Spent Mushroom Substrate Improves Microbial Quantities and Enzymatic Activity in Soils of Different Farming Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Experimental Design
- Soil without fertilizer (C: control);
- Soil with single dose of composted spent mushroom substrate (SMS);
- Soil with single dose of standard fertilization (SF);
- Soil with half dose of SF and half dose of SMS (SF.SMS).
2.2. Soil Sampling
2.3. Processing Soil Samples for Soil Moisture
2.4. Processing Soil Samples for Microbial Population Analysis
2.5. Processing Soil Samples for Enzyme Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Different Fertilization Types and Seasonal Changes in Three Farms
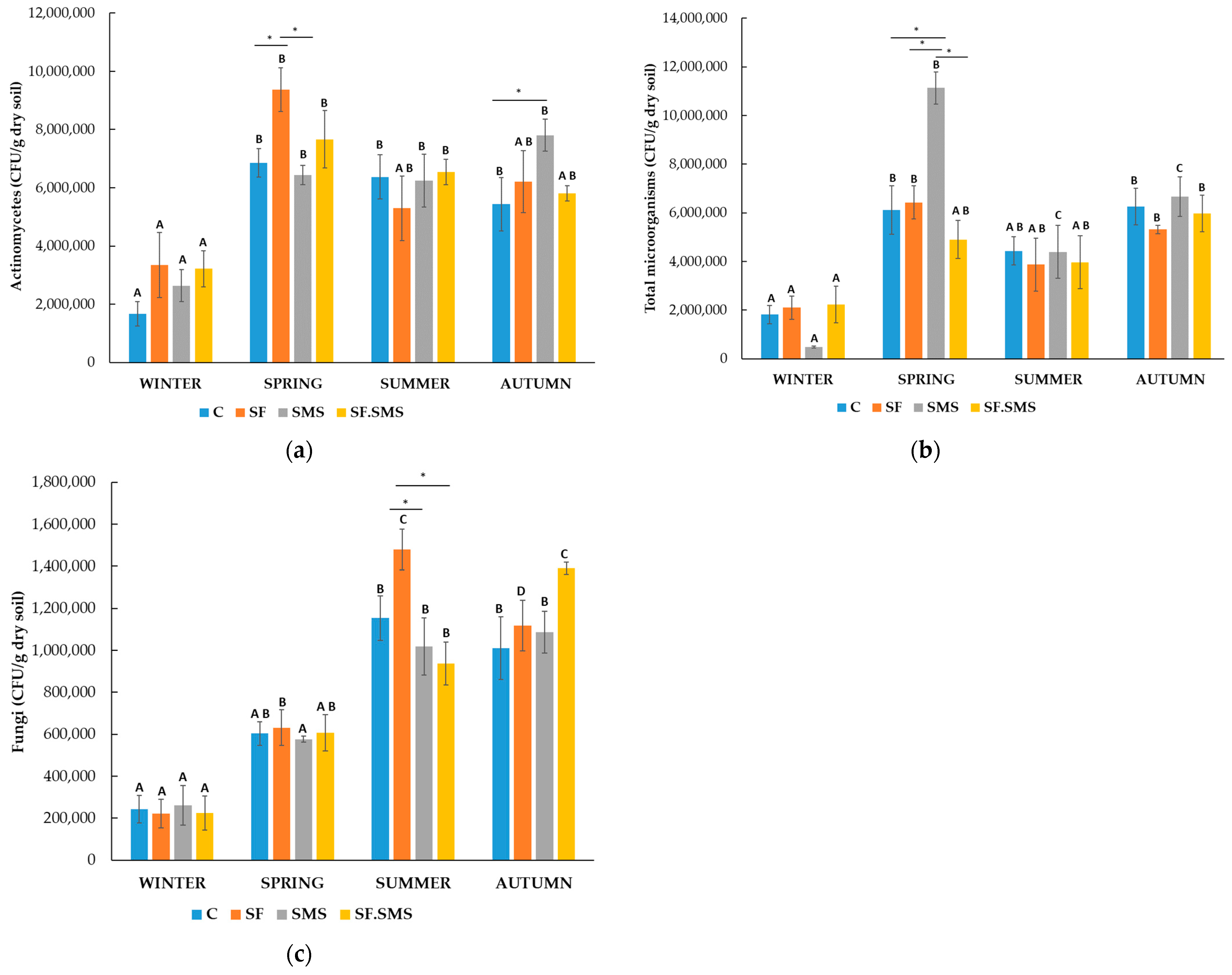
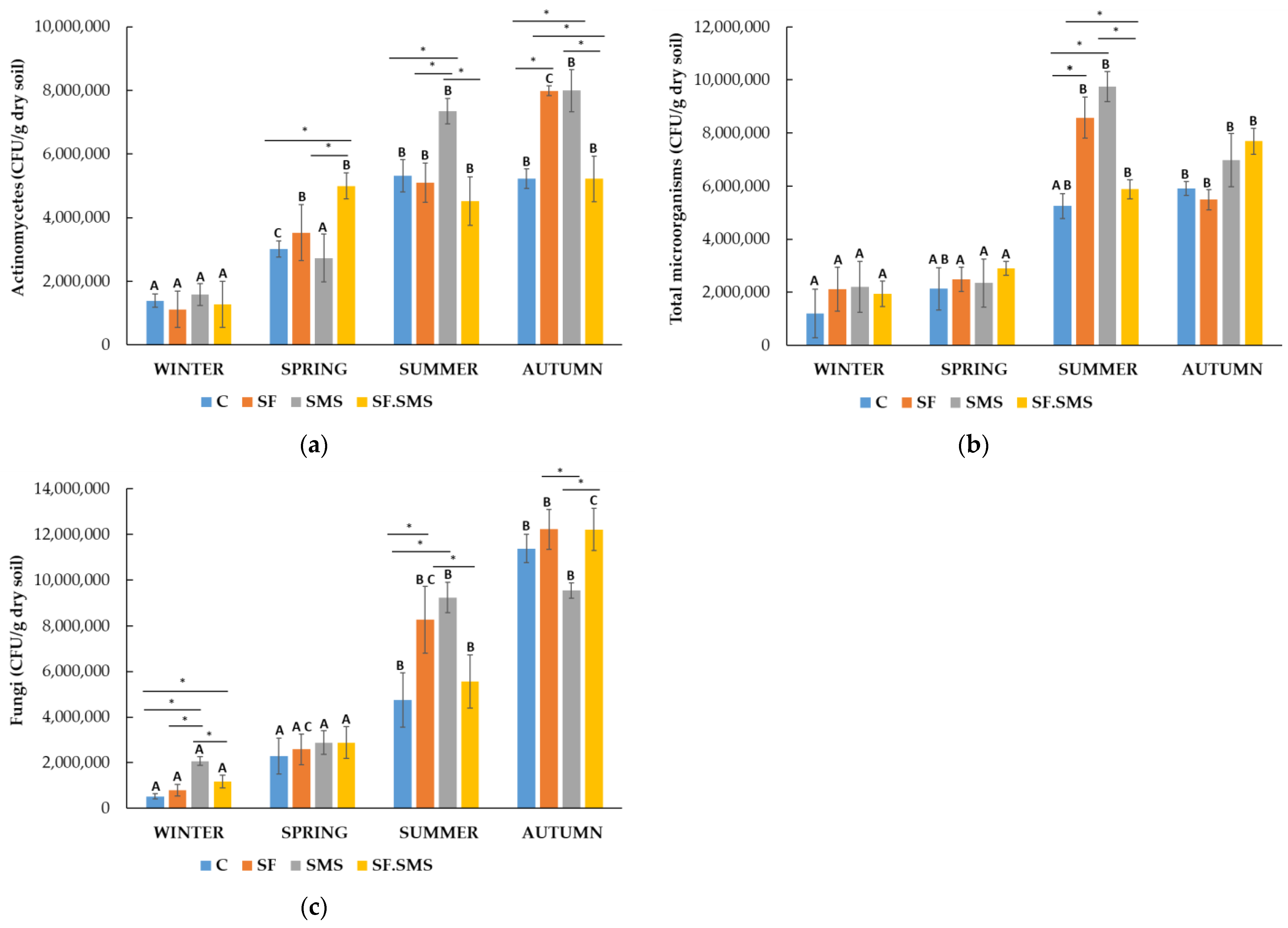
3.1.1. Microorganism Quantities
- Farm M
- Farm T
- Farm S
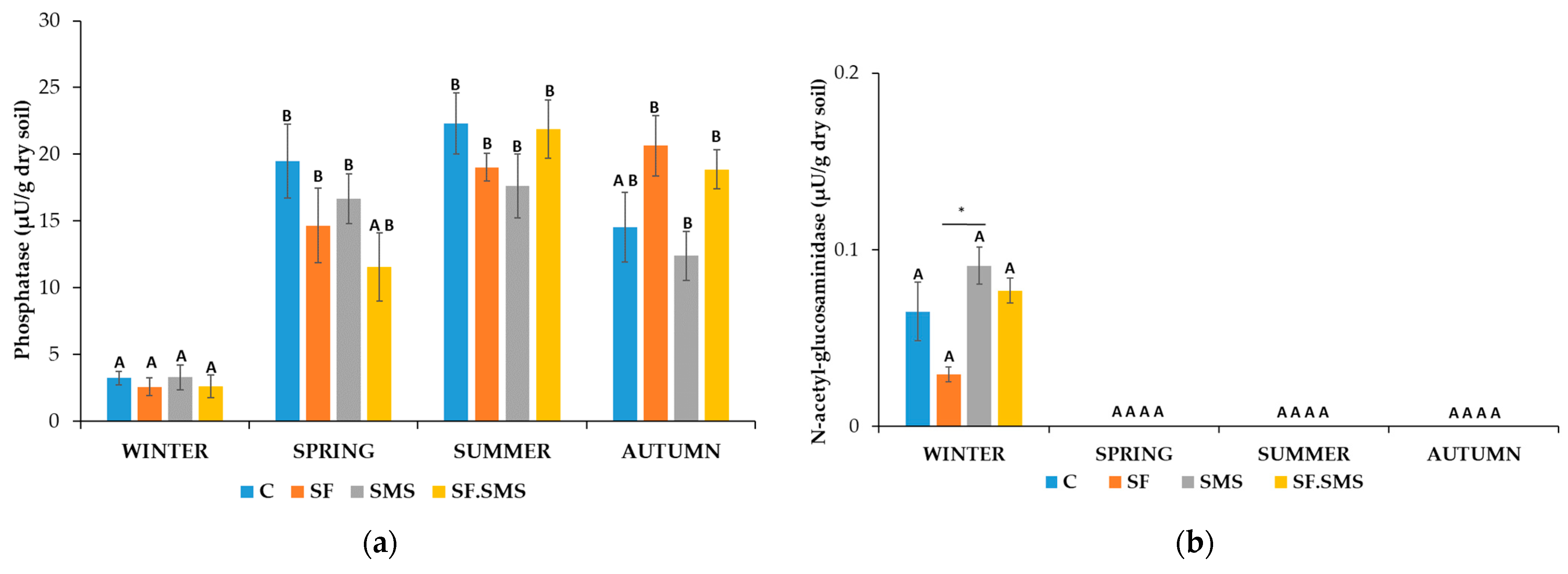
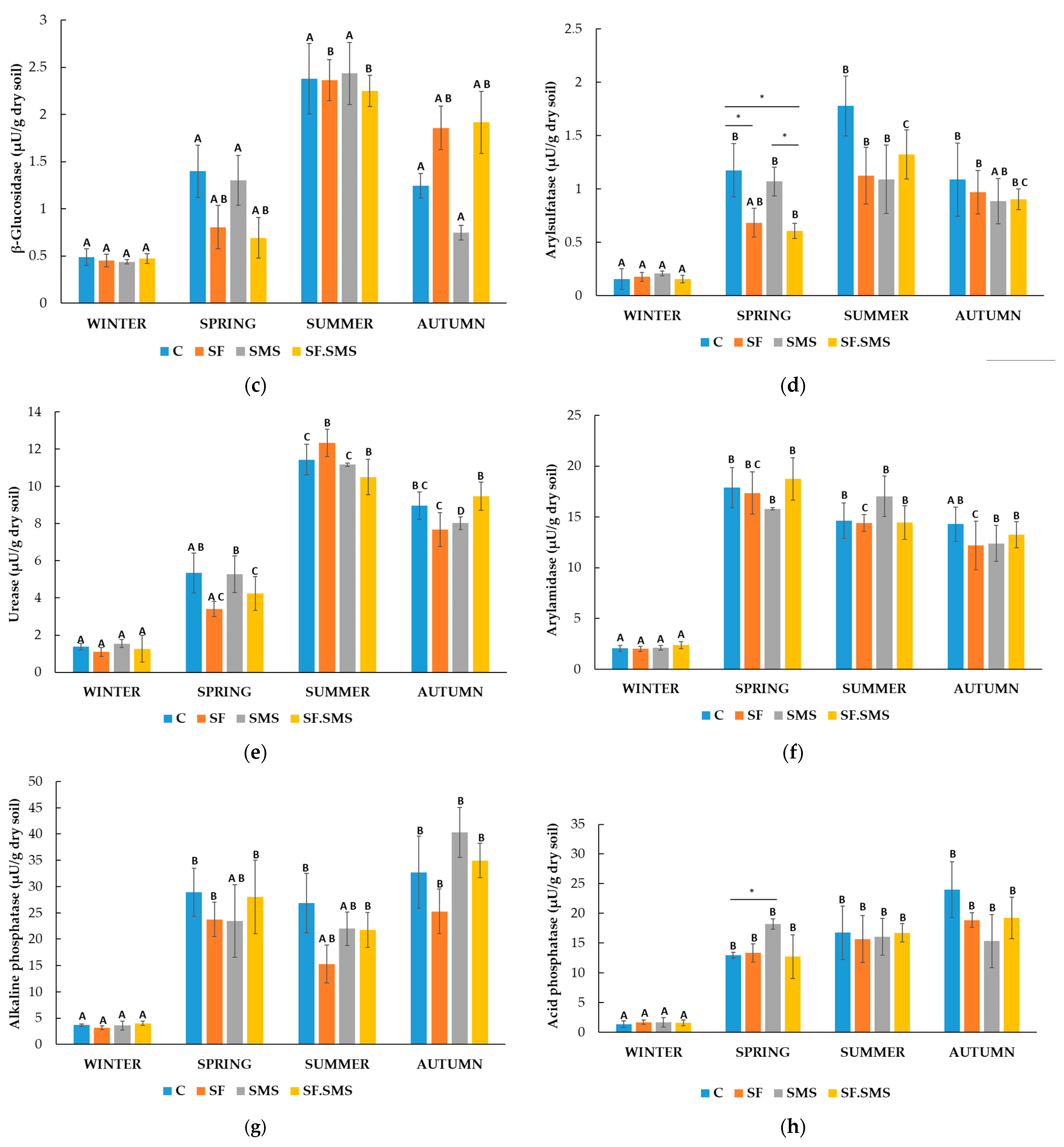
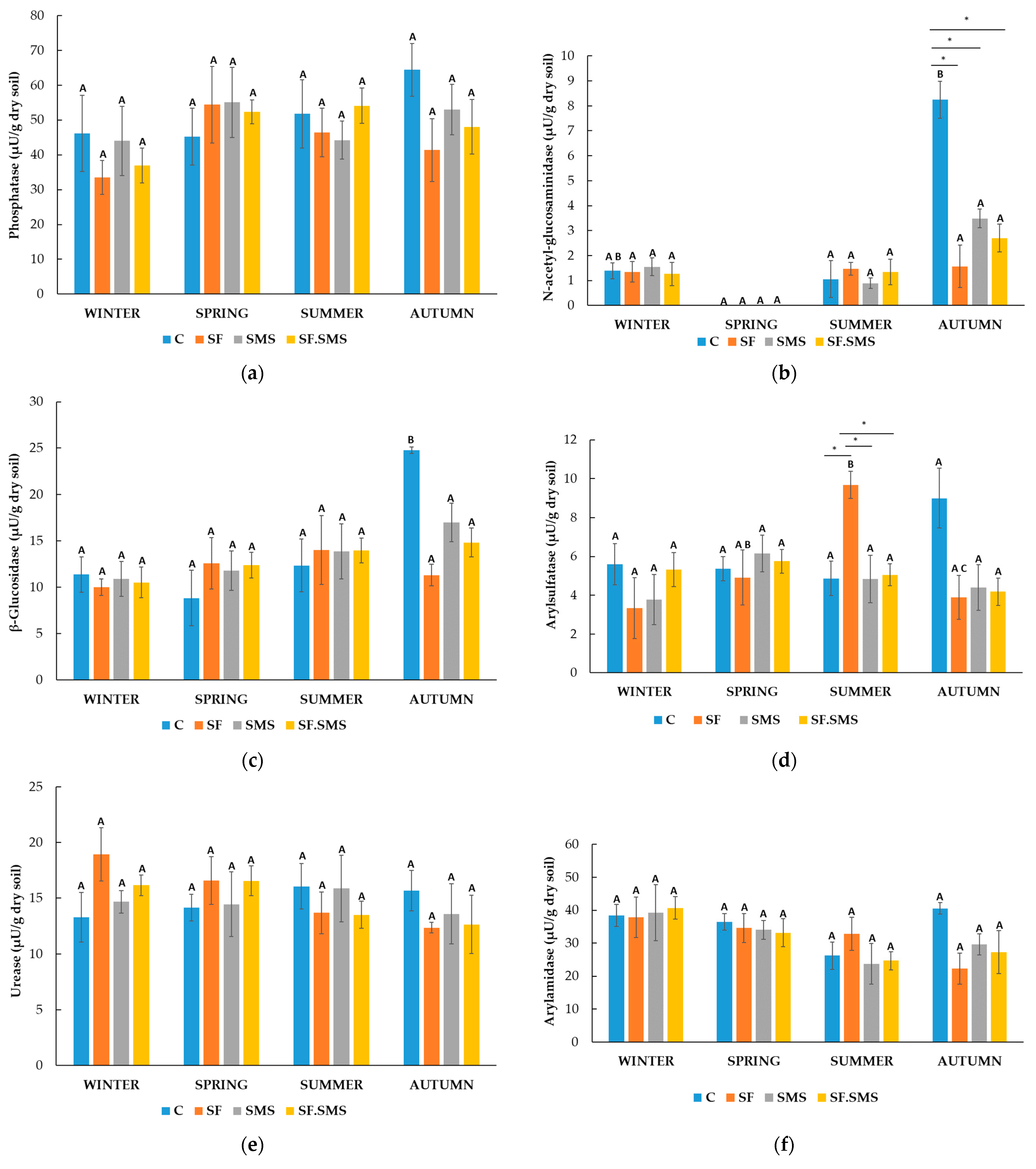
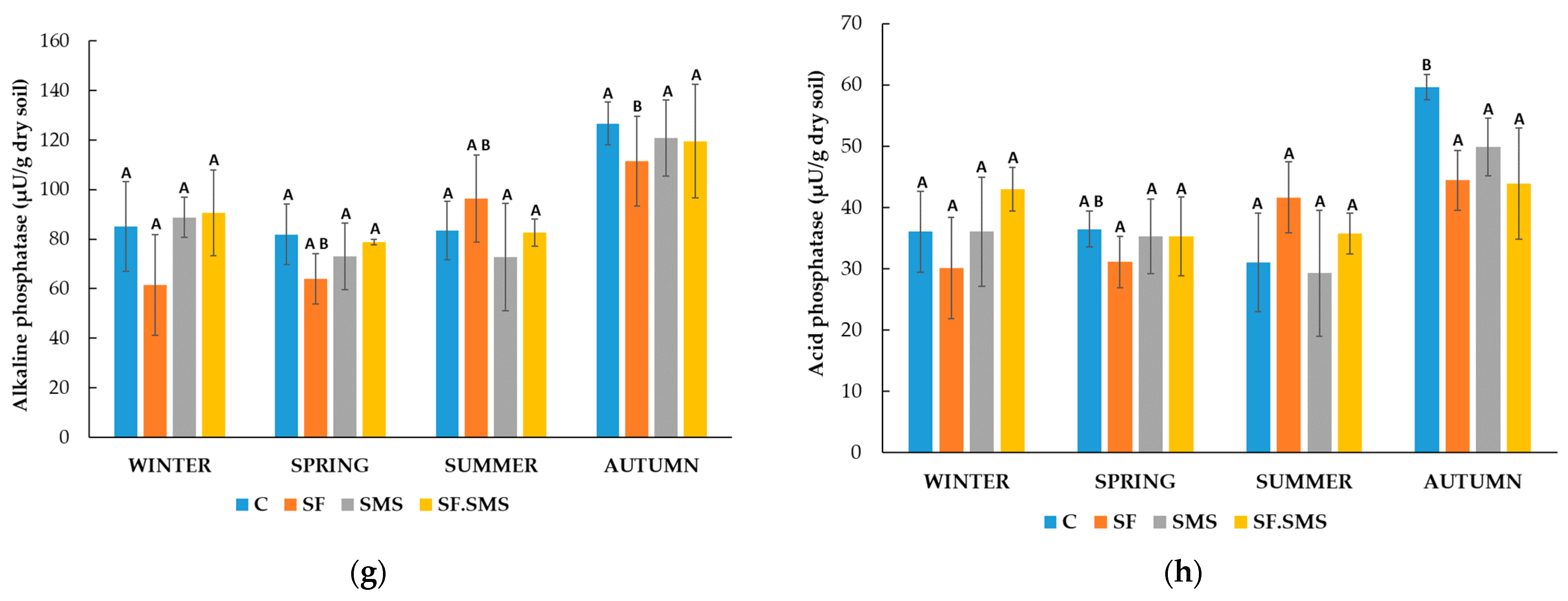
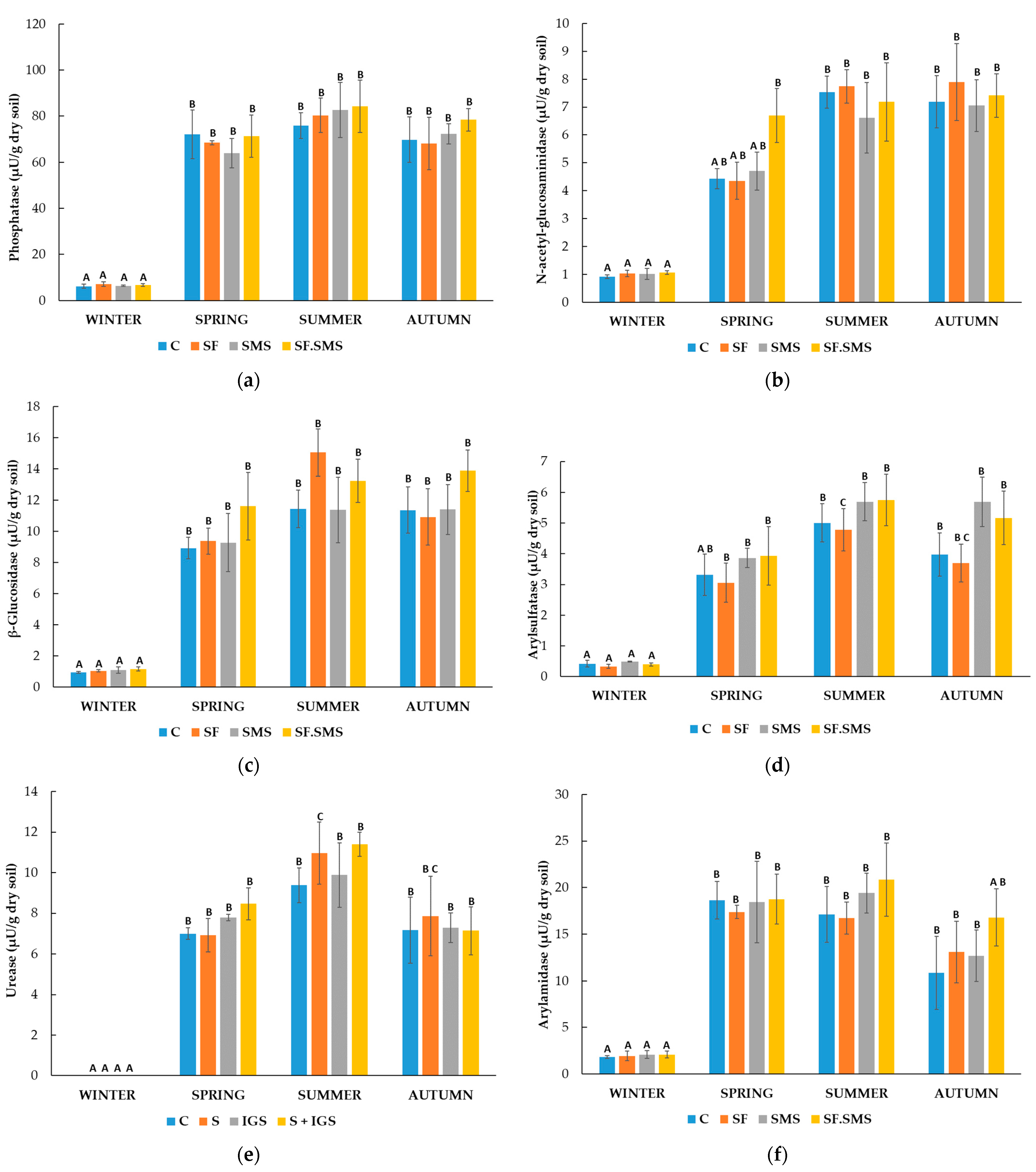
3.1.2. Enzymatic Activity
- Farm M
- Farm T
- Farm S
4. Discussion
4.1. Effect of Different Fertilization Types and Seasonal Changes on the Microorganism Quantities
4.2. Effect of Different Fertilization Types and Seasonal Changes on the Enzyme Activity in Three Farms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nielsen, M.N.; Winding, A. Microorganisms as Indicator of Soil Health; NERI Techn: Roskilde, Denmark, 2002; ISBN 8777726588. [Google Scholar]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y.H. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Ayiti, O.E.; Babalola, O.O. Factors Influencing Soil Nitrification Process and the Effect on Environment and Health. Front. Sustain. Food Syst. 2022, 6, 821994. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic Nitrogen Fertilizers Deplete Soil Nitrogen: A Global Dilemma for Sustainable Cereal Production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of fertilization on soil microorganisms in paddy rice systems—A meta-analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hanafi, F.H.; Rezania, S.; Mat Taib, S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent substrate from mushroom cultivation: Exploitation potential toward various applications and value-added products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef]
- Paula, F.S.; Tatti, E.; Abram, F.; Wilson, J.; O’Flaherty, V. Stabilisation of spent mushroom substrate for application as a plant growth-promoting organic amendment. J. Environ. Manag. 2017, 196, 476–486. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Anthony, M.A.; Bender, S.F.; van der Heijden, M.G.A. Enumerating soil biodiversity. Proc. Natl. Acad. Sci. USA 2023, 120, e2304663120. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Varma, A. Role of Enzymes in Maintaining Soil Health. In Soil Enzymology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 25–42. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, R.; Kumar, A.; Kumar, U.; Singh, D.K.; Mondal, S.; Kumawat, N.; Singh, A.K.; Raman, R.K.; Sundaram, P.K.; et al. Role of Soil Microbes to Assess Soil Health. In Structure and Functions of Pedosphere; Springer: Berlin/Heidelberg, Germany, 2022; pp. 339–363. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Salam, N.; Jiao, J.Y.; Zhang, X.T.; Li, W.J. Update on the classification of higher ranks in the phylum Actinobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 1331–1355. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, A.M.; Anwar, Y.; Abo-Aba, S.E.M.; Bataweel, N.M. A review on Actinomycetes distribution, isolation, and their medical applications. Nov. Res. Microbiol. J. 2023, 7, 1918–1931. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Williams, S.T. Actinomycetes as agents of biodegradation in the environment—A review. Gene 1992, 115, 189–192. [Google Scholar] [CrossRef]
- Zanne, A.E.; Abarenkov, K.; Afkhami, M.E.; Aguilar-Trigueros, C.A.; Bates, S.; Bhatnagar, J.M.; Busby, P.E.; Christian, N.; Cornwell, W.K.; Crowther, T.W.; et al. Fungal functional ecology: Bringing a trait-based approach to plant-associated fungi. Biol. Rev. Camb. Philos. Soc. 2020, 95, 409–433. [Google Scholar] [CrossRef]
- Chambergo, F.S.; Valencia, E.Y. Fungal biodiversity to biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Stone, W. Biochemistry, Proteins Enzymes; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- PISRS. Available online: https://pisrs.si/pregledPredpisa?id=PRAV14922 (accessed on 21 July 2024).
- The Organisation and Functioning of the State Administration. Available online: https://www.gov.si/en/ (accessed on 21 July 2024).
- EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2018/848/oj (accessed on 21 July 2023).
- Biodynamic Federation Demeter. Available online: https://demeter.net/biodynamics/biodynamic-approach/ (accessed on 21 July 2024).
- Brooks, J. Soil Sampling for Microbial Analyses. In Manual of Environmental Microbiology, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 246–256. [Google Scholar] [CrossRef]
- ISO 20130:2018; Soil Quality—Measurement of Enzyme Activity Patterns in Soil Samples Using Colori-Metric Substrates in Micro-Well Plates. ISO: Geneva, Switzerland, 2018.
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Ouhdouch, Y.; Hafidi, M.; Kouisni, L. Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol. Res. 2022, 261, 127059. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.T.; Machado, B.S.; Marchezi, G.; Crestani, L.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Use of soil actinomycetes for pharmaceutical, food, agricultural, and environmental purposes. 3 Biotech 2022, 12, 232. [Google Scholar] [CrossRef]
- Santoni, M.; Ferretti, L.; Migliorini, P.; Vazzana, C.; Pacini, G.C. A review of scientific research on biodynamic agriculture. Org. Agric. 2022, 12, 373–396. [Google Scholar] [CrossRef]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef]
- Kong, C.; Zhang, S.; Yuan, S.; Wang, W.; Song, X.; Guo, D.; Lawi, A.S. Soil bacterial community characteristics and its effect on organic carbon under different fertilization treatments. Front. Microbiol. 2024, 15, 1356171. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, D.; Chen, Y.; Wu, J.; Zhang, J. Effects of Long-Term Straw Returning and Nitrogen Fertilizer Reduction on Soil Microbial Diversity in Black Soil in Northeast China. Agronomy 2023, 13, 2036. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of nitrogen fertilization and root interaction on the agronomic traits of intercropped maize, and the quantity of microorganisms and activity of enzymes in the rhizosphere. Plant Soil 2013, 368, 407–417. [Google Scholar] [CrossRef]
- Chen, Q.; Xin, Y.; Liu, Z. Long-term fertilization with potassium modifies soil biological quality in K-rich soils. Agronomy 2020, 10, 771. [Google Scholar] [CrossRef]
- Kai-Lou, L.; Ya-Zhen, L.; Li-Jun, Z.; Yan, C.; Qing-Hai, H.; Xi-Chu, Y.; Da-Ming, L. Comparison of crop productivity and soil microbial activity among different fertilization patterns in red upland and paddy soils. Acta Ecol. Sin. 2018, 38, 262–267. [Google Scholar] [CrossRef]
- Chaudhry, V.; Rehman, A.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Changes in Bacterial Community Structure of Agricultural Land Due to Long-Term Organic and Chemical Amendments. Microb. Ecol. 2012, 64, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cassman, N.; de Hollander, M.; Mendes, L.W.; Korevaar, H.; Geerts, R.H.E.M.; van Veen, J.A.; Kuramae, E.E. Impact of long-term N, P, K, and NPK fertilization on the composition and potential functions of the bacterial community in grassland soil. FEMS Microbiol. Ecol. 2014, 90, 195–205. [Google Scholar] [CrossRef]
- Becher, M.; Banach-Szott, M.; Godlewska, A. Organic Matter Properties of Spent Button Mushroom Substrate in the Context of Soil Organic Matter Reproduction. Agronomy 2021, 11, 204. [Google Scholar] [CrossRef]
- Joniec, J.; Kwiatkowska, E.; Kwiatkowski, C.A. Assessment of the Effects of Soil Fertilization with Spent Mushroom Substrate in the Context of Microbial Nitrogen Transformations and the Potential Risk of Exacerbating the Greenhouse Effect. Agriculture 2022, 12, 1190. [Google Scholar] [CrossRef]
- Frąc, M.; Pertile, G.; Panek, J.; Gryta, A.; Oszust, K.; Lipiec, J.; Usowicz, B. Mycobiome Composition and Diversity under the Long-Term Application of Spent Mushroom Substrate and Chicken Manure. Agronomy 2021, 11, 410. [Google Scholar] [CrossRef]
- Ntougias, S.; Zervakis, G.I.; Kavroulakis, N.; Ehaliotis, C.; Papadopoulou, K.K. Bacterial diversity in spent mushroom compost assessed by amplified rDNA restriction analysis and sequencing of cultivated isolates. Syst. Appl. Microbiol. 2004, 27, 746–754. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Joniec, J.; Kwiatkowski, C.A.; Kowalczyk, K.; Nowak, M.; Leśniowska-Nowak, J. Assessment of the impact of spent mushroom substrate on biodiversity and activity of soil bacterial and fungal populations based on classical and modern soil condition indicators. Int. Agrophys. 2024, 38, 139–154. [Google Scholar] [CrossRef]
- Yang, W.; Su, Y.; Wang, R.; Zhang, H.; Jing, H.; Meng, J.; Zhang, G.; Huang, L.; Guo, L.; Wang, J.; et al. Microbial production and applications of β-glucosidase—A review. Int. J. Biol. Macromol. 2024, 256, 127915. [Google Scholar] [CrossRef] [PubMed]
- Joniec, J.; Gąsior, J.; Voloshanska, S.; Nazarkiewicz, M.; Hoivanovych, N. Evaluation of the effectiveness of land reclamation based on microbiological and biochemical parameters assessed in an ozokerite mining and processing landfill sown with Trifolium hybridum and Dactylis glomerata. J. Environ. Manag. 2019, 242, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Liu, X.; Sun, L.; He, X.; Wu, Z. Effects of two types nitrogen sources on humification processes and phosphorus dynamics during the aerobic composting of spent mushroom substrate. J. Environ. Manag. 2022, 317, 115453. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, W.; Luo, L.; Li, Y.; Chen, Z.; Gu, Y.; Chen, Q.; Deng, O.; Xu, X.; Lan, T.; et al. Short-term responses of soil nutrients, heavy metals and microbial community to partial substitution of chemical fertilizer with spent mushroom substrates (SMS). Sci. Total Environ. 2022, 844, 157064. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Y.; Zhang, P.; Yu, F.; Perez-Moreno, J. Macrofungi cultivation in shady forest areas significantly increases microbiome diversity, abundance and functional capacity in soil furrows. J. Fungi 2021, 7, 775. [Google Scholar] [CrossRef] [PubMed]
- Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils. Plants 2021, 10, 2648. [Google Scholar] [CrossRef] [PubMed]
- Kátai, J.; Zsuposné, Á.O.; Tállai, M.; Alshaal, T. Would fertilization history render the soil microbial communities and their activities more resistant to rainfall fluctuations? Ecotoxicol. Environ. Saf. 2020, 201, 110803. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, D.; Liu, D.; Sun, J.; Wang, X.; Zhou, W.; Liang, G. Soil Aggregation Shaped the Distribution and Interaction of Bacterial-Fungal Community Based on a 38-Year Fertilization Experiment in China. Front. Microbiol. 2022, 13, 824681. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Joniec, J. Effects of Agricultural Management of Spent Mushroom Waste on Phytotoxicity and Microbiological Transformations of C, P, and S in Soil and Their Consequences for the Greenhouse Effect. Int. J. Environ. Res. Public Health 2022, 19, 12915. [Google Scholar] [CrossRef]
- Hernandez, T.; Berlanga, J.G.; Tormos, I.; Garcia, C.; Hernandez, T.; Berlanga, J.G.; Tormos, I.; Garcia, C. Organic versus inorganic fertilizers: Response of soil properties and crop yield. AIMS Geosci. 2021, 7, 415–439. [Google Scholar] [CrossRef]
- Gryndler, M.; Hršelová, H.; Klír, J.; Kubát, J.; Votruba, J. Long-term fertilization affects the abundance of saprotrophic microfungi degrading resistant forms of soil organic matter. Folia Microbiol. 2003, 48, 76–82. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Jiang, L.; Yang, Y.; Shi, J.; Guan, X.; Sun, T.; Zhao, H.; Wang, Y.; Liu, Y. Effect of Mild Organic Substitution on Soil Quality and Microbial Community. Agronomy 2024, 14, 888. [Google Scholar] [CrossRef]
- Yang, F.; Tian, J.; Fang, H.; Gao, Y.; Xu, M.; Lou, Y.; Zhou, B.; Kuzyakov, Y. Functional Soil Organic Matter Fractions, Microbial Community, and Enzyme Activities in a Mollisol Under 35 Years Manure and Mineral Fertilization. J. Soil Sci. Plant Nutr. 2019, 19, 430–439. [Google Scholar] [CrossRef]
- Perotto, S.; Angelini, P.; Bianciotto, V.; Bonfante, P.; Girlanda, M.; Kull, T.; Mello, A.; Pecoraro, L.; Perini, C.; Persiani, A.M.; et al. Interactions of fungi with other organisms. Plant Biosyst. 2013, 147, 208–218. [Google Scholar] [CrossRef]
- Warnasuriya, S.D.; Udayanga, D.; Manamgoda, D.S.; Biles, C. Fungi as environmental bioindicators. Sci. Total Environ. 2023, 892, 164583. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic microbes and their potential applications in crop management. Pest Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Midgley, G.F. Biodiversity and Ecosystem Function. Science 2012, 335, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Fan, M.; Zhao, C. The Novel Compounds with Biological Activity Derived from Soil Fungi in the Past Decade. Drug Des. Dev. Ther. 2022, 16, 3493–3555. [Google Scholar] [CrossRef] [PubMed]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D.; et al. Linking Soil Microbial Diversity to Modern Agriculture Practices: A Review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Liu, W.; Huang, H.; Ye, Q.; Zhu, S.; Peng, Z.; Li, Y.; Deng, L.; Yang, Z.; Chen, H.; et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants 2023, 12, 3801. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, K.; Łysiak, G.P.; Zydlik, Z. Effect of Nitrogen Fertilization in the Sour Cherry Orchard on Soil Enzymatic Activities, Microbial Population, and Fruit Quality. Agriculture 2022, 12, 2069. [Google Scholar] [CrossRef]
- Asadu, C.L.A.; Obasi, S.C.; Dixon, A.G.O. Variations in soil physical properties in a cleared forestland continuously cultivated for seven years in eastern nsukka, Nigeria. Commun. Soil Sci. Plant Anal. 2010, 41, 123–132. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar] [CrossRef]
- Fan, L.; Tarin, M.W.K.; Zhang, Y.; Han, Y.; Rong, J.; Cai, X.; Chen, L.; Shi, C.; Zheng, Y. Patterns of soil microorganisms and enzymatic activities of various forest types in coastal sandy land. Glob. Ecol. Conserv. 2021, 28, e01625. [Google Scholar] [CrossRef]
- Barros-Rodríguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-Aree, W.; Manzanera, M. Impacts of Agriculture on the Environment and Soil Microbial Biodiversity. Plants 2021, 10, 2325. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of Beneficial Microorganisms in Soil Remediation and Quality Improvement of Medicinal Plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhu, Z.; Jiang, Y.; Ge, S.; Zhu, Z.; Jiang, Y. Long-term impact of fertilization on soil pH and fertility in an apple production system. J. Soil Sci. Plant Nutr. 2018, 18, 282–293. [Google Scholar] [CrossRef]
- Souza, J.L.B.; Antonangelo, J.A.; Zhang, H.; Reed, V.; Finch, B.; Arnall, B. Impact of long-term fertilization in no-till on the stratification of soil acidity and related parameters. Soil Tillage Res. 2023, 228, 105624. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Paredes, C.; Medina, E.; Moral, R.; Pérez-Murcia, M.D.; Moreno-Caselles, J.; Bustamante, A.M.; Cecilia, J.A. Characterization of the different organic matter fractions of spent mushroom substrate. Commun. Soil Sci. Plant Anal. 2009, 40, 150–161. [Google Scholar] [CrossRef]
- Huang, C.; Han, X.; Luo, Q.; Nie, Y.; Kang, M.; Chen, Y.; Tang, M.; Fu, Y.; Li, X.; Chen, Y. Agro-Based Spent Mushroom Compost Substrates Improve Soil Properties and Microbial Diversity in Greenhouse Tomatoes. Agronomy 2023, 13, 2291. [Google Scholar] [CrossRef]
- Sun, C.; Wei, Y.; Kou, J.; Han, Z.; Shi, Q.; Liu, L.; Sun, Z. Improve spent mushroom substrate decomposition, bacterial community and mature compost quality by adding cellulase during composting. J. Clean. Prod. 2021, 299, 126928. [Google Scholar] [CrossRef]
- Bauke, S.L.; Amelung, W.; Bol, R.; Brandt, L.; Brüggemann, N.; Kandeler, E.; Meyer, N.; Or, D.; Schnepf, A.; Schloter, M.; et al. Soil water status shapes nutrient cycling in agroecosystems from micrometer to landscape scales. J. Plant Nutr. Soil Sci. 2022, 185, 773–792. [Google Scholar] [CrossRef]
- Qian, X.; Bi, X.; Xu, Y.; Yang, Z.; Wei, T.; Xi, M.; Li, J.; Chen, L.; Li, H.; Sun, S. Variation in community structure and network characteristics of spent mushroom substrate (SMS) compost microbiota driven by time and environmental conditions. Bioresour. Technol. 2022, 364, 127915. [Google Scholar] [CrossRef] [PubMed]
- Kleyn, J.G.; Wetzler, T.F. The microbiology of spent mushroom compost and its dust. Can. J. Microbiol. 1981, 27, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.; Claassens, A.S.; Wehner, F.C. Effect of direct nitrogen and potassium and residual phosphorus fertilizers on soil chemical properties, microbial components and maize yield under long-term crop rotation. Biol. Fertil. Soils 2002, 35, 420–427. [Google Scholar] [CrossRef]
- Xiong, C.; Lu, Y. Microbiomes in agroecosystem: Diversity, function and assembly mechanisms. Environ. Microbiol. Rep. 2022, 14, 833–849. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, Q.; Zhou, J.; Rengel, Z.; Feng, G. Soil phosphorus availability determines the preference for direct or mycorrhizal phosphorus uptake pathway in maize. Geoderma 2021, 403, 115261. [Google Scholar] [CrossRef]
- Sobucki, L.; Ramos, R.F.; Meireles, L.A.; Antoniolli, Z.I.; Jacques, R.J.S. Contribution of enzymes to soil quality and the evolution of research in Brazil. Rev. Bras. Cienc. Solo 2021, 45, e0210109. [Google Scholar] [CrossRef]
- Banerjee, A.; Sanyal, S.; Sen, S. Soil phosphatase activity of agricultural land: A possible index of soil fertility. Agric. Sci. Res. J. 2012, 2, 412–419. [Google Scholar]
- Chaurasiya, A.; Dutta, S.K.; Prasad, S.; Singh, G.; Kumar, S.; Chaurasiya, A. Maintenance of sustainability of agriculture and soil organic carbon through biodynamic agriculture practices. In Agricultural Soil Sustainability and Carbon Management; Academic Press: Cambridge, MA, USA, 2023; pp. 327–340. [Google Scholar] [CrossRef]
- Muhie, S.H. Concepts, Principles, and Application of Biodynamic Farming: A Review. Circ. Econ. Sustain. 2023, 3, 291–304. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Lee, S.A.; Goddard, M.R. Fungal communities are differentially affected by conventional and biodynamic agricultural management approaches in vineyard ecosystems. Agric. Ecosyst. Environ. 2017, 246, 306–313. [Google Scholar] [CrossRef]
- Cao, D.; Lan, Y.; Yang, X.; Chen, W.; Jiang, L.; Wu, Z.; Li, N.; Han, X. Phosphorus fractions in biochar-amended soil—Chemical sequential fractionation, 31P NMR, and phosphatase activity. Arch. Agron. Soil Sci. 2023, 69, 169–181. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Uwituze, Y.; Nyiraneza, J.; Fraser, T.D.; Dessureaut-Rompré, J.; Ziadi, N.; Lafond, J. Carbon, Nitrogen, Phosphorus, and Extracellular Soil Enzyme Responses to Different Land Use. Front. Soil Sci. 2022, 2, 814554. [Google Scholar] [CrossRef]
- Symanowicz, B.; Toczko, R.; Toczko, M. Enzymatic Activity of Soil after Applying Mineral Fertilizers and Waste Lignite to Maize Grown for Silage. Agriculture 2022, 12, 2146. [Google Scholar] [CrossRef]
- Touhami, D.; Condron, L.M.; Mcdowell, R.W.; Moss, R. Effects of long-term phosphorus fertilizer inputs and seasonal conditions on organic soil phosphorus cycling under grazed pasture. Soil Use Manag. 2023, 39, 385–401. [Google Scholar] [CrossRef]
- Krämer, S.; Green, D.M. Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar] [CrossRef]
- Wang, H.W.; Xu, M.; Cai, X.Y.; Tian, F. Evaluation of soil microbial communities and enzyme activities in cucumber continuous cropping soil treated with spent mushroom (Flammulina velutipes) substrate. J. Soils Sediments 2021, 21, 2938–2951. [Google Scholar] [CrossRef]
- Rinker, D.L. Spent mushroom substrate uses. In Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 427–454. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A. Enzymatic activity as a popular parameter used to determine the quality of the soil environment. Polish J. Agron. 2019, 37, 22–30. [Google Scholar] [CrossRef]
- Schaap, K.J.; Fuchslueger, L.; Quesada, C.A.; Hofhansl, F.; Valverde-Barrantes, O.; Camargo, P.B.; Hoosbeek, M.R. Seasonal fluctuations of extracellular enzyme activities are related to the biogeochemical cycling of C, N and P in a tropical terra-firme forest. Biogeochemistry 2023, 163, 1–15. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 2000, 49, 175–190. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Kluber, L.A.; Myrold, D.D. The importance of amino sugar turnover to C and N cycling in organic horizons of old-growth Douglas-fir forest soils colonized by ectomycorrhizal mats. Biogeochemistry 2013, 112, 679–693. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Naves, E.R.; Mota, R.P. da Soil quality: Enzymatic activity of soil β-glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–150. [Google Scholar]
- Sørensen, A.; Lübeck, M.; Lübeck, P.S.; Ahring, B.K. Fungal Beta-Glucosidases: A Bottleneck in Industrial Use of Lignocellulosic Materials. Biomolecules 2013, 3, 612. [Google Scholar] [CrossRef]
- Ribas, L.C.C.; de Mendonça, M.M.; Camelini, C.M.; Soares, C.H.L. Use of spent mushroom substrates from Agaricus subrufescens (syn. A. blazei, A. brasiliensis) and Lentinula edodes productions in the enrichment of a soil-based potting media for lettuce (Lactuca sativa) cultivation: Growth promotion and soil bioremediati. Bioresour. Technol. 2009, 100, 4750–4757. [Google Scholar] [CrossRef]
- Davies, B.; Coulter, J.A.; Pagliari, P.H. Soil Enzyme Activity Behavior after Urea Nitrogen Application. Plants 2022, 11, 2247. [Google Scholar] [CrossRef] [PubMed]
- Schnecker, J.; Spiegel, F.; Li, Y.; Richter, A.; Sandén, T.; Spiegel, H.; Zechmeister-Boltenstern, S.; Fuchslueger, L. Microbial responses to soil cooling might explain increases in microbial biomass in winter. Biogeochemistry 2023, 164, 521–535. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Li, D.; Xiao, K.; Wang, K. Controls on soil arylsulfatase activity at a regional scale. Eur. J. Soil Biol. 2019, 90, 9–14. [Google Scholar] [CrossRef]
- Li, X.; Sarah, P. Arylsulfatase activity of soil microbial biomass along a Mediterranean-arid transect. Soil Biol. Biochem. 2003, 35, 925–934. [Google Scholar] [CrossRef]
- Stressler, T.; Seitl, I.; Kuhn, A.; Fischer, L. Detection, production, and application of microbial arylsulfatases. Appl. Microbiol. Biotechnol. 2016, 100, 9053–9067. [Google Scholar] [CrossRef]
- Yu, M.; Wu, M.; Secundo, F.; Liu, Z. Detection, production, modification, and application of arylsulfatases. Biotechnol. Adv. 2023, 67, 108207. [Google Scholar] [CrossRef]
- Shafreen, M.; Vishwakarma, K.; Shrivastava, N.; Kumar, N. Physiology and Distribution of Nitrogen in Soils. In Soil Nitrogen Ecology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–31. [Google Scholar] [CrossRef]
- Yang, Z.X.; Liu, S.Q.; Zheng, D.W.; Feng, S.D. Effects of cadium, zinc and lead on soil enzyme activities. J. Environ. Sci. 2006, 18, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Tarin, M.W.K.; Fan, L.; Cai, Y.; Tayyab, M.; Chen, L.; He, T.; Rong, J.; Zheng, Y. Biochar amendment regulated growth, physiological, and biochemical responses of conifer in red soil. iForest-Biogeosci. For. 2020, 13, 490. [Google Scholar] [CrossRef]
- Jabborova, D.; Sulaymanov, K.; Sayyed, R.Z.; Alotaibi, S.H.; Enakiev, Y.; Azimov, A.; Jabbarov, Z.; Ansari, M.J.; Fahad, S.; Danish, S.; et al. Mineral Fertilizers Improves the Quality of Turmeric and Soil. Sustainability 2021, 13, 9437. [Google Scholar] [CrossRef]
- Isobe, K.; Oka, H.; Watanabe, T.; Tateno, R.; Urakawa, R.; Liang, C.; Senoo, K.; Shibata, H. High soil microbial activity in the winter season enhances nitrogen cycling in a cool-temperate deciduous forest. Soil Biol. Biochem. 2018, 124, 90–100. [Google Scholar] [CrossRef]
- Dodor, D.E.; Tabatabai, M.A. Arylamidase activity as an index of nitrogen mineralization in soils. Commun. Soil Sci. Plant Anal. 2007, 38, 2197–2207. [Google Scholar] [CrossRef]
- Kanté, M.; Riah-Anglet, W.; Cliquet, J.B.; Trinsoutrot-Gattin, I. Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition. Agronomy 2021, 11, 2131. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Arylamidase Activity of Soils. Soil Sci. Soc. Am. J. 2000, 64, 215–221. [Google Scholar] [CrossRef]
- Adekanmbi, A.A.; Dale, L.; Shaw, L.; Sizmur, T. Differential temperature sensitivity of intracellular metabolic processes and extracellular soil enzyme activities. Biogeosciences 2023, 20, 2207–2219. [Google Scholar] [CrossRef]
- Grunert, O.; Robles-Aguilar, A.A.; Hernandez-Sanabria, E.; Schrey, S.D.; Reheul, D.; Van Labeke, M.C.; Vlaeminck, S.E.; Vandekerckhove, T.G.L.; Mysara, M.; Monsieurs, P.; et al. Tomato plants rather than fertilizers drive microbial community structure in horticultural growing media. Sci. Rep. 2019, 9, 9561. [Google Scholar] [CrossRef]


| Characteristic | Farm M | Farm S | Farm T |
|---|---|---|---|
| Location site | 46°30′34.7″ N 15°44′08.3″ E | 46°24′54.2″ N 15°39′12.1″ E | 46°37′52.4″ N 15°41′50.8″ E |
| Type of farming | Conventional–integrated | Organic | Biodynamic |
| Standard fertilization | Mineral fertilizer | Commercial fertilizer suitable for organic farming | Own fertilizer (compost) |
| Basic | Organic fertilizer “Bioorganik” | Pelleted manure | Compost/sunflower seed cake |
| Additional | Mineral fertilizer “Rosasol K” | None | None |
| Parameter | Farm M | Farm S | Farm T | SMS |
|---|---|---|---|---|
| pH | 7 | 4.2 | 7.1 | 7.6 |
| Moisture | 17.1 | 28.1 | 26.3 | 84.4 |
| P2O5 (mg/100 g) | 21 | 19.5 | 23 | 345 |
| K2O (mg/100 g) | 12 | 17.5 | 30.5 | 2015 |
| Mg (mg/100 g) | 32 | 14 | 37.5 | 102 |
| Ca (mg/100 g) | 446 | 48 | 557 | 987.5 |
| Total N (%) | 0.16 | 0.20 | 0.31 | 1.52 |
| N-NH+4 (mg/kg) | 1.2 | 0.7 | 0.5 | 48 |
| N-NO−3 (mg/kg) | 144.6 | 1.0 | 60.6 | 0.5 |
| Total organic C (%) | 1.6 | 2.1 | 3.4 | 32.4 |
| Organic matter (%) | 2.7 | 3.5 | 5.9 | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintarič, M.; Štuhec, A.; Tratnik, E.; Langerholc, T. Spent Mushroom Substrate Improves Microbial Quantities and Enzymatic Activity in Soils of Different Farming Systems. Microorganisms 2024, 12, 1521. https://doi.org/10.3390/microorganisms12081521
Pintarič M, Štuhec A, Tratnik E, Langerholc T. Spent Mushroom Substrate Improves Microbial Quantities and Enzymatic Activity in Soils of Different Farming Systems. Microorganisms. 2024; 12(8):1521. https://doi.org/10.3390/microorganisms12081521
Chicago/Turabian StylePintarič, Maša, Ana Štuhec, Eva Tratnik, and Tomaž Langerholc. 2024. "Spent Mushroom Substrate Improves Microbial Quantities and Enzymatic Activity in Soils of Different Farming Systems" Microorganisms 12, no. 8: 1521. https://doi.org/10.3390/microorganisms12081521
APA StylePintarič, M., Štuhec, A., Tratnik, E., & Langerholc, T. (2024). Spent Mushroom Substrate Improves Microbial Quantities and Enzymatic Activity in Soils of Different Farming Systems. Microorganisms, 12(8), 1521. https://doi.org/10.3390/microorganisms12081521








