Phenanthrene-Degrading and Nickel-Resistant Neorhizobium Strain Isolated from Hydrocarbon-Contaminated Rhizosphere of Medicago sativa L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Species Identification
2.3. Cultural, Morphological, Physiological, and Biochemical Characteristics
2.4. DNA Extraction
2.5. DNA Library Preparation
2.6. Whole-Genome Sequencing, Assembly and Annotation
2.7. 16S rRNA Gene Amplification and Sequencing
2.8. Nickel Resistance
2.9. Degradation of Phenanthrene in the Presence of Nickel
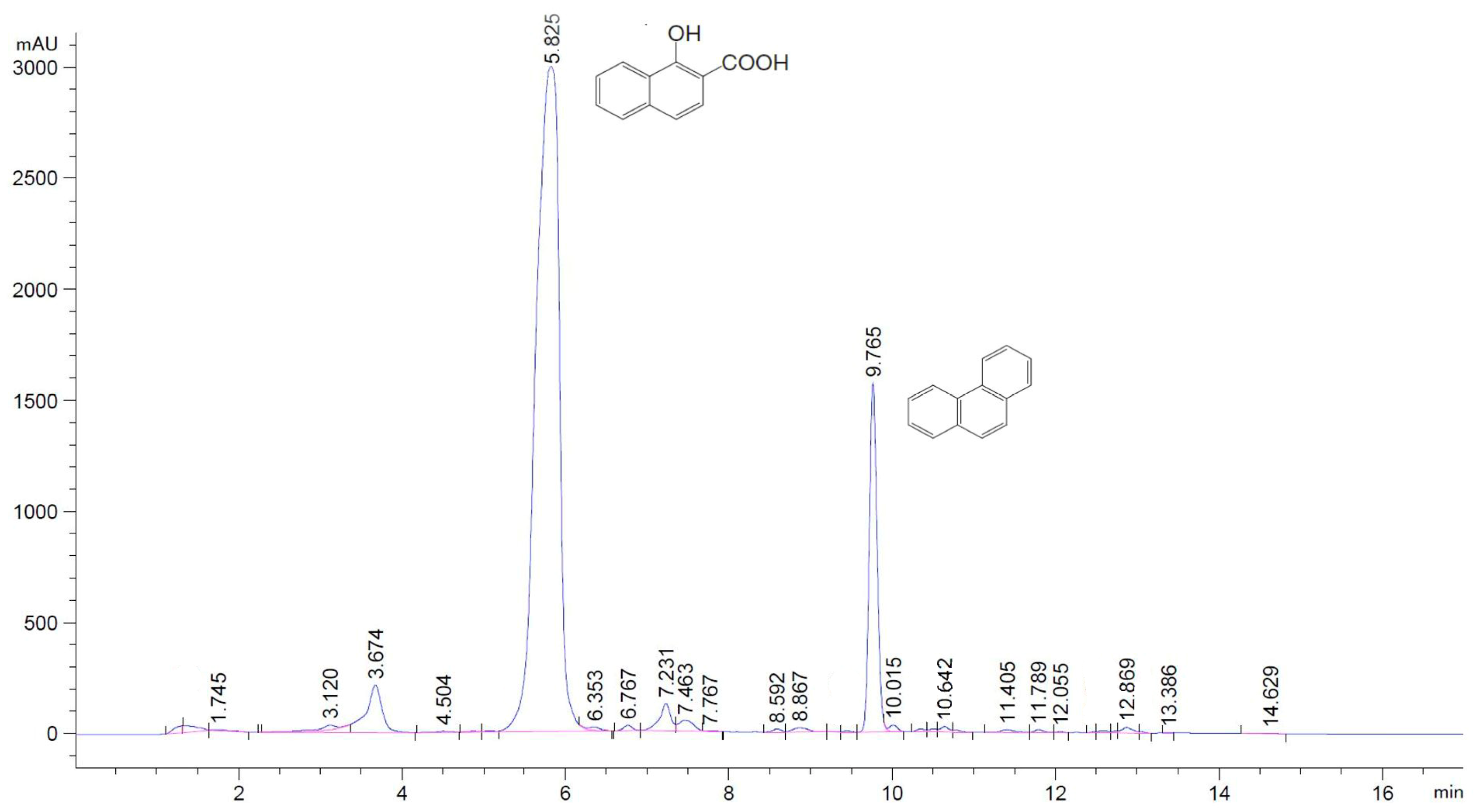
2.10. HPLC Analyses of Phenanthrene and Its Metabolites
2.11. Enzyme Assays
2.12. Statistics
3. Results
3.1. Taxonomic Characteristics of Microorganisms
3.1.1. 16S rRNA Gene Sequence Analysis
3.1.2. Whole-Genome-Based Taxonomic Analysis
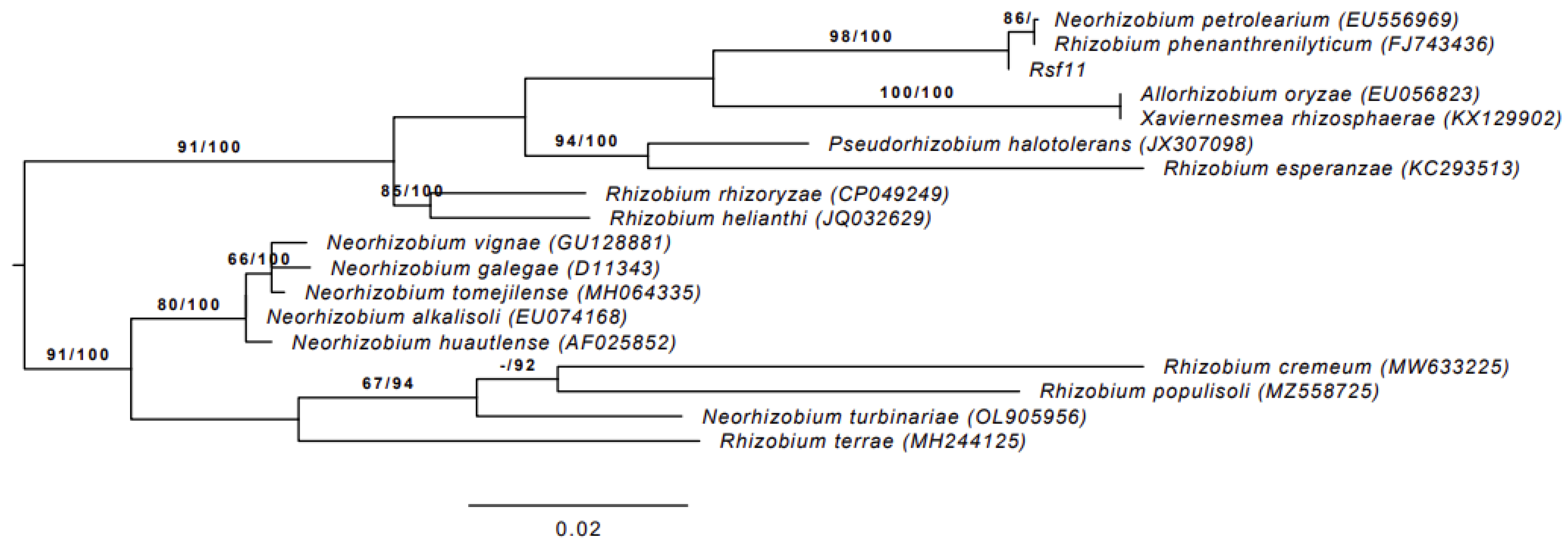
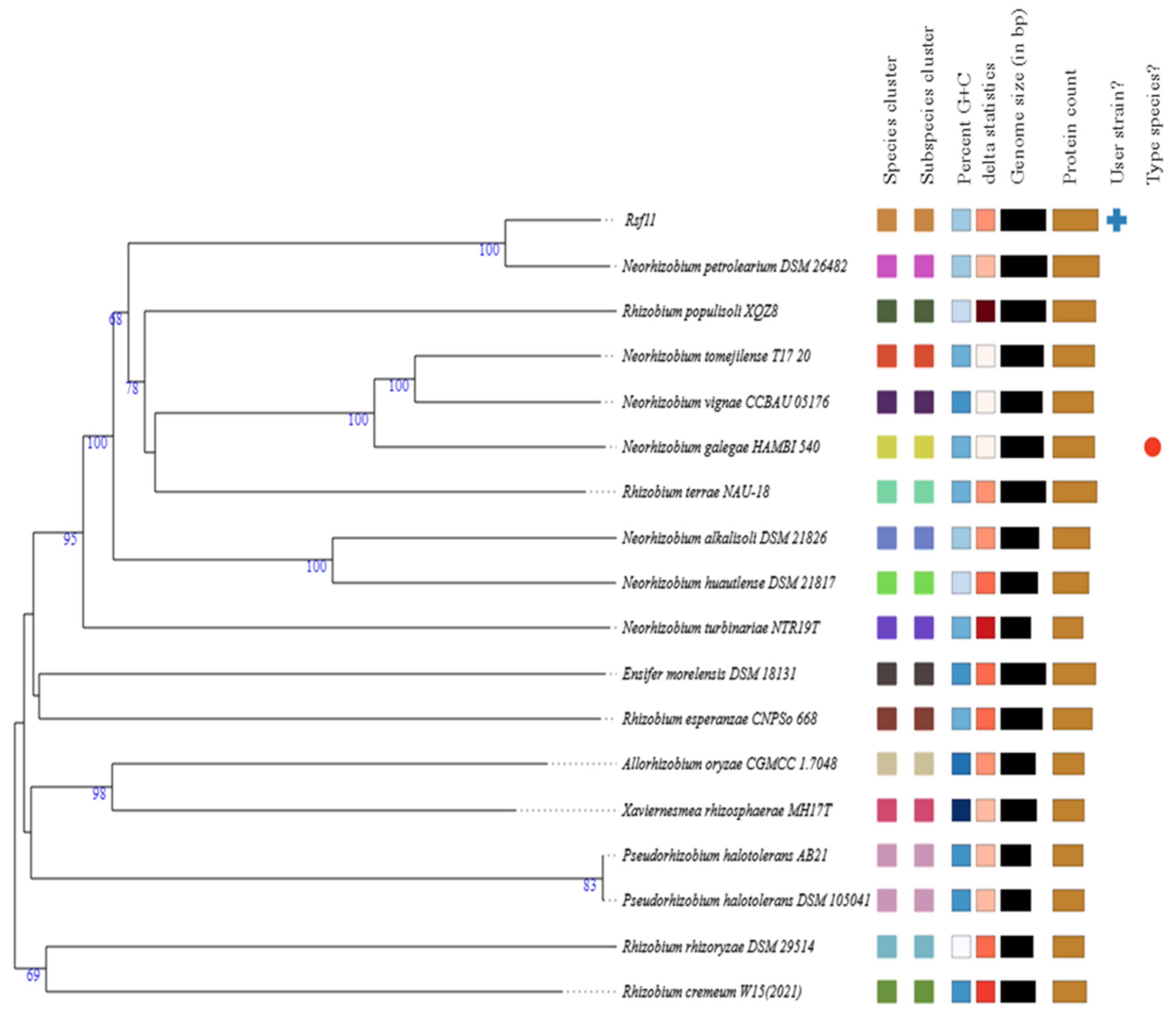
3.1.3. Cultural, Morphological, Physiological, and Biochemical Characteristics
3.1.4. Differential Characteristics
3.1.5. Description of N. phenanthreniclasticum sp. nov.
3.2. Remediating and Plant-Growth-Promoting Potential of Rsf11
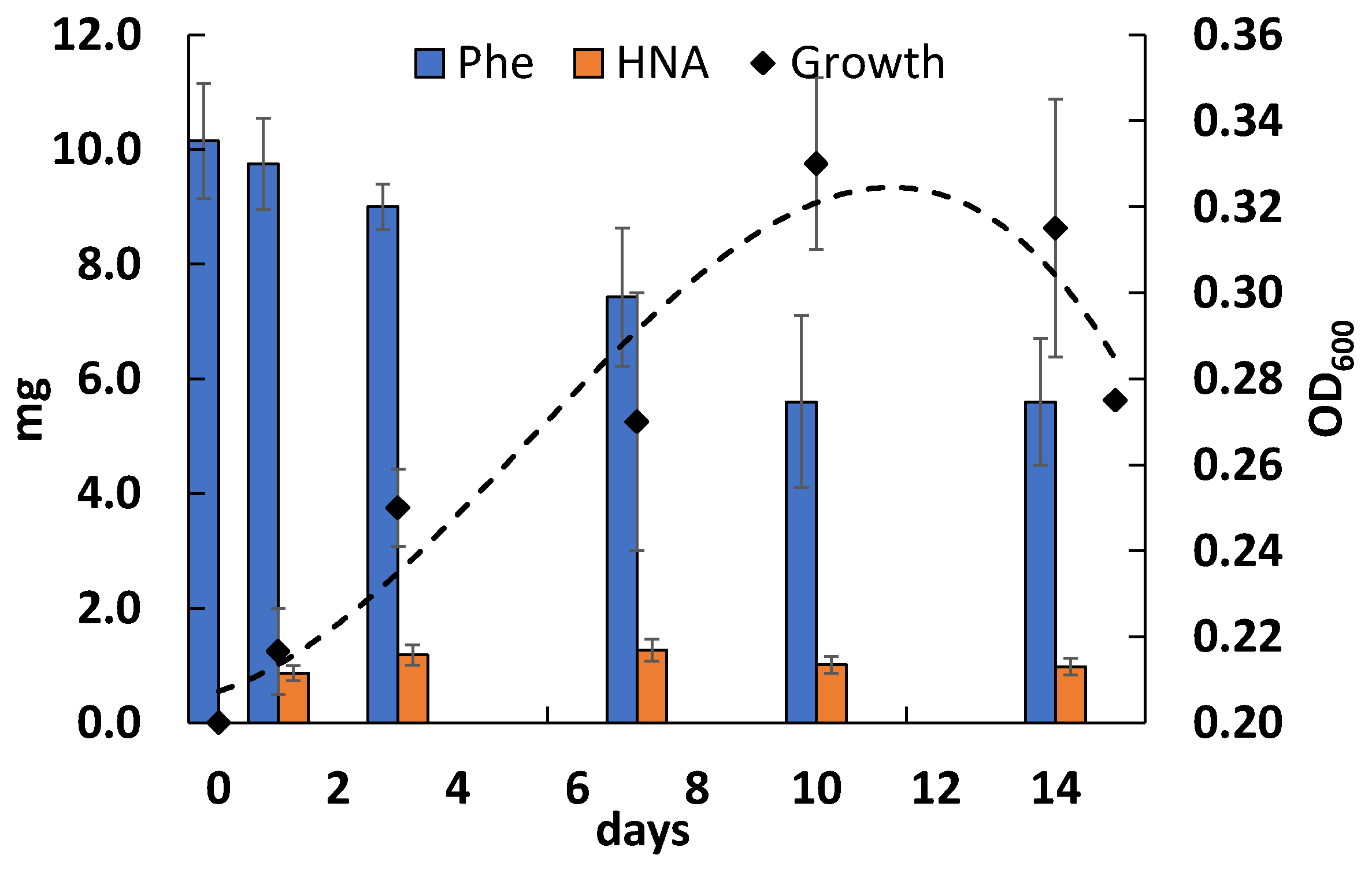
3.3. Phenanthrene Degradation
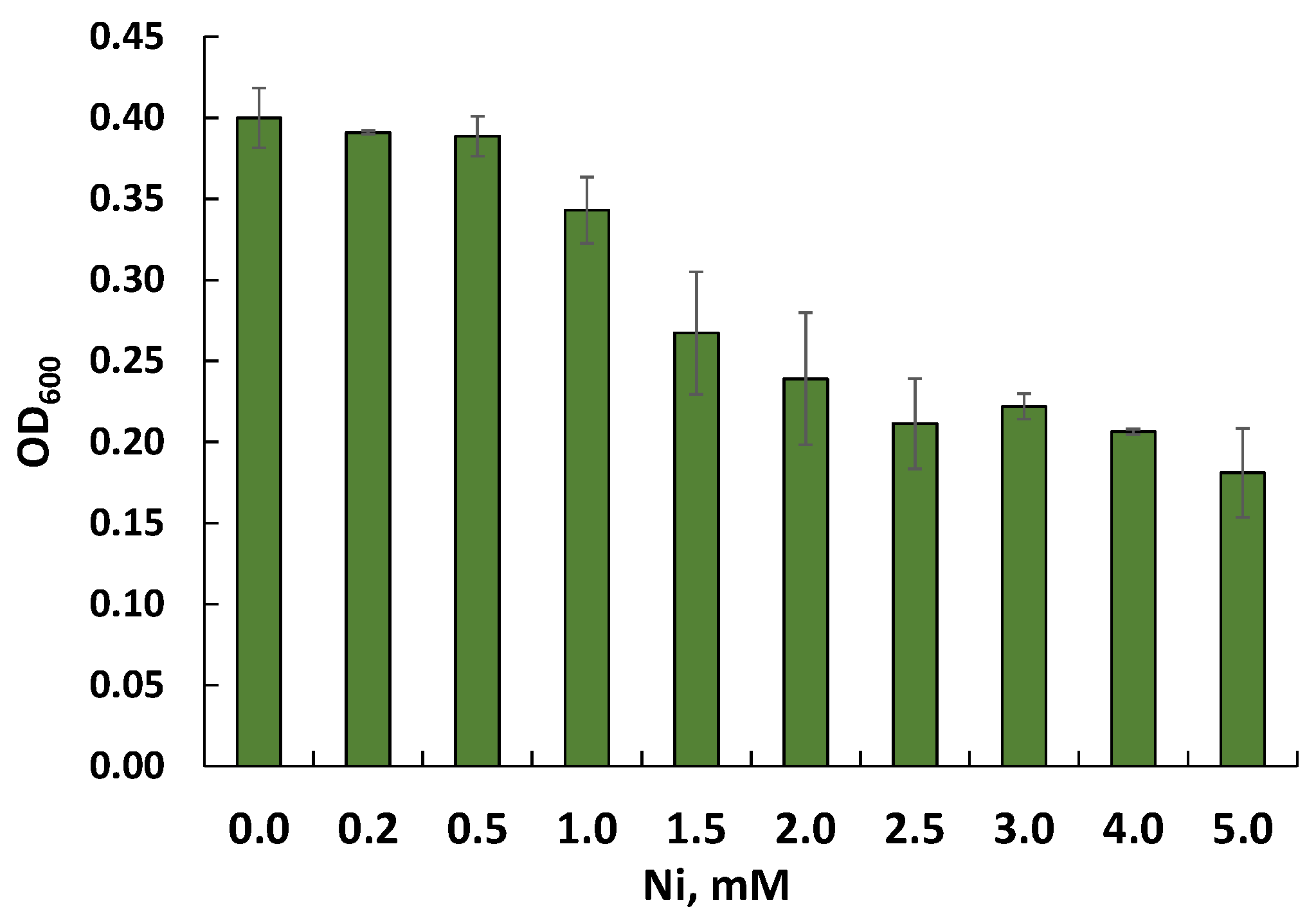
3.4. Nickel Resistance
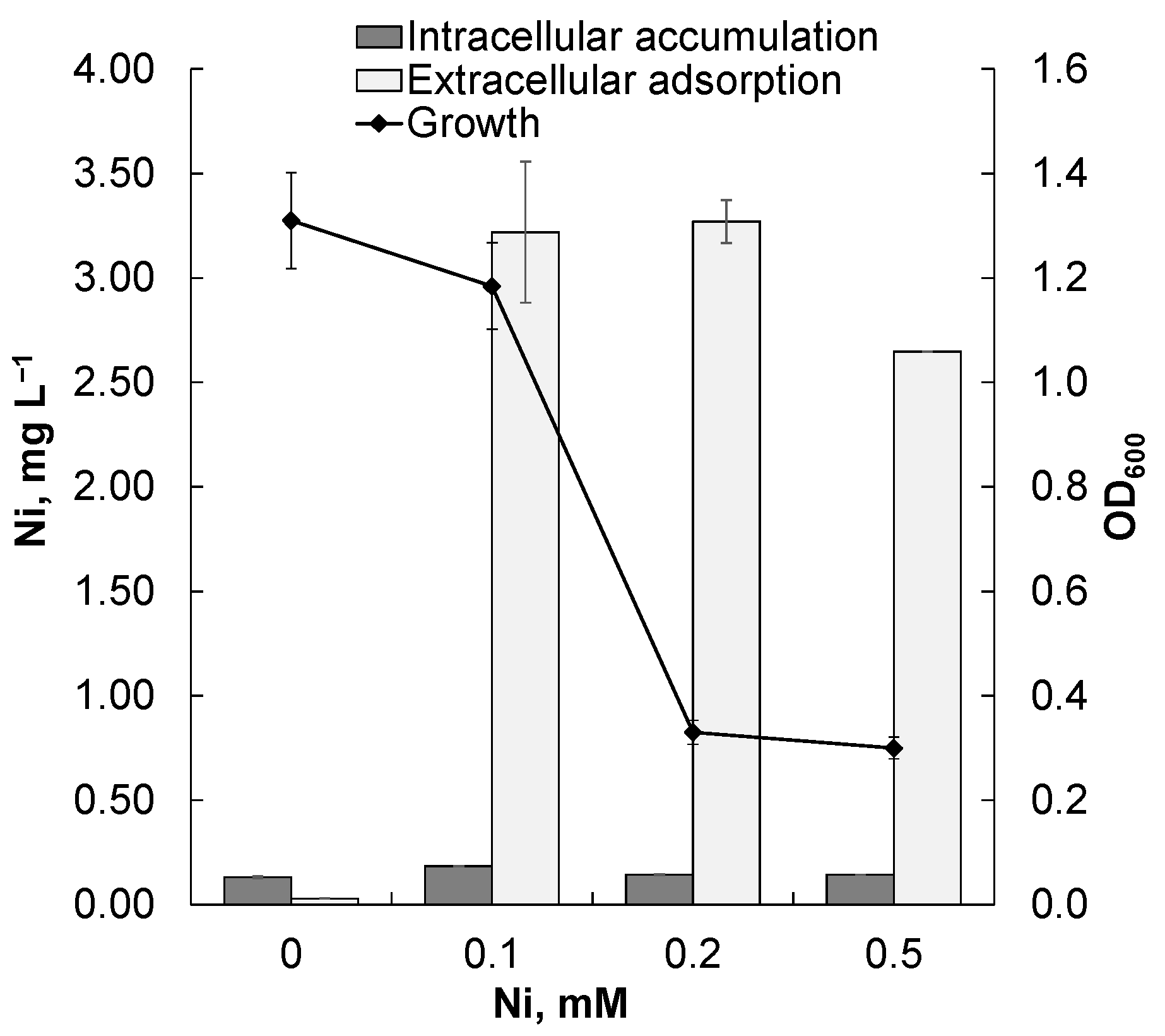
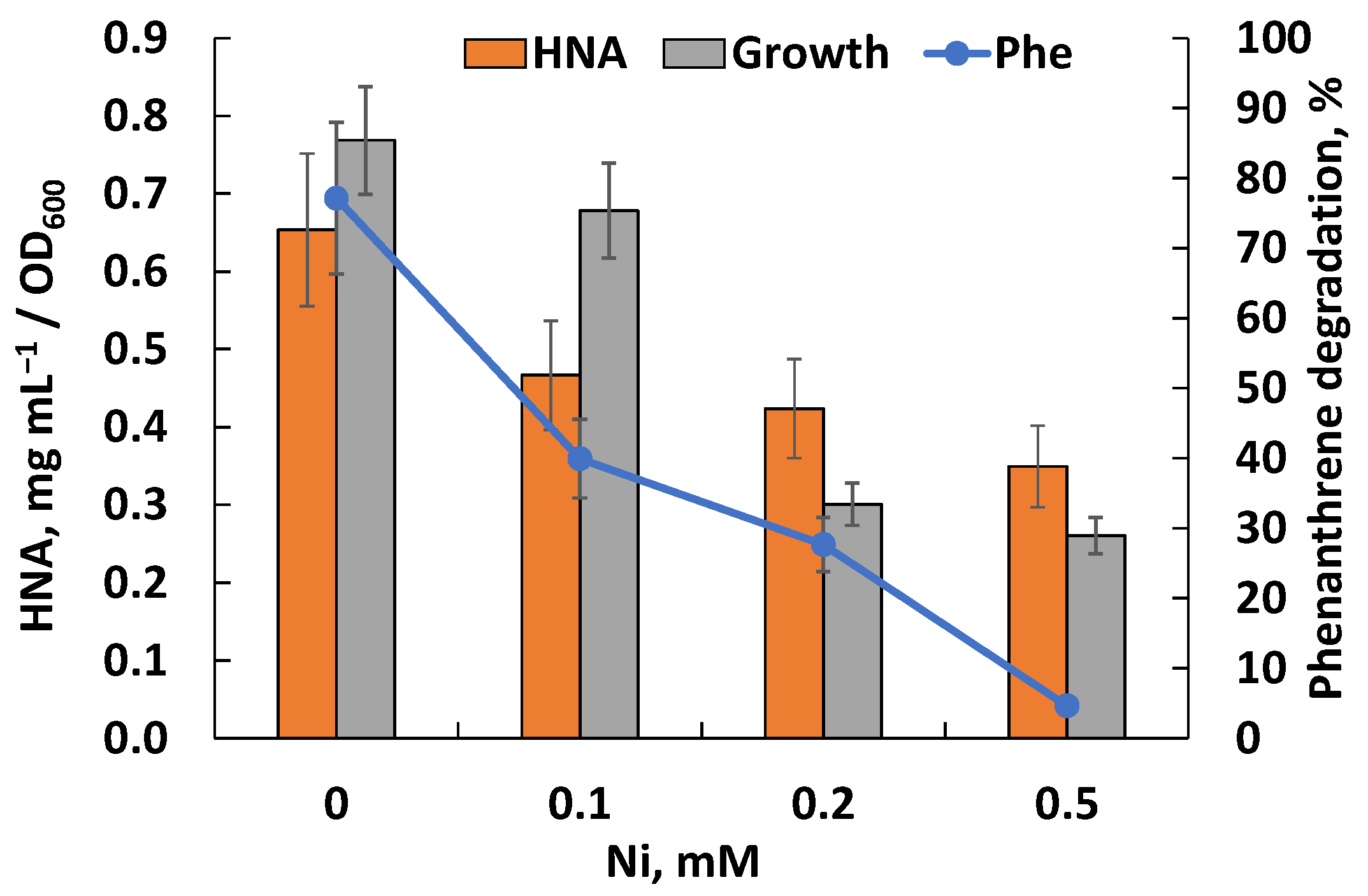
3.5. Effect of Nickel Ions on Phenanthrene Degradation
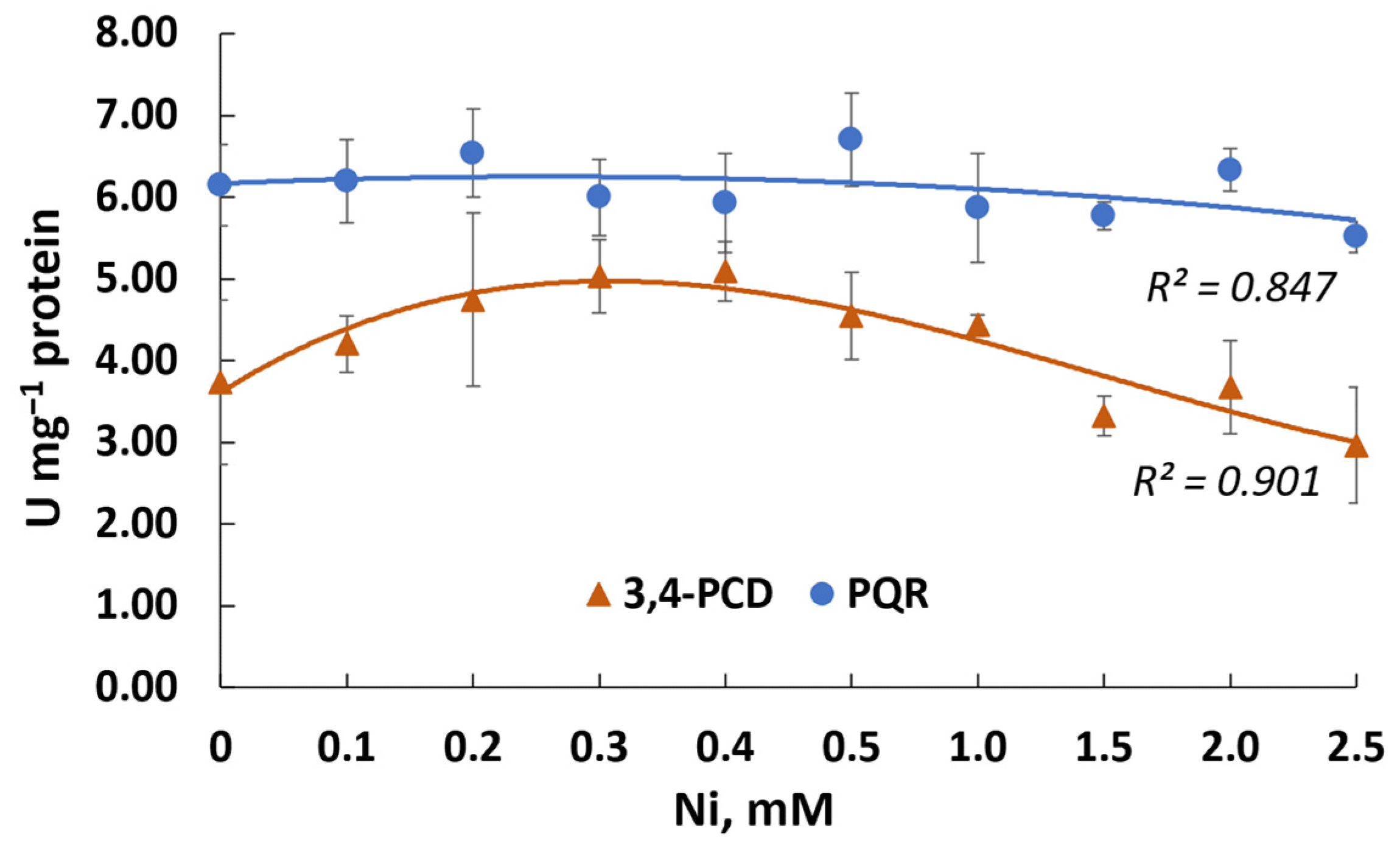
3.6. Genetic Potential of PAH Transformation and Nickel Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tilley, S.K.; Fry, R.C. Priority environmental contaminants: Understanding their sources of exposure, biological mechanisms, and impacts on health. In Systems Biology in Toxicology and Environmental Health; Fry, R.C., Ed.; Academic Press: New York, NY, USA, 2015; pp. 117–169. [Google Scholar]
- GN 2.1.7.2041-06; Predel’no Dopustimye Koncentracii (PDK) Himicheskih Veshchestv v Pochve: Gigienicheskie Normativy—M.: Federal’nyj Centr Gigieny i Epidemiologii Rospotrebnadzora. Maximum Permissible Concentrations (MPCs) of Chemicals in the Soil: Moscow, Russia, 2006; 15p. Available online: https://files.stroyinf.ru/Data2/1/4293850/4293850511.pdf (accessed on 5 July 2024). (In Russian)
- U.S. EPA. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAH). U.S. Environmental Protection Agency, Office of Research and Development, Office of Health and Environmental Assessment: Washington, DC, USA, 1993. [Google Scholar]
- Merhaby, D.; Rabodonirina, S.; Net, S.; Ouddane, B.; Halwani, J. Overview of sediments pollution by PAHs and PCBs in mediterranean basin: Transport, fate, occurrence, and distribution. Mar. Pollut. Bull. 2019, 149, 110646. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, S.; Bao, H.; Chen, D.; Wang, C.; Li, B.; Tong, G.; Yuan, Y.; Xu, B. Improving risk management by using the spatial interaction relationship of heavy metals and PAHs in urban soil. J. Hazard. Mater. 2019, 364, 108–116. [Google Scholar] [CrossRef]
- Mehr, M.R.; Keshavarzi, B.; Moore, F.; Fooladivanda, S.; Sorooshian, A.; Biester, H. Spatial distribution, environmental risk and sources of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in surface sediments-northwest of Persian Gulf. Cont. Shelf Res. 2020, 193, 104036. [Google Scholar] [CrossRef]
- Akinsete, S.J.; Olatimehin, A.D. Priority polycyclic aromatic hydrocarbons and heavy metals in urban roadside soils of heavy-traffic density areas in Ibadan, Nigeria: Levels, sources and health risk assessment. Environ. Forensics 2023, 25, 239–253. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, J.; Huang, Q.; Yang, Y. Occurrence of heavy metals and polycyclic aromatic hydrocarbons in typical used mineral oil from China: Implications for risk management. Environ. Sci. Pollut. Res. 2020, 27, 33065–33074. [Google Scholar] [CrossRef]
- Xiong, D.; Wang, C. Risk assessment of human exposure to heavy metals, polycyclic aromatic hydrocarbons, and radionuclides in oil-based drilling cutting residues used for roadbed materials in Chongqing, China. Environ. Sci. Pollut. Res. 2021, 28, 48171–48183. [Google Scholar] [CrossRef]
- Vishnyakov, A. Vanadium and nickel recovery from the products of heavy petroleum feedstock processing: A review. Metals 2023, 13, 1031. [Google Scholar] [CrossRef]
- Pampanin, D.M.; Sydnes, M.O. Polycyclic aromatic hydrocarbons a constituent of petroleum: Presence and influence in the aquatic environment. Hydrocarbon 2013, 5, 83–118. [Google Scholar]
- Barwise, A.J.G. Role of nickel and vanadium in petroleum classification. Energ. Fuel. 1990, 4, 647–652. [Google Scholar] [CrossRef]
- Ovesen, J.L.; Fan, Y.; Chen, J.; Medvedovic, M.; Xia, Y.; Puga, A. Long-term exposure to low-concentrations of Cr (VI) induce DNA damage and disrupt the transcriptional response to benzo [a] pyrene. Toxicology 2014, 316, 14–24. [Google Scholar] [CrossRef]
- Hu, S.; Gu, H.; Cu, C.; Ji, R. Toxicity of combined chromium (VI) and phenanthrene pollution on the seed germination, stem lengths, and fresh weights of higher plants. Environ. Sci. Pollut. Res. 2016, 23, 15227–15235. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, S.; Zou, Y.; Liu, J.; Zhao, R.; Yin, X.; Yind, X.; Zhang, H.; Li, Y. Quantitative evaluation of in-situ bioremediation of compound pollution of oil and heavy metal in sediments from the Bohai Sea, China. Mar. Pollut. Bull. 2020, 150, 110787. [Google Scholar] [CrossRef] [PubMed]
- Ibarrolaza, A.; Coppotelli, B.M.; Del Panno, M.T.; Donati, E.R.; Morelli, I.S. Application of the knowledge-based approach to strain selection for a bioaugmentation process of phenanthrene-and Cr(VI)-contaminated soil. J. Appl. Microbiol. 2011, 111, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, K.; Khan, A.; Jiang, Y.; Ling, Z.; Liu, P.; Chen, Y.; Tao, X.; Li, X. A novel Pseudomonas gessardii strain LZ-E simultaneously degrades naphthalene and reduces hexavalent chromium. Bioresour. Technol. 2016, 207, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, L.; Liu, X.; Ge, S.; Chen, T.; Hu, X. Removal of pyrene in simulated wetland by joint application of Kyllinga brevifolia Rottb. and immobilized microbes. Int. Biodeterior. Biodegrad. 2016, 114, 228–233. [Google Scholar] [CrossRef]
- Yin, C.; Xiong, W.; Qiu, H.; Peng, W.; Deng, Z.; Lin, S.; Liang, R. Characterization of the phenanthrene-degrading Sphingobium yanoikuyae SJTF8 in heavy metal co-existing liquid medium and analysis of its metabolic pathway. Microorganisms 2020, 8, 946. [Google Scholar] [CrossRef]
- Checcucci, A.; Bazzicalupo, M.; Mengoni, A. Exploiting nitrogen-fixing rhizobial symbionts genetic resources for improving phytoremediation of contaminated soils. In Enhancing Cleanup of Environmental Pollutants; Anjum, N.A., Gill, S.S., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 275–288. [Google Scholar]
- Fagorzi, C.; Checcucci, A.; DiCenzo, G.C.; Debiec-Andrzejewska, K.; Dziewit, L.; Pini, F.; Mengoni, A. Harnessing rhizobia to improve heavy-metal phytoremediation by legumes. Genes 2018, 9, 542. [Google Scholar] [CrossRef]
- Keum, Y.S.; Seo, J.S.; Hu, Y.; Li, Q.X. Degradation pathways of phenanthrene by Sinorhizobium sp. C4. Appl. Microbiol. Biotechnol. 2006, 71, 935–941. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, H.; Sui, X.; Ma, X.; Hong, Q.; Jiang, R. Rhizobium petrolearium sp. nov., isolated from oil-contaminated soil. Int. J. Syst. Evol. Microbiol. 2012, 62, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Muratova, A.; Pozdnyakova, N.; Makarov, O.; Baboshin, M.; Baskunov, B.; Myasoedova, N.; Golovleva, L.; Turkovskaya, O. Degradation of phenanthrene by the rhizobacterium Ensifer meliloti. Biodegradation 2014, 25, 787–795. [Google Scholar] [CrossRef]
- Raina, V.; Nayak, T.; Ray, L.; Kumari, K.; Suar, M. A Polyphasic taxonomic approach for designation and description of novel microbial species. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 137–152. [Google Scholar]
- Meier-Kolthoff, J.P.; Sardà Carbasse, J.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acid Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete genome sequence of DSM 30083T, the type strain (U5/41T) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genom. Sci. 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.P.; Moret, B.M.E.; Stamatakis, A. How many bootstrap replicates are necessary? J. Comput. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0 b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Meier-Kolthoff, J.P.; Göker, M.; Spröer, C.; Klenk, H.-P. When should a DDH experiment be mandatory in microbial taxonomy? Arch. Microbiol. 2013, 195, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Mash, A.P. Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef]
- Farris, J.S. Estimating phylogenetic trees from distance matrices. Am. Nat. 1972, 106, 645–667. [Google Scholar] [CrossRef]
- Kreft, L.; Botzki, A.; Coppens, F.; Vandepoele, K.; Van Bel, M. PhyD3: A phylogenetic tree viewer with extended hyloXML support for functional genomics data visualization. Bioinformatics 2017, 33, 2946–2947. [Google Scholar] [CrossRef]
- Garrity, G.M.; Bell, J.A.; Lilburn, T. Class I. Alphaproteobacteria class. nov. In Bergey’s Manual of Systematic Bacteriology, Vol. 2. The Protobacteria, Part C, the Alpha-, Beta-, Delta-, and Epsilonproteobacteria, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: Boston, MA, USA, 2005; pp. 1–574. [Google Scholar]
- Gerhardt, P.; Murray, R.G.E.; Costilow, R.N.; Nester, E.W.; Wood, W.A.; Krieg, N.R.; Phillips, G.B. (Eds.) Manual of Methods for General Bacteriology; American Society for Microbiology: Washington, DC, USA, 1981. [Google Scholar]
- Tindall, B.J.; Sikorski, J.; Smibert, R.A.; Krieg, N.R. Phenotypic characterization and the principles of comparative systematics. In Methods for General and Molecular Microbiology; Reddy, C.A., Beveridge, T.J., Breznak, J.A., Marzluf, G., Eds.; American Society for Microbiology Press: Washington, DC, USA, 2007; pp. 330–393. [Google Scholar]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. iMeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 127, 2068–2069. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- Golubev, S.N.; Muratova, A.Y.; Panchenko, L.V.; Shchyogolev, S.Y.; Turkovskaya, O.V. Mycolicibacterium sp. strain PAM1, an alfalfa rhizosphere dweller, catabolizes PAHs and promotes partner-plant growth. Microbiol. Res. 2021, 253, 126885. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Guo, C.L.; Lu, G.N.; Yi, X.Y.; Zhu, L.D.; Dang, Z. Bioaccumulation characterization of cadmium by growing Bacillus cereus RC-1 and its mechanism. Chemosphere 2014, 109, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Moody, J.D.; Freeman, J.P.; Brezna, B.; Engesser, K.-H.; Cerniglia, C.E. Evidence for the existence of PAH-quinone reductase and catechol-O-methyltransferase in Mycobacterium vanbaalenii PYR-1. J. Ind. Microbiol. Biotechnol. 2004, 31, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Bubinas, A.; Giedraitytė, G.; Kalėdienė, L.; Nivinskiene, O.; Butkiene, R. Degradation of naphthalene by thermophilic bacteria via a pathway, through protocatechuic acid. Open Life Sci. 2008, 3, 61–68. [Google Scholar] [CrossRef]
- Kasai, D.; Fujinami, T.; Abe, T.; Mase, K.; Katayama, Y.; Fukuda, M.; Masai, E. Uncovering the protocatechuate 2, 3-cleavage pathway genes. J. Bacteriol. 2009, 191, 6758–6768. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, S.G.; Yang, K.; Davies, J. Characterization of the protocatechuic acid catabolic gene cluster from Streptomyces sp. strain 2065. Appl. Environ. Microbiol. 2000, 66, 1499–1508. [Google Scholar] [PubMed]
- Ono, K.; Nozaki, M.; Hayaishi, O. Purification and some properties of protocatechuate 4, 5-dioxygenase. Biochim. Biophys. Acta 1970, 220, 224–238. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović, N.; Fagorzi, C.; Mengoni, A.; Lassalle, F.; DiCenzo, G.C. Taxonomy of Rhizobiaceae revisited: Proposal of a new framework for genus delimitation. Int. J. Syst. Evol. Microbiol. 2022, 72, 005243. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Klenk, H.P.; Göker, M. Taxonomic use of DNA G+C content and DNA-DNA hybridization in the genomic age. Int. J. Syst. Evol. Microbiol. 2014, 64, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Xue, H.; Piao, C.; Jiang, N.; Li, Y. Phylogenomic reappraisal of the family Rhizobiaceae at the genus and species levels, including the description of Ectorhizobium quercum gen. nov., sp. nov. Front. Microbiol. 2023, 3, 1207256. [Google Scholar] [CrossRef]
- Ren, D.W.; Chen, W.F.; Sui, X.H.; Wang, E.T.; Chen, W.X. Rhizobium vignae sp. nov., a symbiotic bacterium isolated from multiple legume species. Int. J. Syst. Evol. Microbiol. 2011, 61, 580–586. [Google Scholar] [CrossRef]
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef]
- Lindstrom, K. Rhizobium galegae, a new species of legume root nodule bacteria. Int. J. Syst. Bacteriol. 1989, 39, 365–367. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Willems, A.; Nesme, X.; de Lajudie, P.; Lindström, K. Revised phylogeny of Rhizobiaceae: Proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst. Appl. Microbiol. 2015, 38, 84–90. [Google Scholar] [CrossRef]
- Ruan, Z.P.; Cao, W.M.; Zhang, X.; Liu, J.T.; Zhu, J.C.; Hu, B.; Jiang, J.D. Rhizobium terrae sp. nov., Isolated from an Oil-Contaminated Soil in China. Curr. Microbiol. 2020, 77, 1117–1124. [Google Scholar] [CrossRef]
- Soenens, A.; Gomila, M.; Imperial, J. Neorhizobium tomejilense sp. nov., first non-symbiotic Neorhizobium species isolated from a dryland agricultural soil in southern Spain. Syst. Appl. Microbiol. 2019, 42, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Li Lu, Y.; Chen, W.F.; Li Han, L.; Wang, E.T.; Chen, W.X. Rhizobium alkalisoli sp. nov., isolated from Caragana intermedia growing in saline-alkaline soils in the north of China. Int. J. Syst. Evol. Microbiol. 2009, 59, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; van Berkum, P.; Beyene, D.; Sui, X.H.; Dorado, O.; Chen, W.X.; Martínez-Romero, E. Rhizobium huautlense sp. nov., a symbiont of Sesbania herbacea that has a close phylogenetic relationship with Rhizobium galegae. Int. J. Syst. Bacteriol. 1998, 48, 687–699. [Google Scholar] [CrossRef]
- Shen, L.; Liu, J.J.; Liu, P.X.; An, M.M.; He, X.W.; Zhao, G.Z. A non-symbiotic novel species, Rhizobium populisoli sp. nov., isolated from rhizosphere soil of Populus popularis. Arch. Microbiol. 2022, 204, 50. [Google Scholar] [CrossRef] [PubMed]
- Golubev, S.N.; Schelud’ko, A.V.; Muratova, A.Y.; Makarov, O.E.; Turkovskaya, O.V. Assessing the potential of rhizobacteria to survive under phenanthrene pollution. Water Air Soil Pollut. 2009, 198, 5–16. [Google Scholar] [CrossRef]
- Patterson, S.J.; Hunt, E.C.; Tucker, K.B.E. The determination of commonly used non-ionic detergents in sewage effluents and river waters by a thin-layer chromatographic method. J. Proc. Inst. Sew. Purif. 1966, 2, 191–198. [Google Scholar]
- Panchenko, L.V.; Turkovskaya, O.V.; Shub, G.M. Isolation and characterization of microorganisms capable of degrading nonionic surfactants. Microbiology 1997, 66, 178–183. [Google Scholar]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo[a]pyrene. Int. Biodeterior. Biodegrad. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Shahsavari, E.; Schwarz, A.; Aburto-Medina, A.; Ball, A.S. Biological degradation of polycyclic aromatic compounds (PAHs) in soil: A current perspective. Curr. Pollut. Rep. 2019, 5, 84–92. [Google Scholar] [CrossRef]
- Muratova, A.; Turkovskaya, O. Association of plants and microorganisms for degradation of polycyclic aromatic hydrocarbons. Chapter 18. In Advances in Microbe-Assisted Phytoremediation of Polluted Sites; Bauddh, K., Ma, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 435–476. [Google Scholar]
- Raheem, A.S.A.; Hentati, D.; Bahzad, D.; Abed, R.M.; Ismail, W. Biocatalytic upgrading of unconventional crude oil using oilfield-inhabiting bacterial consortia. Int. Biodeterior. Biodegrad. 2022, 174, 105468. [Google Scholar]
- Shi, S.; Zhang, H.; Zhang, S.; Yi, L.; Yeerkenbieke, G.; Lu, X. Degradation of benzo[a]pyrene and 2,2′,4,4′-tebrabrominated diphenyl ether in cultures originated from an agricultural soil. Toxics 2024, 12, 33. [Google Scholar] [CrossRef]
- Yessica, G.P.; Alejandro, A.; Ronald, F.C.; José, A.J.; Esperanza, M.R.; Samuel, C.S.J.; Remedios, M.L.M.; Ormeño-Orrillo, E. Tolerance, growth and degradation of phenanthrene and benzo[a]pyrene by Rhizobium tropici CIAT 899 in liquid culture medium. Appl. Soil Ecol. 2013, 63, 105–111. [Google Scholar] [CrossRef]
- Huang, X.; Shi, J.; Cui, C.; Yin, H.; Zhang, R.; Ma, X.; Zhang, X. Biodegradation of phenanthrene by Rhizobium petrolearium SL-1. J. Appl. Microbiol. 2016, 121, 1616–1626. [Google Scholar] [CrossRef]
- Keum, Y.S.; Seo, J.S.; Li, Q.X.; Kim, J.H. Comparative metabolomic analysis of Sinorhizobium sp. C4 during the degradation of phenanthrene. Appl. Microbiol. Biotechnol. 2008, 80, 863–872. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, J.; Yan, Q.; Li, S.; Hong, Q. Rhizobium phenanthrenilyticum sp. nov., a novel phenanthrene-degrading bacterium isolated from a petroleum residue treatment system. J. Gen. Appl. Microbiol. 2011, 57, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Osadebe, A.U.; Chukwu, C.B. Degradation properties of Rhizobium petrolearium on different concentrations of crude oil and its derivative fuels. Environ. Exp. Biol. 2023, 21, 83–92. [Google Scholar] [CrossRef]
- Muratova, A.; Dubrovskaya, E.; Golubev, S.; Grinev, V.; Chernyshova, M.; Turkovskaya, O. The coupling of the plant and microbial catabolisms of phenanthrene in the rhizosphere of Medicago sativa. J. Plant Physiol. 2015, 188, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Engesser, K.H.; Cerniglia, C.E. Two polycyclic aromatic hydrocarbon o-quinone reductases from a pyrene-degrading Mycobacterium. Arch. Biochem. Biophys. 2003, 416, 209–217. [Google Scholar] [CrossRef]
- Mohapatra, B.; Phale, P.S. Microbial degradation of naphthalene and substituted naphthalenes: Metabolic diversity and genomic insight for bioremediation. Front. Bioeng. Biotechnol. 2021, 9, 602445. [Google Scholar] [CrossRef]
- Allocati, N.; Federici, L.; Masulli, M.; Di Ilio, C. Glutathione transferases in bacteria. FEBS J. 2009, 276, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Zafra, G.; Taylor, T.D.; Absalón, A.E.; Cortés-Espinosa, D.V. Comparative metagenomic analysis of PAH degradation in soil by a mixed microbial consortium. J. Hazard. Mater. 2016, 318, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.A.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; pp. 125–167. [Google Scholar]
- Pishchik, V.; Mirskaya, G.; Chizhevskaya, E.; Chebotar, V.; Chakrabarty, D. Nickel stress-tolerance in plant-bacterial associations. PeerJ 2021, 9, e12230. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Phys. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Liu, H.; Cui, Y.; Zhou, J.; Penttinen, P.; Liu, J.; Zeng, L.; Chen, Q.; Gu, Y.; Zou, L.; Zhao, K.; et al. Nickel mine soil is a potential source for soybean plant growth promoting and heavy metal tolerant rhizobia. PeerJ 2022, 10, e13215. [Google Scholar] [CrossRef] [PubMed]
- Edulamudi, P.; Antony Masilamani, A.J.; Vanga, U.R.; Divi, V.R.S.G.; Konada, V.M. Nickel tolerance and biosorption potential of rhizobia associated with horse gram [Macrotyloma uniflorum (Lam.) Verdc.]. Int. J. Phytoremediation 2021, 23, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Milicic, B.; Delic, D.; Stajkovic, O.; Rasulic, N.; Kuzmanovic, D.; Josic, D. Effects of heavy metals on rhizobial growth. Rom. Biotechnol. Lett. 2006, 11, 2995–3003. [Google Scholar]
- Chaintreuil, C.; Rigault, F.; Moulin, L.; Jaffre, T.; Fardoux, J.; Giraud, E.; Dreyfus, B.; Xavier, X. Nickel resistance determinants in Bradyrhizobium strains from nodules of the endemic new Caledonia legume Serianthes calycina. Appl. Environ. Microbiol. 2007, 73, 8018–8022. [Google Scholar] [CrossRef] [PubMed]
- Jobby, R.; Jha, P.; Desai, N. Sinorhizobium, a potential organism for bioremediation of nickel. Int. J. Adv. Res. 2015, 3, 706–717. [Google Scholar]
- Rahal, S.; Menaa, B.; Chekireb, D. Characterization of heavy metal-resistant rhizobia and non-rhizobia isolated from root nodules of Trifolium sp. in a lead and zinc mining area. 20 March 2023, PREPRINT (Version 1). Research Square. Available online: https://www.researchsquare.com/article/rs-2692408/v1 (accessed on 4 July 2024).
- Li, Y.; Pang, H.D.; He, L.Y.; Wang, Q.; Sheng, X.F. Cd immobilization and reduced tissue Cd accumulation of rice (Oryza sativa wuyun-23) in the presence of heavy metal-resistant bacteria. Ecotoxicol. Environ. Saf. 2017, 138, 56–63. [Google Scholar] [CrossRef]
- Klonowska, A.; Chaintreuil, C.; Tisseyre, P.; Miche, L.; Melkonian, R.; Ducousso, M.; Laguerre, G.; Brunel, B.; Moulin, L. Biodiversity of Mimosa pudica rhizobial symbionts (Cupriavidus taiwanensis, Rhizobium mesoamericanum) in New Caedonia and their adaptation to heavy metal-rich soils. FEMS Microbiol. Ecol. 2012, 81, 618–635. [Google Scholar] [CrossRef] [PubMed]
- von Rozycki, T.; Nies, D.H. Cupriavidus metallidurans: Evolution of a metal-resistant bacterium. Antonie Leeuwenhoek 2009, 96, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Castellane, T.C.L.; Lemos, M.V.F.; de Macedo Lemos, E.G. Evaluation of the biotechnological potential of Rhizobium tropici strains for exopolysaccharide production. Carbohydr. Polym. 2014, 111, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, R.; Srivastava, N.; Kumari, S.; Pinnaka, A.K.; Choudhury, A.R. Isolation of an exopolysaccharide from a novel marine bacterium Neorhizobium urealyticum sp. nov. and its utilization in nanoemulsion formation for encapsulation and stabilization of astaxanthin. LWT 2021, 151, 112105. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (eps) resulting from activated sludge according to their type: Soluble or bound. Process Biochem. 2006, 41, 815–823. [Google Scholar] [CrossRef]
- Volbeda, A.; Fontecilla-Camps, J.C. Nickel-Binding Proteins, Overview. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Mikolay, A.; Nies, D.H. The ABC-transporter AtmA is involved in nickel and cobalt resistance of Cupriavidus metallidurans strain CH34. Antonie Leeuwenhoek 2009, 96, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Munkelt, D.; Grass, G.; Nies, D.H. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 2004, 186, 8036–8043. [Google Scholar] [CrossRef] [PubMed]
- Iwig, J.S.; Rowe, J.L.; Chivers, P.T. Nickel homeostasis in Escherichia coli—The rcnR-rcnA efflux pathway and its linkage to NikR function. Mol. Microbiol. 2006, 62, 252–262. [Google Scholar] [CrossRef]
- Cordeiro, A.B.; Ribeiro, R.A.; Helene, L.C.F.; Hungria, M. Rhizobium esperanzae sp. nov., a N2-fixing root symbiont of Phaseolus vulgaris from Mexican soils. Int. J. Syst. Evol. Microbiol. 2017, 67, 3937–3945. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Österman, J.; Wahlberg, N.; Nesme, X.; Lavire, C.; Vial, L.; Paulin, L.; de Lajudie, P.; Lindström, K. Phylogeny of the Rhizobium-Allorhizobium-Agrobacterium clade supports the delineation of Neorhizobium gen. nov. Syst. Appl. Microbiol. 2014, 37, 208–215. [Google Scholar] [CrossRef]
- Sun, H.; Miao, Z.; Liu, S.; Liu, X.; Chen, B.; Liao, B.; Xiao, B. Neorhizobium turbinariae sp. nov., a coral-beneficial bacterium isolated from Turbinaria peltata. Int. J. Syst. Evol. Microbiol. 2023, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Tan, Z.Y.; Willems, A.; Fernández-López, M.; Reinhold-Hurek, B.; Martínez-Romero, E. Sinorhizobium morelense sp. nov., a Leucaena leucocephala-associated bacterium that is highly resistant to multiple antibiotics. Int. J. Syst. Evol. Microbiol. 2002, 52, 1687–1693. [Google Scholar] [PubMed]
- Wang, Y.C.; Wang, F.; Hou, B.C.; Wang, E.T.; Chen, W.F.; Sui, X.H.; Chen, W.X.; Li, Y.; Zhang, Y.B. Proposal of Ensifer psoraleae sp. nov., Ensifer sesbaniae sp. nov., Ensifer morelense comb. nov. and Ensifer americanum comb. nov. Syst. Appl. Microbiol. 2013, 36, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Liu, J.; Chen, D.; Wang, S.; Xu, L.; Ma, K.; Zhang, X.; Sun, L.; Wang, W. Rhizobium cremeum sp. nov., isolated from sewage and capable of acquisition of heavy metal and aromatic compounds resistance genes. Syst. Appl. Microbiol. 2022, 45, 126322. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, F.; Dastgheib, S.M.M.; Zhao, F.J.; Zhang, J.; Verbarg, S.; Frühling, A.; Brinkmann, H.; Osborne, T.H.; Sikorski, J.; Balloux, F.; et al. Phylogenomics reveals the basis of adaptation of Pseudorhizobium species to extreme environments and supports a taxonomic revision of the genus. Syst. Appl. Microbiol. 2021, 44, 126165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tang, X.; Sheirdil, R.A.; Sun, L.; Ma, X.T. Rhizobium rhizoryzae sp. nov., isolated from rice roots. Int. J. Syst. Evol. Microbiol. 2014, 64, 1373–1377. [Google Scholar] [CrossRef]
- Peng, G.; Yuan, Q.; Li, H.; Zhang, W.; Tan, Z. Rhizobium oryzae sp. nov., isolated from the wild rice Oryza alta. Int. J. Syst. Evol. Microbiol. 2008, 58, 2158–2163. [Google Scholar] [CrossRef]


| Type Strain | dDDH (%) 1 | OrthoANIu(%) | G+C Content Difference (%) | |||||
|---|---|---|---|---|---|---|---|---|
| d0 | C.I. d0 | d4 | C.I. d4 | d6 | C.I. d6 | |||
| Neorhizobium petrolearium DSM 26482 | 78.2 | [74.2–81.7] | 66.7 | [63.7–69.5] | 78.8 | [75.3–81.8] | 95.92 | 0.06 |
| Neorhizobium vignae CCBAU 05176 | 33.2 | [29.8–36.7] | 24.7 | [22.4–27.2] | 30.1 | [27.2–33.2] | 82.01 | 1.02 |
| Neorhizobium galegae HAMBI 540 | 32.6 | [29.2–36.2] | 24.7 | [22.4–27.2] | 29.6 | [26.7–32.8] | 81.72 | 0.62 |
| Rhizobium terrae NAU-18 | 34.1 | [30.7–37.6] | 24.7 | [22.4–27.2] | 30.7 | [27.8–33.8] | 81.85 | 0.84 |
| Neorhizobium tomejilense T17 20 | 32.8 | [29.4–36.3] | 24.7 | [22.4–27.2] | 29.8 | [26.9–32.9] | 81.80 | 0.85 |
| Neorhizobium alkalisoli DSM 21826 | 30.5 | [27.1–34.1] | 23.7 | [21.4–26.2] | 27.8 | [24.9–30.9] | 81.20 | 0.3 |
| Neorhizobium huautlense DSM 21817 | 30.1 | [26.7–33.7] | 23.5 | [21.2–26.0] | 27.5 | [24.6–30.6] | 80.63 | 0.57 |
| Rhizobium populisoli XQZ8 | 27.7 | [24.3–31.3] | 23.0 | [20.7–25.5] | 25.6 | [22.7–28.7] | 80.33 | 0.54 |
| Xaviernesmea rhizosphaerae MH17T | 14.4 | [11.6–17.8] | 21.8 | [19.5–24.2] | 14.7 | [12.2–17.5] | 74.85 | 3.9 |
| Neorhizobium turbinariae NTR19T | 23.3 | [20.0–26.9] | 21.6 | [19.4–24.1] | 22.0 | [19.2–25.0] | 78.85 | 0.62 |
| Rhizobium cremeum W15(2021) | 16.2 | [13.2–19.7] | 21.2 | [19.0–23.7] | 16.2 | [13.6–19.1] | 76.03 | 1.04 |
| Allorhizobium oryzae CGMCC 1.7048 | 14.4 | [11.5–17.8] | 20.6 | [18.4–23.1] | 14.6 | [12.1–17.4] | 74.21 | 2.18 |
| Rhizobium esperanzae CNPSo 668 | 16.3 | [13.3–19.8] | 20.6 | [18.4–23.0] | 16.2 | [13.7–19.2] | 75.91 | 0.45 |
| Ensifer morelensis DSM 18131 | 15.0 | [12.1–18.4] | 20.4 | [18.2–22.8] | 15.1 | [12.6–18.0] | 74.64 | 1.25 |
| Pseudorhizobium halotolerans DSM 105041 | 18.1 | [15.0–21.7] | 20.3 | [18.1–22.7] | 17.7 | [15.1–20.7] | nd | 1.09 |
| Pseudorhizobium halotolerans AB21 | 18.1 | [15.0–21.7] | 20.3 | [18.1–22.7] | 17.7 | [15.1–20.7] | 76.65 | 1.08 |
| Rhizobium rhizoryzae DSM 29514 | 14.9 | [12.0–18.3] | 20.1 | [17.9–22.5] | 15.0 | [12.6–17.9] | 74.35 | 2.64 |
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Cell size, µm | 1.9 × 0.8 | 1.75 × 0.75 | 1.25 × 0.6 | 1.45 × 0.65 | 2.0 × 0.4 | ||||
| Halotolerance: | |||||||||
| 2% NaCl | − | + | + | − | + | + | |||
| 3% NaCl | − | − | + | ||||||
| Temperatures: | |||||||||
| 37 °C | + | + | + | − | − | + | |||
| 42 °C | + | − | + | + | − | ||||
| Production: | |||||||||
| phenylalanine deaminase | − | + | + | ||||||
| catalase | + | + | + | − | + | + | |||
| oxidase | + | + | + | + | − | + | + | ||
| urease | + | + | + | + | − | + | |||
| nitrate reductase | + | + | + | + | + | − | + | − | |
| nitrite reductase | + | − | + | ||||||
| indole | − | − | − | − | − | + | − | − | |
| Hydrolysis: | |||||||||
| arginine | − | − | + | − | |||||
| starch | − | − | |||||||
| esculin | + | + | + | + | − | ||||
| Assimilation: | |||||||||
| maltose | − | + | + | − | |||||
| rhamnose | + | + | + | + | − | ||||
| sucrose | − | + | + | + | − | − | |||
| fructose | + | + | − | + | + | + | |||
| mannitol | + | + | + | + | − | ||||
| inositol | − | − | + | ||||||
| sorbitol | − | + | + | − | + | + | − | − | |
| N-acetylglucosamine | + | + | − | − | |||||
| malate | − | − | + | + | |||||
| G+C content (%) | 60.6 | 60.5 | 61.6 | 61.2 | 61.4 | 61.4 | 60.3 | 60.0 | 60.1 |
| PGPR Potential | Remediation Potential | ||
|---|---|---|---|
| Nitrogen fixation | 0.08 ± 0.01 nmol C2H4 h−1 mL−1 | PAH degradation 1 | Phe, Ant, Flu |
| P-solubilization | − | Synthetic surfactant degradation 2 | − |
| Siderophore production | + | Oil degradation | − |
| IAA production | 3.3 ± 0.3 μg mL−1 | Heavy-metal resistance 3 | Ni2+ (1.5 mM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubev, S.; Rasterkovskaya, M.; Sungurtseva, I.; Burov, A.; Muratova, A. Phenanthrene-Degrading and Nickel-Resistant Neorhizobium Strain Isolated from Hydrocarbon-Contaminated Rhizosphere of Medicago sativa L. Microorganisms 2024, 12, 1586. https://doi.org/10.3390/microorganisms12081586
Golubev S, Rasterkovskaya M, Sungurtseva I, Burov A, Muratova A. Phenanthrene-Degrading and Nickel-Resistant Neorhizobium Strain Isolated from Hydrocarbon-Contaminated Rhizosphere of Medicago sativa L. Microorganisms. 2024; 12(8):1586. https://doi.org/10.3390/microorganisms12081586
Chicago/Turabian StyleGolubev, Sergey, Margarita Rasterkovskaya, Irina Sungurtseva, Andrey Burov, and Anna Muratova. 2024. "Phenanthrene-Degrading and Nickel-Resistant Neorhizobium Strain Isolated from Hydrocarbon-Contaminated Rhizosphere of Medicago sativa L." Microorganisms 12, no. 8: 1586. https://doi.org/10.3390/microorganisms12081586





