First Description of Mycoplasma agalactiae Anatomical Localization in Naturally Infected Hard Ticks (Rhipicephalus bursa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Tick Identification
2.2. Tick Dissection
2.3. Microbiological Analysis and Molecular Analysis
2.4. Histology
2.5. Hematoxylin and Eosin Staining
2.6. IHC for Mycoplasma agalactiae
3. Results
3.1. Ethical Statement
3.2. Ticks Collection and Identification
3.3. Isolation and Molecular Identification of Mycoplasma agalactiae from Ticks
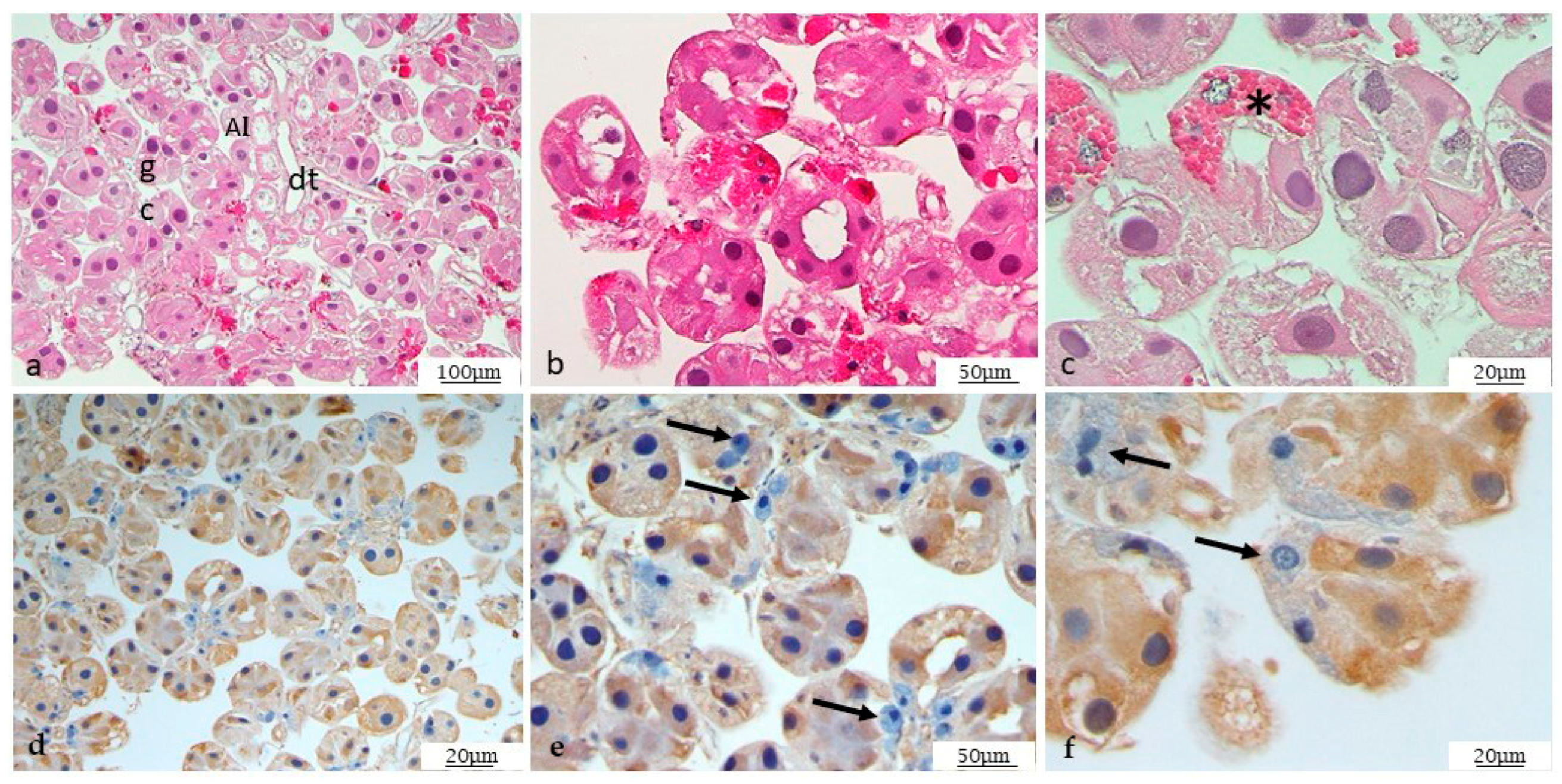
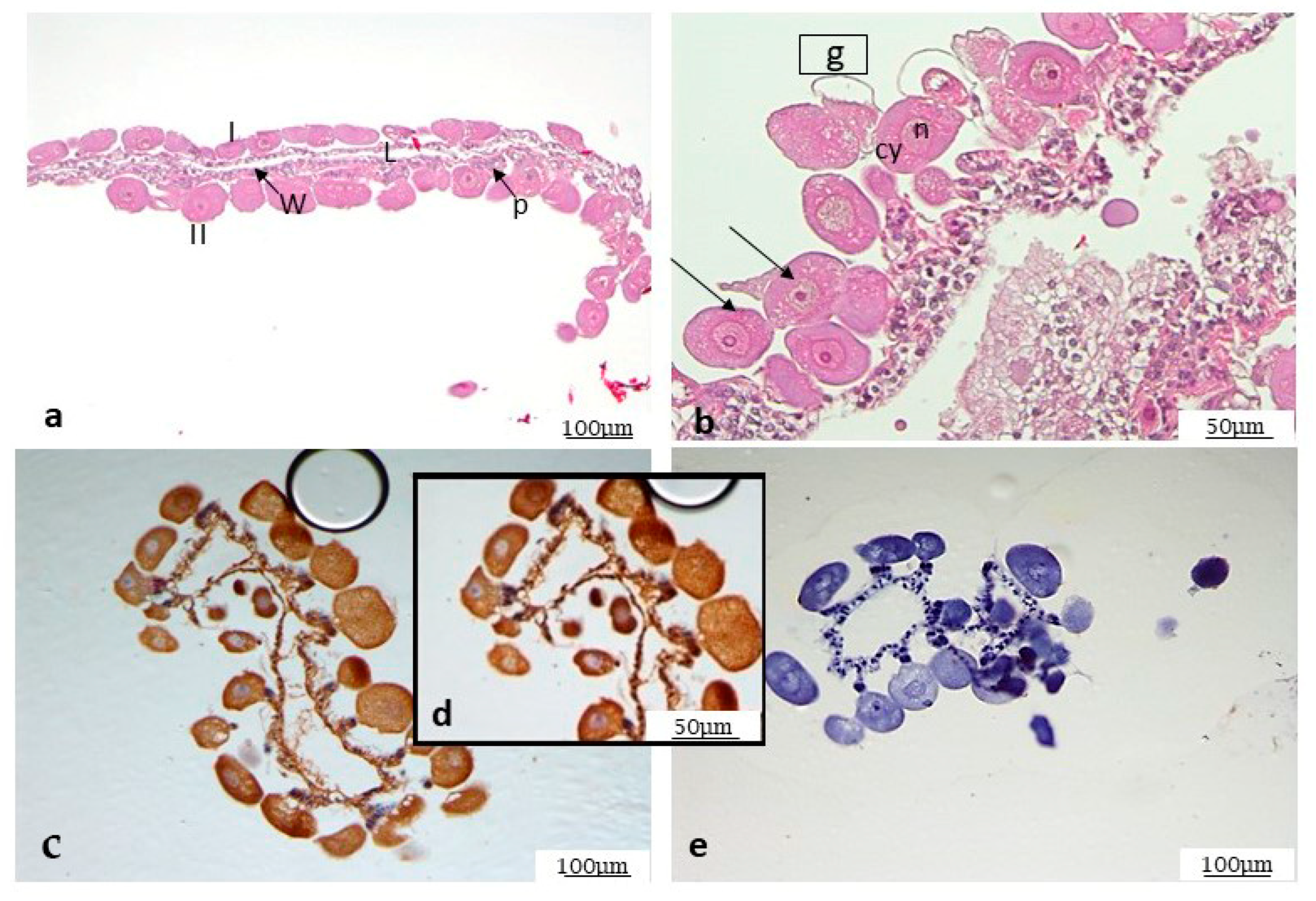
3.4. Histological Analysis
3.5. Immunohistochemistry on Mycoplasma agalactiae Positive Ticks
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migliore, S.; Puleio, R.; Nicholas, R.A.J.; Loria, G.R. Mycoplasma agalactiae: The Sole Cause of Classical Contagious Agalactia? Animals 2021, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Bergonier, D.; Berthelot, X.; Poumarat, F. Contagious agalactia of small ruminants: Current knowledge concerning epidemiology, diagnosis and control. OIE Rev. Scienific Techique 1997, 16, 848–873. [Google Scholar] [CrossRef] [PubMed]
- Loria, G.R.; Nicholas, R.A. Contagious agalactia: The shepherd’s nightmare. Vet. J. 2013, 198, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Jay, M.; Tardy, F. Contagious agalactia in sheep and goats: Current perspectives. Vet. Med. Res. Rep. 2019, 10, 229–247. [Google Scholar]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Biology of Ticks; Oxford University Press: Oxford, UK, 1991; p. 447. [Google Scholar]
- McCoy, K.D.; Léger, E.; Dietrich, M. Host specialization in ticks and transmission of tick-borne diseases: A review. Front. Cell. Infect. Microbiol. 2013, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region: A Guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004. [Google Scholar]
- Neimark, H.; Johansson, K.E.; Rikihisa, Y.; Tully, J.G. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis’, ‘Candidatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii’. Int. J. Syst. Evol. Microbiol. 2001, 51, 891–899. [Google Scholar] [PubMed]
- Nayak, N.C.; Blownik, M.K. Goat flea (order Siphonaptera) as a possible vector for the transmission of caprine mycoplasmal polyarthritis with septicaemia. Prev. Vet. Med. 1990, 9, 259–266. [Google Scholar] [CrossRef]
- Foley, J.E.; Pedersen, N.C. Candidatus Mycoplasma haemominutum, a low-virulence erpierythrocytic parasite of cats. Int. J. Syst. Evol. Microbiol. 2001, 51, 815–817. [Google Scholar] [CrossRef]
- Willi, B.; Boretti, F.S.; Cattori, V.; Tasker, S.; Meli, M.L.; Reusch, C.; Lutz, H.; Hofmann-Lehmann, R. Identification, molecular characterization, and experimental transmission of a new hemoplasma isolate from a cat with hemolytic anemia in Switzerland. J. Clin. Microbiol. 2005, 43, 2581–2585. [Google Scholar] [CrossRef]
- Willi, B.; Boretti, F.S.; Baumgartner, C.; Cattori, V.; Meli, M.L.; Doherr, M.G.; Reusch, C.E.; Hofmann-Lehmann, R. Feline hemoplasmas in Switzerland: Identification of a novel species, diagnosis, prevalence, and clinical importance. Schweiz. Arch. Tierheilk. 2006, 148, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Seneviratna, P.; Weerasinghe, N.; Ariyadasa, S. Transmission of Haemobartonella canis by the dog tick, Rhipicephalus sanguineus. Res. Vet. Sci. 1973, 14, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Novacco, M.; Meli, M.L.; Gentilini, F.; Marsilio, F.; Ceci, C.; Pennisi, M.G.; Lombardo, G.; Lloret, A.; Santos, L.; Carrapiço, T.; et al. Prevalence and geographical distribution of canine hemotropic mycoplasma infections in Mediterranean countries and analysis of risk factors for infection. Vet. Microbiol. 2010, 142, 276–284. [Google Scholar] [CrossRef]
- Hornok, S.; Micsutka, A.; Meli, M.L.; Lutz, H.; Hofmann-Lehmann, R. Molecular investigation of transplacental and vector-borne transmission of bovine haemoplasmas. Vet. Microbiol. 2011, 152, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Ayling, R.D.; Bisgaard-Frantzen, S.; Adler, A.; Blowey, R.W.; Barlow, A.M.; Millar, M.F.; Van Der Burgt, G.M. Detection of ‘Candidatus Mycoplasma haemobos’, Mycoplasma wenyonii and Anaplasma phagocytophilum from cattle in England. Vet. Rec. 2012, 170, 543. [Google Scholar] [CrossRef] [PubMed]
- Girotto, A.; Zangirólamo, A.F.; Bogado, A.L.; Souza, A.S.; Silva, G.C.; Garcia, J.L.; Vilas Boas, L.A.; Biondo, A.W.; Vidotto, O. Molecular detection and occurrence of ‘Candidatus Mycoplasma haemobos’ in dairy cattle of Southern Brazil. Rev. Bras. Parasitol. Vet. 2012, 21, 342–344. [Google Scholar] [CrossRef]
- Hornok, S.; Sugár, L.; Fernández de Mera, I.G.; De La Fuente, J.; Horváth, G.; Kovács, T.; Micsutka, A.; Gönczi, E.; Flaisz, B.; Takács, N.; et al. Tick-and fly-borne bacteria in ungulates: The prevalence of Anaplasma phagocytophilum, haemoplasmas and rickettsiae in water buffalo and deer species in Central Europe, Hungary. BMC Vet. Res. 2018, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.J.; Brito, D.R.; Abate, H.L.; Paixão, S.F.; Soares, E.D.; Vieira, T.S.; Garcia, J.L.; Vieira, R.F.; Vidotto, O. Hemotropic mycoplasmas infection in water buffaloes (Bubalus bubalis) from northeastern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2018, 56, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hu, Y.; Leng, C.; Shi, H.; Jiao, Z.; Chen, X.; Peng, Y.; Yang, H.; Kan, Y.; Yao, L. Molecular investigation of “Candidatus Mycoplasma haemobos” in goats and sheep in central China. Transbound. Emerg. Dis. 2019, 66, 22–27. [Google Scholar] [CrossRef]
- Neimark, H.; Hoff, B.; Ganter, M. Mycoplasma ovis comb. nov. (formerly Eperythrozoon ovis), an eperythrocytic agent of haemolytic anaemia in sheep and goats. Int. J. Syst. Evol. Microbiol. 2004, 54, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Migliore, S.; Puleio, R.; Galuppo, L.; La Russa, F.; Blanda, V.; Tumino, S.; Torina, A.; Ridley, A.; Loria, G.R. Detection of Mycoplasma agalactiae in Ticks (Rhipicephalus bursa) Collected by Sheep and Goats in Sicily (South-Italy), Endemic Area for Contagious Agalactia. Microorganisms 2021, 9, 2312. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK, 2000; p. 643. ISBN 9780521019774. [Google Scholar]
- Rodríguez, F.; Ramírez, G.A.; Ramírez, A.S.; Ball, H.J.; Espinosa de los Monteros, A.; Fernandez, A. Immunohistochemical detection of Mycoplasma agalactiae in formalin-fixed, paraffin-embedded tissues from naturally and experimentally infected goats. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Friis, N.F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord. Vet. Med. 1975, 27, 337–339. [Google Scholar] [PubMed]
- Torina, A.; Khoury, C.; Caracappa, S.; Maroli, M. Ticks infesting livestock on farms in Western Sicily, Italy. Exp. Appl Acarol. 2006, 38, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Raele, D.A.; Galante, D.; Pugliese, N.; De Simone, E.; Cafiero, M.A. Coxiella-like endosymbiont associated to the Anatolian brown tick Rhipicephalus bursa in Southern Italy. Microbes Infect. 2015, 17, 799–805. [Google Scholar] [CrossRef]
- Matei, I.A.; Ionică, A.M.; Corduneanu, A.; Domșa, C.; Sándor, A.D. The Presence of Ehrlichia Canis in Rhipicephalus bursa Ticks Collected from Ungulates in Continental Eastern Europe. J. Vet. Res. 2021, 65, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Ferrolho, J.; Antunes, S.; Santos, A.S.; Velez, R.; Padre, L.; Cabezas-Cruz, A.; Santos-Silva, M.M.; Domingos, A. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick Borne Dis. 2016, 7, 443–448. [Google Scholar] [CrossRef]
- Renneker, S.; Abdo, J.; Salih, D.E.A.; Karagenc, T.; Bilgic, H.; Torina, A.; Oliva, A.G.; Campos, J.; Kullmann, B.; Ahmed, J.; et al. Can Anaplasma ovis in small ruminants be neglected any longer? Transbound. Emerg. Dis. 2013, 60 (Suppl. S2), 105–112. [Google Scholar] [CrossRef]
- Masala, G.; Chisu, V.; Foxi, C.; Socolovschi, C.; Raoult, D.; Parola, P. First detection of Ehrlichia canis in Rhipicephalus bursa ticks in Sardinia Italy. Ticks Tick Borne Dis. 2012, 3, 396–397. [Google Scholar] [CrossRef]
- Agnone, A.; La Manna, M.; Sireci, G.; Puleio, R.; Usticano, A.; Ozdemir, U.; Nicholas, R.A.J.; Chiaracane, V.; Dieli, F.; Di Marco, V.; et al. A comparison of the efficacy of commercial and experimental vaccines for contagious agalactia in sheep. Small Rum. Res. 2013, 112, 230–234. [Google Scholar]
- Enayati, A.A.; Asgarian, F.; Sharif, M.; Boujhmehrani, H.; Amouei, A.; Vahedi, N.; Boudaghi, B.; Piazak, N.; Hemingway, J. Propetamphos resistance in Rhipicephalus bursa (Acari, Ixodidae). Vet. Parasitol. 2009, 162, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Capone, A.; Valzano, M.; Bozic, J.; Preziuso, S.; Mensah, P.; Varotto Boccazzi, I.; Rinaldi, L.; Favia, G.; Ricci, I. Denaturing Gradient Gel Electrophoresis Analysis of Bacteria in Italian Ticks and First Detection of Streptococcus equi in Rhipicephalus bursa from the Lazio Region. Vector Borne Zoonotic Dis. 2019, 19, 328–332. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.D.; Davies, C.R.; Steel, G.M.; Nuttall, P.A. Vector capacity of Rhipicephalus appendiculatus and Amblyomma variegatum for Thogoto and Dhori viruses. Med. Vet. Entomol. 1989, 3, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. Tick-borne disease systems emerge from the shadows: The beauty lies in molecular detail, the message in epidemiology. Parasitology 2009, 136, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E.; Gern, L.; Nuttall, P.A. Co-feeding ticks: Epidemiological significance for tick-borne pathogen transmission. Parasitol Today. 1996, 12, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Sevimli, U.; Migliore, S.; Jafarizadeh, A.; Loria, G.R.; Nicholas, R.A.J. Vaccines for Mycoplasma Diseases of Small Ruminants: A Neglected Area of Research. Pathogens 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]

| Sheep | Mycoplasma spp. Isolation from Milk | No. of Collected Ticks | Ticks Identification | Mycoplasma spp. Isolation from Half Ticks (No. of Positive/Total) | Ma Detection from Positive Samples by VETMAXTM Real-Time PCR | Ma Antigen Detection from Positive Samples by IHC |
|---|---|---|---|---|---|---|
| #1 | - | 3 | R. bursa | 0/2 | - | - |
| #2 | - | 5 | R. bursa | 2/2 | 2/2 | 2/2 |
| #3 | - | 3 | R. bursa | 0/3 | - | - |
| #4 | - | 4 | R. bursa | 0/1 | - | - |
| #5 | - | 5 | R. bursa | 0/3 | - | - |
| 20 | 2/20 (10%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliore, S.; Condorelli, L.; Galluzzo, P.; Galuppo, L.; Corrente, A.; Lepri, E.; Ridley, A.; Loria, G.R.; Puleio, R. First Description of Mycoplasma agalactiae Anatomical Localization in Naturally Infected Hard Ticks (Rhipicephalus bursa). Microorganisms 2024, 12, 1390. https://doi.org/10.3390/microorganisms12071390
Migliore S, Condorelli L, Galluzzo P, Galuppo L, Corrente A, Lepri E, Ridley A, Loria GR, Puleio R. First Description of Mycoplasma agalactiae Anatomical Localization in Naturally Infected Hard Ticks (Rhipicephalus bursa). Microorganisms. 2024; 12(7):1390. https://doi.org/10.3390/microorganisms12071390
Chicago/Turabian StyleMigliore, Sergio, Lucia Condorelli, Paola Galluzzo, Lucia Galuppo, Angelica Corrente, Elvio Lepri, Anne Ridley, Guido Ruggero Loria, and Roberto Puleio. 2024. "First Description of Mycoplasma agalactiae Anatomical Localization in Naturally Infected Hard Ticks (Rhipicephalus bursa)" Microorganisms 12, no. 7: 1390. https://doi.org/10.3390/microorganisms12071390
APA StyleMigliore, S., Condorelli, L., Galluzzo, P., Galuppo, L., Corrente, A., Lepri, E., Ridley, A., Loria, G. R., & Puleio, R. (2024). First Description of Mycoplasma agalactiae Anatomical Localization in Naturally Infected Hard Ticks (Rhipicephalus bursa). Microorganisms, 12(7), 1390. https://doi.org/10.3390/microorganisms12071390





