Comparative Analysis of Phlebotomus argentipes Vector of Leishmaniasis in India and Sri Lanka
Abstract
1. Introduction
2. Materials and Methods
2.1. Sandfly Collection
2.2. Morphological Identification of Sandflies
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Sequence Alignment and Phylogenetic Analysis
2.5. Salivary Gland Dissection
2.6. Salivary Gland Homogenate (SGH) Preparation
2.7. Electrophoretic Separation of Salivary Proteins
2.8. Immunogenic Proteins
2.9. Identification of Highly Expressed Immunogenic Salivary Proteins
3. Results
3.1. Sandflies of Field Collections
3.2. Morphology of Indian and Sri Lankan Ph. argentipes
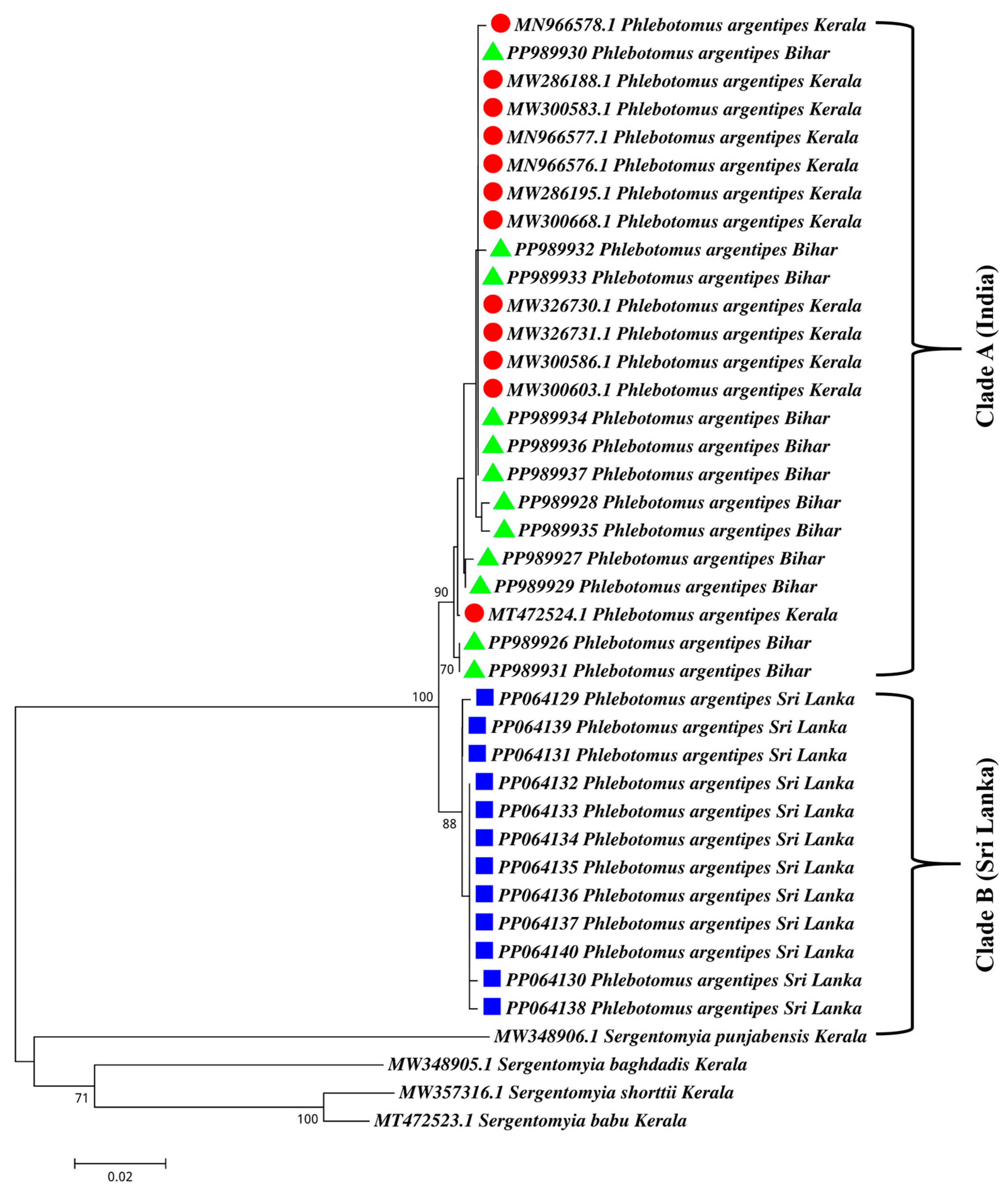
3.3. Phylogenetic Analysis of Indian and Sri Lankan Ph. argentipes
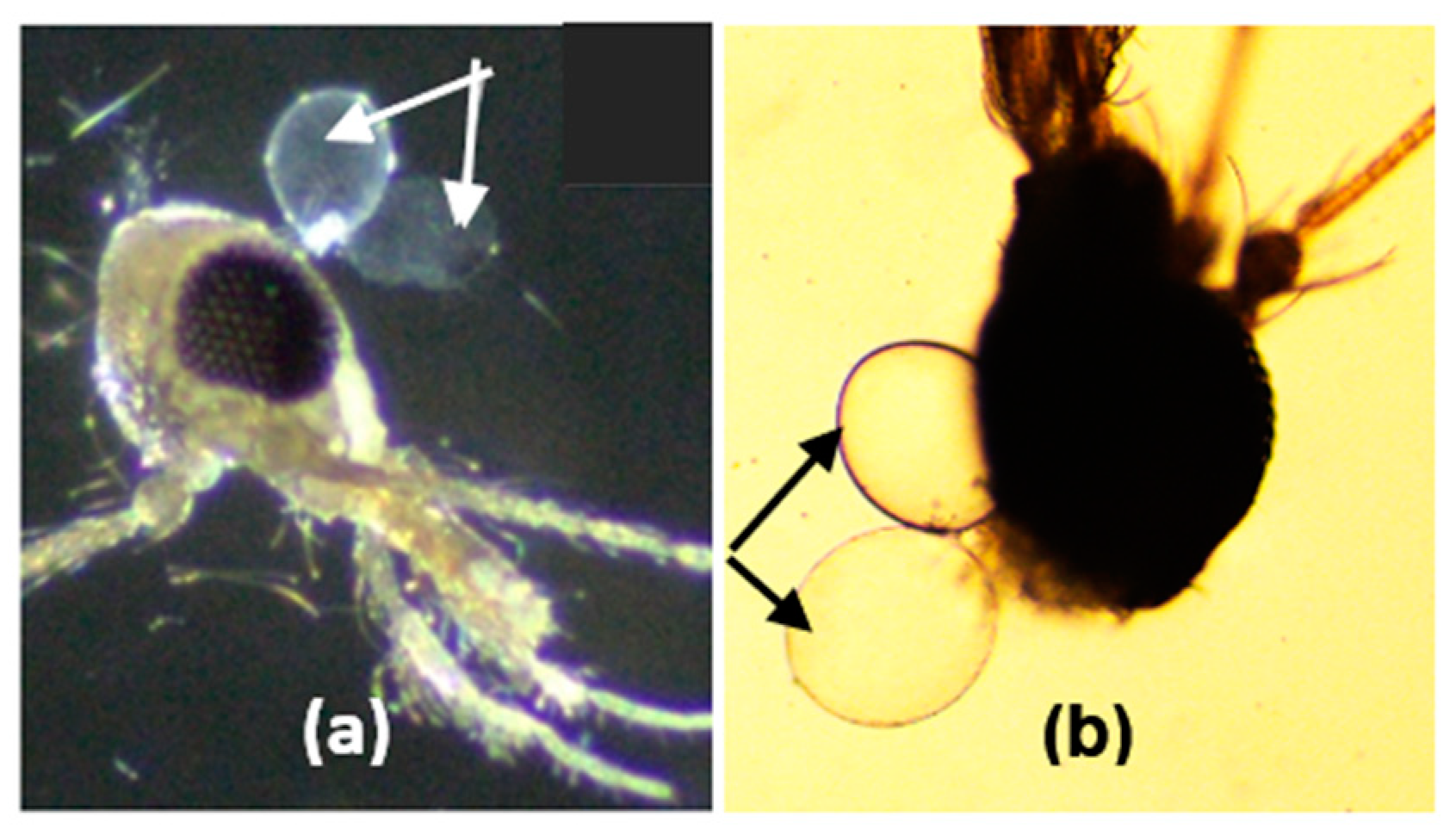
3.4. Morphology of Salivary Glands
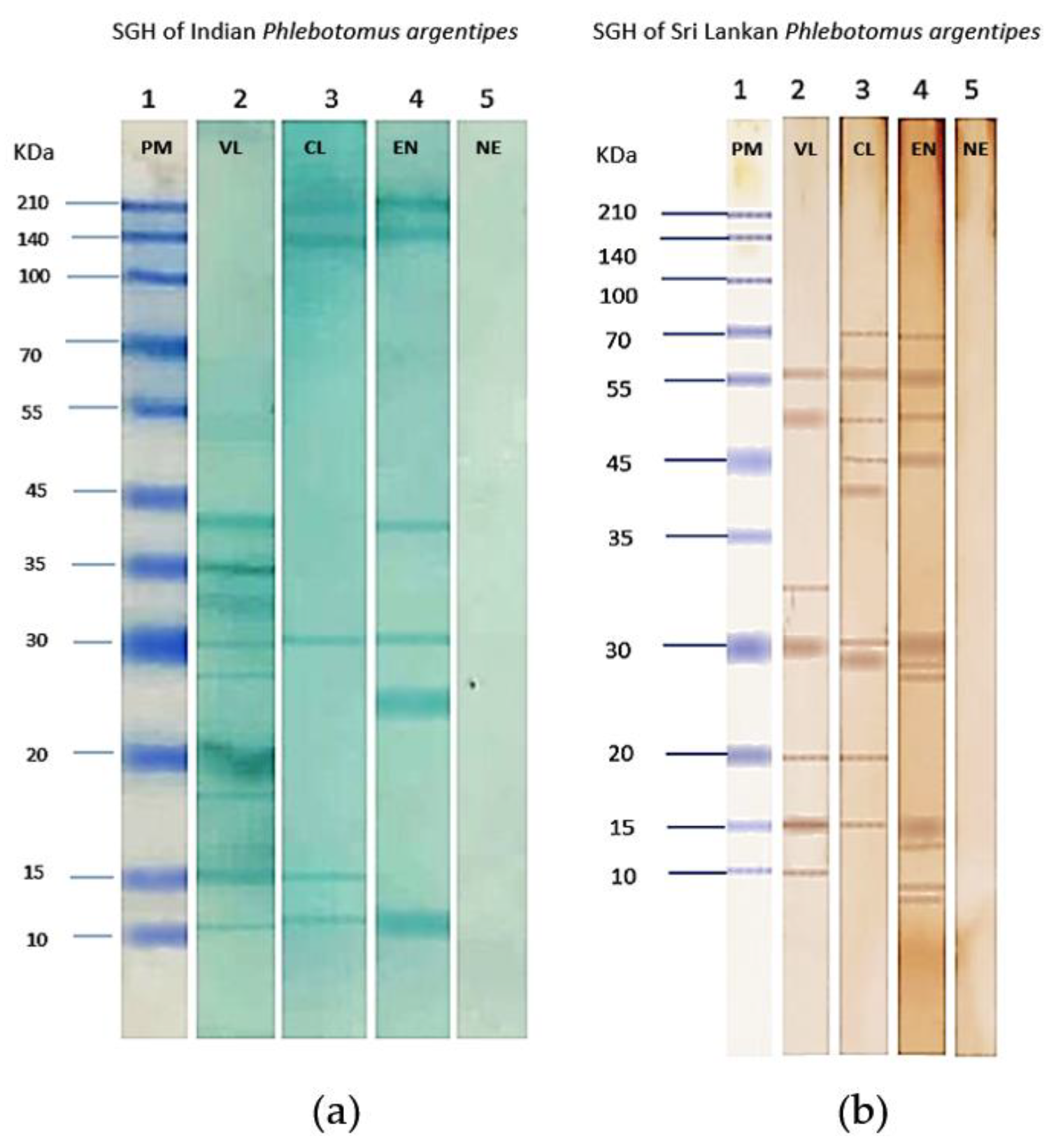
3.5. Salivary Gland Protein Profiles
3.6. Immunogenic Salivary Proteins
3.7. Proteomics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Health Topics. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 9 April 2024).
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.A. Transmission of Leishmania metacyclic promastigotes by phlebotomine sandflies. Int. J. Parasitol. 2007, 37, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Dostálová, A.; Volf, P. Leishmania development in sandflies: Parasite-vector interactions overview. Parasites Vectors 2012, 1, 276. [Google Scholar] [CrossRef] [PubMed]
- Anoopa, S.D.; Bern, C.; Varghese, B.; Chowdhury, R.; Haque, R.; Ali, M.; Amann, J.; Ahluwalia, I.B.; Wagatsuma, Y.; Breiman, R.; et al. The economic impact of visceral leishmaniasis on households in Bangladesh. Trop. Med. Int. Health 2006, 5, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Rijal, S.; Koirala, S.; Van der Stuyft, P.; Boelaert, M. The economic burden of visceral leishmaniasis for households in Nepal. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.C.; Yadav, R.S. Insecticide resistance in phlebotomine sandflies in Southeast Asia with emphasis on the Indian subcontinent. Infect. Dis. Poverty 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kumar, V.; Mondal, D.; Das, M.L.; Das, P.; Dash, A.P.; Kroeger, A. Implication of vector characteristics of Phlebotomus argentipes in the kala-azar elimination programme in the Indian sub-continent. Pathog. Glob. Health 2016, 110, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Thakur, L.; Singh, K.K.; Shanker, V.; Negi, A.; Jain, A.; Matlashewski, G.; Jain, M. Atypical leishmaniasis: A global perspective with emphasis on the Indian subcontinent. PLoS Negl. Trop. Dis. 2018, 12, e0006659. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Srinivasan, R.; Anish, T.S.; Nandakumar, G.; Jambulingam, P. Cutaneous leishmaniasis caused by Leishmania donovani in the tribal population of the Agasthyamala Biosphere Reserve forest, Western Ghats, Kerala, India. J. Med. Microbiol. 2015, 64, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Thakur, L.; Singh, K.K.; Kushwaha, H.R.; Sharma, S.K.; Shankar, V.; Negi, A.; Verma, G.; Kumari, S.; Jain, A.; Jain, M. Leishmania donovani infection with atypical cutaneous manifestations, Himachal Pradesh, India, 2014–2018. Emerg. Infect. Dis. 2020, 8, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Kumari, S.; Das, R.; Pasi, S.; Dhiman, R.C. Typical and atypical cutaneous leishmaniasis in Himachal Pradesh (India). Heliyon 2021, 7, e07282. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Kumar, N.P.; Jambulingam, P. Detection of natural infection of Leishmania donovani (Kinetoplastida: Trypanosomatidae) in Phlebotomus argentipes (Diptera: Psychodidae) from a forest ecosystem in the Western Ghats, India, endemic for cutaneous leishmaniasis. Acta Trop. 2016, 156, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.D.; Pratlong, F.; Siriwardane, H.V.; Ihalamulla, R.L.; Dedet, J.P. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, H.V.; Chandrawansa, P.H.; Sirimanna, G.; Karunaweera, N.D. Leishmaniasis in Sri Lanka: A decade old story. Sri Lankan J. Infect. Dis. 2012, 2, 2–12. [Google Scholar] [CrossRef]
- Athukorale, D.N.; Seneviratne, J.K.; Ihalamulla, R.L.; Premaratne, U.N. Locally acquired cutaneous leishmaniasis in Sri Lanka. J. Trop. Med. Hyg. 1992, 95, 432–433. [Google Scholar] [PubMed]
- Gajapathy, K.; Jude, P.J.; Surendran, S.N. Morphometric and meristic characterization of Phlebotomus argentipes species complex in northern Sri Lanka: Evidence for the presence of potential leishmaniasis vectors in the country. Trop. Biomed. 2011, 28, 259–268. [Google Scholar] [PubMed]
- Ilango, K. A taxonomic reassessment of the Phlebotomus argentipes species complex (Diptera: Psychodidae: Phlebotominae). J. Med. Entomol. 2010, 47, 1–5. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sandflies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Volf, P.; Tesařová, P.; Nohýnková, E. Salivary proteins and glycoproteins in phlebotomine sandflies of various species, sex and age. Med. Vet. Entomol. 2000, 3, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Volf, P.; Rohoušová, I. Species-specific antigens in salivary glands of phlebotomine sandflies. Parasitology 2001, 122, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Collin, N.; Assumpção, T.C.; Mizurini, D.M.; Gilmore, D.C.; Dutra-Oliveira, A.; Kotsyfakis, M.; Sá-Nunes, A.; Teixeira, C.; Ribeiro, J.M.; Monteiro, R.Q.; et al. Lufaxin, a novel factor Xa inhibitor from the salivary gland of the sandfly Lutzomyia longipalpis blocks protease-activated receptor 2 activation and inhibits inflammation and thrombosis in vivo. Arter. Thromb. Vasc. Biol. 2012, 32, 2185–2198. [Google Scholar] [CrossRef] [PubMed]
- Thiakaki, M.; Rohousova, I.; Volfova, V.; Volf, P.; Chang, K.P.; Soteriadou, K. Sandfly specificity of saliva-mediated protective immunity in Leishmania amazonensis-BALB/c mouse model. Microbes Infect. 2005, 7, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Lestinova, T.; Rohousova, I.; Sima, M.; de Oliveira, C.I.; Volf, P. Insights into the sandfly saliva: Blood-feeding and immune interactions between sandflies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017, 11, e0005600. [Google Scholar] [CrossRef] [PubMed]
- Rohoušová, I.; Volfová, V.; Nová, S.; Volf, P. Individual variability of salivary gland proteins in three Phlebotomus species. Acta Trop. 2012, 122, 80–86. [Google Scholar] [CrossRef]
- Ramalho-Ortigão, M.; Coutinho-Abreu, I.V.; Balbino, V.Q.; Figueiredo, C.A.; Mukbel, R.; Dayem, H.; Hanafi, H.A.; El-Hossary, S.S.; Fawaz, E.E.; Abo-Shehada, M.; et al. Phlebotomus papatasi SP15: mRNA expression variability and amino acid sequence polymorphisms of field populations. Parasites Vectors 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Karunaweera, N.D.; Senanayake, S.; Ginige, S.; Silva, H.; Manamperi, N.; Samaranayake, N.; Dewasurendra, R.; Karunanayake, P.; Gamage, D.; de Silva, N.; et al. Spatiotemporal distribution of cutaneous leishmaniasis in Sri Lanka and future case burden estimates. PLoS Negl. Trop. Dis. 2021, 15, e0009346. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J. The phlebotomine sandflies (Diptera: Psychodidae) of the Oriental region. Bull. Br. Mus. (Nat. Hist.) Entomol. 1978, 37, 217–343. [Google Scholar] [CrossRef]
- Kalra, N.L.; Bang, Y.H. Manual on Entomology in Visceral Leishmaniasis; World Health Organization: New Delhi, India, 1988; p. 88. [Google Scholar]
- Kumar, N.P.; Srinivasan, R.; Jambulingam, P. DNA barcoding for identification of sandflies (Diptera: Psychodidae) in India. Mol. Ecol. Resour. 2012, 3, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.N.; Kajatheepan, A.; Hawkes, N.J.; Ramasamy, R. First report on the presence of morphospecies A and B of Phlebotomus argentipes sensu lato (Diptera: Psychodidae) in Sri Lanka–implications for leishmaniasis transmission. J. Vector Borne Dis. 2005, 42, 155–158. Available online: http://repo.lib.jfn.ac.lk/ujrr/handle/123456789/9110 (accessed on 15 July 2024). [PubMed]
- Gajapathy, K.; Peiris, L.B.; Goodacre, S.L.; Silva, A.; Jude, P.J.; Surendran, S.N. Molecular identification of potential leishmaniasis vector species within the Phlebotomus (Euphlebotomus) argentipes species complex in Sri Lanka. Parasites Vectors 2013, 6, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palaniyandi, M. Spatial and temporal analysis of vector borne disease epidemics for mapping the hotspot region, risk assessment, and control for sustainable health. Indian J. Public Health Res. Dev. 2021, 12, 151–161. [Google Scholar] [CrossRef]
- Pathirage, D.R.; Weeraratne, T.C.; Senanayake, S.C.; Karunaratne, S.P.; Karunaweera, N.D. Genetic diversity and population structure of Phlebotomus argentipes: Vector of Leishmania donovani in Sri Lanka. PLoS ONE 2021, 16, e0256819. [Google Scholar] [CrossRef]
- Sarkar, A.; Panati, K.; Narala, V.R. Code inside the codon: The role of synonymous mutations in regulating splicing machinery and its impact on disease. Rev. Mutat. Res. 2022, 790, 108444. [Google Scholar] [CrossRef] [PubMed]
- Warburg, A.; Saraiva, E.; Lanzaro, G.C.; Titus, R.G.; Neva, F. Saliva of Lutzomyia longipalpis sibling species differs in its composition and capacity to enhance leishmaniasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 223–230. [Google Scholar] [CrossRef]
- Lanzaro, G.C.; Lopes, A.H.; Ribeiro, J.M.; Shoemaker, C.B.; Warburg, A.; Soares, M. Variation in the salivary peptide, maxadilan, from species in the Lutzomyia longipalpis complex. Insect. Mol. Biol. 1999, 8, 267–275, PMID: 10380110. Available online: https://www.arca.fiocruz.br/handle/icict/9909 (accessed on 15 July 2024). [PubMed]
- Hosseini-Vasoukolaei, N.; Idali, F.; Khamesipour, A.; Yaghoobi-Ershadi, M.R.; Kamhawi, S.; Valenzuela, J.G.; Edalatkhah, H.; Arandian, M.H.; Mirhendi, H.; Emami, S.; et al. Differential expression profiles of the salivary proteins SP15 and SP44 from Phlebotomus papatasi. Parasites Vectors 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.F.; Gidwani, K.; Kumar, R.; Hostomska, J.; Dinesh, D.S.; Kumar, V.; Das, P.; Müller, I.; Hamilton, G.; Volfova, V.; et al. Measurement of recent exposure to Phlebotomus argentipes, the vector of Indian visceral leishmaniasis, by using human antibody responses to sandfly saliva. Am. J. Trop. Med. Hyg. 2010, 82, 801. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Scorza, B.M.; Petersen, C. Visceral Leishmaniasis and the Skin: Dermal Parasite Transmission to Sandflies. Pathogens 2022, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Oliveira, F.; Kamhawi, S.; Mans, B.J.; Reynoso, D.; Seitz, A.E.; Lawyer, P.; Garfield, M.; Pham, M.; Valenzuela, J.G. Comparative salivary gland transcriptomics of sandfly vectors of visceral leishmaniasis. BMC Genom. 2006, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Iniguez, E.; Saha, S.; Petrellis, G.; Menenses, C.; Herbert, S.; Gonzalez-Rangel, Y.; Rowland, T.; Aronson, N.E.; Rose, C.; Rafuse, H.L.; et al. Composite Recombinant Salivary Proteins Biomarker for Phlebotomus argentipes Provides a Surveillance Tool Postelimination of Visceral Leishmaniasis in India. J. Infect. Dis. 2022, 226, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Sumova, P.; Sanjoba, C.; Willen, L.; Polanska, N.; Matsumoto, Y.; Noiri, E.; Paul, S.K.; Ozbel, Y.; Volf, P. PpSP32-like protein as a marker of human exposure to Phlebotomus argentipes in Leishmania donovani foci in Bangladesh. Int. J. Parasitol. 2021, 51, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Marzouki, S.; Abdeladhim, M.; Abdessalem, C.B.; Oliveira, F.; Ferjani, B.; Gilmore, D.; Louzir, H.; Valenzuela, J.G.; Ahmed, M.B. Salivary antigen SP32 is the immunodominant target of the antibody response to Phlebotomus papatasi bites in humans. PLoS Negl. Trop. Dis. 2012, 6, e1911. [Google Scholar] [CrossRef] [PubMed]
- Marzouki, S.; Kammoun-Rebai, W.; Bettaieb, J.; Abdeladhim, M.; Hadj Kacem, S.; Abdelkader, R.; Gritli, S.; Chemkhi, J.; Aslan, H.; Kamhawi, S.; et al. Validation of recombinant salivary protein PpSP32 as a suitable marker of human exposure to Phlebotomus papatasi, the vector of Leishmania major in Tunisia. PLoS Negl. Trop. Dis. 2015, 9, e0003991. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.; Thakur, A.K.; Jamal, F.; Singh, M.K.; Kumar, A.; Singh, S.K.; Bimal, S.; Das, P.; Narayan, S. Exploring new immunological insight on SP15 (~14 kDa) family protein in saliva of Indian sand-fly (Phlebotomus argentipes) in experimental visceral leishmaniasis. Cell. Immunol. 2018, 332, 51–57. [Google Scholar]




| Features | Protein Identified/Sequence Name | ||
|---|---|---|---|
| SP17 | SP06 | SP05 | |
| Molecular weight of the protein/KDa | 31.702 | 27.000 | 32.357 |
| Total number of peptides matched to each protein | 6 | 6 | 16 |
| Number of unique peptides matched to each protein | 6 | 6 | 16 |
| Confidence score | 34.662 | 30.948 | 90.228 |
| Peptide sequences | NVVNDIVEVTFGDEEPLN TGVFIVK GFNNSNYIK VNYNLYREELEQK YDYNTNK DILQLQGFIR | LFETNKAEEVIAK DFPVPTADEK ELVIDESTAR DAPATYVFK VRQNSQDR | LRDR LAEYNVR WNDELAK EWFLEYK GSMSPFQSAAK DAQLMPITEDTKK LCTFGPGLPARPHIGCK AIGHFTAFIHEK |
| Best match to protein database | 29 kDa salivary protein PagSP17 of Phlebotomus argentipes | 25 kDa salivary protein PagSP06 of Phlebotomus argentipes | 29 kDa salivary antigen 5-related protein PagSP05 of Phlebotomus argentipes |
| Best match UniProtKB accession # | Q0ZST4 | Q0ZSU3 | Q0ZSU4 |
| Best match Gen Bank accession # | ABA12147 | ABA12138 | ABA12137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piyasiri, S.B.; Fathima, P.A.; Shah, H.K.; Senanyake, S.; Samaranayake, N.; Karunaweera, N.D.; Rahi, M.; Saini, P. Comparative Analysis of Phlebotomus argentipes Vector of Leishmaniasis in India and Sri Lanka. Microorganisms 2024, 12, 1459. https://doi.org/10.3390/microorganisms12071459
Piyasiri SB, Fathima PA, Shah HK, Senanyake S, Samaranayake N, Karunaweera ND, Rahi M, Saini P. Comparative Analysis of Phlebotomus argentipes Vector of Leishmaniasis in India and Sri Lanka. Microorganisms. 2024; 12(7):1459. https://doi.org/10.3390/microorganisms12071459
Chicago/Turabian StylePiyasiri, Sachee Bhanu, P.A. Fathima, Harish Kumar Shah, Sanath Senanyake, Nilakshi Samaranayake, Nadira Darshani Karunaweera, Manju Rahi, and Prasanta Saini. 2024. "Comparative Analysis of Phlebotomus argentipes Vector of Leishmaniasis in India and Sri Lanka" Microorganisms 12, no. 7: 1459. https://doi.org/10.3390/microorganisms12071459
APA StylePiyasiri, S. B., Fathima, P. A., Shah, H. K., Senanyake, S., Samaranayake, N., Karunaweera, N. D., Rahi, M., & Saini, P. (2024). Comparative Analysis of Phlebotomus argentipes Vector of Leishmaniasis in India and Sri Lanka. Microorganisms, 12(7), 1459. https://doi.org/10.3390/microorganisms12071459








