Anti-Leishmania amazonensis Activity of Morolic Acid, a Pentacyclic Triterpene with Effects on Innate Immune Response during Macrophage Infection
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Drugs
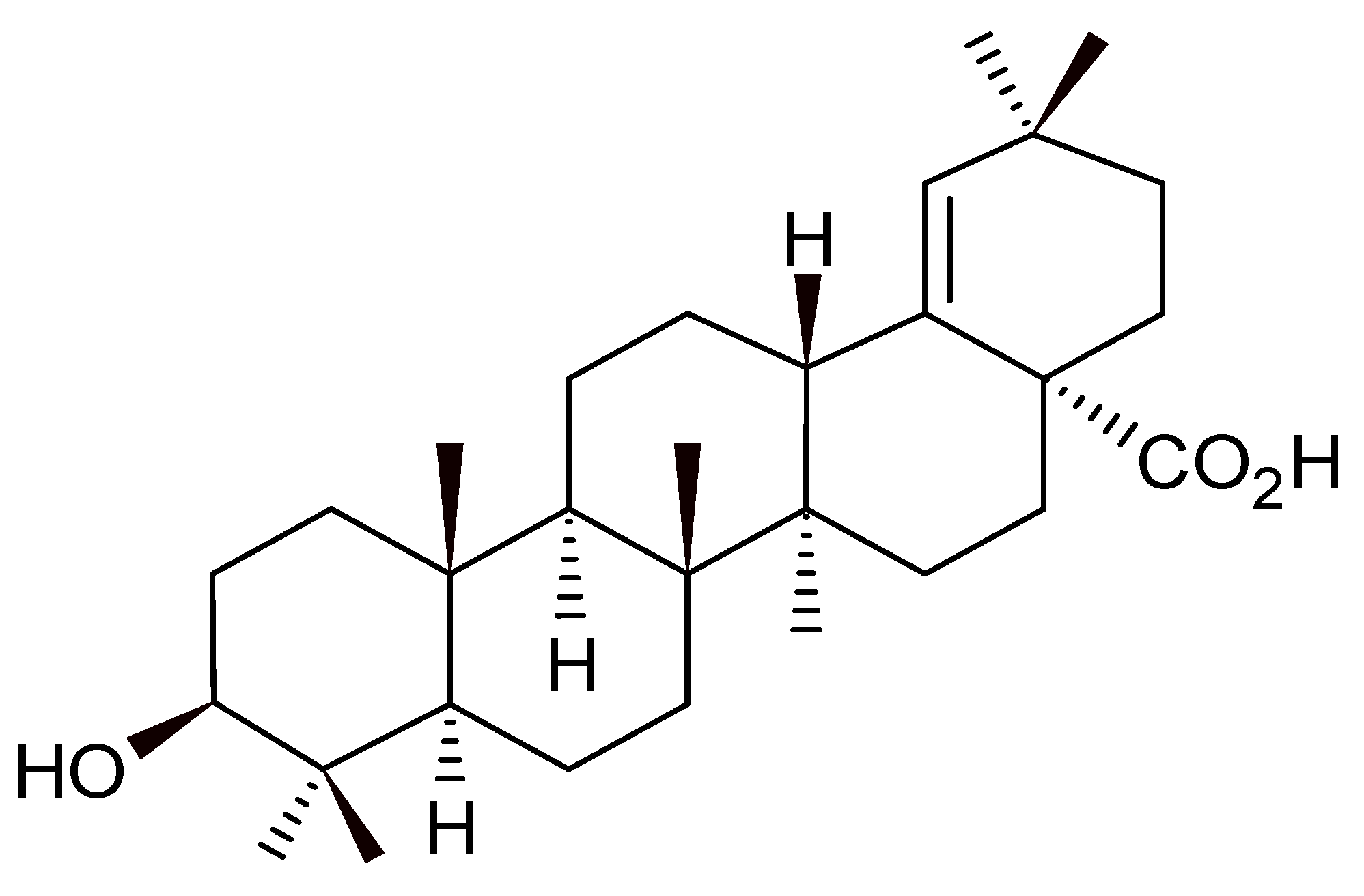
2.2. Morolic Acid
2.3. Parasite Cultivation
2.4. Murine Macrophages and VERO Lineage Cells
2.5. Antileishmanial Activity of Morolic Acid on Axenic Strains of L. (L.) amazonensis—Promastigotes and Amastigotes
2.6. Cytotoxicity of Morolic Acid on J774A.1 Macrophages and VERO CCL 81 Cells
2.7. Ultrastructural Analysis
2.8. In Vitro Activity of Morolic Acid against Intramacrophageal Amastigotes
2.9. Production of Reactive Oxygen Species (ROS)
2.10. Production of Nitric Oxide by Infected Macrophages
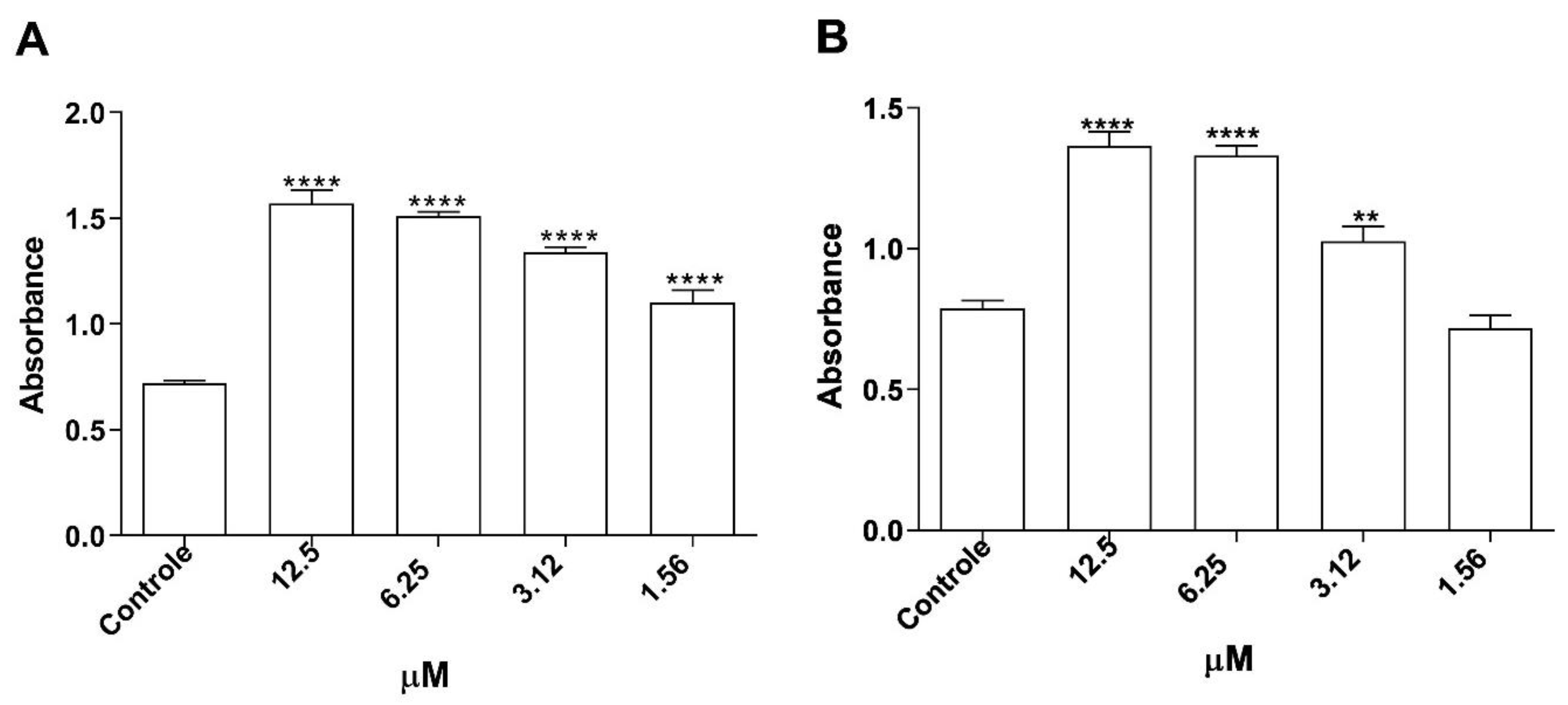
2.11. Assessment of Lysosomal Activity in Murine Macrophages
2.12. Phagocytic Capacity of Infected Macrophages
2.13. Assessment of Cytokine Production
2.14. Statistical Analysis
3. Results
3.1. Evaluation of Antileishmanial Activity of Morolic Acid on Promastigotes and Axenic Amastigotes from L. (L.) amazonensis
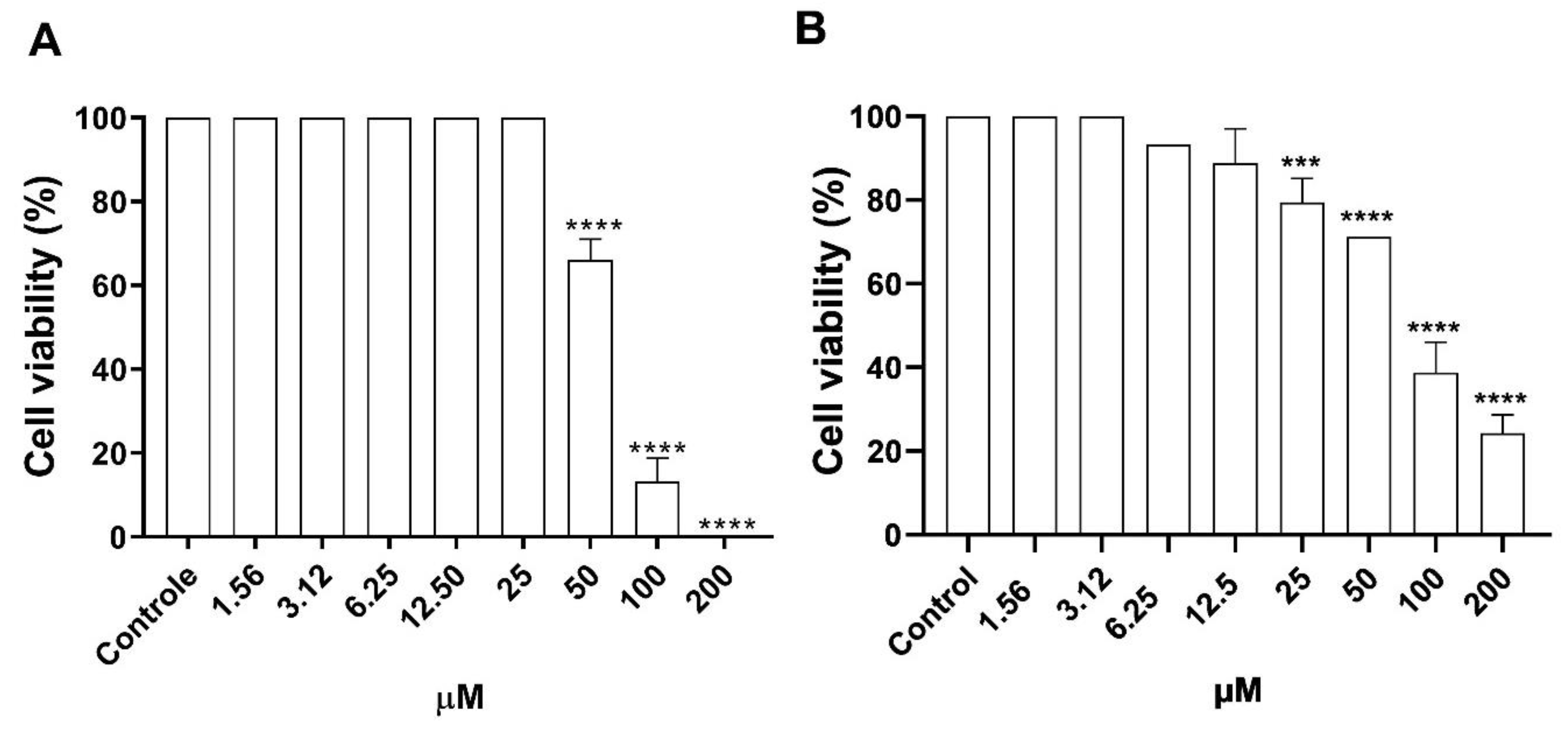
3.2. Cytotoxicity of Morolic Acid on Murine Macrophages and VERO Cells
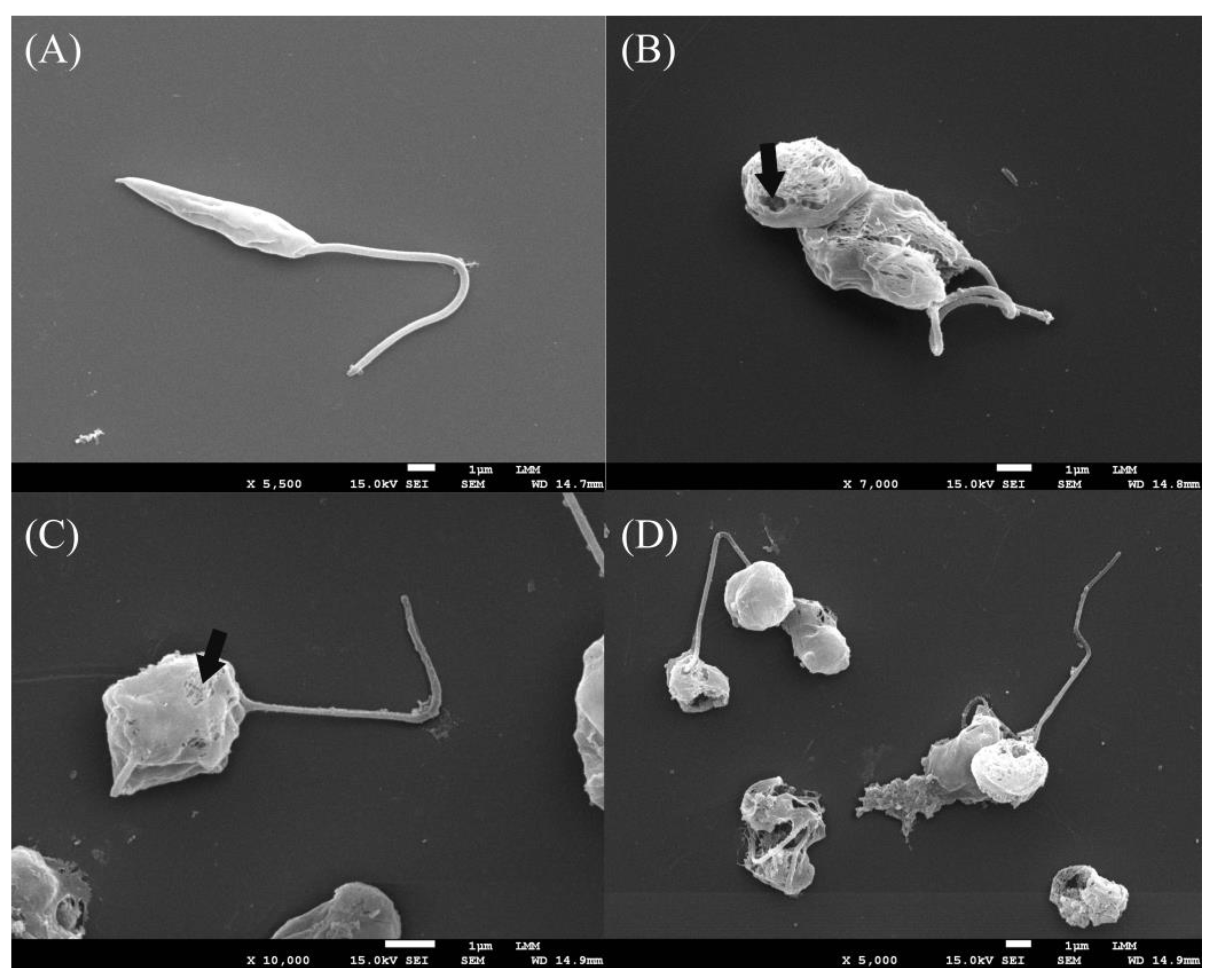
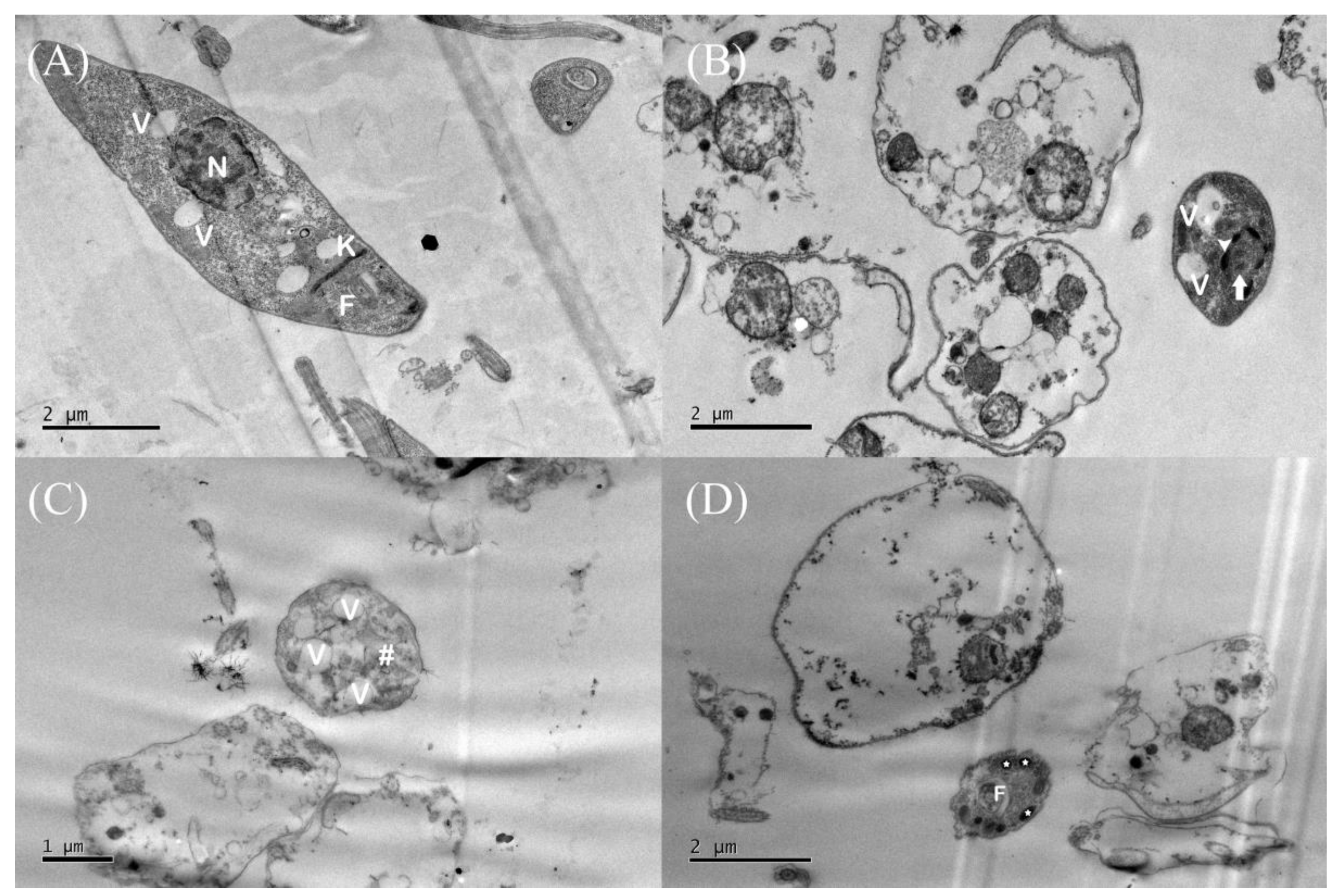
3.3. Assessment of Promastigote form Ultrastructures
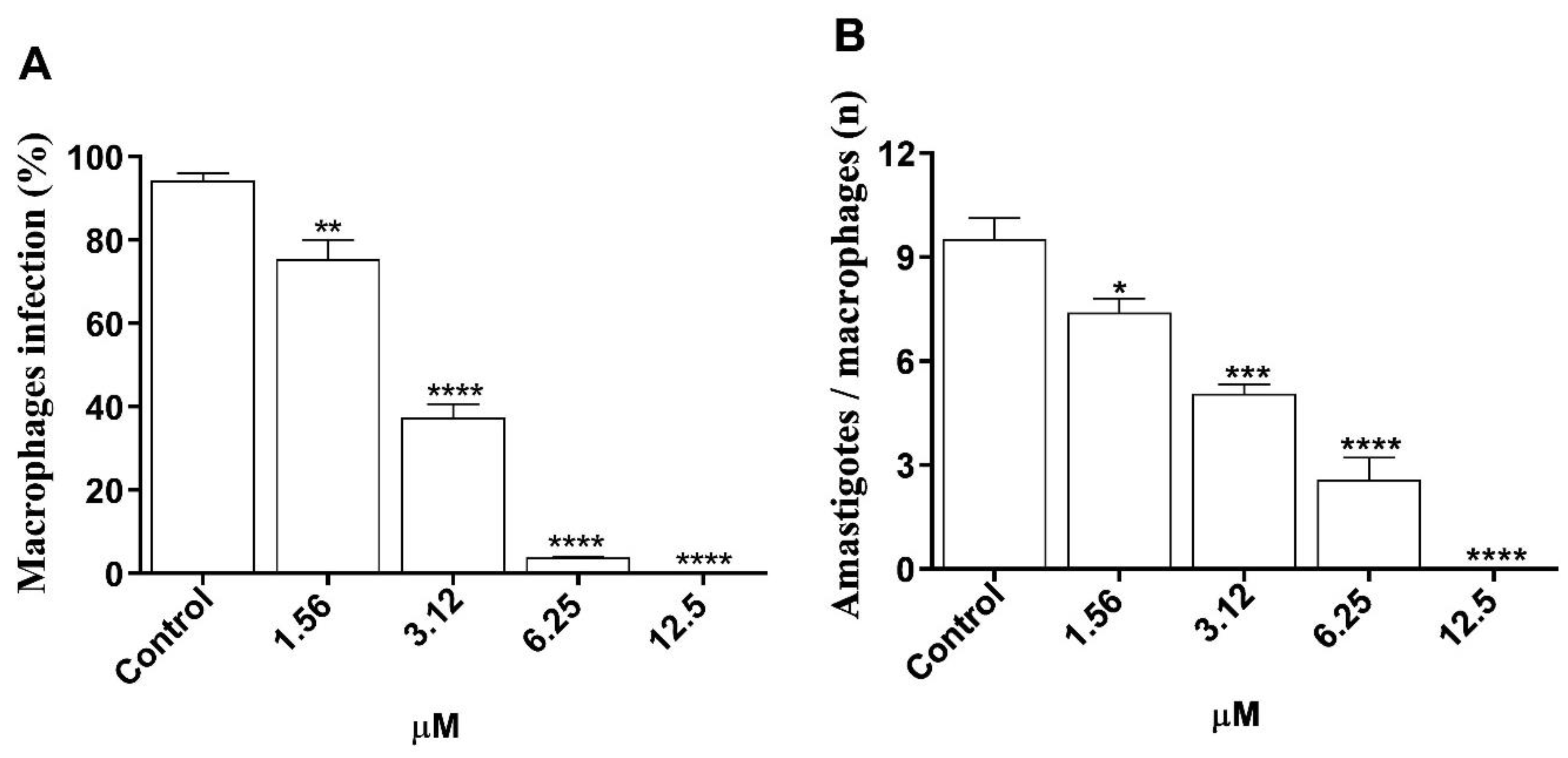
3.4. In Vitro Efficacy of Morolic Acid on Infection of J774A.1 Macrophages by L. (L.) amazonensis
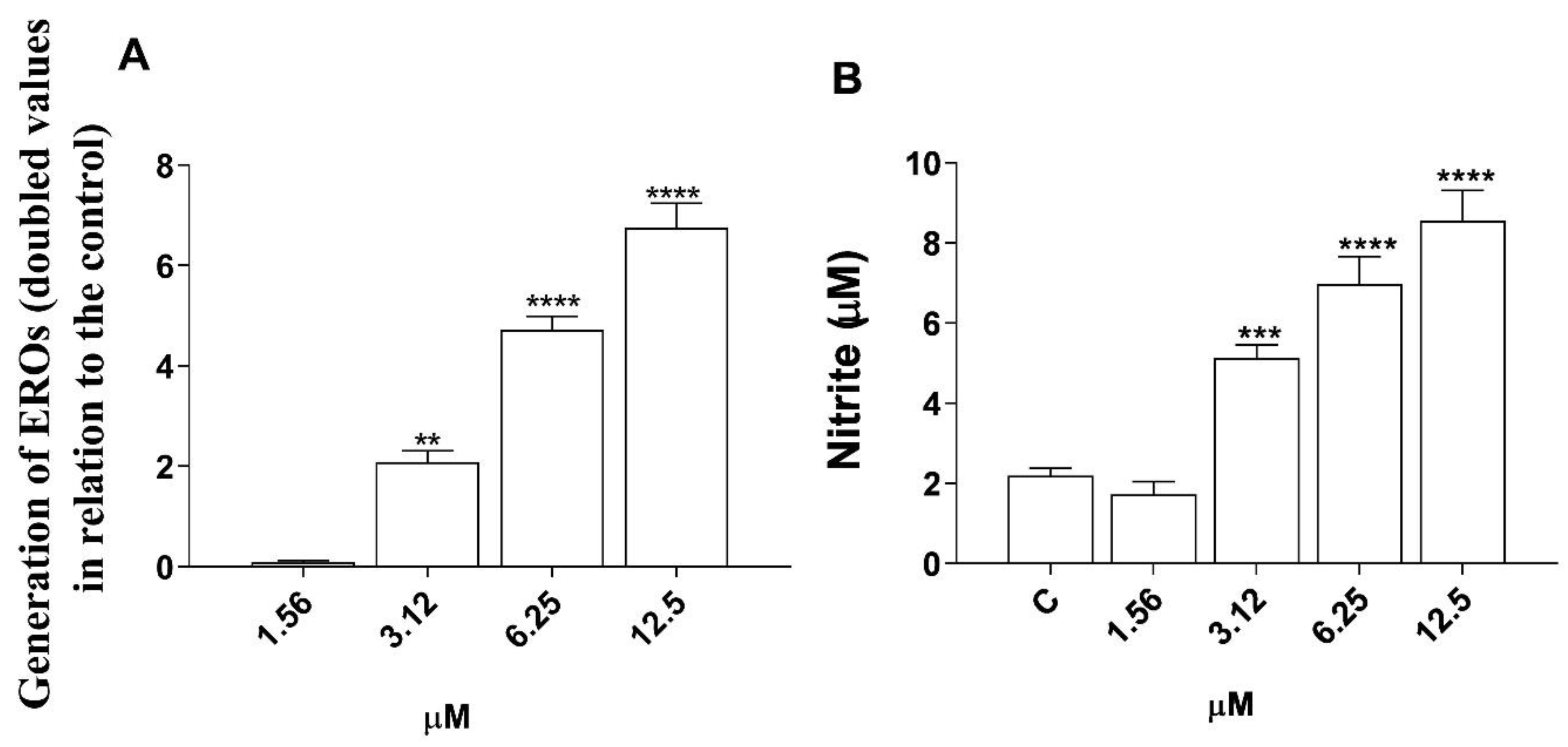
3.5. Induction of Reactive Oxygen Species (ROS) and Nitric Oxide (NO) Production by Morolic Acid
3.6. Assessment of Cytokine Production in Infected Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McArthur, D.B. Emerging Infectious Diseases. Nurs. Clin. N. Am. 2019, 54, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Shahmanesh, M.; Harling, G.; Coltart, C.E.M.; Bailey, H.; King, C.; Gibbs, J.; Seeley, J.; Phillips, A.; Sabin, C.A.; Aldridge, R.W.; et al. From the micro to the macro to improve health: Microorganism ecology and society in teaching infectious disease epidemiology. Lancet Infect. Dis. 2020, 20, e142–e147. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.R.; Nascimento-Júnior, N.M. Leishmaniasis: General Aspects Related with the Disease, the Parasite Cycle, Available Drugs, Novel Prototypes and Vaccines. Rev. Virtual Química. 2017, 9, 861–876. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- da Franca Rodrigues, K.A.; Amorim, L.V.; Dias, C.N.; Moraes DF, C.; Carneiro SM, P.; de Amorim Carvalho, F.A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.E.S.; Sobrinho-Junior, E.P.C.; Brito, L.M.; Nicolau, L.A.D.; Carvalho, T.P.; Moura, A.K.S.; Rodrigues, K.A.F.; Carneiro, S.M.P.; Arcanjo, D.D.R.; Citó, A.M.G.L.; et al. Anti-Leishmania activity of essential oil of Myracrodruon urundeuva (Engl.) Fr. All.: Composition, cytotoxity and possible mechanisms of action. Exp. Parasitol. 2017, 175, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Herrera, I.; Satyal, P.; Setzer, W. In-Vitro Evaluation of 52 Commercially-available essential oils Against Leishmania amazonensis. Molecules 2019, 24, 1248. [Google Scholar] [CrossRef]
- Dawé, A.; Mbiantcha, M.; Yakai, F.; Jabeen, A.; Ali, M.S.; Lateef, M.; Ngadjui, B.T. Flavonoids and triterpenes from Combretum fragrans with anti-inflammatory, antioxidant and antidiabetic potential. Z. Für Naturforsch. C. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Lacouth-Silva, F.; Xavier, C.V.; da SSetúbal, S.; Pontes, A.S.; Nery, N.M.; de Castro, O.B.; Fernandes, C.F.; Honda, E.R.; Zanchi, F.B.; Calderon, L.A.; et al. The effect of 3β, 6β, 16β-trihydroxylup-20(29)-ene lupane compound isolated from Combretum leprosum Mart. on peripheral blood mononuclear cells. BMC Complement. Altern. Med. 2015, 15, 420. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Webster, D.; Johnson, J.A.; Gray, C.A. Anti-mycobacterial triterpenes from the Canadian medicinal plant Alnus incana. J. Ethnopharmacol. 2015, 165, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, A.; Rufino-Palomares, E.E.; Fernández-Gallego, N.; Ortuño-Costela, M.C.; Reyes-Zurita, F.J.; Peragón, J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A. Target molecules in 3T3-L1 adipocytes differentiation are regulated by maslinic acid, a natural triterpene from Olea europaea. Phytomedicine 2016, 23, 1301–1311. [Google Scholar] [CrossRef]
- Soares, I.C.R.; Santos, S.A.A.R.; Coelho, R.F.; Alves, Y.A.; Vieira-Neto, A.E.; Tavares, K.C.S.; Magalhaes, F.E.A.; Campos, A.R. Oleanolic acid promotes orofacial antinociception in adult zebrafish (Danio rerio) through TRPV1 receptors. Chem. Biol. Interact. 2019, 299, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.-H.; Sun, W.-J.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. New hydroperoxylated and 20,24-epoxylated dammarane triterpenes from the rot roots of Panax notoginseng. J. Ginseng Res. 2020, 44, 405–412. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, J.A.; Laurenti, M.D.; Antonangelo, L.; Faria, C.S.; Lago, J.H.G.; Passero, L.F.D. related pentacyclic triterpenes have immunomodulatory activity in chronic experimental visceral leishmaniasis. J. Immunol. Res. 2021, 2021, 6671287. [Google Scholar] [CrossRef]
- Srisawat, P.; Yasumoto, S.; Fukushima, E.O.; Robertlee, J.; Seki, H.; Muranaka, T. Production of the bioactive plant-derived triterpenoid morolic acid in engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2020, 117, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Dorr, C.R.; Yemets, S.; Kolomitsyna, O.; Krasutsky, P.; Mansky, L.M. Triterpene derivatives that inhibit human immunodeficiency virus type 1 replication. Bioorg. Med. Chem. Lett. 2011, 21, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; García-Jiménez, S.; Rios, M.Y.; Medina-Franco, J.L.; López-Vallejo, F.; Webster, S.P.; Binnie, M.; Ibarra-Barajas, M.; Ortiz-Andrade, R.; Estrada-Soto, S. Antihyperglycemic and sub-chronic antidiabetic actions of morolic and moronic acids, in vitro and in silico inhibition of 11β-HSD 1. Phytomedicine 2013, 20, 571–576. [Google Scholar] [CrossRef]
- Dias, C.N.; de Lima Nunes, T.A.; de Sousa, J.M.S.; Costa, L.H.; Rodrigues, R.R.L.; Araújo, A.J.; Marinho Filho, J.D.B.; da Silva, M.V.; Oliveira, M.R.; de Amorim Carvalho, F.A.; et al. Rodrigues, Methyl gallate: Selective antileishmanial activity correlates with host-cell directed effects. Chem. Biol. Interact. 2020, 320, 109026. [Google Scholar] [CrossRef]
- de Lima Nunes, T.A.; Santos, M.M.; de Oliveira, M.S.; de Sousa, J.M.S.; Rodrigues, R.R.L.; de Araujo Sousa, P.S.; de Araújo, A.R.; da Cunha Pereira, A.C.T.; Ferreira, G.P.; Rocha, J.A.; et al. Curzerene antileishmania activity: Effects on Leishmania amazonensis and possible action mechanisms. Int. Immunopharmacol. 2021, 100, 108130. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Paiva, K.L.R.; Radicchi, M.A.; Báo, S.N. In Vitro Evaluation of NLS-DTX Activity in Triple-Negative Breast Cancer. Molecules 2022, 27, 4920. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.M.; Rabelo, R.E.; Assis, B.M.; Baó, S.N.; Neto, A.F.G.; Oliveira, L.P.; Jesus, L.O.; Helfenstein, K.K.; Vulcani, V.A.S. Morphological and morphometric characterization of the preputial ostium, internal preputial leaflet, and free part of the penises of Aberdeen Angus and Nellore bulls. Arq. Bras. Med. Veterinária e Zootec. 2022, 74, 1–10. [Google Scholar] [CrossRef]
- Amorim, F.M.; Rodrigues, Y.K.S.; Barbosa, T.P.; Néris, P.L.N.; Caldas, J.P.A.; Sousa, S.C.O.; Leite, J.A.; Rodrigues-Mascarenhas, S.; Vasconcellos, M.L.A.A.; Oliveira, M.R. Morita-Baylis-Hillman adduct shows in vitro activity against Leishmania (Viannia) braziliensis associated with a reduction in IL-6 and IL-10 but independent of nitric oxide. Parasitology 2013, 140, 29–38. [Google Scholar] [CrossRef]
- Rodrigues, R.R.L.; Nunes, T.A.L.; de Araujo, A.R.; Marinho Filho, J.D.B.; da Silva, M.V.; de Amorim Carvalho, F.A.; Pessoa, O.D.L.; Freitas, H.P.S.; da Franca Rodrigues, K.A.; Araújo, A.J. Antileishmanial activity of cordiaquinone E towards Leishmania (Leishmania) amazonensis. Int. Immunopharmacol. 2021, 90, 107124. [Google Scholar] [CrossRef]
- Rodrigo, C.; Weeratunga, P.; Fernando, S.D.; Rajapakse, S. Amphotericin B for treatment of visceral leishmaniasis: Systematic review and meta-analysis of prospective comparative clinical studies including dose-ranging studies. Clin. Microbiol. Infect. 2018, 24, 591–598. [Google Scholar] [CrossRef]
- Andre, C.M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J.M.; Lateur, M.; Hausman, J.; Larondelle, Y.; et al. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids. New Phytol. 2016, 211, 1279–1294. [Google Scholar] [CrossRef]
- López-Martínez, S.; Navarrete-Vázquez, G.; Estrada-Soto, S.; León-Rivera, I.; Rios, M.Y. Chemical constituents of the hemiparasitic plant Phoradendron brachystachyum DC Nutt (Viscaceae). Nat. Prod. Res. 2013, 27, 130–136. [Google Scholar] [CrossRef]
- Ranalli, P.; Trono, D. Strategie per L’accumulo Nelle Piante di Metaboliti di Interesse Nutrizionale e Industriale; Georgofili Quaderni: Florence, Italy, 2013; Volume VII, pp. 7–17. [Google Scholar]
- da Silveira Regueira-Neto, M.; Tintino, S.R.; Rolón, M.; Coronal, C.; Vega, M.C.; de Queiroz Balbino, V.; de Melo Coutinho, H.D. Antitrypanosomal, antileishmanial and cytotoxic activities of Brazilian red propolis and plant resin of Dalbergia ecastaphyllum (L) Taub. Food Chem. Toxicol. 2018, 119, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Suarez, J.; Riveros, I.; Delgado, G. Evaluation of the leishmanicidal and cytotoxic potential of essential oils derived from ten colombian plants. Iran. J. Parasitol. 2013, 8, 129–136. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23682270 (accessed on 13 April 2024).
- Khan, I.; Ahmad, K.; Khalil, A.T.; Khan, J.; Khan, Y.A.; Saqib, M.S.; Umar, M.N.; Ahmad, H. Evaluation of antileishmanial, antibacterial and brine shrimp cytotoxic potential of crude methanolic extract of Herb Ocimum basilicum (Lamiacea). J. Tradit. Chin. Med. 2015, 35, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.M.; Rosa TD, D.S.; Mouchrek, A.N.; Abreu-Silva, A.L.; Calabrese KD, S.; Almeida-Souza, F. Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa essential oils: Chemical composition, antimicrobial and antileishmanial activity. Evid.-Based Complement Altern. Med. 2019, 2019, 2421695. [Google Scholar] [CrossRef]
- Chae, S.I.; Yi, S.A.; Nam, K.H.; Park, K.J.; Yun, J.; Kim, K.H.; Lee, J.; Han, J.-W. morolic acid 3-o-caffeate inhibits adipogenesis by regulating epigenetic gene expression. Molecules 2020, 25, 5910. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Ramos, P.; Serrano, D.R.; Saldaña, H.K.R.; Torrado, J.J.; Bolás-Fernández, F.; Dea-Ayuela, M.A. Evaluating the potential of ursolic acid as bioproduct for cutaneous and visceral leishmaniasis. Molecules 2020, 25, 1394. [Google Scholar] [CrossRef]
- Souza, A.C.; Alves, M.M.D.M.; Brito, L.M.; Oliveira, L.G.D.C.; Sobrinho-Júnior, E.P.C.; Costa, I.C.G.; Freitas, S.D.L.; Rodrigues, K.A.D.F.; Chaves, M.H.; Arcanjo, D.D.R.; et al. Platonia insignis Mart. a brazilian amazonian plant: The stem barks extract and its main constituent lupeol exert antileishmanial effects involving macrophages activation. Evid.-Based Complement Altern. Med. 2017, 2017, 3126458. [Google Scholar] [CrossRef]
- Alakurtti, S.; Heiska, T.; Kiriazis, A.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Yli-Kauhaluoma, J. Synthesis and anti-leishmanial activity of heterocyclic betulin derivatives. Bioorg. Med. Chem. 2010, 18, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, M.; Miroslaw, B.; Sikora, E.; Ogonowski, J.; Wojtkiewicz, A.M.; Szaleniec, M.; Pasikowska-Piwko, M.; Eris, I. New lupeol esters as active substances in the treatment of skin damage. PLoS ONE 2019, 14, e0214216. [Google Scholar] [CrossRef]
- Furtado, N.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef]
- Saudagar, P.; Dubey, V.K. Carbon nanotube based betulin formulation shows better efficacy against Leishmania parasite. Parasitol. Int. 2014, 63, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, W.; López, A.S.; Alakurtti, S.; Tuononen, M.-L.; Yli-Kauhaluoma, J.; Ponte-Sucre, A. Betulin derivatives impair Leishmania braziliensis viability and host–parasite interaction. Bioorg. Med. Chem. 2014, 22, 6220–6226. [Google Scholar] [CrossRef]
- Magalhães, T.B.D.S.; Silva, D.K.C.; Teixeira, J.D.S.; De Lima, J.D.T.; Barbosa-Filho, J.M.; Moreira, D.R.M.; Guimarães, E.T.; Soares, M.B.P. A Betulinic acid derivative, ba5, induces g0/g1 cell arrest, apoptosis like-death, and morphological alterations in Leishmania sp. Front. Pharmacol. 2022, 13, 846123. [Google Scholar] [CrossRef]
- Prajeeth, C.K.; Haeberlein, S.; Sebald, H.; Schleicher, U.; Bogdan, C. Leishmania-infected macrophages are targets of nk cell-derived cytokines but not of nk cell cytotoxicity. Infect. Immun. 2011, 79, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.B.; Gangadhar, K.N.; Morais, T.R.; Conserva, G.A.A.; Vizetto-Duarte, C.; Pereira, H.; Laurenti, M.D.; Campino, L.; Levy, D.; Uemi, M.; et al. Antileishmanial activity of meroditerpenoids from the macroalgae Cystoseira baccata. Exp. Parasitol. 2017, 174, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Islamuddin, M.; Tabrez, S.; Alsaweed, M.; Alaidarous, M.A.; Alshehri, B.M.; Banawas, S.; Dukhyil, A.A.B.; Rub, A. Embilica officinalis L. inhibits the growth and proliferation of Leishmania donovani through the induction of ultrastructural changes, mitochondrial dysfunction, oxidative stress and apoptosis-like cell death. Biomed. Pharmacother. 2021, 143, 112156. [Google Scholar] [CrossRef]
- Vannier-Santos, M.; De Castro, S. Electron microscopy in antiparasitic chemotherapy: A (close) View to a Kill. Curr. Drug Targets. 2009, 10, 246–260. [Google Scholar] [CrossRef]


| J774A.1 | VERO CCL 81 | Promastigotes | Axenic Amastigotes | Intracellular Amastigotes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC50 µM | CC50 µM | IC50 µM | SIJ774A.1 | SIVERO | EC50 µM | SIJ774A.1 | SIVERO | EC50 µM | SIJ774A.1 | SIVERO | |
| Morolic acid | 68.61 ± 2.46 | 82.94 ± 1.84 | 1.13 ± 0.04 | 60.7 | 73.39 | 2.74 ± 0.03 | 25.04 | 30.27 | 2.56 ± 0.08 | 26.8 | 32.39 |
| Amphotericin B | 0.38 ± 0.06 | 0.32 ± 0.08 | 0.36 ± 0.03 | 1.05 | 0.9 | 0.23 ± 0.01 | 0.78 | 360.6 | 0.29 ± 0.04 | 1.31 | 1.10 |
| Meglumine antimoniate | 16433 ± 43.2 | 17363 ± 94.7 | 21564 ± 90.85 | 0.76 | 0.80 | 1017.4 ± 25.72 | 1.01 | 82.11 | 541 ± 13.5 | 30.37 | 32.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, V.M.R.; Maciel, N.B.; Machado, Y.A.A.; de Sousa, J.M.S.; Rodrigues, R.R.L.; dos Santos, A.L.S.; Gonçalves da Silva, M.G.; Martins da Silva, I.G.; Barros-Cordeiro, K.B.; Báo, S.N.; et al. Anti-Leishmania amazonensis Activity of Morolic Acid, a Pentacyclic Triterpene with Effects on Innate Immune Response during Macrophage Infection. Microorganisms 2024, 12, 1392. https://doi.org/10.3390/microorganisms12071392
de Souza VMR, Maciel NB, Machado YAA, de Sousa JMS, Rodrigues RRL, dos Santos ALS, Gonçalves da Silva MG, Martins da Silva IG, Barros-Cordeiro KB, Báo SN, et al. Anti-Leishmania amazonensis Activity of Morolic Acid, a Pentacyclic Triterpene with Effects on Innate Immune Response during Macrophage Infection. Microorganisms. 2024; 12(7):1392. https://doi.org/10.3390/microorganisms12071392
Chicago/Turabian Stylede Souza, Vanessa Maria Rodrigues, Nicolle Barreira Maciel, Yasmim Alves Aires Machado, Julyanne Maria Saraiva de Sousa, Raiza Raianne Luz Rodrigues, Airton Lucas Sousa dos Santos, Maria Gabrielly Gonçalves da Silva, Ingrid Gracielle Martins da Silva, Karine Brenda Barros-Cordeiro, Sônia Nair Báo, and et al. 2024. "Anti-Leishmania amazonensis Activity of Morolic Acid, a Pentacyclic Triterpene with Effects on Innate Immune Response during Macrophage Infection" Microorganisms 12, no. 7: 1392. https://doi.org/10.3390/microorganisms12071392
APA Stylede Souza, V. M. R., Maciel, N. B., Machado, Y. A. A., de Sousa, J. M. S., Rodrigues, R. R. L., dos Santos, A. L. S., Gonçalves da Silva, M. G., Martins da Silva, I. G., Barros-Cordeiro, K. B., Báo, S. N., Tavares, J. F., & Rodrigues, K. A. d. F. (2024). Anti-Leishmania amazonensis Activity of Morolic Acid, a Pentacyclic Triterpene with Effects on Innate Immune Response during Macrophage Infection. Microorganisms, 12(7), 1392. https://doi.org/10.3390/microorganisms12071392






