Antibacterial Activity of Brass against Antibiotic-Resistant Bacteria following Repeated Exposure to Hydrogen Peroxide/Peracetic Acid and Quaternary Ammonium Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Metal Specimens
2.2. Aging Process with Disinfectants Routinely Used in Healthcare Settings
2.3. Bacterial Strains
2.4. Detection of Genes Involved in Bacterial Copper Homeostasis and Resistance
2.5. Antimicrobial Efficacy Testing
2.6. Statistical Analysis
2.7. Data Availability
3. Results
3.1. Detection of Genes Involved in Copper Homeostasis and Resistance in Antibiotic-Resistant Strains
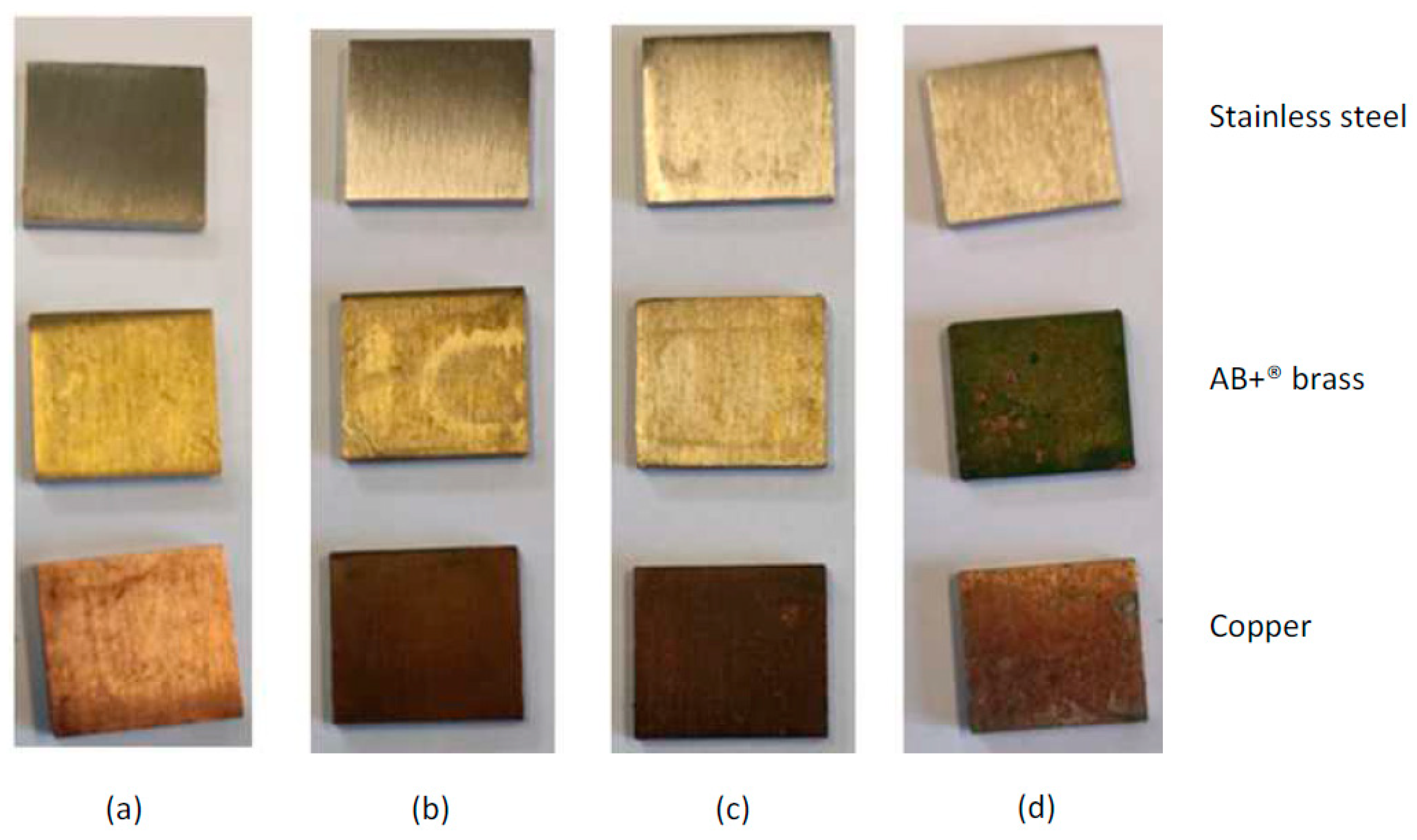
3.2. Visual Aspect of Metal Surfaces Post-Aging Treatment
3.3. Antibacterial Efficacy Post-Aging with a Single Disinfectant
3.4. Antibacterial Efficacy Post-Aging with a Combination of Quaternary Ammonium Compound and Peraceticacid/Hydrogen Peroxide Mix
3.5. Comparison of Antibacterial Efficacies following the Different Aging Processes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewnard, J.A.; Charani, E.; Gleason, A.; Hsu, L.Y.; Khan, W.A.; Karkey, A.; Chandler, C.I.R.; Mashe, T.; Khan, E.A.; Bulabula, A.N.H.; et al. Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: An evidence review and modelling analysis. Lancet 2024, 403, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Carling, P.C. Healthcare environmental hygiene: New insights and centers for disease control and prevention guidance. Infect. Dis. Clin. N. Am. 2021, 35, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Janc, J.; Łysenko, L.; Leśnik, P.; Słabisz, N.; Oleksy-Wawrzyniak, M.; Uchmanowicz, I. How to defeat multidrug-resistant bacteria in intensive care units. A lesson from the COVID-19 pandemic. Prevention, reservoirs, and implications for clinical practice. Int. J. Med. Sci. 2024, 21, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Rutala, W.A.; Sickbert-Bennett, E.E.; Weber, D.J. Role of the contaminated environment in transmission of multidrug-resistant organisms in nursing homes and infection prevention. Am. J. Infect. Control 2023, 51, A151–A157. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Pailhoriès, H.; Aviat, F.; Lepelletier, D.; Le Pape, P.; Dubreil, L.; Irle, M.; Buchner, J.; Eveillard, M.; Federighi, M.; et al. Hygienic perspectives of wood in healthcare buildings. Hygiene 2021, 1, 12–23. [Google Scholar] [CrossRef]
- Dauvergne, E.; Mullié, C. Brass alloys: Copper-bottomed solutions against hospital-acquired infections? Antibiotics 2021, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Dawson, W.R. The Egyptian medical papyri. In Diseases in Antiquity; Brothwell, D., Sandison, A.T., Eds.; Charles C Thomas Publisher: Springfield, IL, USA, 1967; pp. 98–114. [Google Scholar]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity mechanisms of copper nanoparticles and copper surfaces on bacterial cells and viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef] [PubMed]
- EPA. Registration Copper Stewardship Site. Available online: https://www.copperalloystewardship.com/ (accessed on 8 April 2024).
- Russel, A.D. Mechanisms of bacterial resistance to antibiotics and biocides. In Progress in Medicinal Chemistry; Ellis, G.P., Luscombe, D.K., Oxford, A.W., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1998; Volume 35, pp. 133–197. [Google Scholar] [CrossRef]
- McDonald, M.; Wesgate, R.; Rubiano, M.; Holah, J.; Denyer, S.P.; Jermann, C.; Maillard, J.Y. Impact of a dry inoculum deposition on the efficacy of copper-based antimicrobial surfaces. J. Hosp. Infect. 2020, 106, 465–472. [Google Scholar] [CrossRef]
- Dauvergne, E.; Lacquemant, C.; Adjidé, C.; Mullié, C. Validation of a worst-case scenario method adapted to the healthcare environment for testing the antibacterial effect of brass surfaces and implementation on hospital antibiotic-resistant strains. Antibiotics 2020, 9, 245. [Google Scholar] [CrossRef]
- Airey, P.; Verran, J. Potential use of copper as a hygienic surface; problems associated with cumulative soiling and cleaning. J. Hosp. Infect. 2007, 67, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Hayashi, T.; Nishikubo, H.; Morikawa, A.; Suzuki, S.; Sato, Y.; Kikuchi, Y. Effects of surface contamination and cleaning with hypochlorite wipes on the antibacterial activity of copper-alloyed antibacterial stainless steel. Biocontrol Sci. 2014, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Nishikubo, H.; Hirayama, K.; Suzuki, S.; Sato, Y.; Kikuchi, Y. Effects of NaOCl aqueous solutions and ethyl alcohol solutions on removing protein surface contaminants and re-establishing antibacterial activities of copper-alloyed stainless steel. Biocontrol Sci. 2015, 20, 193–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bryce, E.A.; Velapatino, B.; Akbari Khorami, H.; Donnelly-Pierce, T.; Wong, T.; Dixon, R.; Asselin, E. In vitro evaluation of antimicrobial efficacy and durability of three copper surfaces used in healthcare. Biointerphases 2020, 15, 011005. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.K.; Williams, T.C.; Nakhaie, D.; Woznow, T.; Velapatino, B.; Lorenzo-Leal, A.C.; Bach, H.; Bryce, E.A.; Asselin, E. In vitro assessment of antibacterial and antiviral activity of three copper products after 200 rounds of simulated use. Biometals 2023. [Google Scholar] [CrossRef]
- Andrei, A.; Öztürk, Y.; Khalfaoui-Hassani, B.; Rauch, J.; Marckmann, D.; Trasnea, P.I.; Daldal, F.; Koch, H.G. Cu homeostasis in bacteria: The ins and outs. Membranes 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Fiche Technique Surfa’Safe Premium Anios. Available online: https://www.robe-materiel-medical.com/fiches-techniques/-Surfa-safe-Premium-Anios-Spray-750-ml--SURFA.pdf (accessed on 8 April 2024).
- Aseptanios AD. Available online: https://www.robe-materiel-medical.com/images/files/ft_aseptanios_ad_fr.pdf (accessed on 8 April 2024).
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- ISO 7581:2023; Évaluation de L’Activité Bactéricide D’Une Surface Antimicrobienne Non Poreuse Utilisee Dans un Environnement. International Standard Organization: Geneva, Switzerland, 2023. Available online: https://norminfo.afnor.org/consultation/nf-iso-7581/evaluation-de-lactivite-bactericide-dune-surface-antimicrobienne-non-poreuse-utilisee-dans-un-environnement-sec/332102 (accessed on 11 April 2024).
- Perron, K.; Caille, O.; Rossier, C.; Van Delden, C.; Dumas, J.L.; Köhler, T. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J. Biol. Chem. 2004, 279, 8761–8768. [Google Scholar] [CrossRef] [PubMed]
- Goodman, E.R.; Piatt, R.; Bass, R.; Onderdonk, A.B.; Yokoe, D.S.; Huang, S.S. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect. Control Hosp. Epidemiol. 2008, 29, 593–599. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Havill, H.L.; Mangione, E.; Dumigan, D.G.; Moore, B.A. Comparison of fluorescent marker systems with 2 quantitative methods of assessing terminal cleaning practices. Infect. Control Hosp. Epidemiol. 2011, 32, 1187–1193. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, Y.-C.; Chen, M.-L.; Cheng, A.; Hung, I.-C.; Wang, J.-T.; Sheng, W.-H.; Chang, S.-C. Comparing visual inspection, aerobic colony counts, and adenosine triphosphate bioluminescence assay for evaluating surface cleanliness at a medical center. Am. J. Infect. Control 2015, 43, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Mathews, S.; Mücklich, F.; Solioz, M. Physicochemical properties of copper important for its antibacterial activity and development of a unified model. Biointerphases. 2015, 11, 018902. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, S.; Jennings, M.C.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.P.C.; Carnevale, V. Analysis of the destabilization of bacterial membranes by quaternary ammonium compounds: A combined experimental and computational study. ChemBioChem 2020, 21, 1510–1516. [Google Scholar] [CrossRef]
- Zhou, P.; Ogle, K. The corrosion of copper and copper alloy. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6, pp. 478–489. [Google Scholar] [CrossRef]
- Harrison, J.J.; Turner, R.J.; Joo, D.A.; Stan, M.A.; Chan, C.S.; Allan, N.D.; Vrionis, H.A.; Olson, M.E.; Ceri, H. Copper and quaternary ammonium cations exert synergistic bactericidal and antibiofilm activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Bondarczuk, K.; Piotrowska-Seget, Z. Molecular basis of active copper resistance mechanisms in Gram-negative bacteria. Cell. Biol. Toxicol. 2013, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Bouharkat, B.; Tir Touil-Meddah, A.; Guénin, S.; Mullié, C. MexXY multidrug efflux system is more frequently overexpressed in ciprofloxacin resistant French clinical isolates compared to hospital environment ones. Front. Microbiol. 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Caille, O.; Rossier, C.; Perron, K. A copper-activated two-component system interacts with zinc and imipenem resistance in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 4561–4568. [Google Scholar] [CrossRef]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal resistance and its association with antibiotic resistance. Adv. Microb. Physiol. 2017, 70, 261–313. [Google Scholar] [CrossRef]
- Mourão, J.; Novais, C.; Machado, J.; Peixe, L.; Antunes, P. Metal tolerance in emerging clinically relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- clones circulating in Europe. Int. J. Antimicrob. Agents. 2015, 45, 610–616. [Google Scholar] [CrossRef]
- Brown, N.L.; Barrett, S.R.; Camakaris, J.; Lee, B.T.; Rouch, D.A. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol. Microbiol. 1995, 17, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Ovreås, L.; Forney, L.; Daae, F.L.; Torsvik, V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Roosa, S.; Wattiez, R.; Prygiel, E.; Lesven, L.; Billon, G.; Gillan, D.C. Bacterial metal resistance genes and metal bioavailability in contaminated sediments. Environ. Pollut. 2014, 189, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Besaury, L.; Bodilis, J.; Delgas, F.; Andrade, S.; De la Iglesia, R.; Ouddane, B.; Quillet, L. Abundance and diversity of copper resistance genes cusA and copA in microbial communities in relation to the impact of copper on Chilean marine sediments. Mar. Pollut. Bull. 2013, 67, 16–25. [Google Scholar] [CrossRef]
- Laverde Gomez, J.A.; van Schaik, W.; Freitas, A.R.; Coque, T.M.; Weaver, K.E.; Francia, M.V.; Witte, W.; Werner, G. A multiresistance megaplasmid pLG1 bearing a hylEfm genomic island in hospital Enterococcus faecium isolates. Int. J. Med. Microbiol. 2011, 301, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.; Freitas, A.R.; Antunes, P.; Barros, M.; Campos, J.; Coque, T.M.; Peixe, L.; Novais, C. Co-transfer of resistance to high concentrations of copper and first-line antibiotics among Enterococcus from different origins (humans, animals, the environment and foods) and clonal lineages. J. Antimicrob. Chemother. 2014, 69, 899–906. [Google Scholar] [CrossRef]
- Zimmermann, M.; Udagedara, S.R.; Sze, C.M.; Ryan, T.M.; Howlett, G.J.; Xiao, Z.; Wedd, A.G. PcoE—A metal sponge expressed to the periplasm of copper resistance Escherichia coli. Implication of its function role in copper resistance. J. Inorg. Biochem. 2012, 115, 186–197. [Google Scholar] [CrossRef]
- Reyes-Jara, A.; Latorre, M.; López, G.; Bourgogne, A.; Murray, B.E.; Cambiazo, V.; González, M. Genome-wide transcriptome analysis of the adaptive response of Enterococcus faecalis to copper exposure. Biometals 2010, 23, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
| Strain Reference | Bacterial Species | Antibiotic Resistance Profile 1 | Sample Origin (Isolation Year) |
|---|---|---|---|
| ABAM41 | Acinetobacter baumannii | Oxa-23, AmpC, ArmA | Environment (2017) |
| AM85 | Pseudomonas aeruginosa | EPO | Rectal swab (2009) |
| ECLOAM1 | Enterobacter cloacae | Oxa-48, ESBL | External quality control (2019) |
| EFUMAM2 | Enterococcus faecium | vanA | Sputum (2008) |
| KPNAM2 | Klebsiella pneumoniae | KPC | Rectal swab (2017) |
| SAAM33 | Staphylococcus aureus | mecA, EPO | Tracheal (2012) |
| Gene | Function | ABAM41 | AM85 | ECLOAM1 | KPNAM2 | EFUMAM2 | SAAM33 |
|---|---|---|---|---|---|---|---|
| copA | ATPase pump | −/+ a | +/+ | −/− | −/− | −/− | −/− |
| tcrB | ATPase pump | +/+ | −/− | −/− | −/− | −/− | +/− |
| cusA | RND 1 pump | −/− | −/− | −/− | +/+ | −/− | −/− |
| pcoD | Inner membrane pump | −/− | −/− | +/+ | +/+ | −/− | −/− |
| czcA | Zn2+ pump | +/+ | +/+ | −/− | +/+ | +/− | +/− |
| cueO | Multicopper oxidase | −/− | −/− | −/− | −/− | −/− | −/− |
| pcoE | Chaperone | −/− | −/− | +/+ | +/+ | −/− | −/− |
| copZ | Chaperone | −/− | −/− | −/− | −/− | −/− | +/− |
| Bacterial Strain | Copper | AB+® Brass | ||||
|---|---|---|---|---|---|---|
| Untreated | QA | QA and PA/HP | Untreated | QA | QA and PA/HP | |
| ABAM 41 | 93.15 ± 11.517 1,* | 99.99 ± 0.019 * | 100 ± 0 * | 99.95 ± 0.051 * | 99.27 ± 0.420 *$ | 99.91 ± 0.137 *† |
| AM85 | 99.95 ± 0.068 * | 99.99 ± 0.008 * | 100 ± 0 * | 100 ± 0 * | 99.86 ± 0.087 * | 99.99 ± 0.017 *† |
| ECLOAM1 | 99.73 ± 0.342 * | 99.93 ± 0.059 * | 100 ± 0 * | 99.44 ± 0.913 * | 99.63 ± 0.072 *$ | 99.99 ± 0.001 *† |
| KPNAM2 | 98.03 ± 2.343 * | 99.54 ± 0.200 * | 96.75 ± 5.622 * | 99.16 ± 0.582 * | 99.20 ± 0.255 *$ | 99.77 ± 0.398 *† |
| EFUMAM2 | 76.15 ± 27.228 ** | 99.03 ± 0.587 * | 96.45 ± 3.133 * | 99.94 ± 0.050 * | 97.96 ± 0.046 * | 99.38 ± 0.936 * |
| SAAM33 | 99.97 ± 0.053 *,£ | 100 ± 0 * | 99.89 ± 0.175 * | 99.85 ± 0.129 *,£ | 100 ± 0 * | 99.81 ± 0.090 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauvergne, E.; Lacquemant, C.; Mullié, C. Antibacterial Activity of Brass against Antibiotic-Resistant Bacteria following Repeated Exposure to Hydrogen Peroxide/Peracetic Acid and Quaternary Ammonium Compounds. Microorganisms 2024, 12, 1393. https://doi.org/10.3390/microorganisms12071393
Dauvergne E, Lacquemant C, Mullié C. Antibacterial Activity of Brass against Antibiotic-Resistant Bacteria following Repeated Exposure to Hydrogen Peroxide/Peracetic Acid and Quaternary Ammonium Compounds. Microorganisms. 2024; 12(7):1393. https://doi.org/10.3390/microorganisms12071393
Chicago/Turabian StyleDauvergne, Emilie, Corinne Lacquemant, and Catherine Mullié. 2024. "Antibacterial Activity of Brass against Antibiotic-Resistant Bacteria following Repeated Exposure to Hydrogen Peroxide/Peracetic Acid and Quaternary Ammonium Compounds" Microorganisms 12, no. 7: 1393. https://doi.org/10.3390/microorganisms12071393
APA StyleDauvergne, E., Lacquemant, C., & Mullié, C. (2024). Antibacterial Activity of Brass against Antibiotic-Resistant Bacteria following Repeated Exposure to Hydrogen Peroxide/Peracetic Acid and Quaternary Ammonium Compounds. Microorganisms, 12(7), 1393. https://doi.org/10.3390/microorganisms12071393





