Biodegradation of Crude Oil by Nitrate-Reducing, Sulfate-Reducing, and Methanogenic Microbial Communities under High-Pressure Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Pressure and Atmospheric-Pressure Incubations
2.2. Analysis of the Family Composition of Crude Oil
2.3. Gas Chromatography Analysis of Saturates
2.4. Gas Chromatography Mass Spectrometry Analysis of Aromatics
3. Results
3.1. Nitrate-Reducing Enrichment Group
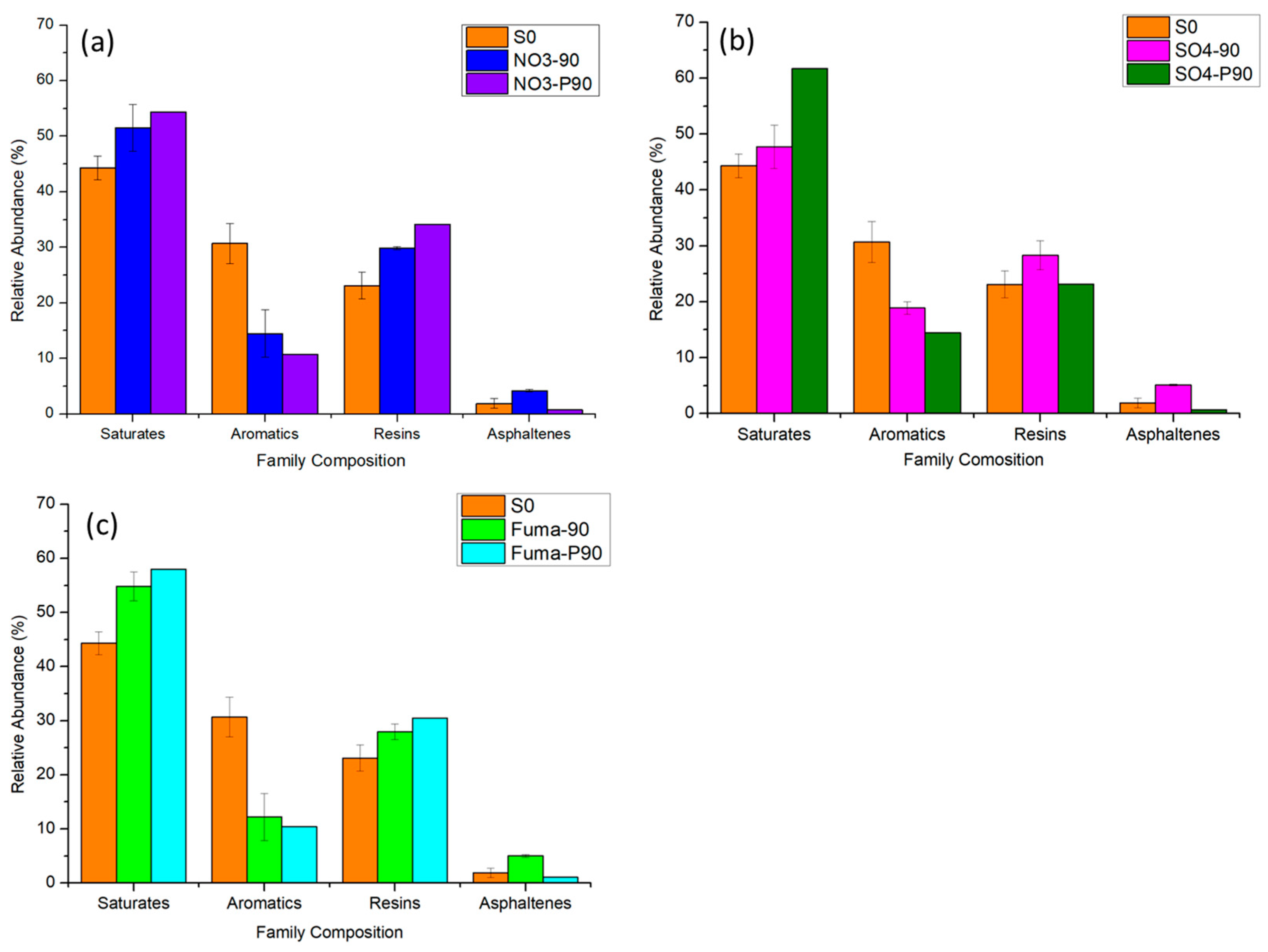
3.1.1. Family Composition of Crude Oil
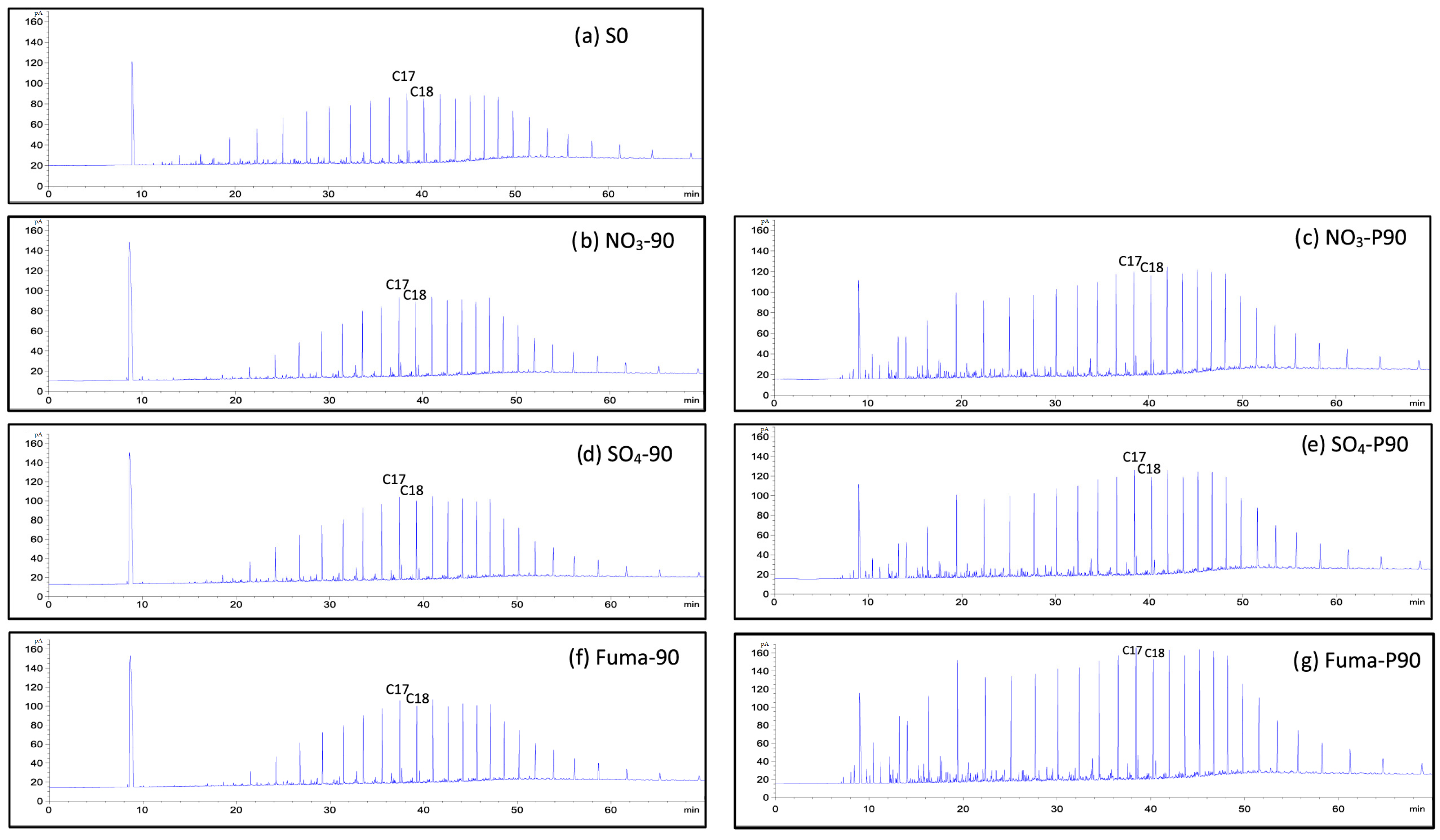
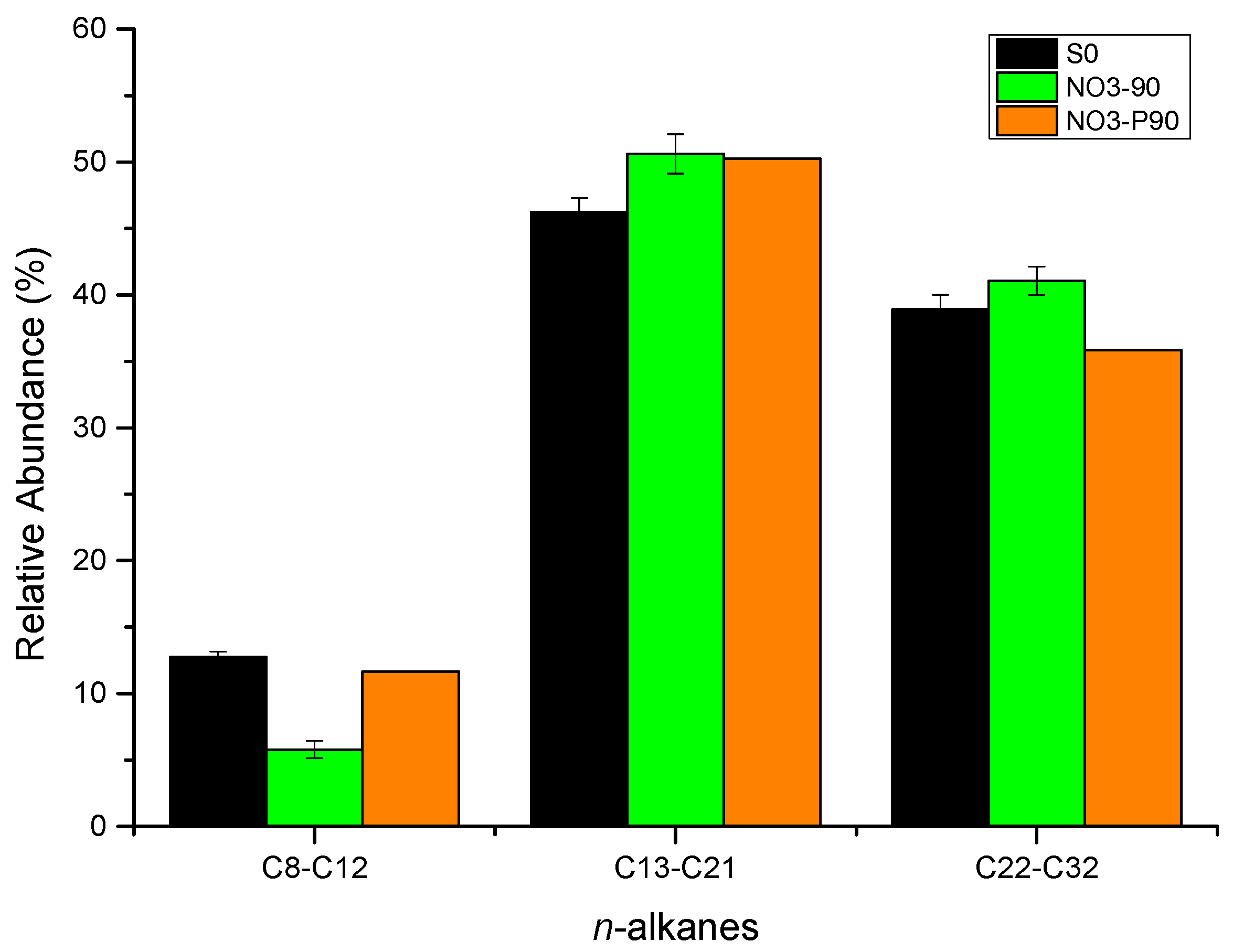
3.1.2. Gas Chromatography of Saturates
3.1.3. Gas Chromatography and Mass Spectrometry of Aromatics
3.2. Sulfate-Reducing Enrichment Group
3.2.1. Family Composition of Crude Oil
3.2.2. Gas Chromatography of Saturates
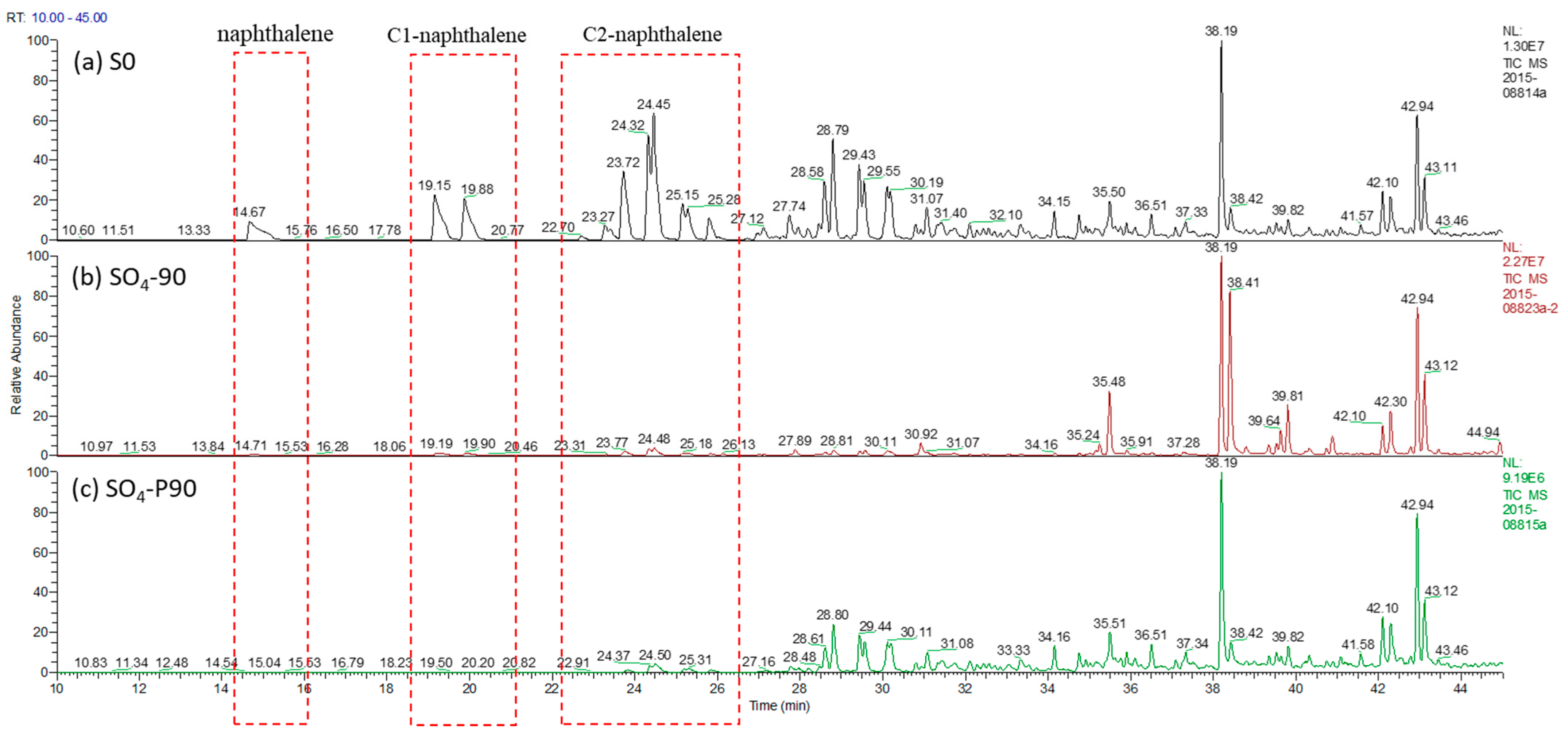
3.2.3. Gas Chromatography and Mass Spectrometry of Aromatics
3.3. Methanogenic Enrichment Group
3.3.1. Family Composition of Crude Oil
3.3.2. Gas Chromatography of Saturates
3.3.3. Gas Chromatography and Mass Spectrometry of Aromatics
4. Discussion
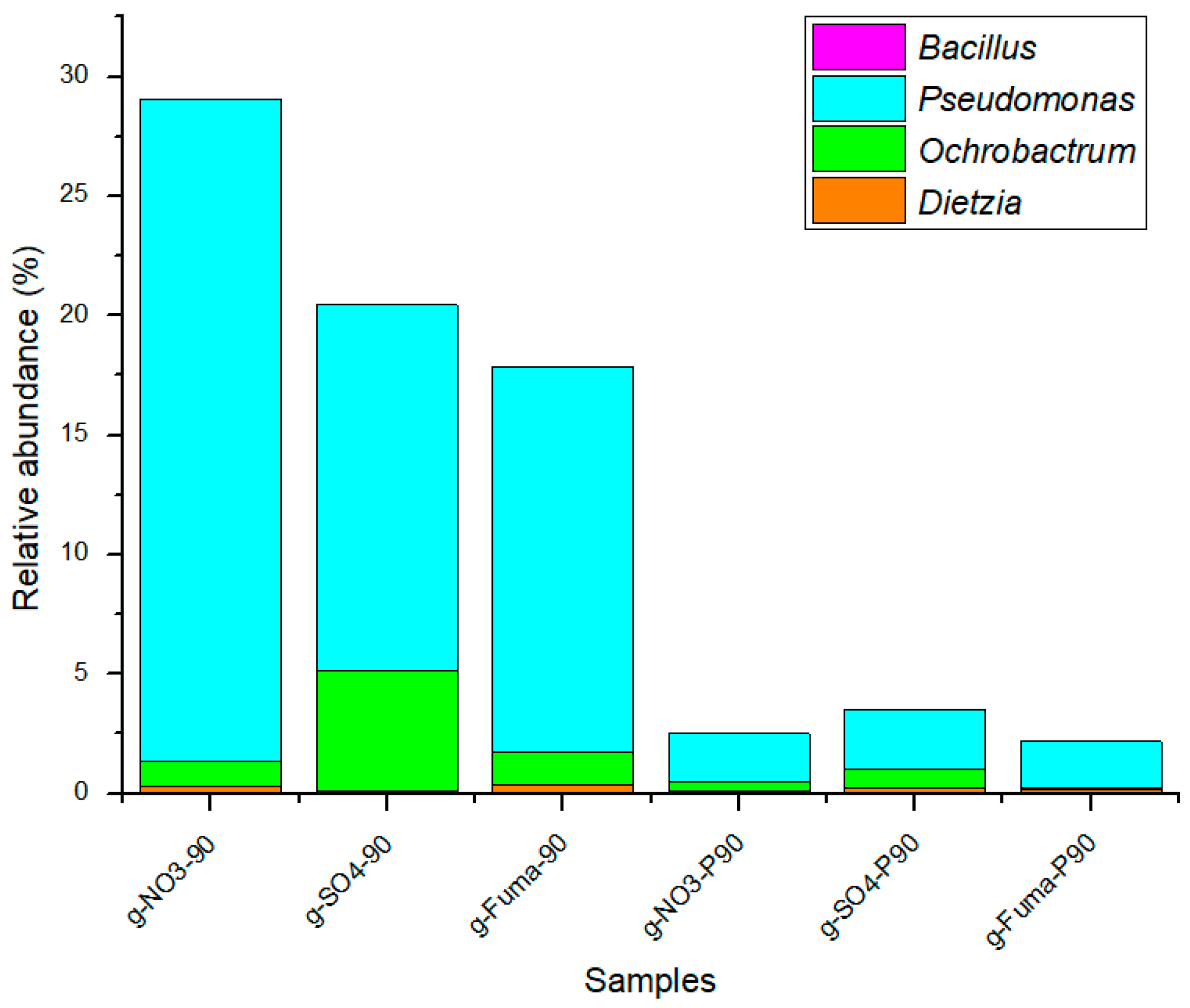
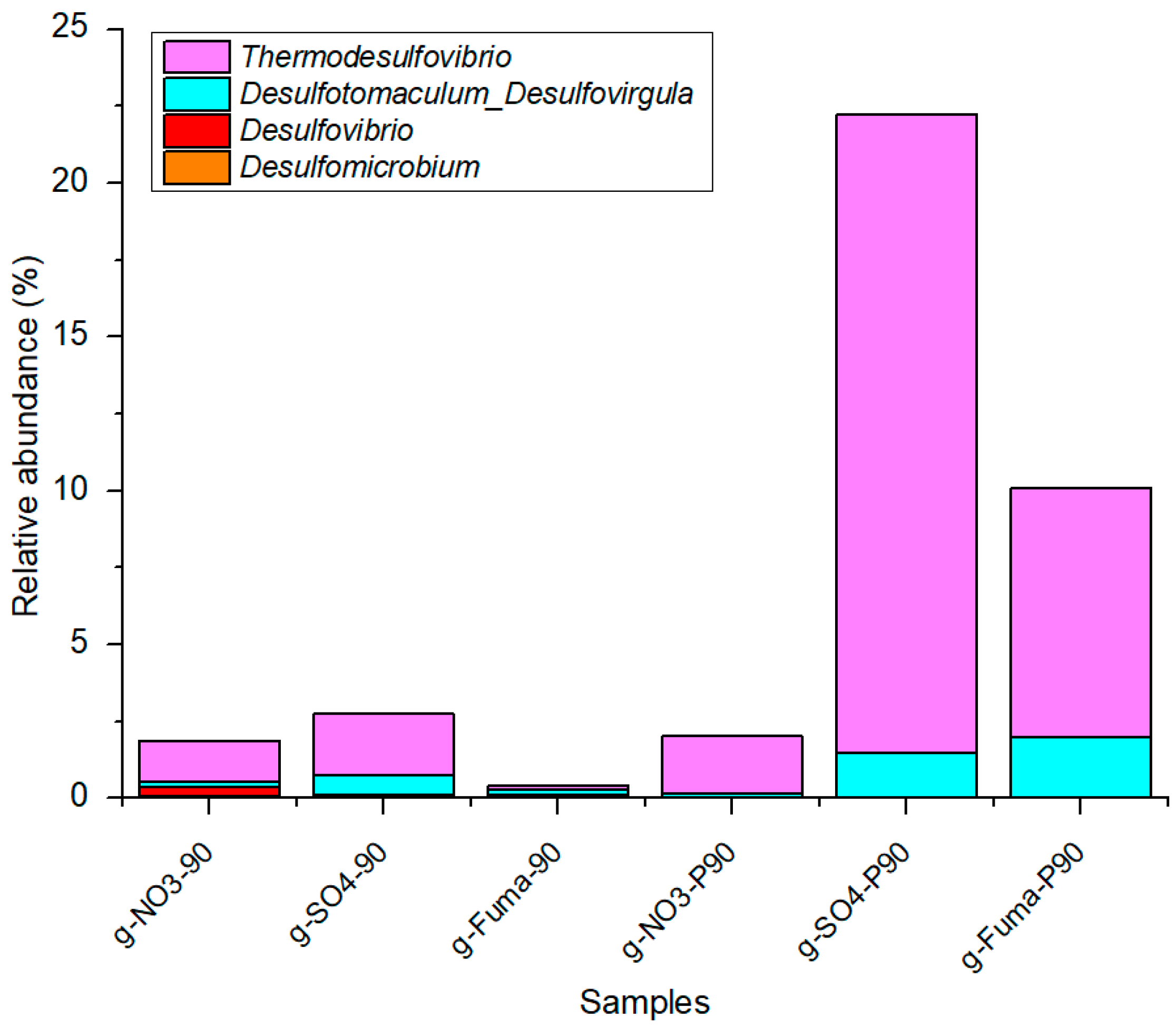
4.1. Effect of the Biodegradation Efficiency of Oil Family
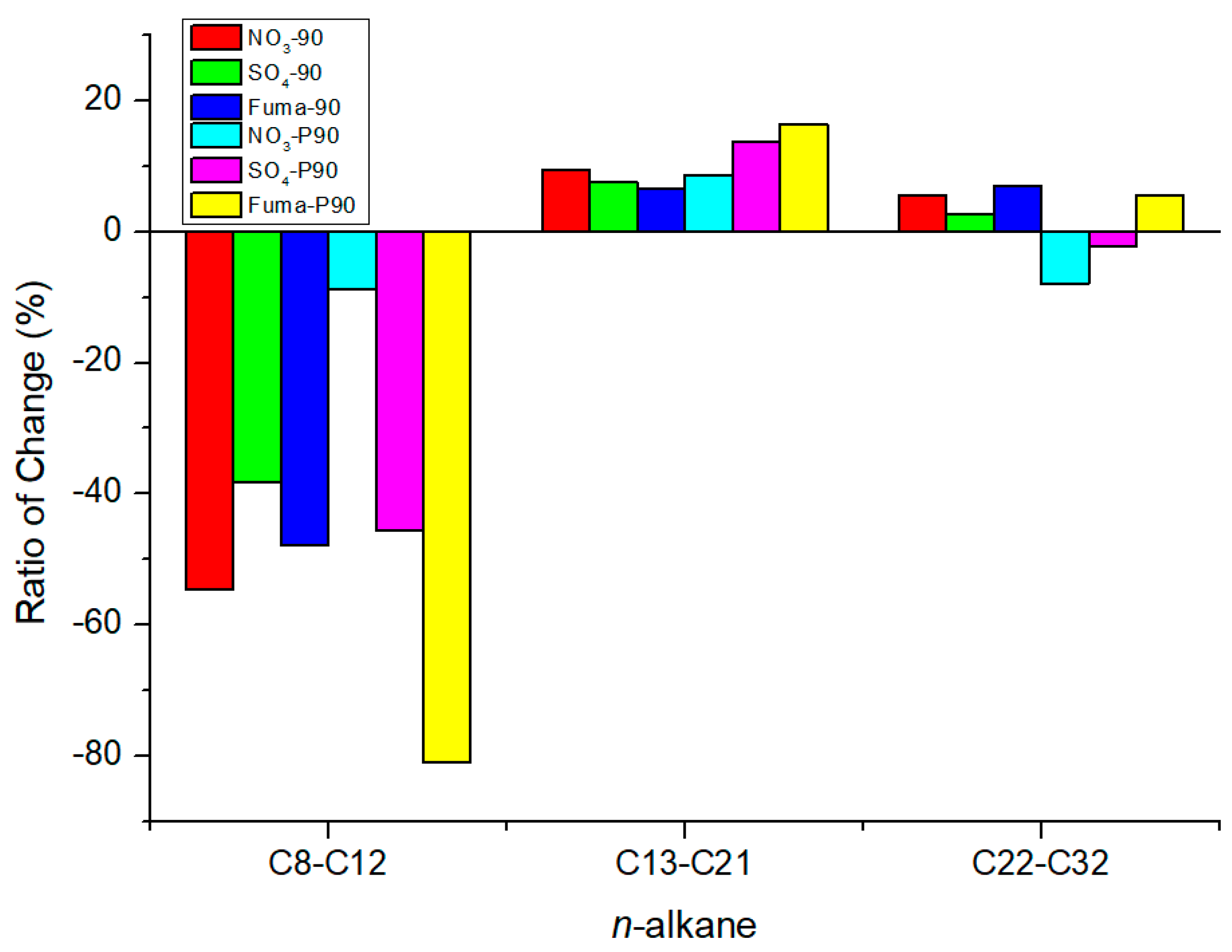
4.2. Effect of Oil Saturate Hydrocarbon Biodegradation Efficiency
4.3. Effect of Oil Aromatic Hydrocarbon Biodegradation Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsimplis, M.; Noussia, K. The Use of Ships Within a CCUS System: Regulation and Liability. Resour. Conserv. Recycl. 2022, 181, 106218. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Gao, L.; Li, S.; Wang, D. A Comprehensive Evaluation Model for Full-chain CCUS Performance Based on the Analytic Hierarchy Process Method. Energy 2022, 239, 122033. [Google Scholar] [CrossRef]
- Chen, S.Y.; Liu, J.F.; Zhang, Q.; Teng, F.; McLellan, B.C. A Critical Review on Deployment Planning and Risk Analysis of Carbon Capture, Utilization, and Storage (CCUS) Toward Carbon Neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Cai, B.F.; Li, Q.; Zhang, X.; Cao, C.; Cao, L.B.; Chen, W.H.; Chen, Z.J.; Dong, J.C.; Fan, J.L.; Jiang, Y.; et al. Annual Report of China’s CO2 Capture, Utilization and Storage (CCUS) (2021)—China CCUS Path Study; Chinese Academy of Environmental Planning of the Ministry of Ecology and Environment, Institute of Rock and Soil Mechanics, Chinese Academy of Science, the Administration Center for China’s Agenda 21: Beijing, China, 2021. [Google Scholar]
- Tyne, R.L.; Barry, P.H.; Lawson, M.; Lloyd, K.G.; Giovannelli, D.; Summers, Z.M.; Ballentine, C.J. Identifying and Understanding Microbial Methanogenesis in CO2 Storage. Environ. Sci. Technol. 2023, 57, 9459–9473. [Google Scholar] [CrossRef]
- Mayumi, D.; Dolfing, J.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Tamaki, H.; Takeuchi, M.; Nakatsu, C.H.; Kamagata, Y.C. Carbon Dioxide Concentration Dictates Alternative Methanogenic Pathways in Oil Reservoirs. Nat. Commun. 2013, 2998, 1998. [Google Scholar] [CrossRef]
- Vilcaez, J.; York, J.; Youssef, N.; Elshahed, M. Stimulation of Methanogenic Crude Oil Biodegradation in Depleted Oil Reservoirs. Fuel 2018, 232, 581–590. [Google Scholar] [CrossRef]
- Morozova, D.; Wandrey, M.; Alawi, M.; Zimmer, M.; Vieth, A.; Zettlitzer, M.; Würdemann, H. Monitoring of the Microbial Community Composition in Saline Aquifers During CO2 Storage by Fluorescence in situ Hybridization. Int. J. Greenh. Gas Control 2010, 4, 981–989. [Google Scholar] [CrossRef]
- Ma, L.; Liang, B.; Wang, L.Y.; Zhou, L.; Mbadinga, S.M.; Gu, J.D.; Mu, B.Z. Microbial Reduction of CO2 from Injected NaH13CO3 with Degradation of n-hexadecane in the Enrichment Culture Derived from a Petroleum Reservoir. Int. Biodeterior. Biodegrad. 2018, 127, 192–200. [Google Scholar] [CrossRef]
- Liang, B.; Wang, L.Y.; Zhou, Z.C.; Mbadinga, S.M.; Zhou, L.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. High Frequency of Thermodesulfovibrio spp. and Anaerolineaceae in Association with Methanculleus spp. In a Long-term Incubation of n-alkanes-degrading Methanogenic Enrichment Culture. Front. Microbiol. 2016, 7, 1431. [Google Scholar] [CrossRef]
- Ning, D.; Wang, Y.J.; Fan, Y.P.; Wang, J.J.; Van Nostrand, J.D.; Wu, L.Y.; Zhang, P.; Curtis, D.J.; Tian, R.M.; Lui, L.; et al. Environmental Stress Mediates Groundwater Microbial Community Assembly. Nat. Microbiol. 2024, 9, 490–501. [Google Scholar] [CrossRef]
- Rojo, F. Degradation of Alkanes by Bacteria. Environ. Microbiol. 2009, 11, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Zhu, Z.W.; Song, X.; Cai, Q.H.; Chen, B.; Dong, G.H.; Ye, X.D. Microbial Eco-physiological Strategies for Salinity-mediated Crude Oil Biodegradation. Sci. Total Environ. 2020, 727, 138723. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, S.; Sorial, G.A.; Weaver, J.W. Dispersant Effectiveness on Oil Spills-Impact of Salinity. ICES J. Mar. Sci. 2006, 63, 1418–1430. [Google Scholar] [CrossRef]
- Tavassoli, T.; Mousavi, S.M.; Shojaosadati, S.A.; Salehizadeh, H. Asphaltene Biodegradation Using Microorganisms Isolated from Oil Samples. Fuel 2012, 93, 142–148. [Google Scholar] [CrossRef]
- Korenblum, E.; Souza, D.B.; Penna, M.; Seldin, L. Molecular Analysis of the Bacterial Communities in Crude Oil Samples from Two Brazilian Offshore Petroleum Platforms. Int. J. Microbiol. 2012, 2012, 156537. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Kim, M.; Zengler, K.; Chin, K.J.; Overholt, W.A.; Gieg, L.M.; Konstantinidis, K.T.; Kostka, J.E. Anaerobic Degradation of Hexadecane and Phenanthrene Coupled to Sulfate Reduction by Enriched Consortia from Northern Gulf of Mexio Seafloor Sediment. Sci. Rep. 2019, 9, 26567. [Google Scholar]
- Zhang, K.; Hu, Z.; Zeng, F.F.; Yang, X.J.; Wang, J.J.; Jing, R.; Zhang, H.N.; Li, Y.T.; Zhang, Z. Biodegradation of Petroleum Hydrocarbons and Changes in Microbial Community Structure in Sediment Under Nitrate-, Ferric-, Sulfate-reducing and Methanogenic Conditions. J. Environ. Manag. 2019, 249, 109425. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, O.N.; Izosimova, O.N.; Chernitsyna, S.M.; Ivanov, V.G.; Pogodaeva, T.V.; Khabuev, A.V.; Gorshkov, A.G.; Zemskaya, T.I. Anaerobic Oxidation of Petroleum Hydrocarbons in Enrichment Cultures from Sediments of the Gorevoy Utes Natural Oil Seep under Methanogenic and Sulfate-reducing Conditions. Microb. Ecol. 2022, 83, 899–915. [Google Scholar] [CrossRef]
- Ma, T.T.; Liu, L.Y.; Rui, J.P.; Yuan, Q.; Feng, D.S.; Zhou, Z.; Dai, L.R.; Zeng, W.Q.; Zhang, H.; Cheng, L. Coexistence and Competition of Sulfate-reducing and Methanogenic Populations in an Anaerobic Hexadecane-degrading Culture. Biotechnol. Biofuels 2017, 10, 207. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhang, K.; Liang, B.; Zhou, Z.C.; Yang, S.Z.; Li, W.; Hou, Z.W.; Wu, X.L.; Gu, J.D.; Mu, B.Z. Key Players in the Methanogenic Biodegradation of n-hexadecane Identified by DNA-Stable Isotope Probing. Int. Biodeterior. Biodegrad. 2019, 143, 104709. [Google Scholar] [CrossRef]
- Hasinger, M.; Scherr, K.E.; Lundaa, T.; Brauer, L.; Zach, C.; Loibner, A.P. Changes in Iso- and n-alkane Distribution During Biodegradation of Crude Oil Under Nitrate and Sulphate Reducing Conditions. J. Biotechnol. 2012, 157, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.B.; Lu, W.; Lv, W.F.; Song, X.M.; Nie, Y.; Wu, X.L. Metabolic Profiling of Petroleum-degrading Microbial Communities Incubated under High-pressure Conditions. Front. Microbiol. 2023, 14, 1305731. [Google Scholar] [CrossRef] [PubMed]
- Marietou, A.; Chastain, R.; Beulig, F.; Scoma, A.; Hazen, T.C.; Bartlett, D.H. The Effect of Hydrostatic Pressure on Enrichments of Hydrocarbon Degrading Microbes from the Gulf of Mexico Following the Deepwater Horizon Oil Spill. Front. Microbiol. 2018, 9, 808. [Google Scholar]
- Fasca, H.; Castilho, L.V.A.; Castilho, J.F.M.; Pasqualino, I.P.; Alvarez, V.M.; Azevedo Jurelevicius, D.; Seldin, L. Response of Marine Bacteria to Oil Contamination and to High Pressure and Low Temperature Deep Sea Conditions. MicrobiologyOpen 2018, 7, e00550. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.J.P.; Gontikaki, E.; Potts, L.D.; Shaw, S.; Gallego, A.; Anderson, J.A.; Witte, U. Pressure and Temperature Effects on Deep-sea Hydrocarbon-degrading Microbial Communities in Subarctic Sediments. MicrobiologyOpen 2019, 8, e00768. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, D.; Mochimaru, H.; Yoshioka, H.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Takeuchi, M.; Kamagata, Y. Evidence for Syntrophic Acetate Oxidation Coupled to Hydrogenotrophic Methanogenesis in the High-temperature Petroleum Reservoir of Yabase Oil Field (Japan). Environ. Microbiol. 2010, 13, 1995–2006. [Google Scholar] [CrossRef]
- Suda, K.; Ikarashi, M.; Tamaki, H.; Tamazawa, S.; Sakata, S.; Haruo, M.; Kamagata, Y.; Kaneko, M.; Ujiie, T.; Shinotsuka, Y.; et al. Methanogenic Crude Oil Degradation Induced by an Exogenous Microbial Community and Nutrient Injections. J. Pet. Sci. Eng. 2021, 201, 108458. [Google Scholar] [CrossRef]
- Barbato, M.; Scoma, A. Mild Hydrostatic-pressure (15 MPa) Affects the Assembly, But Not the Growth, of Oil-degrading Coastal Microbial Communities Tested under Limiting Conditions (5 °C, no added nutrients). FEMS Microbiol. Ecol. 2020, 96, fiaa160. [Google Scholar] [CrossRef]
- Crescenzo, C.D.; Marzocchella, A.; Karatza, D.; Chianese, S.; Musmarra, D. Autogenerative High-pressure Anaerobic Digestion Modelling of Volatile Fatty Acids: Effect of Pressure Variation and Substrate Composition on Volumetric Mass Transfer Coefficients, Kinetic Parameters, and Process Performance. Fuel 2024, 358, 130144. [Google Scholar] [CrossRef]
- Merkle, W.; Baer, K.; Lindner, J.; Zielonka, S.; Ortloff, F.; Graf, F.; Kolb, T.; Jungbluth, T.; Lemmer, A. Influence of Pressures Up to 50 Bar on Two-stage Anaerobic Digestion. Bioresour. Technol. 2017, 232, 72–78. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M. Perfromance Evaluation of Pressurized Anaerobic Digestion (PDA) of Raw Compost Leachate. Fermentation 2022, 8, 15. [Google Scholar] [CrossRef]
- Jones, D.M.; Head, I.M.; Gray, N.D.; Adams, J.J.; Rowan, A.K.; Aitken, C.M.; Bennett, B.; Huang, H.; Brown, A.; Bowler, B.F.J.; et al. Crude-oil Biodegradation via Methanogenesis in Subsurface Petroleum Reservoirs. Nature 2008, 451, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Milkov, A.V. Worldwide Distribution and Significance of Secondary Microbial Methane Formed During Petroleum Biodegradation in Conventional Reservoirs. Org. Geochem. 2011, 42, 184–207. [Google Scholar] [CrossRef]
- Xue, J.L.; Yu, Y.; Bai, Y.; Wang, L.P.; Wu, Y.N. Marine Oil-degrading Microorganisms and Biodegradation Process of Petroleum Hydrocarbon in Marine Environments: A Review. Curr. Microbiol. 2015, 71, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shi, S.; Li, Q.; Chen, J.; Zhang, H.; Lu, Y. Progressive Degradation of Crude Oil n-alkanes Coupled to Methane Production under Mesophilic and Thermophilic Conditions. PLoS ONE 2014, 9, e113253. [Google Scholar] [CrossRef] [PubMed]
- Ehmedan, S.S.; Ibrahim, M.K.; Azzam, A.M.; Hamedo, H.A.; Saeed, A.M. Acceleration the Bacterial Biodegradation of Crude Oil Pollution Sing Fe2O3 and ZnO Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100613. [Google Scholar]
- Harayama, S.; Kishira, H.; Kasai, Y.; Shutsubo, K. Petroleum Biodegradation in Marine Environments. J. Mol. Microbiol. Biotechnol. 1999, 1, 63–70. [Google Scholar] [PubMed]
- Chuah, L.F.; Chew, K.W.; Bokhari, A.; Mubashir, M.; Show, P.L. Biodegradation of Crude Oil in Seawater by Using a Consortium of Symbiotic Bacteria. Environ. Res. 2022, 213, 113721. [Google Scholar] [CrossRef]
- Head, I.M.; Jones, D.M.; Larter, S.R. Biological Activity in the Deep Subsurface and the Origin of Heavy Oil. Nature 2003, 426, 344–352. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Kang, Y.; Hu, Z.; Liang, S.; Xie, H.J.; Ngo, H.H.; Zhang, J. Removal Pathways of Benzofluoranthene in a Constructed Wetland Amended with Metallic Ions Embedded Carbon. Bioresour. Technol. 2020, 311, 123481. [Google Scholar] [CrossRef]
- Han, X.K.; Wang, F.W.; Zhang, D.J.; Feng, T.; Zhang, L.L. Nitrate-assisted Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) in the Water-level-fluctuation Zone of the Three Gorges Reservoir, China: Insights from in Situ Microbial Interaction Analyses and a Microcosmic Experiment. Environ. Pollut. 2021, 268, 115693. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.T.; Wang, H.; Rogers, M.J.; Guo, H.J.; He, J.Z. Exploration of the Biotransformation of Phenanthrene Degradation Coupled with Methanogensis by Metabolites and Enzyme Analyses. Environ. Pollut. 2022, 293, 1184491. [Google Scholar] [CrossRef]
- Bianco, F.; Monteverde, G.; Race, M.; Papirio, S.; Esposito, G. Comparing Performances, Costs and Energy Balance of Ex Situ Remediation Processes for PAH-contaminated Marine Sediments. Environ. Sci. Pollut. Res. 2020, 27, 19363–19374. [Google Scholar] [CrossRef] [PubMed]
- Dhar, K.; Subashchandrabose, S.R.; Venkateswarlu, K.; Krishnan, K.; Megharaj, M. Anaerobic Microbial Degradation of Polycyclic Aromatic Hydrocarbons: A Comprehensive Review. In Reviews of Environmental Contanination and Toxicology; De Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 251, pp. 25–108. [Google Scholar]
- Mu, J.; Chen, Y.; Song, Z.; Liu, M.; Zhu, B.K.; Tao, H.C.; Bao, M.T.; Chen, Q.G. Effect of Terminal Electron Acceptors on the Anaerobic Biodegradation of PAHs in Marine Sediments. J. Hazard. Mater. 2022, 438, 129569. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Xu, P.; Hu, H.Y.; Tang, H.Z. Anaerobic Biodegradtion of Polycyclic Aromatic Hydrocarbons. ACS Earth Space Chem. 2023, 7, 823–837. [Google Scholar] [CrossRef]
- Ji, J.H.; Liu, Y.F.; Zhou, L.; Mbadinga, S.M.; Pan, P.; Chen, J.; Liu, J.F.; Yang, S.Z.; Sand, W.F.; Gu, J.D.; et al. Methanogenic Degradation of Long n-Alkanes Requires Fumarate-dependent Activation. Appl. Environ. Microbiol. 2019, 85, e00985-19. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Liu, Y.F.; Zhou, L.; Irfan, M.; Mbadinga, S.M.; Pan, P.; Chen, J.; Liu, J.F.; Yang, S.Z.; Sand, W.; et al. Methanogenic Biodegradation of C13 and C14 n-alkanes Activated by Addition to Fumarate. Int. Biodeterior. Biodegrad. 2020, 153, 104994. [Google Scholar] [CrossRef]
- Ji, J.H.; Zhou, L.; Mbadinga, S.M.; Irfan, M.; Liu, Y.F.; Pan, P.; Qi, Z.Z.; Chen, J.; Liu, J.F.; Yang, S.Z.; et al. Methanogenic Biodegradation of C9 to C12 n-alkanes Initiated by Smithella via Fumarate Addition Mechanism. AMB Express 2020, 10, 23. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, L.; Liu, Y.F.; Hou, Z.W.; Li, W.; Mbadainga, S.M.; Zhou, J.; Yang, T.; Liu, J.F.; Yang, S.Z.; et al. Synthesis and Mass Spectra of Rearrangement Bio-signature Metabolites of Anaerobic Alkane Degradation via Fumarate Addition. Anal. Biochem. 2020, 600, 113746. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.Y.; Hu, C.X.; Yao, S.; Shi, L.; Niu, T.; Li, X.; Tong, L.H.; Zhang, T.; Xia, W.J. CO2 Improves the Anaerobic Biodegradation Intensity and Selectivity of Heterocyclic Hydrocarbons in Heavy Oil. Environ. Res. 2023, 224, 115541. [Google Scholar] [CrossRef]
- Panda, S.K.; Andersson, J.T.; Schrader, W. Mass-spectrometric Analysis of Complex Volatile and Nonvolatile Crude Oil Components: A Challenge. Anal. Bioanal. Chem. 2007, 389, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- SY/T 5119-2016; Analysis Method for Family Composition of Rock Extracts and Crude Oil. National Energy Administration: Beijing, China, 2016.
- SY/T 5779-2008; Analytical and Method of Hydrocarbons in Petroleum and Sediment by Gas Chromatography. National Development and Reform Commission: Beijing, China, 2008.
- Navas-Cáceres, O.D.; Parada, M.; Zafra, G. Development of a Highly Tolerant Bacterial Consortium for Asphaltene Biodegradation in Soils. Environ. Sci. Pollut. Res. 2023, 30, 123439–123451. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, N.; Choure, K.; Pandey, P. Biodegradation of Asphaltene by Lipopeptide-biosurfactant Producing Hydrocarbonoclastic, Crude Oil Degrading Bacillus spp. Bioresour. Technol. 2023, 382, 129198. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Chi, C.Q.; Nie, Y.; Tang, Y.Q.; Tan, Y.; Wu, G.; Wu, X.L. Degradation of Petroleum Hydrocarbons(C6–C40) and Crude oil by Novel Dietzia Strain. Bioresour. Technol. 2011, 102, 7755–7761. [Google Scholar] [CrossRef]
- Bihari, Z.; Szvetnik, A.; Szabo, Z.; Blastyak, A.; Zombori, Z.; Balazs, M. Functional Analysis of Long-chain n-alkane Degradation by Dietzia spp. FEMS Microbiol. Lett. 2011, 316, 100–107. [Google Scholar] [CrossRef]
- Weid, I.; Marques, J.M.; Cunha, C.D.; Lippi, R.K.; Santos, S.; Rosado, A.S.; Lins, U.; Seldin, L. Identification and Biodegradation Potential of a Novel Strain of Dietzia cinnamea Isolated from a Petroleum Contaminated Tropical Soil. Syst. Appl. Microbiol. 2007, 30, 331–339. [Google Scholar] [CrossRef]
- Venil, C.K.; Malathi, M.; Devi, P.R. Characterization of Dietzia maris AURCCBT01 from Oil-contaminated Soil for Biodegradation of Crude Oil. 3 Biotech 2021, 11, 291. [Google Scholar] [CrossRef]
- Raja Abd Rahman, R.N.Z.; Latip, W.; Adlan, N.A.; Sabri, S.; Mohamad Ali, M.S. Bacteria Consortia Enhanced Hydrocarbon Degradation of Waxy Crude Oil. Arch. Microbiol. 2022, 204, 701. [Google Scholar]
- Tang, F.; Zhang, H.; Cheng, H.; Wang, Y.R.; Liu, Q.Y.; Zhao, C.C.; Gu, Y.Y.; Wang, J.G. New Insights of Crude Oil Biodegradation Construction by Microbial Consortium B10: Responded Substrates, Genomics, Biodegradation Mechanism and Pathways. Chem. Eng. J. 2023, 478, 147143. [Google Scholar] [CrossRef]
- Baltaci, M.O.; Omeroglu, M.A.; Ozkan, H.; Taskin, M.; Adiguzel, A. Enhanced Biodegradation of Crude Oil Contamination by Indigenous Bacterial Consortium under Real Conditions. Biocatal. Biotransform. 2024, 42, 56–67. [Google Scholar] [CrossRef]
- Adlan, N.A.; Sabri, S.; Masomian, M.; Mohamad Ali, M.S.; Raja Abd Rahman, R.N.Z. Microbial Biodegradation of Paraffin Wax in Malaysian Crude Oil Mediated by Degradative Enzymes. Front. Microbiol. 2020, 11, 565608. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Nie, Y.; Chen, X.; Xu, J.; Ji, Z.; Song, W.; Wei, X.; Song, X.; Wu, X.-L. Biodegradation of Crude Oil by Nitrate-Reducing, Sulfate-Reducing, and Methanogenic Microbial Communities under High-Pressure Conditions. Microorganisms 2024, 12, 1543. https://doi.org/10.3390/microorganisms12081543
Wang L, Nie Y, Chen X, Xu J, Ji Z, Song W, Wei X, Song X, Wu X-L. Biodegradation of Crude Oil by Nitrate-Reducing, Sulfate-Reducing, and Methanogenic Microbial Communities under High-Pressure Conditions. Microorganisms. 2024; 12(8):1543. https://doi.org/10.3390/microorganisms12081543
Chicago/Turabian StyleWang, Lu, Yong Nie, Xinglong Chen, Jinbo Xu, Zemin Ji, Wenfeng Song, Xiaofang Wei, Xinmin Song, and Xiao-Lei Wu. 2024. "Biodegradation of Crude Oil by Nitrate-Reducing, Sulfate-Reducing, and Methanogenic Microbial Communities under High-Pressure Conditions" Microorganisms 12, no. 8: 1543. https://doi.org/10.3390/microorganisms12081543
APA StyleWang, L., Nie, Y., Chen, X., Xu, J., Ji, Z., Song, W., Wei, X., Song, X., & Wu, X.-L. (2024). Biodegradation of Crude Oil by Nitrate-Reducing, Sulfate-Reducing, and Methanogenic Microbial Communities under High-Pressure Conditions. Microorganisms, 12(8), 1543. https://doi.org/10.3390/microorganisms12081543







