Microbial Electrolysis Cells Based on a Bacterial Anode Encapsulated with a Dialysis Bag Including Graphite Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
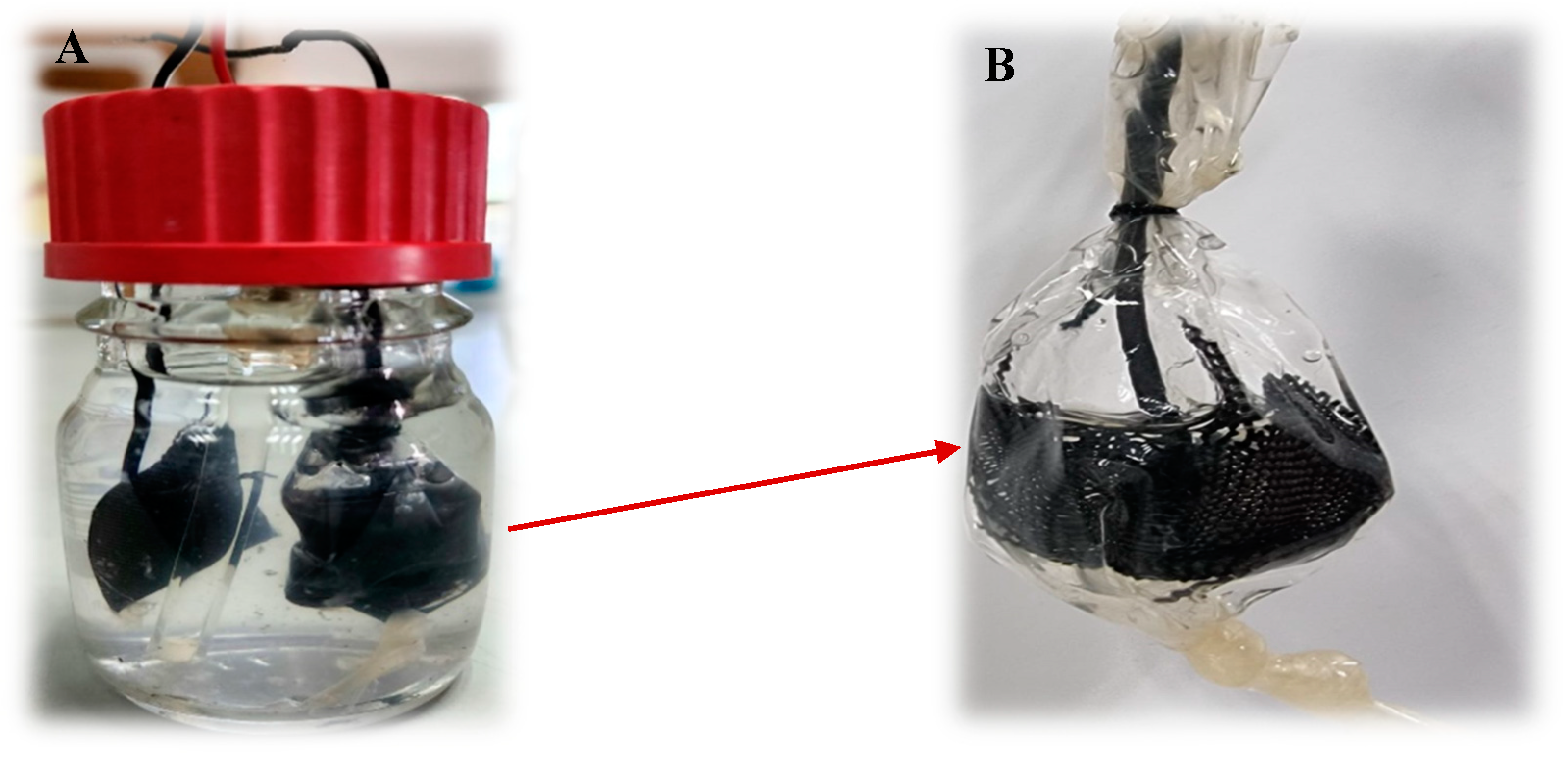
2.2. Dialysis Bag Preparation
2.3. Preparation of Artificial Wastewater
2.4. MEC System Setup
2.5. Inoculation of G. sulfurreducens into the MEC
2.6. Electrochemical Measurements
2.7. Chemical Oxygen Demand Assay
2.8. Measurement of the Bacterial Viability of the Anode Biofilm and the Suspended Bacteria
2.9. Relative Bacterial Distribution Analysis of the Bioanode Community
3. Results and Discussion
3.1. Voltammetric Measurements
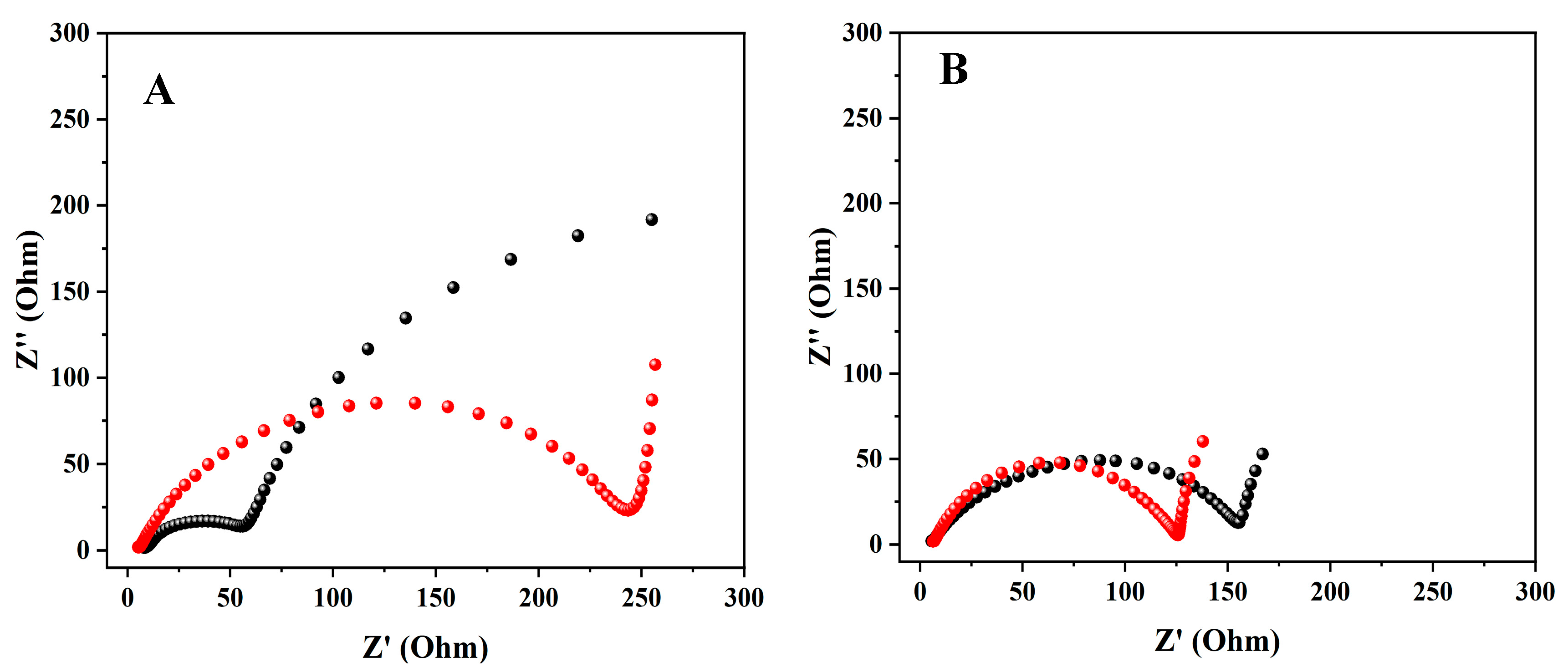
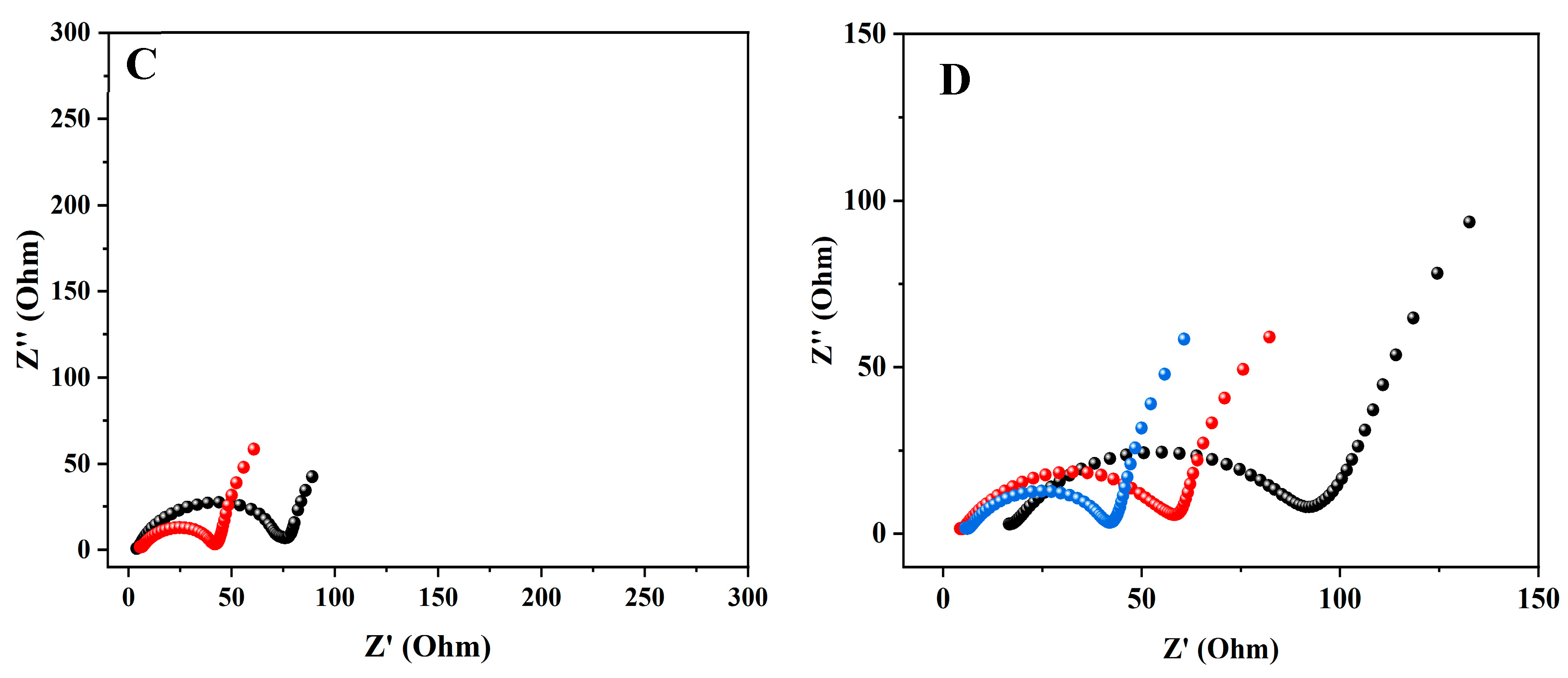
3.2. Electrochemical Impedance Spectroscopy (EIS)
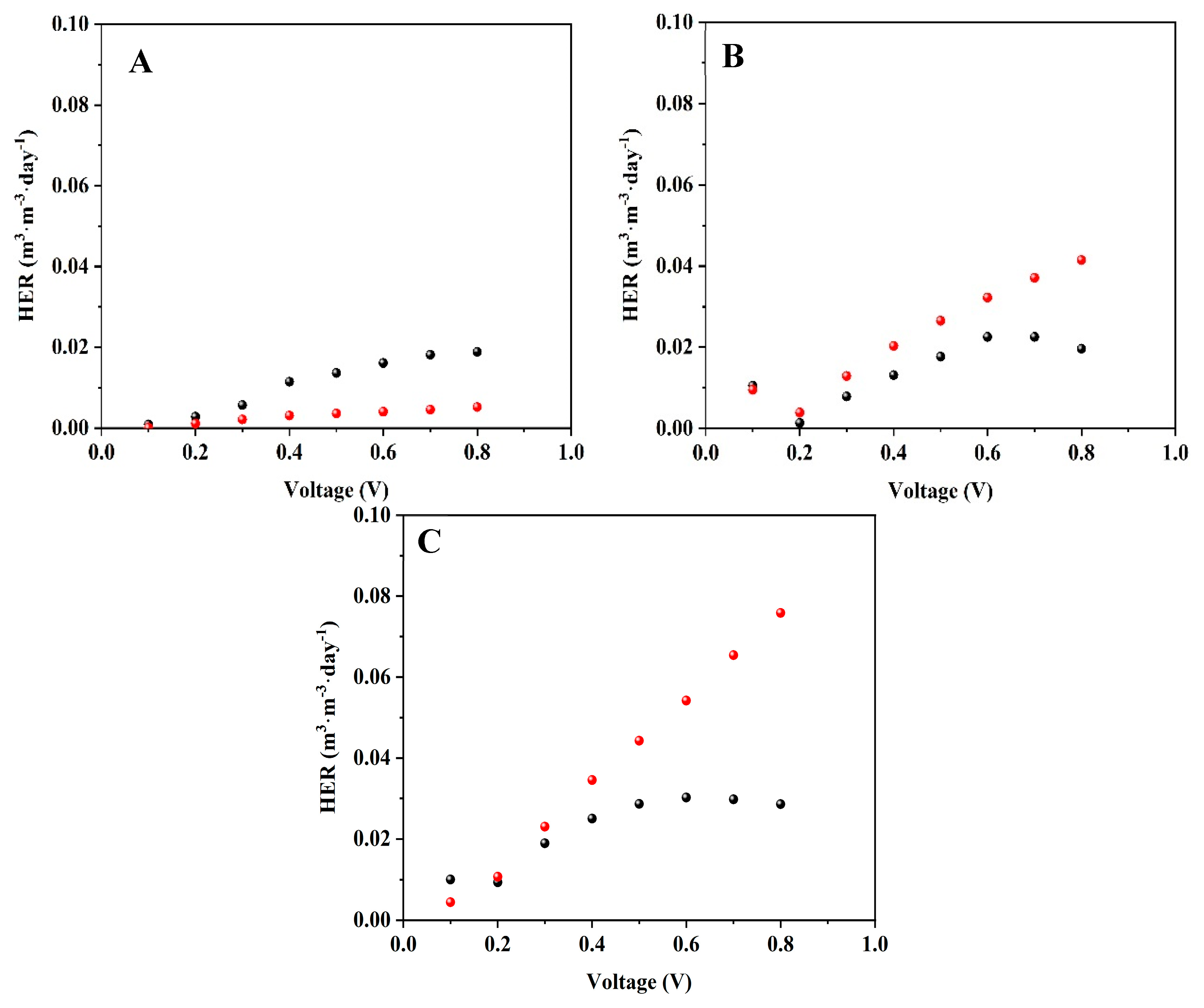
3.3. Hydrogen Production Rate in the MECs
3.4. The Contribution of the Planktonic Bacteria to the Overall Evolved Currents of the MECs
3.5. COD Removal and Coulombic Hydrogen Recovery in the MECs
3.6. Bacterial Biofilm Anode and Planktonic Bacteria Viability
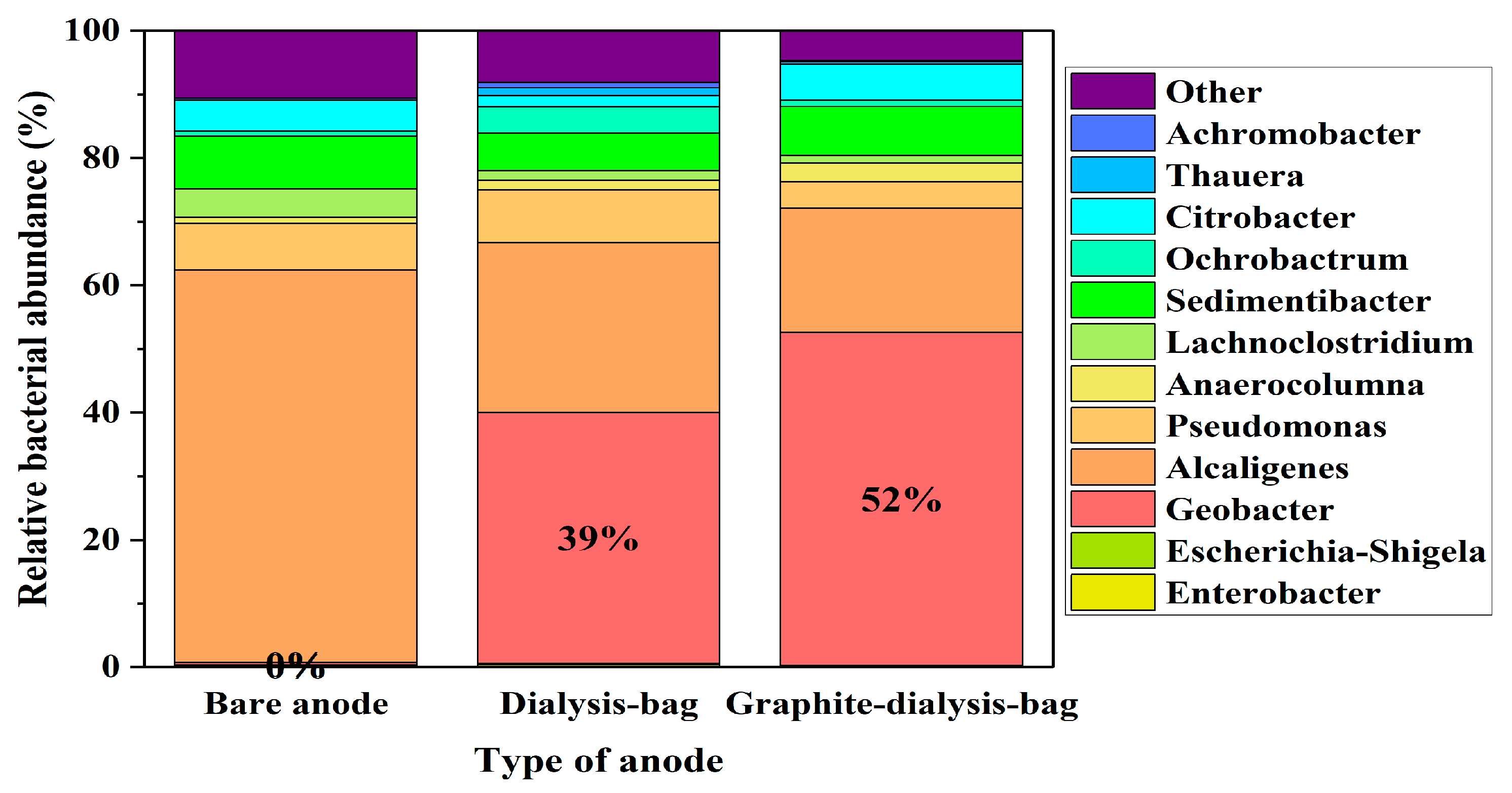
3.7. Microbial Diversity of the Biofilm on the Carbon Cloth Anodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gaur, N.; Sharma, S.; Yadav, N. Environmental Pollution. In Green Chemistry Approaches to Environmental Sustainability: Status, Challenges and Prospective; Elsevier: Amsterdam, The Netherlands, 2024; pp. 23–41. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, Y.; Xu, J. A Clean Optimization Approach for Sustainable Waste-to-Energy Using Integrated Technology. Renew. Energy 2024, 221, 119750. [Google Scholar] [CrossRef]
- Shah, A.V.; Singh, A.; Sabyasachi Mohanty, S.; Kumar Srivastava, V.; Varjani, S. Organic Solid Waste: Biorefinery Approach as a Sustainable Strategy in Circular Bioeconomy. Bioresour. Technol. 2022, 349, 126835. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Prajapati, P.; Shah, A.V.; Varjani, S. Municipal Solid Waste Management: Dynamics, Risk Assessment, Ecological Influence, Advancements, Constraints and Perspectives. Sci. Total Environ. 2022, 814, 152802. [Google Scholar] [CrossRef] [PubMed]
- Baykara, S.Z. Hydrogen: A Brief Overview on Its Sources, Production and Environmental Impact. Int. J. Hydrogen Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Horsfield, B.; Mahlstedt, N.; Weniger, P.; Misch, D.; Vranjes-Wessely, S.; Han, S.; Wang, C. Molecular Hydrogen from Organic Sources in the Deep Songliao Basin, P.R. China. Int. J. Hydrogen Energy 2022, 47, 16750–16774. [Google Scholar] [CrossRef]
- Asif, M.; Sidra Bibi, S.; Ahmed, S.; Irshad, M.; Shakir Hussain, M.; Zeb, H.; Kashif Khan, M.; Kim, J. Recent Advances in Green Hydrogen Production, Storage and Commercial-Scale Use via Catalytic Ammonia Cracking. Chem. Eng. J. 2023, 473, 145381. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Huang, J.M.; Wang, H.; Wen, Y.J.; Zhang, Q.A.; Sun, D.L.; Zhu, M. Excellent Hydrolysis Performances of Mg3RE Hydrides. Int. J. Hydrog. Energy 2013, 38, 2973–2978. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470258590. [Google Scholar]
- Li, M.; Zhang, Q.; Liu, Y.; Zhu, J.; Sun, F.; Cui, M.H.; Liu, H.; Zhang, T.C.; Chen, C. Enhancing Degradation of Organic Matter in Microbial Electrolytic Cells Coupled with Anaerobic Digestion (MEC-AD) Systems by Carbon-Based Materials. Sci. Total Environ. 2023, 900, 165805. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Nayak, J.K.; Ress, N.V.; Steinberger-Wilckens, R.; Ghosh, U.K. Bio-Hydrogen Production through Microbial Electrolysis Cell: Structural Components and Influencing Factors. Chem. Eng. J. 2023, 455, 140535. [Google Scholar] [CrossRef]
- Nosek, D.; Mikołajczyk, T.; Cydzik-Kwiatkowska, A. Anode Modification with Fe2O3 Affects the Anode Microbiome and Improves Energy Generation in Microbial Fuel Cells Powered by Wastewater. Int. J. Environ. Res. Public. Health 2023, 20, 2580. [Google Scholar] [CrossRef]
- Park, S.G.; Rajesh, P.P.; Sim, Y.U.; Jadhav, D.A.; Noori, M.T.; Kim, D.H.; Al-Qaradawi, S.Y.; Yang, E.; Jang, J.K.; Chae, K.J. Addressing Scale-up Challenges and Enhancement in Performance of Hydrogen-Producing Microbial Electrolysis Cell through Electrode Modifications. Energy Rep. 2022, 8, 2726–2746. [Google Scholar] [CrossRef]
- Posadas-Hernández, M.; García-Rojas, J.L.; Khamkure, S.; García-Sánchez, L.; Gutierrez-Macías, T.; Morales-Morales, C.; Estrada-Arriaga, E.B. Enhanced Biohydrogen Production in a Membraneless Single-Chamber Microbial Electrolysis Cell during High-Strength Wastewater Treatment: Effect of Electrode Materials and Configurations. Int. J. Hydrogen Energy 2023, 48, 495–513. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Sangeetha, T.; He, Z.; Guo, Z.; Gao, L.; Wang, A.; Liu, W. Methane Production in a Bioelectrochemistry Integrated Anaerobic Reactor with Layered Nickel Foam Electrodes. Bioresour. Technol. 2020, 313, 123657. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.Z.; Wu, X.W.; Wang, Y.F.; Feng, J.; Hou, S.Y.; Huang, Z.H.; Shen, K.; Chen, Y.X.; Liu, H.B.; Kang, F.Y. Recent Progress in the Research and Development of Natural Graphite for Use in Thermal Management, Battery Electrodes and the Nuclear Industry. New Carbon Mater. 2023, 38, 73–91. [Google Scholar] [CrossRef]
- Frattini, D.; Accardo, G.; Duarte, K.D.Z.; Kim, D.H.; Kwon, Y. Improved Biofilm Adhesion and Electrochemical Properties of a Graphite-Cement Composite with Silica Nanoflowers versus Two Benchmark Carbon Felts. Appl. Energy 2020, 261, 114391. [Google Scholar] [CrossRef]
- Hirsch, L.O.; Dubrovin, I.A.; Gandu, B.; Emanuel, E.; Kjellerup, B.V.; Ugur, G.E.; Schechter, A.; Cahan, R. Anode Amendment with Kaolin and Activated Carbon Increases Electricity Generation in a Microbial Fuel Cell. Bioelectrochemistry 2023, 153, 108486. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Sustainable and Efficient Biohydrogen Production via Electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.C.; Liao, Z.H.; Sun, J.Z.; Zheng, T.; Jiang, R.R.; Song, H. Enhancement of Coulombic Efficiency and Salt Tolerance in Microbial Fuel Cells by Graphite/Alginate Granules Immobilization of Shewanella oneidensis MR-1. Process Biochem. 2013, 48, 1947–1951. [Google Scholar] [CrossRef]
- Dubrovin, I.A.; Hirsch, L.O.; Rozenfeld, S.; Gandu, B.; Menashe, O.; Schechter, A.; Cahan, R. Hydrogen Production in Microbial Electrolysis Cells Based on Bacterial Anodes Encapsulated in a Small Bioreactor Platform. Microorganisms 2022, 10, 1007. [Google Scholar] [CrossRef]
- Rozenfeld, S.; Gandu, B.; Hirsch, L.O.; Dubrovin, I.; Schechter, A.; Cahan, R. Hydrogen Production in a Semi-Single-Chamber Microbial Electrolysis Cell Based on Anode Encapsulated in a Dialysis Bag. Int. J. Energy Res. 2021, 45, 19074–19088. [Google Scholar] [CrossRef]
- Gandu, B.; Rozenfeld, S.; Ouaknin Hirsch, L.; Schechter, A.; Cahan, R. Immobilization of Bacterial Cells on Carbon-Cloth Anode Using Alginate for Hydrogen Generation in a Microbial Electrolysis Cell. J. Power Sources 2020, 455, 227986. [Google Scholar] [CrossRef]
- Yasri, N.G.; Nakhla, G. Electrochemical Behavior of Anode-Respiring Bacteria on Doped Carbon Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 35150–35162. [Google Scholar] [CrossRef] [PubMed]
- Rani, G.; Krishna, K.; Yogalakshmi, K.N. Enhancing the Electrochemical Performance of Fe3O4 Nanoparticles Layered Carbon Electrodes in Microbial Electrolysis Cell. J. Environ. Chem. Eng. 2021, 9, 106326. [Google Scholar] [CrossRef]
- Zikmund, E.; Kim, K.Y.; Logan, B.E. Hydrogen Production Rates with Closely-Spaced Felt Anodes and Cathodes Compared to Brush Anodes in Two-Chamber Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2018, 43, 9599–9606. [Google Scholar] [CrossRef]
- Hirsch, L.O.; Gandu, B.; Chiliveru, A.; Dubrovin, I.A.; Rozenfeld, S.; Schechter, A.; Cahan, R. The Performance of a Modified Anode Using a Combination of Kaolin and Graphite Nanoparticles in Microbial Fuel Cells. Microorganisms 2024, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yu, S.; Liu, G.; Zhang, R.; Teng, W. Effect of In-Situ Immobilized Anode on Performance of the Microbial Fuel Cell with High Concentration of Sodium Acetate. Fuel 2016, 182, 732–739. [Google Scholar] [CrossRef]
- Yu, L.; Yuan, Y.; Rensing, C.; Zhou, S. Combined Spectroelectrochemical and Proteomic Characterizations of Bidirectional Alcaligenes faecalis-Electrode Electron Transfer. Biosens. Bioelectron. 2018, 106, 21–28. [Google Scholar] [CrossRef]
- Wang, X.; Yu, P.; Zeng, C.; Ding, H.; Li, Y.; Wang, C.; Lu, A. Enhanced Alcaligenes faecalis Denitrification Rate with Electrodes as the Electron Donor. Appl. Environ. Microbiol. 2015, 81, 5387–5394. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, T.; Zhu, H.; Cai, L.; Lu, Y.; Wang, J.; Tao, H.; Li, P. Bioelectricity Facilitates Carbon Dioxide Fixation by Alcaligenes faecalis ZS-1 in a Biocathodic Microbial Fuel Cell (MFC). Bioresour. Technol. 2024, 399, 130555. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel Cells Select for Microbial Consortia That Self-Mediate Electron Transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef]
- Semenec, L.; Franks, A.E. Delving through Electrogenic Biofilms: From Anodes to Cathodes to Microbes. AIMS Bioeng. 2015, 2, 222–248. [Google Scholar] [CrossRef]
- Torres, C.I.; Krajmalnik-Brown, R.; Parameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting Anode-Respiring Bacteria Based on Anode Potential: Phylogenetic, Electrochemical, and Microscopic Characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef] [PubMed]




| Areal Capacitance (F·m−2) | |||
|---|---|---|---|
| Type of Anode | Bare Anode | Dialysis-Bag Anode | Graphite-Dialysis-Bag Anode |
| Geobacter medium | 26.25 | 56.25 | 117.75 |
| Artificial wastewater | 13.75 | 82.50 | 134.13 |
| Current Density at an Applied Voltage of 0.8 V (A·m−2) | |||
|---|---|---|---|
| Type of Anode | Bare Anode | Dialysis-Bag Anode | Graphite-Dialysis-Bag Anode |
| Geobacter medium | 0.53 ± 0.25 | 0.81 ± 0.05 | 1.29 ± 0.76 |
| Artificial wastewater | 0.33 ± 0.28 | 1.73 ± 0.97 | 2.73 ± 0.49 |
| Carbon Source | Electrode | Current Source | Current Density (A·m−2) | |||
|---|---|---|---|---|---|---|
| 0.2 V | 0.4 V | 0.6 V | 0.8 V | |||
| Geobacter medium | Bare anode | Complete MEC | 0.39 ± 0.11 | 0.42 ± 0.29 | 0.48 ± 0.32 | 0.53 ± 0.36 |
| Planktonic bacteria | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.17 ± 0.04 | ||
| Dialysis-bag anode | Complete MEC | 0.42 ± 0.30 | 0.35 ± 0.22 | 0.45 ± 0.33 | 0.94 ± 0.42 | |
| Planktonic bacteria | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.03 | 0.13 ± 0.04 | ||
| Graphite-dialysis-bag anode | Complete MEC | 0.58 ± 0.24 | 0.81 ± 0.05 | 1.05 ± 0.02 | 1.54 ± 0.24 | |
| Planktonic bacteria | 0.16 ± 0.08 | 0.20 ± 0.08 | 0.35 ± 0.10 | 0.62 ± 0.11 | ||
| Artificial wastewater | Bare anode | Complete MEC | 0.17 ± 0.06 | 0.21 ± 0.09 | 0.23 ± 0.09 | 0.33 ± 0.16 |
| Planktonic bacteria | 0.03 ± 0.01 | 0.06 ± 0.02 | 0.10 ± 0.03 | 0.13 ± 0.05 | ||
| Dialysis-bag anode | Complete MEC | 0.96 ± 0.50 | 1.08 ± 0.14 | 1.17 ± 0.24 | 1.28 ± 0.22 | |
| Planktonic bacteria | 0.03 ± 0.01 | 0.07 ± 0.01 | 0.14 ± 0.11 | 0.37 ± 0.19 | ||
| Graphite-dialysis-bag anode | Complete MEC | 1.31 ± 0.24 | 1.59 ± 0.10 | 1.81 ± 0.02 | 2.62 ± 0.29 | |
| Planktonic bacteria | 0.11 ± 0.03 | 0.18 ± 0.05 | 0.25 ± 0.06 | 0.65 ± 0.06 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubrovin, I.A.; Hirsch, L.O.; Chiliveru, A.; Jukanti, A.; Rozenfeld, S.; Schechter, A.; Cahan, R. Microbial Electrolysis Cells Based on a Bacterial Anode Encapsulated with a Dialysis Bag Including Graphite Particles. Microorganisms 2024, 12, 1486. https://doi.org/10.3390/microorganisms12071486
Dubrovin IA, Hirsch LO, Chiliveru A, Jukanti A, Rozenfeld S, Schechter A, Cahan R. Microbial Electrolysis Cells Based on a Bacterial Anode Encapsulated with a Dialysis Bag Including Graphite Particles. Microorganisms. 2024; 12(7):1486. https://doi.org/10.3390/microorganisms12071486
Chicago/Turabian StyleDubrovin, Irina Amar, Lea Ouaknin Hirsch, Abhishiktha Chiliveru, Avinash Jukanti, Shmuel Rozenfeld, Alex Schechter, and Rivka Cahan. 2024. "Microbial Electrolysis Cells Based on a Bacterial Anode Encapsulated with a Dialysis Bag Including Graphite Particles" Microorganisms 12, no. 7: 1486. https://doi.org/10.3390/microorganisms12071486







