Hunting Dynamics and Identification of Potentially Pathogenic Bacteria in European Fallow Deer (Dama dama) across Three Hunting Reserves in Western Romania
Abstract
1. Introduction
2. Materials and Methods
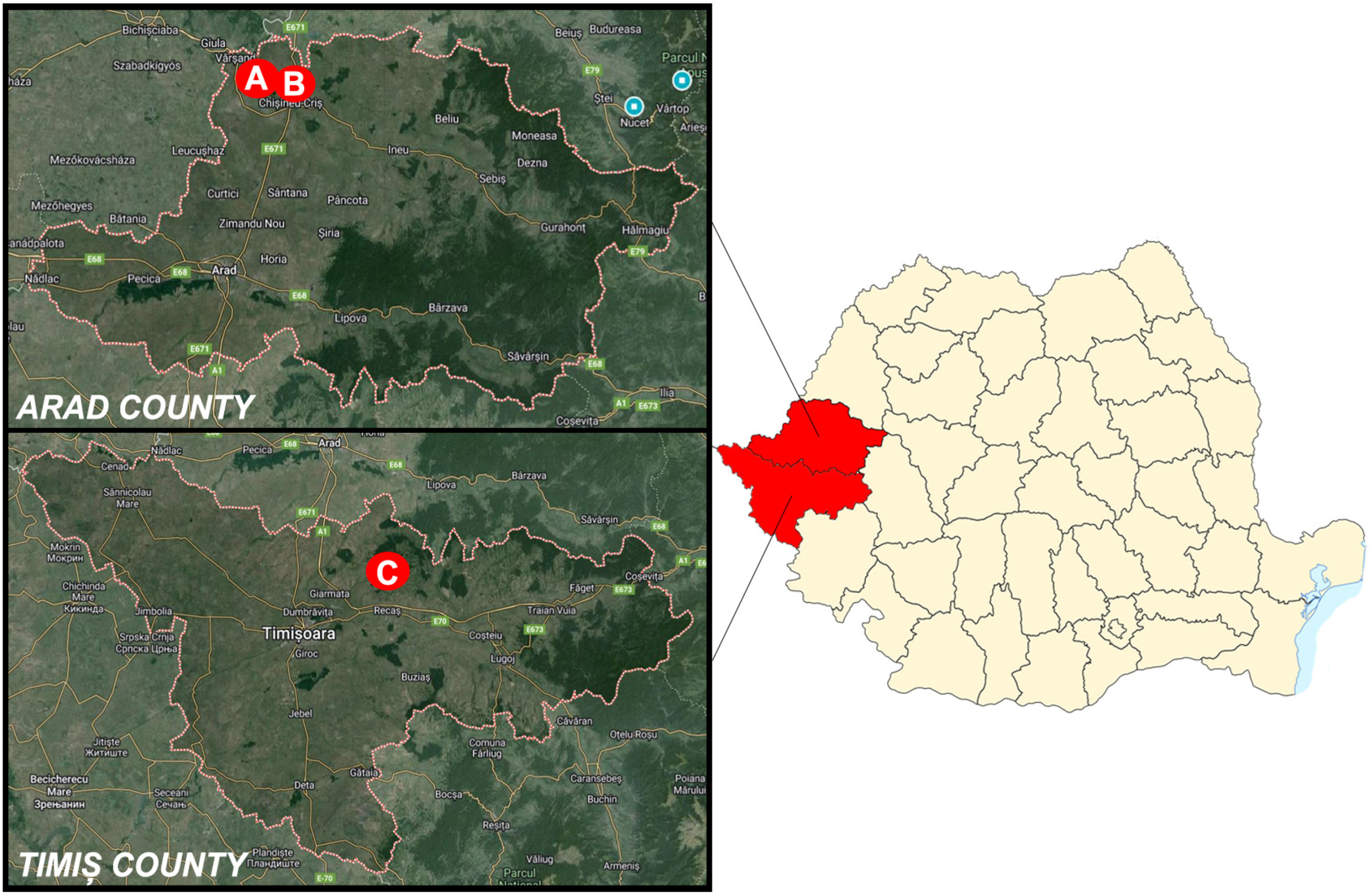
2.1. Harvesting Quota Dynamics and Sample Collection
2.2. Bacterial Preliminary Isolation and Classification
2.3. Bacterial Biochemical Identification
3. Results
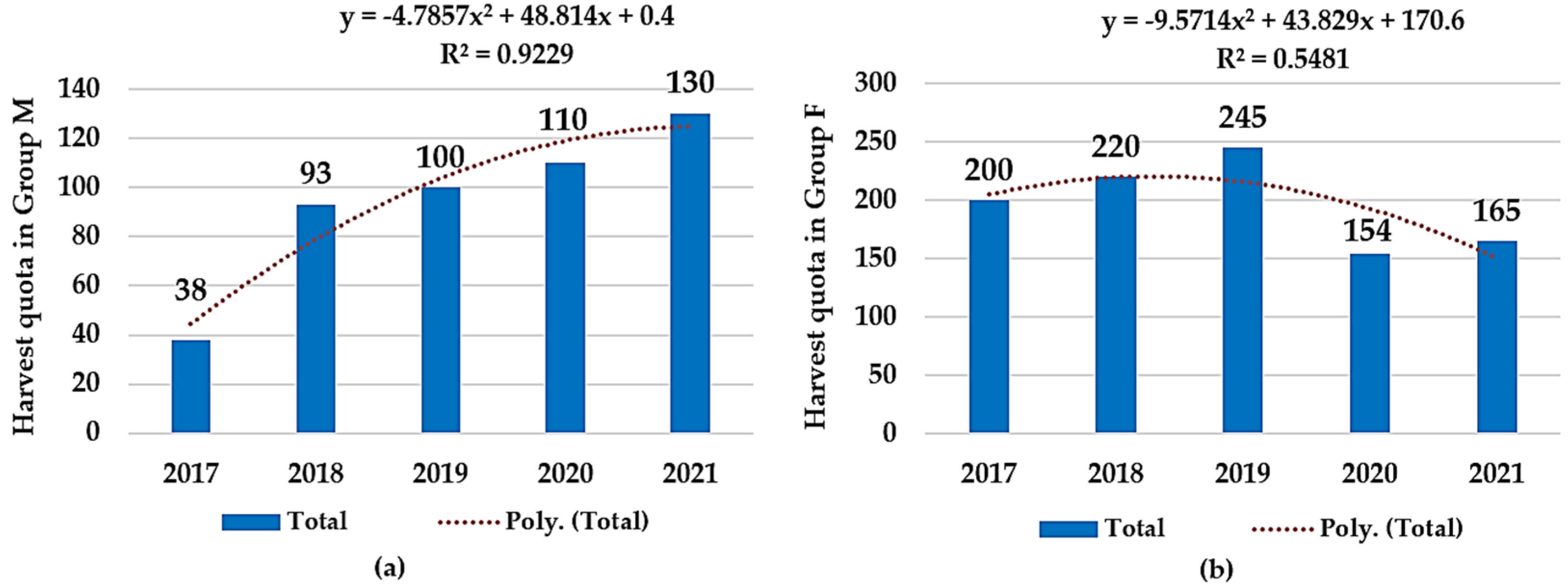
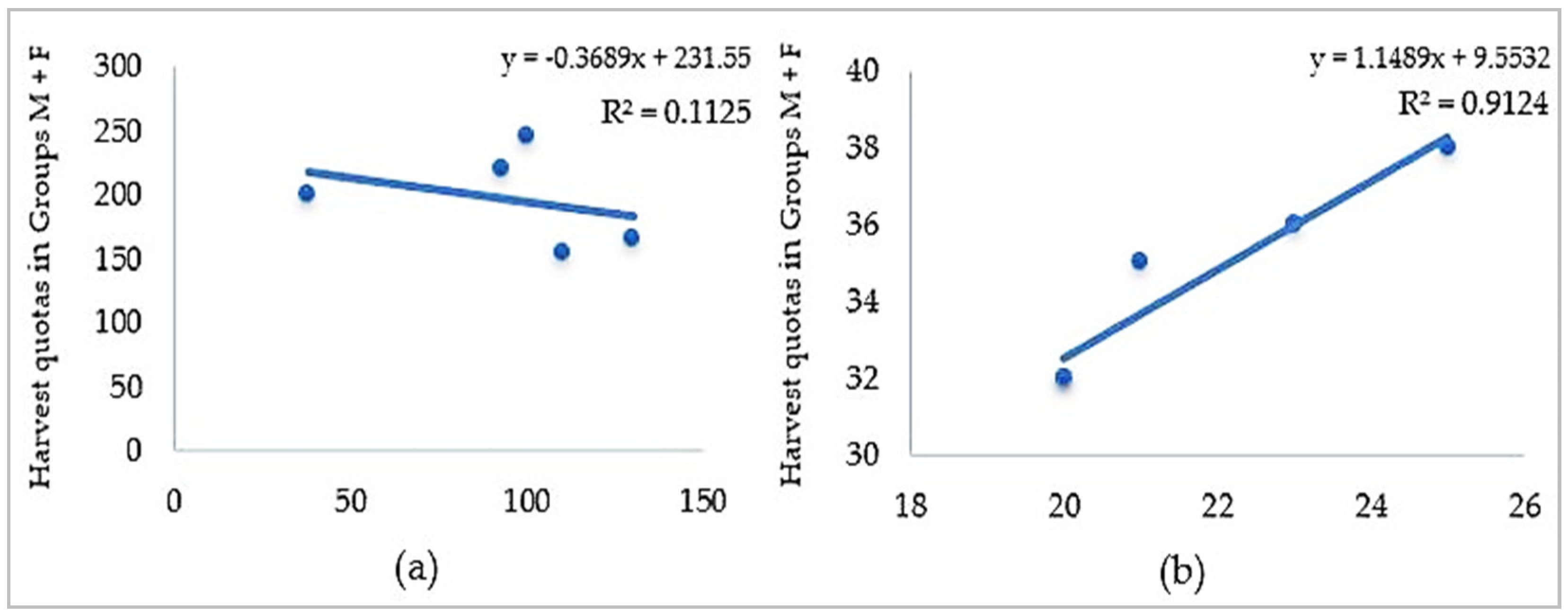
3.1. Harvesting Quota Dynamics and Sample Collection
3.2. Bacterial Preliminary Isolation and Classification
3.3. Bacterial Confirmation with VITEK 2 Compact
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bijl, H.; Csányi, S. Fallow Deer (Dama dama) Population and Harvest Changes in Europe since the Early 1980s. Sustainability 2022, 14, 12198. [Google Scholar] [CrossRef]
- Chakanya, C.; Dokora, A.-E.-M.; Muchenje, V.; Hoffman, L.C. The fallow deer (Dama spp.); endangered or not? Der Zool. Gart. 2016, 85, 160–172. [Google Scholar] [CrossRef]
- Masseti, M.; Mertzanidou, D. Dama dama, Fallow Deer. In The IUCN Red List of Threatened Species; IUCN Red List: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Romanian Parliament. Lege nr. 13 din 11 Martie 1993 Pentru Aderarea României la Convenția Privind Conservarea Vieții Sălbatice și a Habitatelor Naturale din Europa, Adoptată la Berna la 19 Septembrie 1979. Ed. Monitorul Oficial nr. 62 din 25 Martie 1993. 1993. Available online: https://www.cdep.ro/pls/legis/legis_pck.htp_act_text?idt=13681 (accessed on 6 January 2023).
- Romanian Parliament. Lege Nr. 407 din 9 noiembrie 2006 a Vânătorii şi a Protecţiei Fondului Cinegetic. Anexa nr. 1. Valoarea de Despăgubire în Cazul Unor Fapte Ilicite şi Valoarea de Calcul a Tarifului de Gestionare Pentru Speciile la Care Vânarea Este Permisă. Parliament, R., Ed. Monitorul Oficial nr. 944 din 22 Noiembrie 2006. 2006. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/77053 (accessed on 6 January 2023).
- Putman, R.J.; Apollonio, M.; Andersen, R. Ungulate Management in Europe: Problems and Practices; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Ahl, M.; Elmhagen, B.; Bergqvist, G. Survey of Methods for Harvest Reporting of Game Species in Europe: Viltforum 5/2020; Svenska Jägareförbundet: Björnlunda, Sweden, 2021; pp. 1–49. ISBN 978-91-86971-37-3. [Google Scholar]
- Obwegeser, T.; Stephan, R.; Hofer, E.; Zweifel, C. Shedding of foodborne pathogens and microbial carcass contamination of hunted wild ruminants. Vet. Microbiol. 2012, 159, 149–154. [Google Scholar] [CrossRef]
- Kiss, J.; Vasile, A.; Marinov, M.; Bolboaca, L.; Dorosencu, A.; Chişamera, G.; Nanu, C. The current and historical presence of the European fallow deer -Dama dama, (Linnaeus 1758) on the territory of the Danube Delta Biosphere Reserve (Romania). Sci. Ann. Danub. Delta Inst. 2023, 28, 77–84. [Google Scholar] [CrossRef]
- Dias, D.; Costa, S.; Fonseca, C.; Baraúna, R.; Caetano, T.; Mendo, S. Pathogenicity of Shiga toxin-producing Escherichia coli (STEC) from wildlife: Should we care? Sci. Total Environ. 2022, 812, 152324. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Ruan, X.; Duan, Z.; Cao, J.; Liang, J.; Yang, J.; Jiang, Y.; Shi, M.; Xu, J. Wildlife-borne microorganisms and strategies to prevent and control emerging infectious diseases. J. Biosaf. Biosecu. 2021, 3, 67–71. [Google Scholar] [CrossRef]
- Schmeller, D.S.; Courchamp, F.; Killeen, G. Biodiversity loss, emerging pathogens and human health risks. Biodivers. Conserv. 2020, 29, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Pacheco-Torres, I.; Hernández-Sánchez, D.; García-De la Peña, C.; Tarango-Arámbula, L.A.; Crosby-Galván, M.M.; Sánchez-Santillán, P. Analysis of the Intestinal and Faecal Bacterial Microbiota of the Cervidae Family Using 16S Next-Generation Sequencing: A Review. Microorganisms 2023, 11, 1860. [Google Scholar] [CrossRef]
- Gnat, S.; Troscianczyk, A.; Nowakiewicz, A.; Majer-Dziedzic, B.; Ziolkowska, G.; Dziedzic, R.; Zieba, P.; Teodorowski, O. Experimental studies of microbial populations and incidence of zoonotic pathogens in the faeces of red deer (Cervus elaphus). Lett. Appl. Microbiol. 2015, 61, 446–452. [Google Scholar] [CrossRef]
- Syczylo, K.; Platt-Samoraj, A.; Bancerz-Kisiel, A.; Szczerba-Turek, A.; Pajdak-Czaus, J.; Labuc, S.; Procajlo, Z.; Socha, P.; Chuzhebayeva, G.; Szweda, W. The prevalence of Yersinia enterocolitica in game animals in Poland. PLoS ONE 2018, 13, e0195136. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Caetano, T.; Torres, R.T.; Fonseca, C.; Mendo, S. Shiga toxin-producing Escherichia coli in wild ungulates. Sci. Total Environ. 2019, 651, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Pista, A.; Silveira, L.; Ribeiro, S.; Fontes, M.; Castro, R.; Coelho, A.; Furtado, R.; Lopes, T.; Maia, C.; Mixão, V.; et al. Pathogenic Escherichia coli, Salmonella spp. and Campylobacter spp. in Two Natural Conservation Centers of Wildlife in Portugal: Genotypic and Phenotypic Characterization. Microorganisms 2022, 10, 2132. [Google Scholar] [CrossRef]
- Szczerba-Turek, A.; Siemionek, J.; Socha, P.; Bancerz-Kisiel, A.; Platt-Samoraj, A.; Lipczynska-Ilczuk, K.; Szweda, W. Shiga toxin-producing Escherichia coli isolates from red deer (Cervus elaphus), roe deer (Capreolus capreolus) and fallow deer (Dama dama) in Poland. Food Microbiol. 2020, 86, 103352. [Google Scholar] [CrossRef] [PubMed]
- Tirziu, E.; Bulucea, A.V.; Imre, K.; Nichita, I.; Muselin, F.; Dumitrescu, E.; Tirziu, A.; Mederle, N.G.; Moza, A.; Bucur, I.M.; et al. The Behavior of Some Bacterial Strains Isolated from Fallow Deer Compared to Antimicrobial Substances in Western Romania. Antibiotics 2023, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Odyniec, M.; Bancerz-Kisiel, A. Assessment of the Role of Free-Living and Farmed Fallow Deer (Dama dama) as A Potential Source of Human Infection with Multiple-Drug-Resistant Strains of Yersinia enterocolitica and Yersinia pseudotuberculosis. Pathogens 2022, 11, 1266. [Google Scholar] [CrossRef]
- Di Blasio, A.; Varello, K.; Vitale, N.; Irico, L.; Bozzetta, E.; Goria, M.; Chiavacci, L.; Zoppi, S.; Dondo, A. Animal tuberculosis in a free-ranging fallow deer in northwest Italy: A case of “lucky strain survival” or multi-host epidemiological system complexity? Eur. J. Wildl. Research. 2019, 65, 74. [Google Scholar] [CrossRef]
- Șelaru, N. Evoluția populației de cerb lopătar. In Vânătorul și Pescarul; AGVPS: București, Romania, 2020; Volume 88, pp. 6–8. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Buiuc, D.; Neguț, M. Tratat de Microbiologie Clinică, 3rd ed.; Editura Medicala: Bucharest, Romania, 2017; ISBN 978-973-39-0593-6. [Google Scholar]
- Paray, A.A.; Singh, M.; Mir, M.A.; Kaur, D. Gram Staining: A Brief Review. Int. J. Educ. Res. Rev. 2023, 10, 336–341. [Google Scholar] [CrossRef]
- Press Release. Fondul Cinegetic 2008, Nr. 146 Din 31 Iulie 2009. National Institute of Statistics Bucharest Romania. Available online: https://insse.ro/cms/en (accessed on 7 January 2023).
- Burbaitė, L.; Csányi, S. Red deer population and harvest changes in Europe. Acta Zool. Litu. 2010, 20, 179–188. [Google Scholar] [CrossRef]
- Geacu, S. Evoluţia populaţiilor de cerb lopătar colonizate în Subcarpaţii Buzăului (România). Cercet. Științifice Mediu. Ambiant 2010, 1, 1–5. [Google Scholar]
- Menichetti, L.; Agren, G.I.; Barre, P.; Moyano, F.; Katterer, T. Generic parameters of first-order kinetics accurately describe soil organic matter decay in bare fallow soils over a wide edaphic and climatic range. Sci. Rep. 2019, 9, 20319. [Google Scholar] [CrossRef]
- Kenward, R.; Putman, R. Ungulate management in Europe: Towards a sustainable future. In Ungulate Management in Europe: Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 376–395. [Google Scholar] [CrossRef]
- Gheibipour, M.; Ghiasi, S.E.; Bashtani, M.; Bagher, M.; Montazer-Torbati, M.; Motamedi, H. Tannase-producing Bacteria Isolated from the Rumen of Fallow Deer (Dama dama): Livestock Potential Feed Additives. SSRN J. 2023, 12, 27–40. [Google Scholar] [CrossRef]
- Travis, D.A.; Watson, R.P.; Tauer, A. The spread of pathogens through trade in wildlife. Rev. Sci. Tech. 2011, 30, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Wasyl, D.; Zajac, M.; Lalak, A.; Skarzynska, M.; Samcik, I.; Kwit, R.; Jablonski, A.; Bocian, L.; Wozniakowski, G.; Hoszowski, A.; et al. Antimicrobial Resistance in Escherichia coli Isolated from Wild Animals in Poland. Microb. Drug Resist. 2018, 24, 807–815. [Google Scholar] [CrossRef]
- Velhner, M.; Todorovic, D.; Grego, E.; Jovcic, B.; Prunic, B.; Stojanov, I.; Kehrenberg, C. Fluoroquinolone-resistant and extended-spectrum beta-lactamase producing Escherichia coli isolates from free-living wild animals. Vet. Microbiol. 2018, 223, 168–172. [Google Scholar] [CrossRef]
- Frank, E.; Bonke, R.; Drees, N.; Heurich, M.; Märtlbauer, E.; Gareis, M. Shiga toxin-producing Escherichia coli (STEC) shedding in a wild roe deer population. Vet. Microbiol. 2019, 239, 108479. [Google Scholar] [CrossRef] [PubMed]
- Amézquita-López, B.A.; Soto-Beltrán, M.; Lee, B.G.; Yambao, J.C.; Quiñones, B. Isolation, genotyping and antimicrobial resistance of Shiga toxin-producing Escherichia coli. J. Microbiol. Immunol. Infect. 2018, 51, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Pathogenicity assessment of Shiga toxin-producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA J. 2020, 18, e05967. [Google Scholar] [CrossRef]
- Gorski, L.; Parker, C.T.; Liang, A.; Cooley, M.B.; Jay-Russell, M.T.; Gordus, A.G.; Atwill, E.R.; Mandrell, R.E. Prevalence, distribution, and diversity of Salmonella enterica in a major produce region of California. Appl. Environ. Microbiol. 2011, 77, 2734–2748. [Google Scholar] [CrossRef]
- Paulsen, P.; Smulders, F.J.M.; Hilbert, F. Salmonella in meat from hunted game: A Central European perspective. Food Res. Int. 2012, 45, 609–616. [Google Scholar] [CrossRef]
- Magnino, S.; Frasnelli, M.; Fabbi, M.; Bianchi, A.; Zanoni, M.G.; Merialdi, G.; Pacciarini, M.L.; Gaffuri, A. The monitoring of selected zoonotic diseases of wildlife in Lombardy and Emilia-Romagna, northern Italy. In Game Meat Hygiene in Focus: Microbiology, Epidemiology, Risk Analysis and Quality Assurance; Paulsen, P., Bauer, A., Vodnansky, M., Winkelmayer, R., Smulders, F.J.M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 223–244. [Google Scholar] [CrossRef]
- Renter, D.G.; Gnad, D.P.; Sargeant, J.M.; Hygnstrom, S.E. Prevalence and serovars of Salmonella in the feces of free-ranging white-tailed deer (Odocoileus virginianus) in Nebraska. J. Wildl. Dis. 2006, 42, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, N.; Ugarte-Ruiz, M.; Domínguez, L.; Ruiz-Fons, F. A European Perspective on the Transmission of Foodborne Pathogens at the Wildlife–Livestock–Human Interface. In Food Safety Risks from Wildlife: Challenges in Agriculture, Conservation, and Public Health; Jay-Russell, M., Doyle, M.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–88. [Google Scholar] [CrossRef]
- Mateus Vargas, R.; Reich, F.; Klein, G.; Atanassova, V. Bacterial contamination and antibiotic resistance of isolates from packaged game meat. Fleischwirtschaft 2013, 93, 179–182. [Google Scholar]
- Avagnina, A.; Nucera, D.; Grassi, M.A.; Ferroglio, E.; Dalmasso, A.; Civera, T. The microbiological conditions of carcasses from large game animals in Italy. Meat Sci. 2012, 91, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Weindl, L.; Frank, E.; Ullrich, U.; Heurich, M.; Kleta, S.; Ellerbroek, L.; Gareis, M. Listeria monocytogenes in Different Specimens from Healthy Red Deer and Wild Boars. Foodborne Pathog. Dis. 2016, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Mateus-Vargas, R.H.; Lienen, T.; Maaz, D.; Richter, M.; Maurischat, S.; Steinhoff-Wagner, J. Evaluation of the Occurrence of Staphylococcaceae with Reduced Susceptibility to Cefoxitin in Wild Ungulates in Brandenburg, Germany, Based on Land Use-Related Factors. Microbiol. Spectr. 2022, 10, e0256022. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Gavier-Widen, D.; Hotzel, H.; Peters, M.; Guenther, S.; Lazaris, A.; Loncaric, I.; Muller, E.; Reissig, A.; Ruppelt-Lorz, A.; et al. Diversity of Staphylococcus aureus Isolates in European Wildlife. PLoS ONE 2016, 11, e0168433. [Google Scholar] [CrossRef] [PubMed]
- Nowakiewicz, A.; Ziółkowska, G.; Zięba, P.; Gnat, S.; Wojtanowicz-Markiewicz, K.; Trościańczyk, A. Coagulase-positive Staphylococcus isolated from wildlife: Identification, molecular characterization and evaluation of resistance profiles with focus on a methicillin-resistant strain. Comp. Immunol. Microbiol. Infect. Dis. 2016, 44, 21–28. [Google Scholar] [CrossRef]
- Hamarova, L.; Kopcakova, A.; Kocianova-Adamcova, M.; Piknova, M.; Javorsky, P.; Pristas, P. Antimicrobial Resistance of Enterococci from Wild Animals in Slovakia. Pol. J. Environ. 2021, 30, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Lillehaug, A.; Bergsjø, B.; Schau, J.; Bruheim, T.; Vikøren, T.; Handeland, K. Campylobacter spp., Salmonella spp., Verocytotoxic Escherichia coli, and Antibiotic Resistance in Indicator Organisms in Wild Cervids. Acta Vet. Scand. 2005, 46, 23. [Google Scholar] [CrossRef]
- Guerrero-Ramos, E.; Cordero, J.; Molina-Gonzalez, D.; Poeta, P.; Igrejas, G.; Alonso-Calleja, C.; Capita, R. Antimicrobial resistance and virulence genes in enterococci from wild game meat in Spain. Food Microbiol. 2016, 53, 156–164. [Google Scholar] [CrossRef]
- Gautam, L.; Kaur, R.; Kumar, S.; Bansal, A.; Gautam, V.; Singh, M.; Ray, P. Pseudomonas oleovorans Sepsis in a Child: The First Reported Case in India. Jpn. J. Infect. Dis. 2015, 68, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Fridman, D.; Chaudhry, A.; Makaryus, J.; Black, K.; Makaryus, A.N. Rothia dentocariosa Endocarditis: An Especially Rare Case in a Previously Healthy Man. Tex. Heart Inst. J. 2016, 43, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Rajni, E.; Jain, A.; Garg, V.K.; Sharma, R.; Vohra, R.; Jain, S.S. Providencia Causing Urinary Tract Infections: Are We Reaching a Dead End? Indian J. Crit. Care Med. 2022, 26, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.; Zaman, K.; Anand, N.; Taneja, N. Aerococcus Viridans: A Rare Pathogen Causing Urinary Tract Infection. J. Clin. Diagn. Res. 2017, 11, DR01–DR03. [Google Scholar] [CrossRef] [PubMed]

| Category | Harvesting Quotas | ||||
|---|---|---|---|---|---|
| 2017 No. (%) | 2018 No. (%) | 2019 No. (%) | 2020 No. (%) | 2021 No. (%) | |
| Group M | 38 | 93 | 100 | 110 | 130 |
| Group F | 200 | 220 | 245 | 154 | 165 |
| TOTAL (A) | 238 (75.80) | 313 (79.44) | 345 (79.99) | 264 (75.86) | 295 (74.12) |
| Group M | 21 | 23 | 20 | 20 | 25 |
| Group F | 35 | 36 | 32 | 32 | 38 |
| TOTAL (B) | 56 (17.83) | 59 (14.97) | 52 (12.17) | 52 (14.94) | 63 (15.83) |
| Group M | 5 | 7 | 8 | 9 | 10 |
| Group F | 15 | 15 | 22 | 25 | 30 |
| TOTAL (C) | 20 (6.37) | 22 (5.59) | 30 (7.02) | 32 (9.20) | 40 (10.05) |
| TOTAL (A + B + C) | 314 (100) | 394 (100) | 427 (100) | 348 (100) | 398 (100) |
| Sample Origin | No. Positive Samples/Examined (%) | Gram Staining (n = 221) | ||||
|---|---|---|---|---|---|---|
| L | Ra | Ch | Ox | No. of GNB (%) | No. of GPB (%) | |
| Rectal | 46/120 (38.33) | 19/120 (15.83) | 38/120 (31.66) | 3/120 (2.50) | 41 (35.04) | 64 (61.54) |
| Nasal | 47/120 (39.16) | 5/120 (4.16) | 58/120 (48.33) | 5/120 (4.16) | 76 (64.96) | 40 (38.46) |
| Total | 93/240 (38.75) | 24/240 (10) | 96/240 (40) | 8/240 (3.33) | 117 (100) | 104 (100) |
| Sample Origin | Identified Strains | No. of Isolated Bacteria (n = 221) | Prevalence (%) |
|---|---|---|---|
| Rectal (n = 120) | Escherichia coli | 43 | 40.57 |
| Salmonella spp. | 18 | 16.98 | |
| Staphylococcus vitulinus | 13 | 12.26 | |
| Staphylococcus aureus | 7 | 6.61 | |
| Staphylococcus lentus | 5 | 4.72 | |
| Enterobacter spp. | 3 | 2.83 | |
| Enterococcus faecium | 3 | 2.83 | |
| Staphylococcus xylosus | 3 | 2.83 | |
| Rothia dentocariosa | 3 | 2.83 | |
| Aerococcus viridans | 3 | 2.83 | |
| Kocuria kristinae | 2 | 1.89 | |
| Staphylococcus hominis | 1 | 0.94 | |
| Pseudomonas oleovorans | 1 | 0.94 | |
| Leuconostoc mesenteroides ssp. cremoris | 1 | 0.94 | |
| Total | 106 | 100 | |
| Nasal (n = 120) | Escherichia coli | 42 | 36.52 |
| Staphylococcus lentus | 27 | 23.48 | |
| Staphylococcus aureus | 9 | 7.82 | |
| Aerococcus viridans | 5 | 4.35 | |
| Listeria monocytogenes | 5 | 4.35 | |
| Salmonella spp. | 4 | 3.48 | |
| Enterobacter spp. | 4 | 3.48 | |
| Kocuria kristinae | 4 | 3.48 | |
| Staphylococcus vitulinus | 4 | 3.48 | |
| Gemella spp. | 3 | 2.61 | |
| Staphylococcus xylosus | 3 | 2.61 | |
| Staphylococcus sciuri | 2 | 1.73 | |
| Pseudomonas oleovorans | 1 | 0.87 | |
| Providencia rettgeri | 1 | 0.87 | |
| Staphylococcus hominis | 1 | 0.87 | |
| Total | 115 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucur, I.-M.; Moza, A.C.; Pop, M.; Nichita, I.; Gaspar, C.M.; Cojocaru, R.; Gros, R.-V.; Boldea, M.V.; Tirziu, A.; Tirziu, E. Hunting Dynamics and Identification of Potentially Pathogenic Bacteria in European Fallow Deer (Dama dama) across Three Hunting Reserves in Western Romania. Microorganisms 2024, 12, 1236. https://doi.org/10.3390/microorganisms12061236
Bucur I-M, Moza AC, Pop M, Nichita I, Gaspar CM, Cojocaru R, Gros R-V, Boldea MV, Tirziu A, Tirziu E. Hunting Dynamics and Identification of Potentially Pathogenic Bacteria in European Fallow Deer (Dama dama) across Three Hunting Reserves in Western Romania. Microorganisms. 2024; 12(6):1236. https://doi.org/10.3390/microorganisms12061236
Chicago/Turabian StyleBucur, Iulia-Maria, Alex Cristian Moza, Mirel Pop, Ileana Nichita, Cristina Mirabela Gaspar, Răzvan Cojocaru, Radu-Valentin Gros, Marius Valentin Boldea, Andreea Tirziu, and Emil Tirziu. 2024. "Hunting Dynamics and Identification of Potentially Pathogenic Bacteria in European Fallow Deer (Dama dama) across Three Hunting Reserves in Western Romania" Microorganisms 12, no. 6: 1236. https://doi.org/10.3390/microorganisms12061236
APA StyleBucur, I.-M., Moza, A. C., Pop, M., Nichita, I., Gaspar, C. M., Cojocaru, R., Gros, R.-V., Boldea, M. V., Tirziu, A., & Tirziu, E. (2024). Hunting Dynamics and Identification of Potentially Pathogenic Bacteria in European Fallow Deer (Dama dama) across Three Hunting Reserves in Western Romania. Microorganisms, 12(6), 1236. https://doi.org/10.3390/microorganisms12061236








