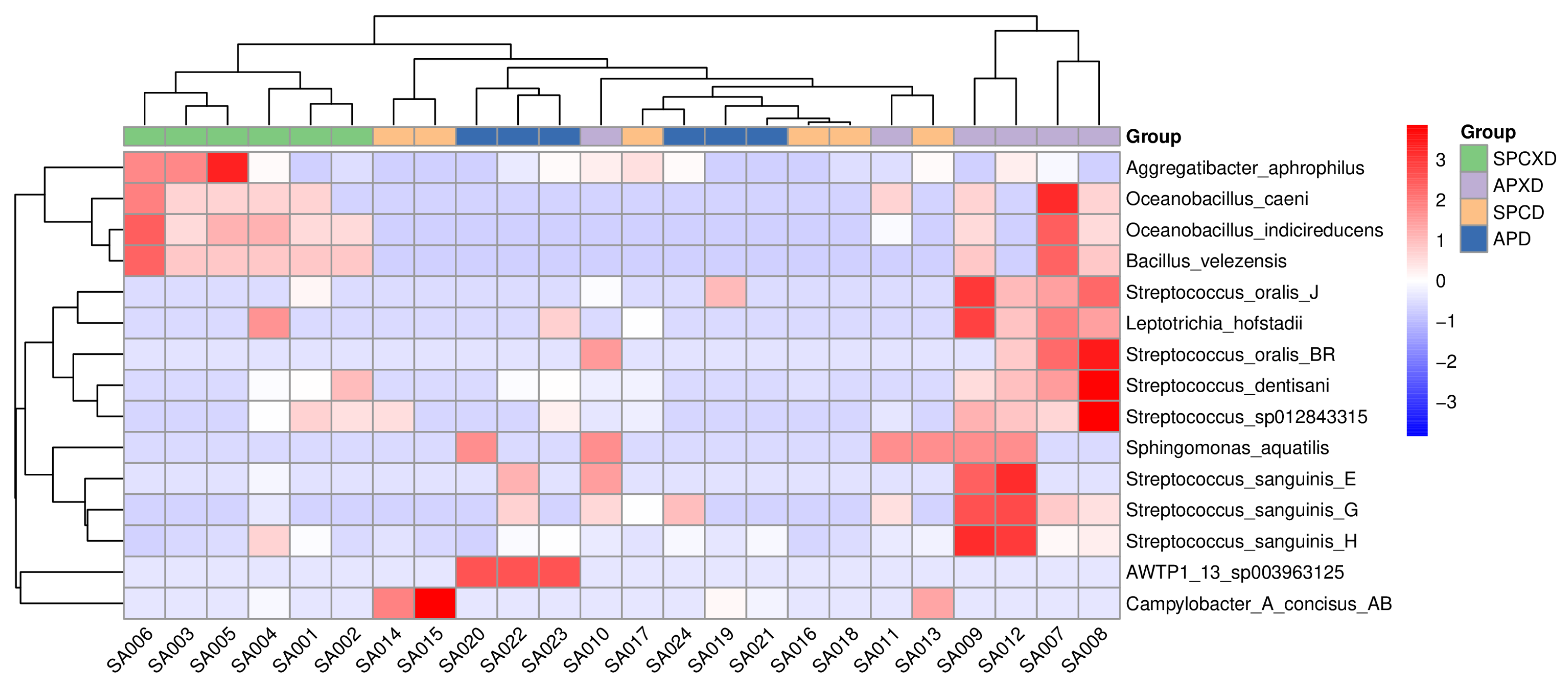
3.1. Relative Bacterial Density and Composition in Denture-Wearing and Non-Denture-Wearing Individuals in Healthy and Diseased Periodontal Conditions
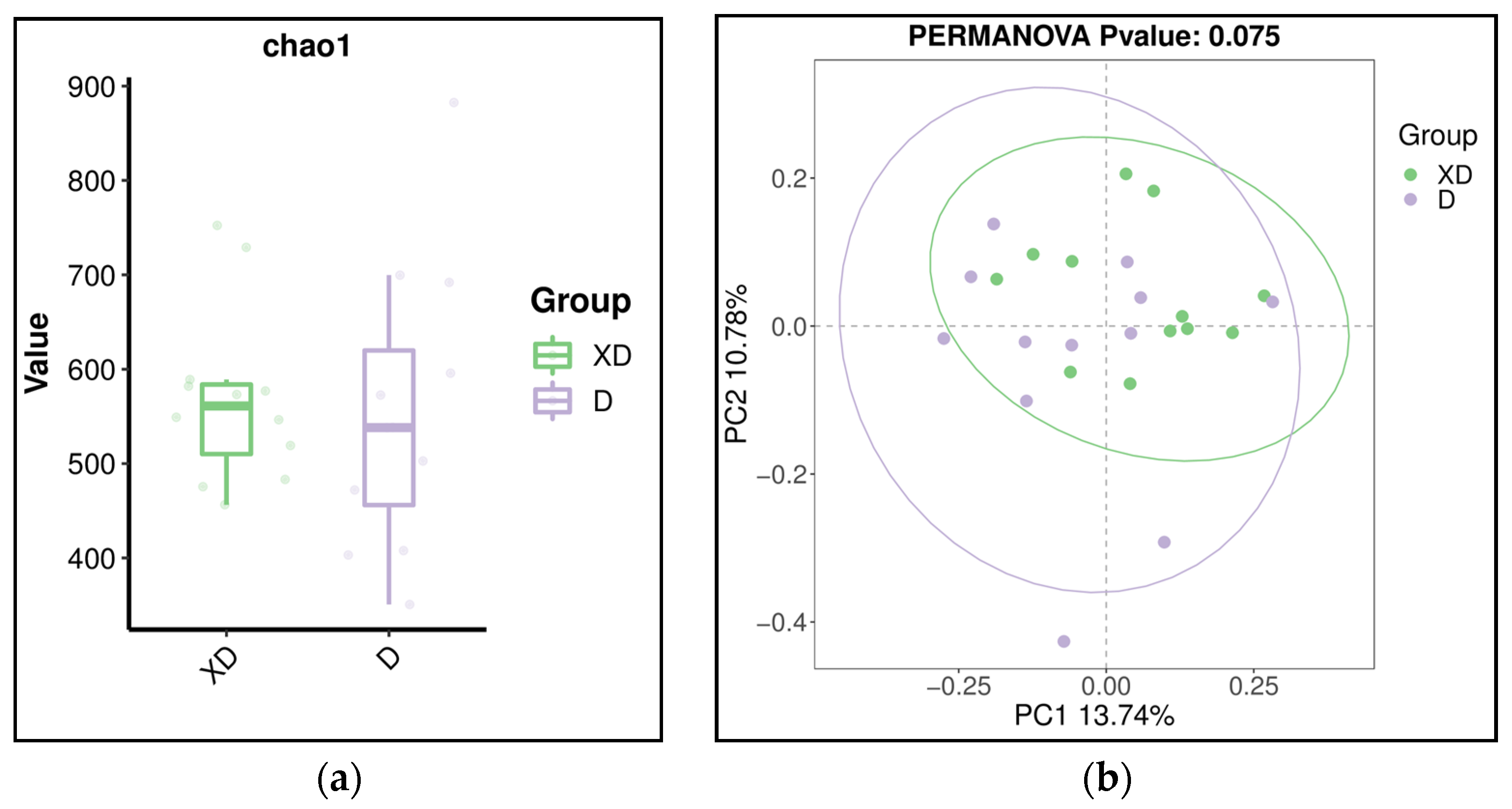
3.2. Diversity of Microbiome in Denture-Wearing and Non-Denture-Wearing Individuals in Healthy and Diseased Periodontal Conditions
3.3. Predictive Function Analysis
3.4. Fungi–Bacteria Ratio and Microbial Index of Pathogenic Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Adaptor | Sequence (5′ to 3′) |
| Adap-1 sense | ACACTCTTTCCCTACACGACGCTCTTCCGATCTNNN |
| Adap-1 antisense | AGATCGGAAGAGC(AminoC6) |
| Adap-2 sense | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNN |
| Adap-2 antisense | AGATCGGAAGAGC(AminoC6) |
| Primer | |
| Primer1 | ACACTCTTTCCCTACACGACGCT |
| Primer2 | GTGACTGGAGTTCAGACGTGTGCT |
| Primer3 | AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCT |
| Index primer | CAAGCAGAAGACGGCATACGAGATXXXXXXGTGACTGGAGTTCAGACGTGT |
Appendix B
References
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149 Pt 2, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.; Lo, E.; Chow, T.; Clark, R. Oral health status of patients 5–6 years after placement of cobalt–chromium removable partial dentures. J. Oral Rehabil. 2000, 27, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yusof, Z.; Isa, Z. Periodontal status of teeth in contact with denture in removable partial denture wearers. J. Oral Rehabil. 1994, 21, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.W.; Burrow, M.F.; McGrath, C. Evaluating risk factors associated with poor removable prosthesis hygiene in community-dwelling elders: A cross-sectional study. J. Prosthet. Dent. 2023, in press. [Google Scholar] [CrossRef]
- Ng, J.Y.M.; Lim, T.W.; Tarib, N.; Ho, T.K. Effect of educational progressive web application on patient’s oral and denture knowledge and hygiene: A randomised controlled trial. Health Inform. J. 2021, 27, 14604582211035821. [Google Scholar] [CrossRef] [PubMed]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Nearing, J.T.; DeClercq, V.; Van Limbergen, J.; Langille, M.G. Assessing the variation within the oral microbiome of healthy adults. Msphere 2020, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Farina, R.; Severi, M.; Carrieri, A.; Miotto, E.; Sabbioni, S.; Trombelli, L.; Scapoli, C. Whole metagenomic shotgun sequencing of the subgingival microbiome of diabetics and non-diabetics with different periodontal conditions. Arch. Oral Biol. 2019, 104, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of oral microbiome in periodontal health and periodontitis: A critical review on prevention and treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Long, J.; Cai, Q.; Steinwandel, M.; Hargreaves, M.K.; Bordenstein, S.R.; Blot, W.J.; Zheng, W.; Shu, X.O. Association of oral microbiome with type 2 diabetes risk. J. Periodontal Res. 2017, 52, 636–643. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A. Poor oral health in the etiology and prevention of aspiration pneumonia. Dent. Clin. 2021, 65, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Redfern, J.; Tosheva, L.; Malic, S.; Butcher, M.; Ramage, G.; Verran, J. The denture microbiome in health and disease: An exploration of a unique community. Lett. Appl. Microbiol. 2022, 75, 195–209. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.E.; Robertson, D.; Nile, C.J.; Cross, L.J.; Riggio, M.; Sherriff, A.; Bradshaw, D.; Lambert, M.; Malcolm, J.; Buijs, M.J.; et al. The oral microbiome of denture wearers is influenced by levels of natural dentition. PLoS ONE 2015, 10, e0137717. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Wu, T.; McLean, J.; Edlund, A.; Young, Y.; He, X.; Lv, H.; Zhou, X.; Shi, W.; Li, H.; et al. The denture-associated oral microbiome in health and stomatitis. MSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.W.; Huang, S.; Jiang, Y.; Zhang, Y.; Burrow, M.F.; McGrath, C. Characterization of pathogenic microbiome on removable prostheses with different levels of cleanliness using 2bRAD-M metagenomic sequencing. J. Oral Microbiol. 2024, 16, 2317059. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.W.; Huang, S.; Zhang, Y.; Burrow, M.F.; McGrath, C. A comparison of the prevalence of respiratory pathogens and opportunistic respiratory pathogenic profile of ‘clean’ and ‘unclean’ removable dental prostheses. J. Dent. 2024, 145, 104968. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.W.; Li, K.Y.; Burrow, M.F.; McGrath, C. Prevalence of respiratory pathogens colonizing on removable dental prostheses in healthy older adults: A systematic review and meta-analysis. J. Prosthodont. 2023; ahead of print. [Google Scholar]
- O’Donnell, L.E.; Smith, K.; Williams, C.; Nile, C.J.; Lappin, D.F.; Bradshaw, D.; Lambert, M.; Robertson, D.P.; Bagg, J.; Hannah, V.; et al. Dentures are a Reservoir for Respiratory Pathogens. J. Prosthodont. 2016, 25, 99–104. [Google Scholar] [CrossRef]
- Sumi, Y.; Miura, H.; Sunakawa, M.; Michiwaki, Y.; Sakagami, N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology 2002, 19, 25–29. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, S.; Zhu, P.; Tzehau, L.; Zhao, H.; Lv, J.; Zhang, R.; Zhou, L.; Niu, Q.; Wang, X.; et al. Species-resolved sequencing of low-biomass or degraded microbiomes using, 2.b.R.A.D.-M. Genome Biol. 2022, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, X.; Jing, G.; Chen, Y.; Jiang, S.; Zhang, M.; Liu, J.; Xu, J.; Su, X. Comprehensive understanding to the public health risk of environmental microbes via a microbiome-based index. J. Genet. Genom. = Yi Chuan Xue Bao 2022, 49, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.A.; Hugenholtz, P. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022, 50, D785–D794. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, S.; Zhu, P.; Yue, F.; Zhao, H.; Yang, M.; Niu, Y.; Jing, G.; Su, X.; Li, H.; et al. A microbiome-based index for assessing skin health and treatment effects for atopic dermatitis in children. Msystems 2019, 4, e00293-19. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, R.; Zeng, X.; He, T.; Zhao, H.; Chang, A.; Bo, C.; Chen, J.; Yang, F.; Knight, R.; et al. Predictive modeling of gingivitis severity and susceptibility via oral microbiota. ISME J. 2014, 8, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Yang, F.; Huang, S.; Bo, C.; Xu, Z.Z.; Amir, A.; Knight, R.; Ling, J.; Xu, J. Prediction of early childhood caries via spatial-temporal variations of oral microbiota. Cell Host Microbe 2015, 18, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. J. Appl. Stat. Pharm. Ind. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. The saliva microbiome profiles are minimally affected by collection method or DNA extraction protocols. Sci. Rep. 2017, 7, 8523. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13, 3–10. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- He, S.; Sun, Y.; Sun, W.; Tang, M.; Meng, B.; Liu, Y.; Kong, Q.; Li, Y.; Yu, J.; Li, J. Oral microbiota disorder in GC patients revealed by, 2.b.-R.A.D.-M. J. Transl. Med. 2023, 21, 831. [Google Scholar] [CrossRef] [PubMed]
- Kharitonova, M.; Vankov, P.; Abdrakhmanov, A.; Mamaeva, E.; Yakovleva, G.; Ilinskaya, O. The composition of microbial communities in inflammatory periodontal diseases in young adults Tatars. AIMS Microbiol. 2021, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Kistler, J.O.; Booth, V.; Bradshaw, D.J.; Wade, W.G. Bacterial community development in experimental gingivitis. PLoS ONE 2013, 8, e71227. [Google Scholar] [CrossRef] [PubMed]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012, 6, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, W.; Nishikawa, K.; Ozawa, S.; Hasegawa, Y.; Takebe, J. Correlation between the relative abundance of oral bacteria and Candida albicans in denture and dental plaques. J. Oral Biosci. 2021, 63, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gomar-Vercher, S.; Simón-Soro, A.; Montiel-Company, J.M.; Almerich-Silla, J.M.; Mira, A. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLoS ONE 2018, 13, e0198021. [Google Scholar] [CrossRef]
- Chahal, G.; Quintana-Hayashi, M.P.; Gaytán, M.O.; Benktander, J.; Padra, M.; King, S.J.; Linden, S.K. Streptococcus oralis employs multiple mechanisms of salivary mucin binding that differ between strains. Front. Cell. Infect. Microbiol. 2022, 12, 889711. [Google Scholar] [CrossRef]
- Campbell, K. Oral microbiome findings challenge dentistry dogma. Nature 2021. ahead of print. [Google Scholar]
- Xu, P.; Alves, J.M.; Kitten, T.; Brown, A.; Chen, Z.; Ozaki, L.S.; Manque, P.; Ge, X.; Serrano, M.G.; Puiu, D.; et al. Genome of the opportunistic pathogen Streptococcus sanguinis. J. Bacteriol. 2007, 189, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Raabe, V.N.; Shane, A.L. Group B streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Garnett, J.A.; Simpson, P.J.; Taylor, J.; Benjamin, S.V.; Tagliaferri, C.; Cota, E.; Chen, Y.Y.; Wu, H.; Matthews, S. Structural insight into the role of Streptococcus parasanguinis Fap1 within oral biofilm formation. Biochem. Biophys. Res. Commun. 2012, 417, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, M.; Fu, T.; Shan, F.; Jiang, L.; Shao, Z. Clinical characteristics of infections caused by Streptococcus anginosus group. Sci. Rep. 2020, 10, 9032. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.d.; Maluta, R.P.; Stella, A.E.; Rigobelo, E.C.; Marin, J.M.; Ávila, F.A.d. Isolation of Pseudomonas aeruginosa strains from dental office environments and units in Barretos, state of São Paulo, Brazil, and analysis of their susceptibility to antimicrobial drugs. Braz. J. Microbiol. 2008, 39, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.; Percival, R.; Challacombe, S. The influence of denture-wearing and age on the oral microflora. J. Dent. Res. 1992, 71, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Slazhneva, E.; Tikhomirova, E.; Tsarev, V.; Orekhova, L.; Loboda, E.; Atrushkevich, V. Candida species detection in patients with chronic periodontitis: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2022, 8, 1354–1375. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, D.; Karkowska-Kuleta, J.; Zawrotniak, M.; Satała, D.; Michalik, K.; Zielinska, G.; Bochenska, O.; Kozik, A.; Ciaston, I.; Koziel, J.; et al. Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci. Rep. 2019, 9, 4376. [Google Scholar] [CrossRef]
- Zhou, M.; Dong, J.; Zha, L.; Liao, Y. Causal Association between Periodontal Diseases and Cardiovascular Diseases. Genes 2021, 13, 13. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]




| Inclusion Criteria (for Participants Aged 60 and above) | |
|---|---|
| (i) Diseased periodontal condition | |
| 1. | Participants without evidence of previous therapy and clinical improvement: |
| |
| |
| 2. | Participants who had been given periodontal therapy and presented with one or more non-responding periodontal pocket sites: |
| |
| |
| (ii) Healthy periodontal condition | |
| 1. | Participants without sign and history of periodontitis, characterized by the absence of detectable attachment and bone loss |
| 2. | Participants with gingival health on a reduced periodontium, characterized by intra-oral BOP < 10%, periodontal pocket depth ≤ 4 mm, and no 4 mm periodontal pocket depth with BOP |
| 3. | Participants with gingival inflammation in a stable periodontitis patient, characterized by intra-oral BOP > 10%, periodontal pocket depth ≤ 4 mm, and no 4 mm periodontal pocket depth with BOP |
| (iii) Removable denture groups | |
| 1. | Denture base made of acrylic resin |
| 2. | Partially dentate |
| 3. | Good denture quality with Kapur index of retention score ≥ 2 and stability score ≥ 1 |
| 4. | Denture cleanliness index ≥ 1 (denture has visible plaque and/or debris with ≥25% fit surface stained) |
| Exclusion Criteria | |
| 1. | Rinse with mouthwash prior to sample collection |
| 2. | Mentally, physically, or medically unfit (dementia and neurological diseases, received radiotherapy or chemotherapy, use of steroid treatment for more than 2 weeks in the past year, use of local and/or systemic antibiotic therapy/antifungal/antiviral/anti-inflammatory/bisphosphonates/phenytoin within 1 month prior to sample collection) |
| 3. | Use of denture adhesive products or the acrylic denture was relined |
| 4. | Currently smoking or quit smoking less than 6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, H.-H.; Hung, C.-H.; Yip, J.; Lim, T.-W. Metagenomic Characterization and Comparative Analysis of Removable Denture-Wearing and Non-Denture-Wearing Individuals in Healthy and Diseased Periodontal Conditions. Microorganisms 2024, 12, 1197. https://doi.org/10.3390/microorganisms12061197
Wong H-H, Hung C-H, Yip J, Lim T-W. Metagenomic Characterization and Comparative Analysis of Removable Denture-Wearing and Non-Denture-Wearing Individuals in Healthy and Diseased Periodontal Conditions. Microorganisms. 2024; 12(6):1197. https://doi.org/10.3390/microorganisms12061197
Chicago/Turabian StyleWong, Ho-Hin, Chun-Ho Hung, Jason Yip, and Tong-Wah Lim. 2024. "Metagenomic Characterization and Comparative Analysis of Removable Denture-Wearing and Non-Denture-Wearing Individuals in Healthy and Diseased Periodontal Conditions" Microorganisms 12, no. 6: 1197. https://doi.org/10.3390/microorganisms12061197






